Published online Apr 21, 2021. doi: 10.3748/wjg.v27.i15.1655
Peer-review started: January 14, 2021
First decision: February 10, 2021
Revised: February 19, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: April 21, 2021
Gastrointestinal cytomegalovirus (CMV) disease occurs commonly in immunocompromised/immunodeficient patients with advanced human immunodeficiency virus infection, neoplasm, solid organ transplantation, hematopoietic stem cell transplantation, or treatment with immunosuppressants, but is rarely reported in association with measles infection.
We describe a case of extensive gastrointestinal CMV disease secondary to measles infection in a 9-mo-old boy who presented with persistent fever and bloody diarrhea. His condition was improved after ganciclovir treatment. Serological analysis of CMV showed negative immunoglobulin (Ig) M and positive IgG. Blood CMV-DNA was 9.26 × 103 copies/mL. The diagnosis of gastrointestinal CMV disease was confirmed by histopathological findings of intranuclear and intracytoplasmic inclusions and Owl’s eye inclusion. This case highlights the differential diagnosis and histopathological characteristics of gastrointestinal CMV infection and laboratory tests.
Extensive gastrointestinal CMV lesions can be induced by the immune suppression secondary to measles infection. Rational, fast, and effective laboratory examinations are essential for suspected patients.
Core Tip: We report a case of gastrointestinal cytomegalovirus (CMV) disease secondary to measles infection in a 9-mo-old Chinese boy who had extensive gastrointestinal lesions; the diagnosis was confirmed by histopathological analysis. His condition was improved by ganciclovir treatment. This case highlights the differential diagnosis and histopathological characteristics of gastrointestinal CMV infection and laboratory tests and sheds light on the difficulty in diagnosing gastrointestinal CMV disease due to its nonspecific clinical presentation and the weak diagnostic value of serologic antibody detection.
- Citation: Yang QH, Ma XP, Dai DL, Bai DM, Zou Y, Liu SX, Song JM. Gastrointestinal cytomegalovirus disease secondary to measles in an immunocompetent infant: A case report. World J Gastroenterol 2021; 27(15): 1655-1663
- URL: https://www.wjgnet.com/1007-9327/full/v27/i15/1655.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i15.1655
Cytomegalovirus (CMV) is an intracellular virus, and CMV infection of the gastrointestinal tract is commonly documented in immunocompromised/ immunodeficient patients. Measles infection induces immune suppression and is associated with an increased susceptibility to secondary infections[1], and this effect has typically been thought to last from a few weeks to a few months[2]. The Th2 response during convalescence of measles might inhibit Th1 responses, increasing susceptibility to other infections in children with measles[3]. The morbidity from diarrhea, one of the most common complications of measles, is reported to increase in the acute phase of measles[4]; however, gastrointestinal CMV infection is rarely reported as a causative etiology of persistent diarrhea secondary to measles infection.
CMV disease of the gastrointestinal tract is associated with significant mortality, mostly resulting from considerable bleeding and perforation when the patient is in an immunodepressed status[5]. However, it is not so common for the lesions to be very large. Limitations of the serologic assay of CMV and nonspecific clinical manifesta
A 9-mo-old Chinese boy was admitted to Shenzhen Children’s hospital because of a persistent high fever and diarrhea that lasted for 17 d.
After 3 d of fever, he had a rash extending from his face to his trunk and limbs, and 5 d later, he suffered watery and bloody diarrhea. Positive immunoglobulin (Ig) M on the 10th day of fever confirmed a diagnosis of measles. Peripheral white blood cells, neutrophils, and C-reactive protein were elevated. Many leukocytes were found in his stool. Cefoperazone-sulbactam was administered intravenously for 4 d before admission, but the frequency of diarrhea increased, along with abdominal distension.
The patient had two episodes of pneumonia 40 d before this admission.
The patient was born by cesarean section after full-term gestation, without complications during gestation or delivery. He had met physical and developmental milestones. Routine childhood immunizations were administered except for the measles vaccine.
On admission, the patient was weak but alert with a dehydrated appearance and typical measles skin (pigmentation and desquamation all over his body). The abdomen was soft and distended, with no tenderness or masses. The remainder of the physical examination was normal. Rapid intravenous rehydration was applied before further evaluation.
