Published online Apr 21, 2021. doi: 10.3748/wjg.v27.i15.1569
Peer-review started: January 17, 2021
First decision: February 9, 2021
Revised: February 13, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: April 21, 2021
Processing time: 87 Days and 0.7 Hours
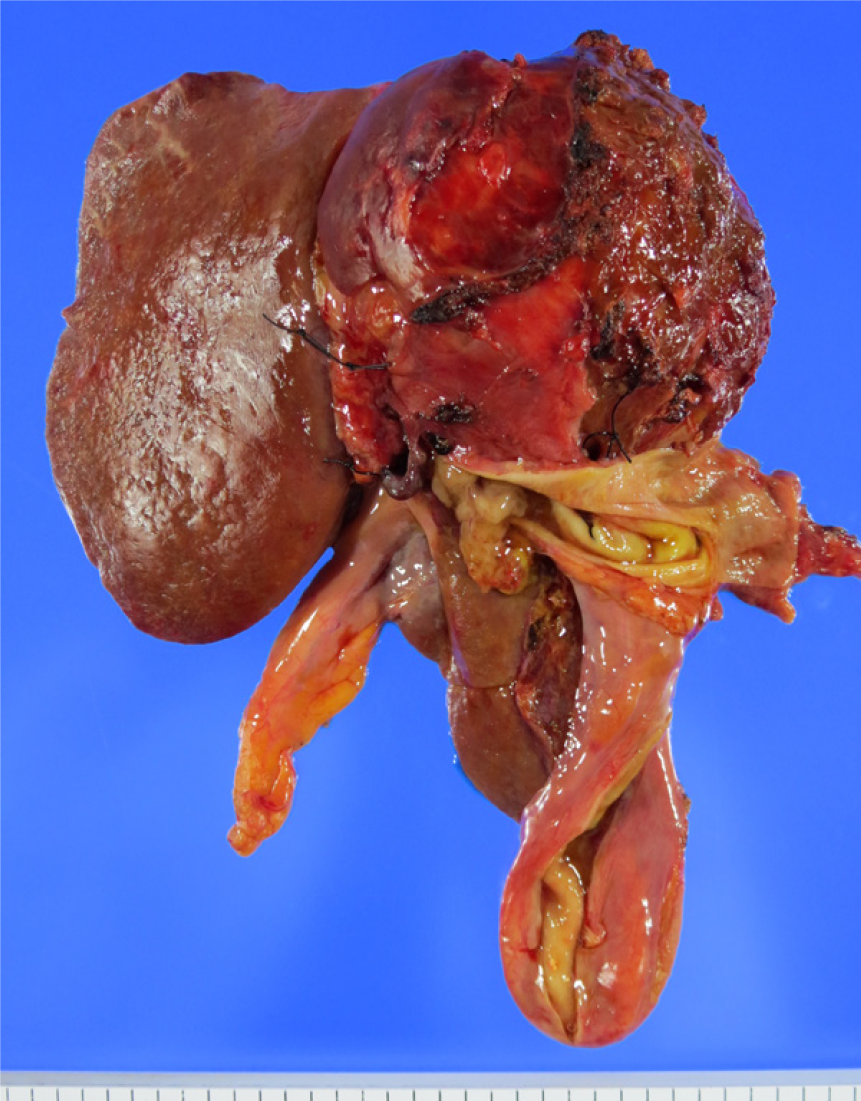
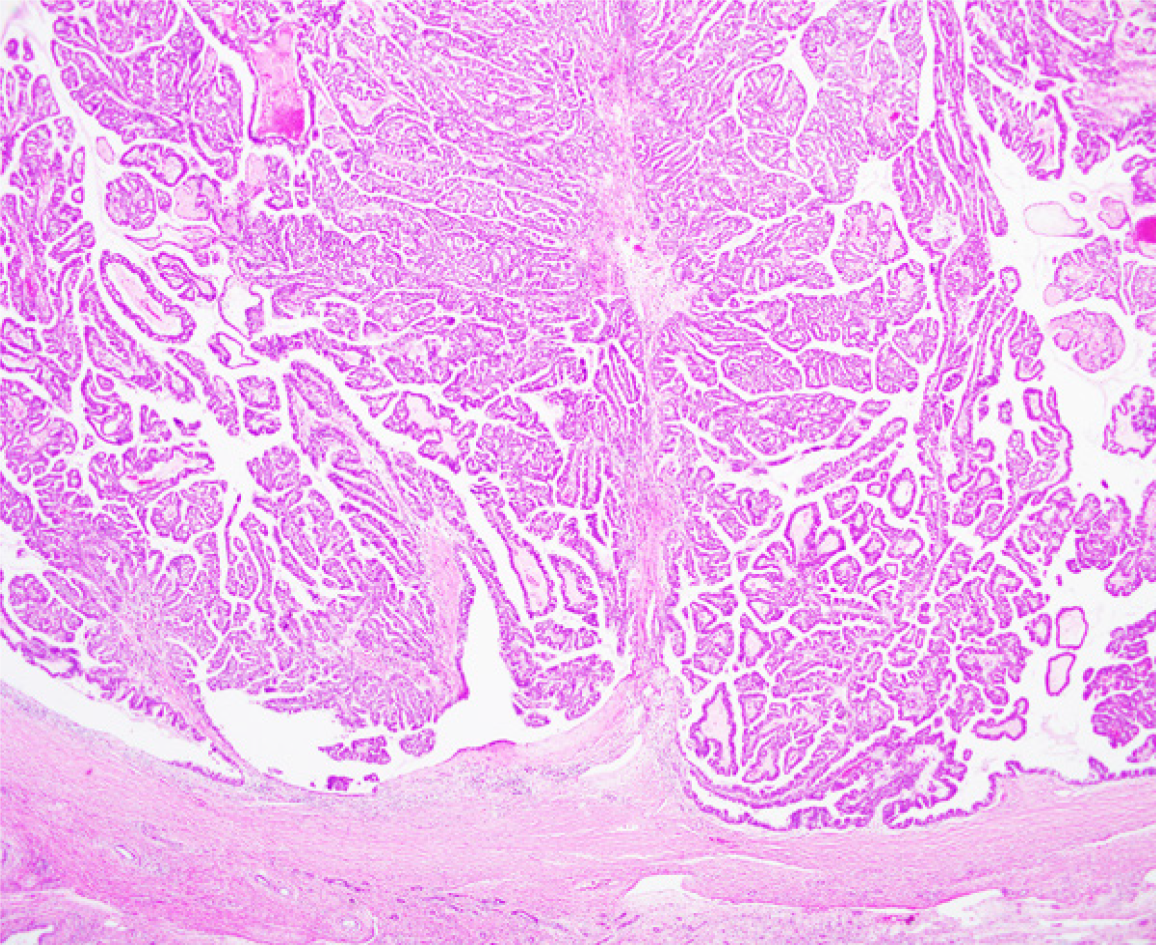
Bile duct epithelial tumours showing papillary neoplasm in the bile duct lumen are present in the intrahepatic and extrahepatic bile ducts. Clinicopathological images of these tumours are distinctive and diverse, including histological images with a low to high grade dysplasia, infiltrating and noninfiltrating characteristics, excessive mucus production, and similarity to intraductal papillary mucinous neoplasm (IPMN) of the pancreas. The World Health Organization Classification of Tumours of the Digestive System in 2010 named these features, intraductal papillary neoplasm of the bile duct (IPNB), as precancerous lesion of biliary carcinoma. IPNB is currently classified into type 1 that is similar to IPMN, and type 2 that is not similar to IPMN. Many of IPNB spreads superficially, and diagnosis with cholangioscopy is considered mandatory to identify accurate localization and progression. Prognosis of IPNB is said to be better than normal bile duct cancer.
Core Tip: Intraductal papillary neoplasm of the bile duct (IPNB) is classified into type 1 that is similar to intraductal papillary mucinous neoplasm (IPMN) and type 2 that is not similar to IPMN. Many of IPNB spreads superficially, and diagnosis with cholangioscopy is considered mandatory to identify accurate localization and progression. Prognosis of IPNB is said to be better than normal bile duct cancer.