Serological analysis for CMV showed negative IgM and positive IgG. PCR blood assay showed an elevation of CMV-DNA. The other laboratory results are shown in Tables 1 and 2.
| Parameter | Reference range | 5 d before admission | 1 d before admission | On admission | Day 6 | Day 12 | Day 23 | Day 37 |
| Hemoglobin (g/L) | 110-160 | 120 | 115 | 79 | 91 | 73 | 126 | 69 |
| Erythrocytes (× 1012/L) | 3.5-5.5 | 4.70 | 4.56 | 2.93 | 3.21 | 2.50 | 3.93 | 2.34 |
| Leucocytes (× 109/L) | 5-12 | 14.96 | 9.36 | 17.87 | 8.37 | 20.42 | 38.68 | 10.35 |
| Neutrophils (%) | 50-70 | 54.9 | 32.6 | 55.1 | 62.7 | 61.6 | 80.4 | 59.9 |
| Lymphocytes (%) | 20-40 | 36.9 | 56.7 | 27 | 29.3 | 28.3 | 15.4 | 33.4 |
| Atypical lymphocytes (%) | 0-2 | 9 | 7 | 0 | 0 | 0 | ||
| Eosinophils (%) | 0.02-0.5 | 1.3 | 1.4 | 0.9 | 0.4 | 0 | 0 | 0 |
| Platelets (× 109/L) | 100-300 | 234 | 226 | 769 | 200 | 276 | 165 | 110 |
| Sedimentation rate (mm/h) | 0-15 | 78 | 1 | |||||
| C-reactive protein (mg/L) | 0-10 | 39.7 | 176 | 80 | 114 | 3.7 | 4.7 | |
| Procalcitonin (ng/L) | 0-0.5 | 31.87 | 0.77 | 0.31 | ||||
| Sodium (mmol/L) | 135-146 | 116.3 | 138.4 | 131.2 | 139.4 | 135 | ||
| Potassium (mmol/L) | 3.5-5.5 | 6.15 | 4.53 | 3.9 | 4.2 | 3.61 | ||
| Chloride (mmol/L) | 101-111 | 91.1 | 102.7 | 97.8 | 116.9 | 110.5 | ||
| Calcium (mmol/L) | 2.25-2.75 | 1.79 | 2.46 | 1.87 | 2.04 | 1.66 | ||
| Magnesium (mmol/L) | 0.7-1.15 | 0.96 | 0.61 | 0.53 | 0.35 | 0.38 | ||
| Albumin (g/L) | 35-55 | 25.8 | 37.3 | 25.9 | 15.5 | 20.6 | ||
| Globulin (g/L) | 20-30 | 28.1 | 17.6 | 19.3 | 13.2 | 5.9 | ||
| Aspartate amino transferase (IU/L) | 0-40 | 70 | 28 | 48 | 85 | 22 | ||
| Alanine amino transferase (IU/L) | 0-40 | 61 | 12 | 41 | 53 | 6 | ||
| Prothrombin time (s) | 9.3-12.9 | 17.4 | 19.6 | 12.5 | 11.4 | 16.2 | ||
| Activated partial thromboplastin time (s) | 26.1-40.7 | 34.5 | 57.8 | 38 | 39.5 | 55.2 | ||
| Fecal occult blood test | - | - | + | + | + | + | ||
| Fecal white blood cell | - | ++ | - | - | ++ | ++++ | ++ | - |
| Peripheral blood | Feces | |
| Day 1 | HBsAg (-); HBsAb (+); HBeAg (-); HBeAb (-); HBcAb (-); TP antibody (-); HIV antibody (-); HCV antibody (-); EBNA IgG (+); EBV-CA IgG (+); EBV-CA IgM (±); EBV-EA IgM (-); EBV DNA < 5.00 × 102 copies/mL (LD) | Norovirus (-); Rotavirus (-); Astrovirus (-); Enteral adenovirus (-); Shigella NA (-); Salmonella NA (-); Widal's test (-) |
| Day 6 | G test (-); EBV DNA < 5.00 × 102 copies/mL (LD) | Norovirus (-); Rotavirus (-); Astrovirus (-); Enteral adenovirus (-) |
| Day 12 | G test (-); Interferon-γ release assay of tuberculosis (-) | Clostridium difficile GDH (-); Clostridium difficile toxin A/B (-) |
| Day 16 | CMV-IgM 11.5 U/mL (ref 0-22); CMV-IgG 18.3 U/mL (ref 0-14); CMV DNA quantitation 9.26 × 103 copies/mL | Clostridium difficile GDH (-); Clostridium difficile toxin A/B (-) |
| Day 36 | CMV DNA quantitation < 5.00 × 102 copies/mL (LD); CMV-IgG 82.2 U/mL (ref 0-14) | |
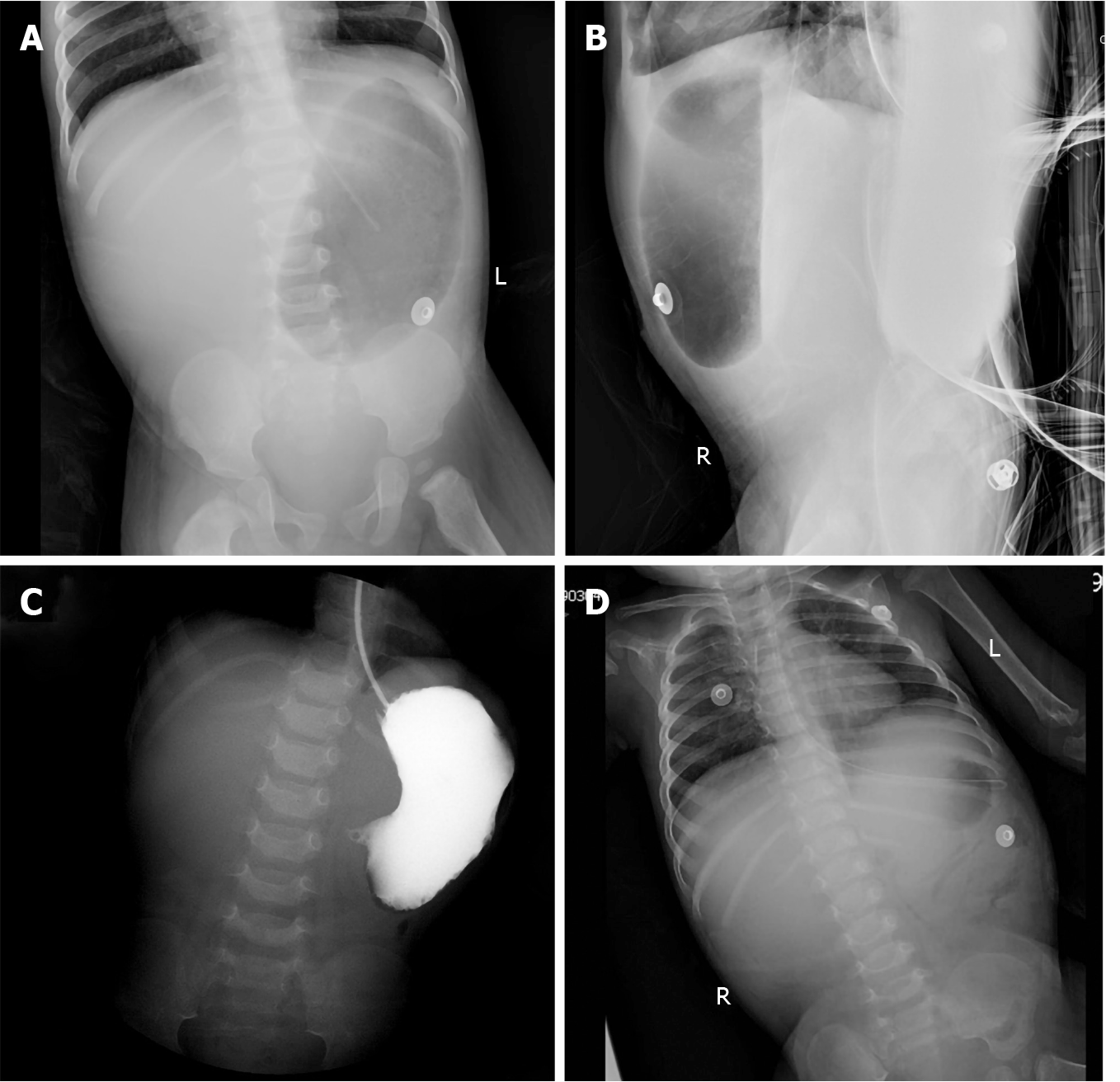
Abdominal radiography (Figure 1) revealed upper gastrointestinal obstruction, while abdominal ultrasonography showed no obvious dilatation of the intestine or free effusion. Enhanced computed tomography demonstrated obstruction in the horizontal duodenum.