- Citation: Sakai Y, Ohtsuka M, Sugiyama H, Mikata R, Yasui S, Ohno I, Iino Y, Kato J, Tsuyuguchi T, Kato N. Current status of diagnosis and therapy for intraductal papillary neoplasm of the bile duct. World J Gastroenterol 2021; 27(15): 1569-1577
- URL: https://www.wjgnet.com/1007-9327/full/v27/i15/1569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i15.1569
Presence of intraductal tumours showing a pattern of spurring papillary neoplasm has been known since before[1-4]. Although the names of these tumours varied depending on the country and region, it is known that the tumours have a better postoperative prognosis than normal infiltrating bile duct cancer. Also known are the facts that quite a few pre-infiltrating phases can be found in these tumours; cases with a low grade dysplasia are present; and some cases have excessive mucus production. Many similarities with intraductal papillary mucinous neoplasm (IPMN) of the pancreas are also pointed out. A recommendation has been made in recent years to call these tumours intraductal papillary neoplasm of the bile duct (IPNB)[1]. Diagnosis of IPNB and the current status of its treatment are hereby reported.
Epithelial tumours demonstrating a variety of images develop in the bile duct. Of these tumours, bile duct epithelial tumours indicate papillary neoplasm in the bile duct lumen[1-4]. These tumours have better prognosis than normal bile duct cancer, and often present with distinguishing and diverse clinical pathophysiology. Yet, no specific name was given to these tumours. In the United States and Europe, these tumours are called biliary papilloma if they are solitary, and biliary papillomatosis if they are multiple[5,6]. These are slow growing, low grade tumours with potential malignancy, and some cases of infiltration and metastasis have been also reported. Separately, hepatobiliary cystadenoma and adenoma also exist, and differences among these diseases were not necessarily clear-cut.
Intrahepatic stone-related intrahepatic bile duct cancer was examined in 2001. There are quite a few bile duct epithelial tumours showing papillary growth in the bile duct, with excessive mucus production. These tumours are morphologically and characteristically similar to IPMN of the pancreas; thus, we consider these cases as neoplastic lesion and reported it as intraductal papillary neoplasm of the liver[7]. As similar tumours were also noted thereafter in the extrahepatic bile duct, the tumours were renamed IPNB[8]. This lesion was officially named IPNB in the revised World Health Organization Classification of Tumours of the Digestive System in 2010[9].
IPNB is epithelial tumour with intraductal papillary proliferation, and defined as having a histologically thin fibrovascular stem[1-4]. Excessive mucus production is often noted but this is not a decisive element for diagnosis of IPNB. IPNB is reported to be a lesion similar to IPMN. Specific lesions are: (1) Papillary proliferation of tumours in the bile duct lumen or in the bile duct; (2) Intestinal, gastric, pancreatobiliary, and oncocytic subtypes noted in IPMN are also common in IPNB; (3) As in IPMN, cancerous cells show intraepithelial and intramucosal progression along the intraductal wall adjacent to the primary site, and some are multiple cases with asynchronous and simultaneous tumours; (4) IRNB has better prognosis compared with normal bile duct cancer or normal pancreatic carcinoma; (5) Some IPNB cases show excessive mucus production; however, the incidence is lower than that of IPMN. In some cases, no clear excessive mucus production is noted. Patients with excessive mucus production often show cystic expansion of the bile duct at the main site of papillary growth; and (6) In the case of infiltrating growth of IPNB, either normal tubular adenocarcinoma or bile duct mucinous adenocarcinoma may result. Similar infiltrating images are also known in IPMN.
The World Health Organization Classification of Tumours of the Digestive System in 2010 added the definition of IPNB as one of precancerous, pre-infiltrating lesions of the bile duct cancer[9]. However, studies since then have revealed that some cases with intraductal noninfiltrating papillary tumours are slightly different from those cases similar to IPMN. These different cases include biliary papillary adenocarcinoma that has been previously reported by Albores-Saavedra[10,11]. Based on these results, IPNB was classified into two types. Type 1 is IPNB similar to IPMN, and mostly classified into gastric and intestinal subtypes. Type 1 shows homogeneous tissue image, and often occurs in the intrahepatic bile duct, with excessive mucus production often experienced by patients. Many of type 1 IPNB is considered a high grade dysplasia; however, some regions often show a low grade dysplasia, and some cases have only a low grade dysplasia. Type 1 includes many of patients undergoing resection in noninfiltrating phase, and those who show cystic expansion in the bile duct with lesion. Type 2 IPNB is slightly different from IPMN; however, it is experienced as IPMN in rare cases. Type 2 IPNB includes lesions previously reported by Albores-Saavedra as bile duct papillary adenocarcinoma and papillary bile duct cancer[7,8]. Tissue images of type 2 IPNB are slightly more complicated, showing some solid sites. Almost all cases are considered a high grade dysplasia, often occurring in the extrahepatic bile duct. Many cases already show infiltrating images at the time of resection, but some cases show noninfiltration. Most of the cases are classified into intestinal and pancreatobiliary subtypes, with few cases of excessive mucus production. Examination of IRNB with macroscopic mucus production revealed that many of tumours with mucus production are noninfiltrating, similar to IPMN, and those with no mucus production tended to show infiltration[12]. IPNB classification is still far from perfect. After classifying into two types, we find it difficult to classify into type 1 IPNB and type 2 IPNB in some cases, or to differentiate progressed papillary bile duct cancer from type 2 IPNB. Further consideration is necessary.