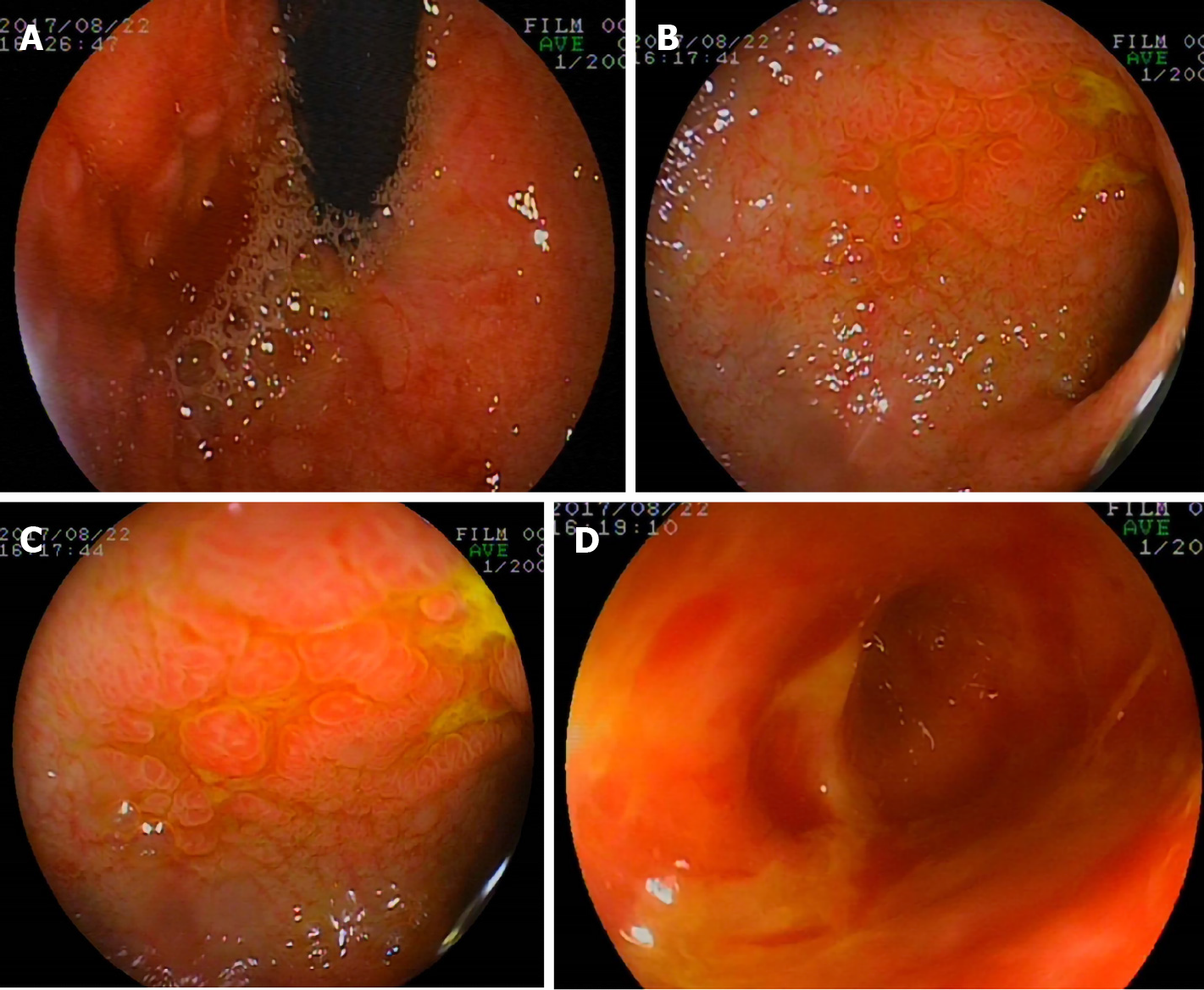
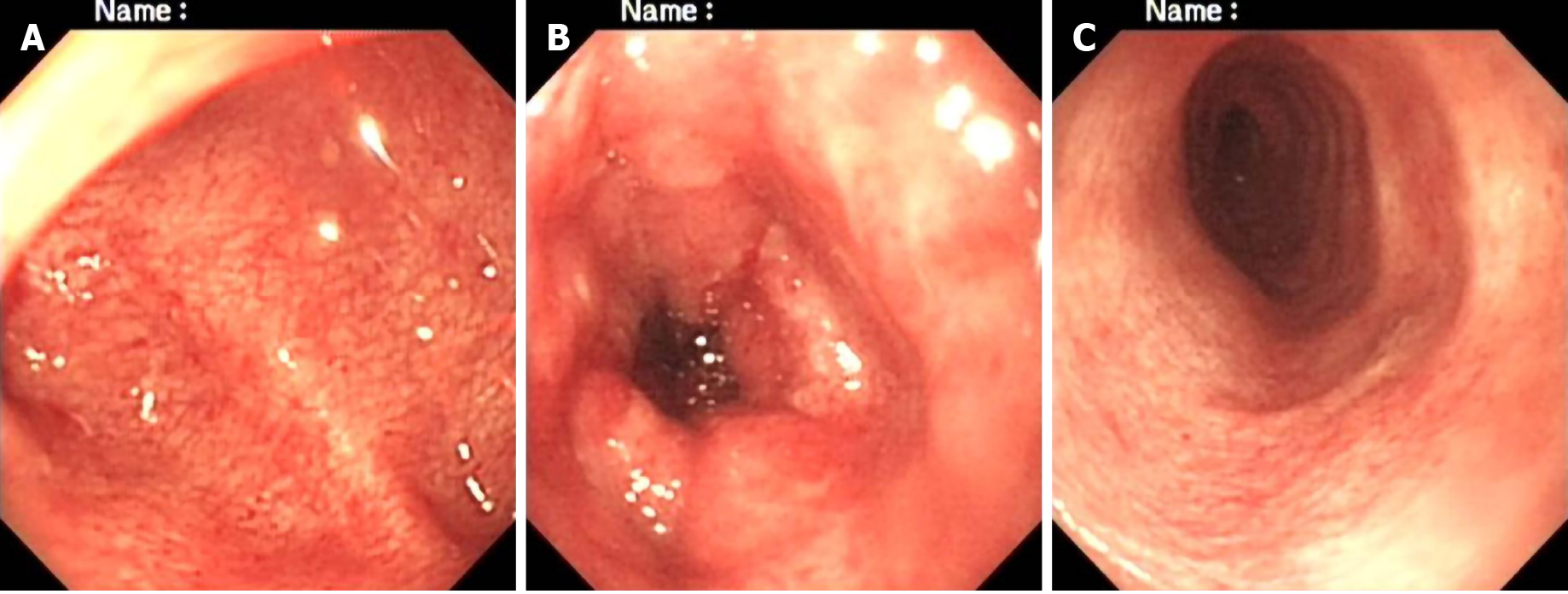
On the 23rd day, gastroscopy (EG-99WR; Fujinon, Tokyo, Japan) and colonoscopy (GIF-XQ240; Olympus, Tokyo, Japan) were performed. Gastroscopy (Figure 2) revealed gastroduodenal mucosal edema and hyperemia, lymphoid hyperplasia, focal ulceration, pseudotumor formation, and stenosis in the horizontal duodenum. Incomplete colonoscopy (Figure 3) displayed diffuse mucosal edema and roughness, stiffness, friability, and white membranoid substances in the descending colon, sigmoid colon, and rectum. The histopathological examination revealed characteristic inclusions suggestive of CMV.
The final diagnosis of the presented case was gastrointestinal CMV disease.
After admission to the hospital, suspected bacterial infection was treated with intravenous vancomycin and meropenem without improvement. On hospital day 12, diarrhea was aggravated with persistent high fever and episodes of vomiting and abdominal distension. Fasting and gastrointestinal decompression did not improve his condition. After the diagnosis of gastrointestinal CMV disease was made, intravenous ganciclovir was administered at 5 mg/kg every 12 h on the 30th hospital day for 2 wk and then reduced to 5 mg/kg each day for 1 wk.