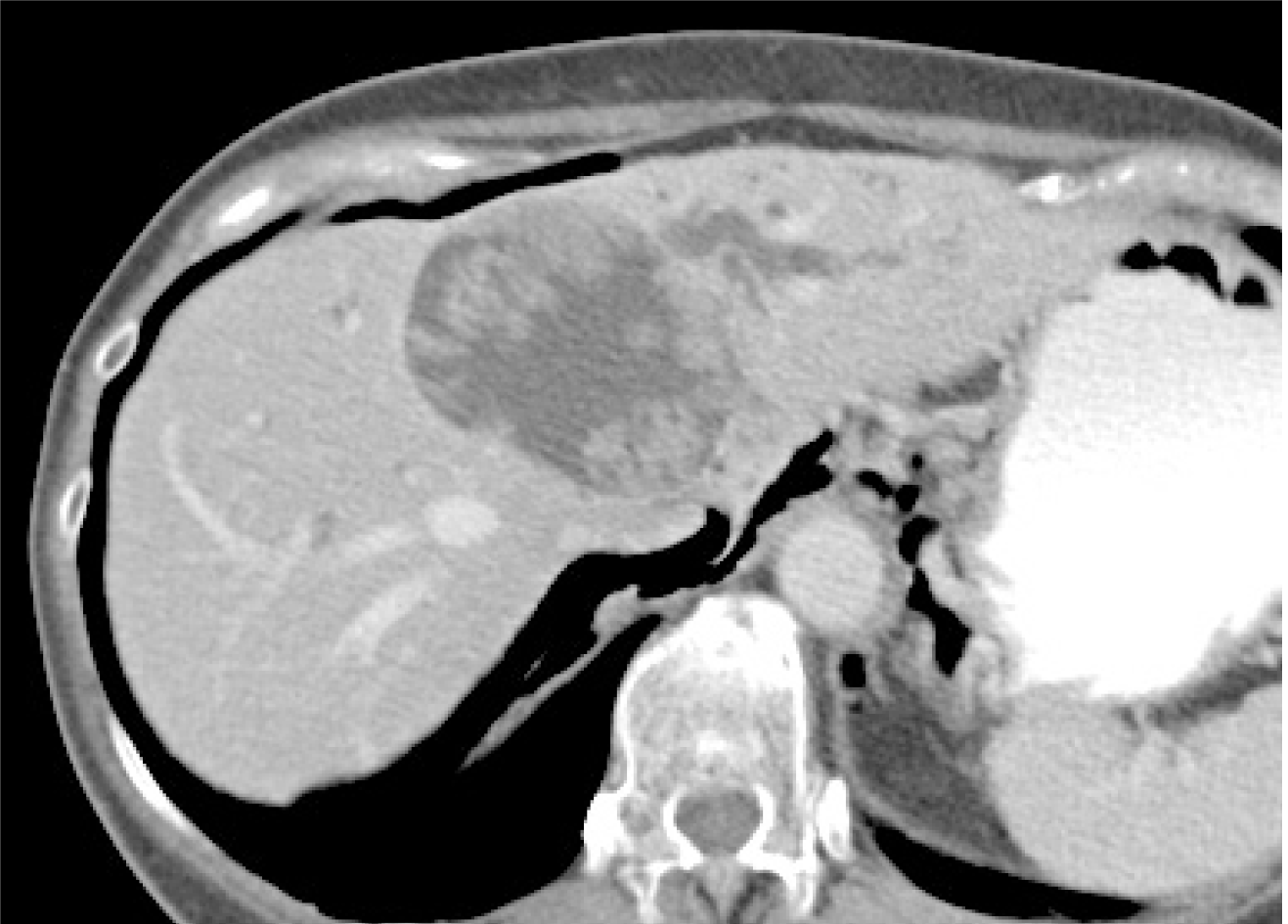
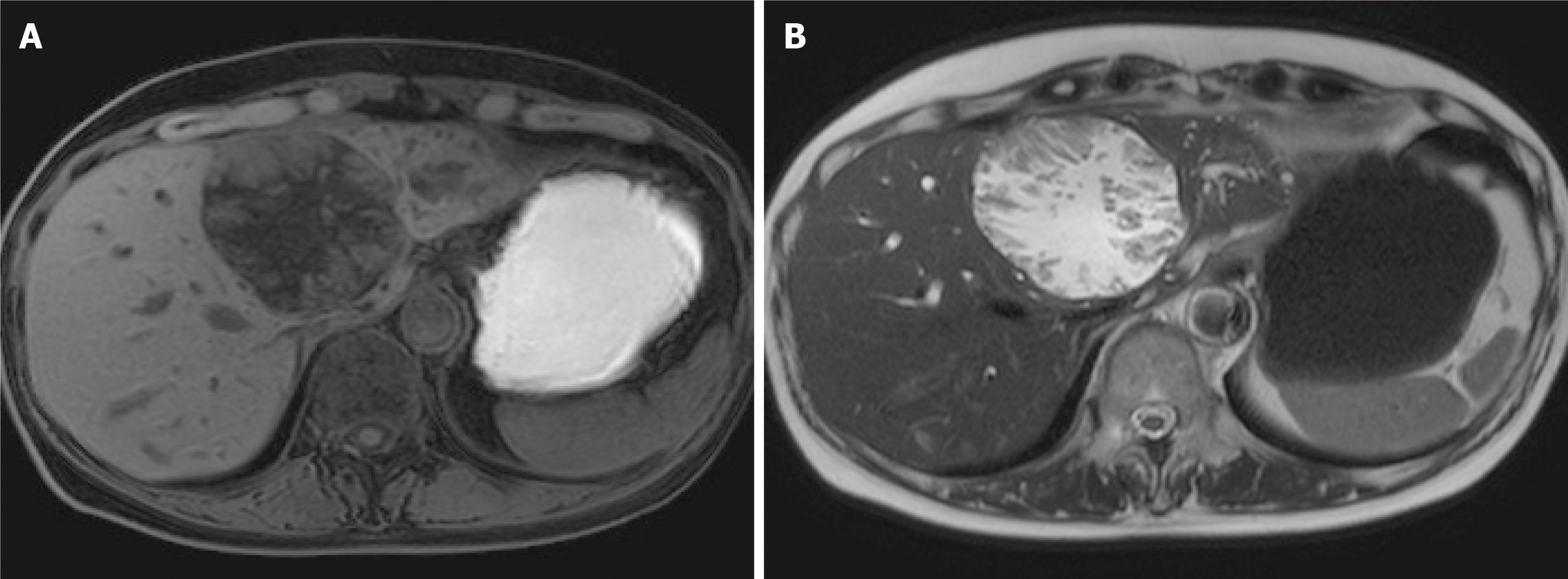
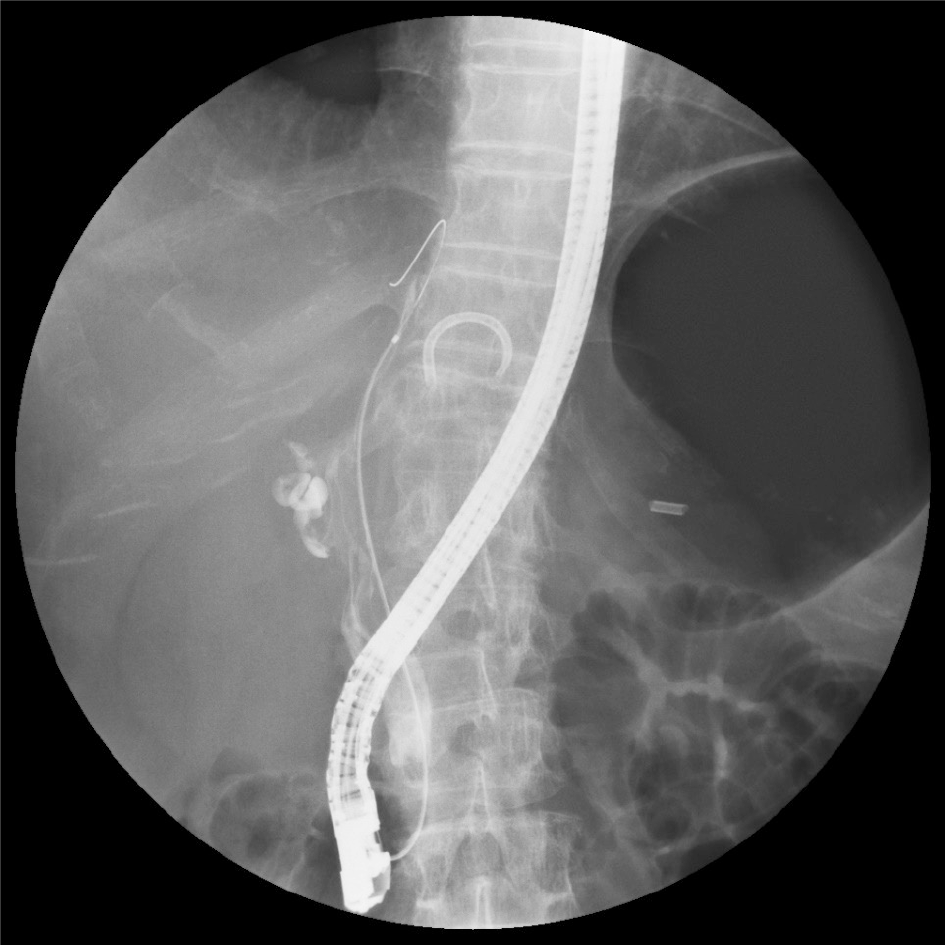
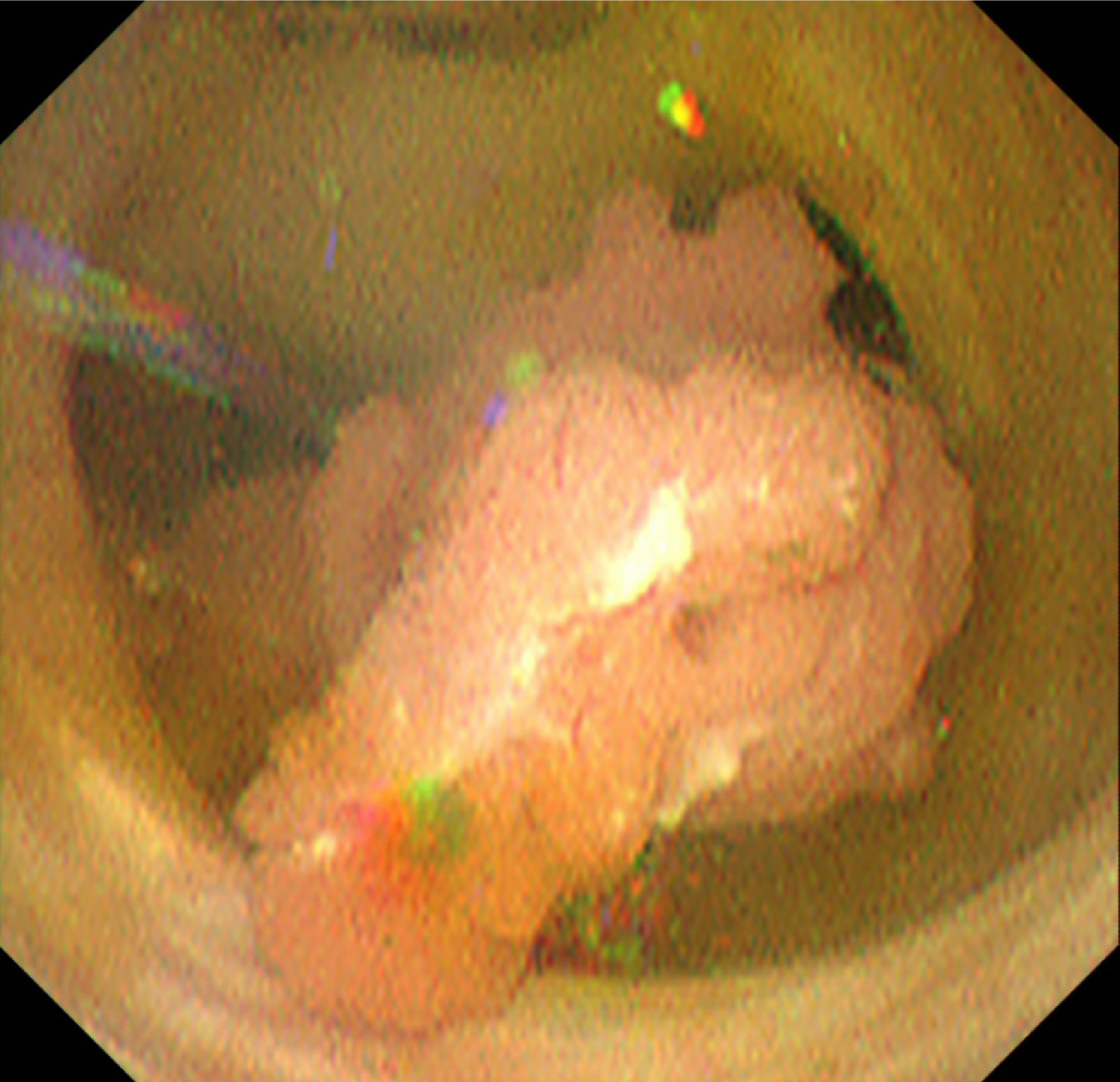
Image diagnosis plays an important role in giving a diagnosis of IPNB. If clinical symptoms show abdominal pain, pyrexia, and elevation of hepatobiliary enzymes in a blood test, an image diagnosis of the abdominal region will be made. In this case, abdominal ultrasonography, contrast computed tomography (Figure 1), Magnetic resonance imaging (Figure 2), and endosonography will enable us to identify the main focus[13]. However, these modalities may not ensure an accurate diagnosis because the presence of mucus, which is characteristics of IPNB, must be verified, and superficial expansion and progression of IPNB are often to the extent beyond our expectation. This is where direct cholangiography, intraductal ultrasonography (IDUS), and cholangioscopy