The patient’s condition improved after treatment with IV ganciclovir and he was discharged on the 54th hospital day. He was followed regularly to 4.5 years old, and he is doing well.
This 9-mo-old Chinese boy had persistent fever and diarrhea after measles infection; ensuing vomiting, abdominal distension, and hematochezia presented at a later stage. All of these symptoms are indicative of digestive system diseases. Fever and diarrhea are common signs of different diagnoses in infants and children, including gastrointestinal infectious and noninfectious causes. In complicated cases with a prolonged course, there are difficulties in distinguishing pathogens; thus, detailed clinical data, exposure history, and pertinent laboratory tests can provide valuable assessment information.
Bacteria are reported as predominant pathogens of measles associated with diarrhea. However, this patient failed to respond to various antibiotics. Some distinct pathogens were considered. Enteroaggregative Escherichia coli (E. coli) and enteropatho
While asymptomatic colonization of Clostridium difficile (C. difficile) is prevalent in infants, for patients with recent exposure to hospitals and antibiotics, microbiological evaluation should focus on the diagnosis of C. difficile infection[8]; however, C. difficile should be sensitive to vancomycin, together with repeated negative bacterial separation and culture and enzyme immunoassay tests for toxins A and B and glutamate dehydrogenase. There was no support for the diagnosis of C. difficile infection in this patient.
The patient lives in China, a country that ranks 3rd in the world for high tuberculosis (TB) morbidity; thus, intestinal TB (ITB) should be considered even though it is more likely to cause miliary TB in infants. ITB is characterized by low-grade fever, abdominal pain, loss of weight, altered bowel habits, and night sweats[9]. The absence of exposure to Mycobacterium tuberculosis and negative chest X-ray and interferon-γ release assay for TB revealed no direct evidence of TB in this patient.
As a noninfectious disease, inflammatory bowel disease (IBD) should be included in the differential diagnosis when lacking evidence of clear pathogenic microorganism infection[7]. The typical clinical presentations of infantile-onset IBD are intermittent fever and bloody diarrhea[10]; some cases are also associated with oral ulcers and perianal abscess and fistula. The diagnosis relies on endoscopic evaluation and histopathological findings of mucosal biopsy other than on clinical characteristics and history. Macroscopic features revealed no contiguous or skip linear ulceration, aphtha ulceration, cobblestoning, fistula, abscesses, or other changes, and microscopic features showed no crypt architectural changes or noncaseating granuloma, so the evidence supporting IBD was insufficient[11]. Granting that very-early onset IBD may have heterogeneous or atypical symptoms and signs, endoscopy and mucosal biopsy were of great benefit to exclude IBD in this patient. There was no evidence of indigestion, malabsorption, autoimmune enteropathy, hemolytic uremic syndrome, allergic or neoplastic disorders, drug- or poison-induced diarrhea, structural diseases, or functional enteropathy in this case (Table 3).
| Gastrointestinal diseases | Digestion and absorption disorder |
| (1) Inflammatory bowel disease; (2) Gastroenteric tumor; (3) Intussusception; (4) Enteric cyst; (5) Duplication of digestive tract; (6) Diverticulosis; (7) Polyposis coli; and (8) Ischemic enteropathy | (1) Short bowel syndrome; (2) Exocrine pancreatic insufficiency; (3) Bile acid-losing syndrome; and (4) Lactose intolerance |
| Systemic disease: (1) Anaphylactoid purpura; (2) Hyperthyroidism; and (3) Primary chronic adrenocortical hypofunction | Neoplastic diseases: (1) Gastrin adenoma; (2) Carcinoid syndrome; (3) Vasoactive intestinal peptide tumor; and (4) Pheochromocytoma |
| Allergic diarrhea: (1) Food protein-induced enterocolitis syndrome; (2) food protein-induced proctocolitis; and (3) eosinophilic gastroenteritis | Primary immunodeficiency diseases: (1) Chronic granulomatous disease; (2) Common variable immunodeficiency; and (3) X linked agammaglobulinemia |
| Secondary immunodeficiency diseases | |
| Functional diarrhea | |
| Drugs/toxicants factor |
Multiple factors may contribute to several weeks of measles-induced immune suppression[1]. There is an epidemiologically detectable long-term increase in suscep
Any part of the alimentary tract can be impacted by CMV infection but the typical CMV lesions are limited to either the upper or the lower gastrointestinal tract, while both the upper and the lower gastrointestinal tracts are rarely involved simultane
In addition to broad clinical presentations and signs, confirmation of the virus via laboratory methods is indispensable in diagnosing CMV disease. In this patient, CMV-IgM was negative, and the absence of evidence of active infection led to an uncertain causal relationship between CMV infection and various symptoms, which demanded histopathology detection for further verification. Nonetheless, the positive CMV-IgG titre was more than 4-fold in 2 wk, and elevated CMV DNA copies provided clues for diagnosis. Nevertheless, false negative IgM results can be obtained in immunocom
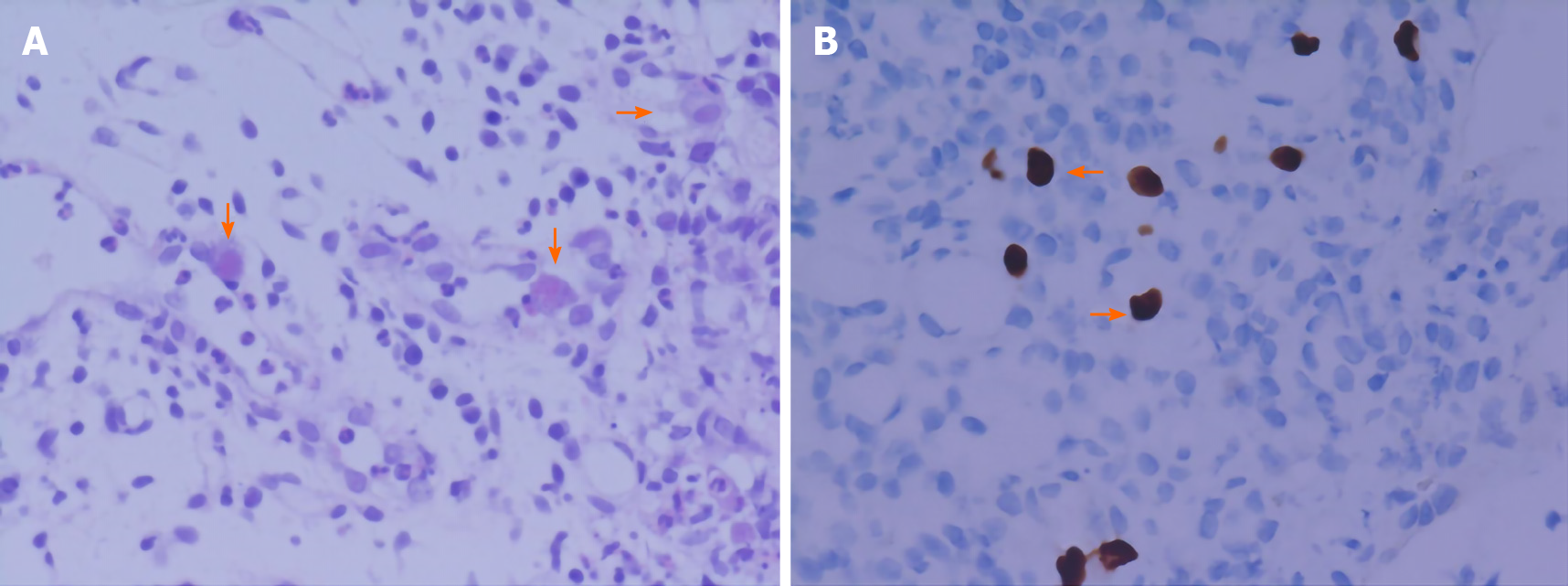
Negative results of CMV PCR do not rule out CMV disease[17]. As a marker of early diagnosis, CMV pp65 antigen is not able to differentiate between latent infection and active disease. Viral culture is the diagnostic gold standard, but its poor sensitivity and slow turn-around time limit its clinical utility, while histopathology assays can provide confirmatory information for invasive disease which is highly specific[18] (Figure 4). Therefore, when gastrointestinal CMV infection is suspected, endoscopy and biopsy must be performed to obtain positive evidence for diagnosis.
The macroscopic features of gastrointestinal CMV disease vary from normal mucosa, focal erythematous, erosion, and pseudotumor formation to deep ulceration, lacking specificity[19]. Based on the patchy distributions and diverse endoscopic findings, biopsy location and number are important to assess CMV[20]. Gastroduodeno
CMV disease requires evidence of parenchymal organ damage before it can be diagnosed and treated with antiviral therapy. Anti-CMV treatment in immunocom
In conclusion, we sought to review the differential diagnosis for similar clinical outcomes in a young child with diarrhea, hematochezia, and fevers, and to clarify endoscopic and histopathological findings as well as outline treatment options for CMV colitis. We highlighted the diagnostic challenges of gastrointestinal CMV disease due to its nonspecific clinical presentation and the weak diagnostic value of serologic antibody detection. We hope to help clinicians improve the understanding of the importance of considering this disease in immunocompromised patients, as well as to consider it in otherwise healthy patients with a prolonged course characterized by fever, diarrhea, and hematochezia who may become immunocompromised due to measles infection.
This is the first case of gastrointestinal CMV infection due to transient immunosuppression secondary to measles infection in a presumably immunocompe
We thank the patient’s family for providing background information and allowing us to publish this manuscript. We wish to thank Dr. Cao WG and Gan YG (MD, Radiologist, Radiology Department of Shenzhen Children's Hospital) for their imaging technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ilic K, Komatsu H, Liatsos GD S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Griffin DE. Measles virus-induced suppression of immune responses. Immunol Rev. 2010;236:176-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Bester JC. Measles and Measles Vaccination: A Review. JAMA Pediatr. 2016;170:1209-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Jackson BD, Black RE. Available studies fail to provide strong evidence of increased risk of diarrhea mortality due to measles in the period 4-26 weeks after measles rash onset. BMC Public Health. 2017;17:783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Arnold M, Itzikowitz R, Young B, Machoki SM, Hsiao NY, Pillay K, Alexander A. Surgical manifestations of gastrointestinal cytomegalovirus infection in children: Clinical audit and literature review. J Pediatr Surg. 2015;50:1874-1879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136:1874-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | DuPont HL. Persistent Diarrhea: A Clinical Review. JAMA. 2016;315:2712-2723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:987-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 703] [Cited by in F6Publishing: 721] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 9. | Donoghue HD, Holton J. Intestinal tuberculosis. Curr Opin Infect Dis. 2009;22:490-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Ye Z, Zhou Y, Huang Y, Wang Y, Lu J, Tang Z, Miao S, Dong K, Jiang Z. Phenotype and Management of Infantile-onset Inflammatory Bowel Disease: Experience from a Tertiary Care Center in China. Inflamm Bowel Dis. 2017;23:2154-2164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology; Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 811] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 12. | Griffin DE. Measles immunity and immunosuppression. Curr Opin Virol. 2021;46:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Moss WJ. Measles. Lancet. 2017;390:2490-2502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 14. | Baumgartner JD, Glauser MP, Burgo-Black AL, Black RD, Pyndiah N, Chiolero R. Severe cytomegalovirus infection in multiply transfused, splenectomized, trauma patients. Lancet. 1982;2:63-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | de Andrade LG, Rodrigues MA, Romeiro FG, Carvalho MF. Gastrointestinal cytomegalovirus disease in renal transplant recipients: a case series. Clin Transplant. 2012;26:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Razonable RR, Paya CV, Smith TF. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J Clin Microbiol. 2002;40:746-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Ruell J, Barnes C, Mutton K, Foulkes B, Chang J, Cavet J, Guiver M, Menasce L, Dougal M, Chopra R. Active CMV disease does not always correlate with viral load detection. Bone Marrow Transplant. 2007;40:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J Gastroenterol. 2016;22:2030-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 19. | Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 295] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Juric-Sekhar G, Upton MP, Swanson PE, Westerhoff M. Cytomegalovirus (CMV) in gastrointestinal mucosal biopsies: should a pathologist perform CMV immunohistochemistry if the clinician requests it? Hum Pathol. 2017;60:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 660] [Article Influence: 132.0] [Reference Citation Analysis (0)] |