come in[14-16]. The presence of mucus can be identified by checking papillary dilation and emission of mucus with endoscopy, or by outlining the mucous plug with cholangiography (Figure 3). Even when cholangiography contrasts the bile duct dilated with mucus, it is difficult in some cases to identify the main focus itself owing to mucus, let alone superficial expansion and progression. Meanwhile, IDUS enables us to identify the main focus and depict mucus; it can be easily conducted at the time of ERCP by following the guide wire; and it allows us to capture a wide scope of the region for observation. The presence of mucus, however, makes it difficult to evaluate the bile duct wall in IDUS. Thus, cholangioscopy plays a central role in the diagnosis of IPNB[14-16]. Cholangioscopy enables us to observe with narrow band imaging in addition to usual white light imaging, and is considered useful for the diagnosis of benignancy and malignancy as well as progression staging[14-16]. As mentioned earlier, the main focus of papillary bulge may be captured by computed tomography and magnetic resonance imaging, but the evaluation of superficial spread and progression from the main focus is difficult. Cholangioscopy allows us to directly observe the superficial spread and progression and to perform an open biopsy, thereby enabling accurate diagnosis of the progression (Figure 4). Depending on a route of approach, percutaneous transhepatic cholangioscopy (PTCS) and peroral cholangiosccopy (POCS) are available in cholangioscopy. Generally, PTCS where percutaneous biliary drainage is performed, has a bigger channel diameter and a shorter effective length. These features make it easy to wash and superior in removing mucus. With great operability, PTCS ensures a sufficient visual field and enables a wider scope of observation and more detailed monitoring. Weak points of PTCS are that it takes time until we can observe as time to fistula formation is required, and that dissemination via fistula has been also reported[15]. Therefore, the current indications for PTCS are limited, and POCS is mostly performed except for the following cases: Difficulty in biliary cannulation with ERCP; difficulty in reaching duodenal papilla after Billroth-2 method or Rou-X-en Y anastomosis; and difficulty in removing mucus with POCS. Benefits of POCS are that it does not require fistula formation, allows direct observation from the initial ERCP, and entails no risk for fistula dissemination. On the other hand, POCS may limit the scope of observation since its approach route is longer and operation is cumbersome and difficult. Nonetheless, sufficient evaluation with POCS is possible because the resection line of distal site of the tumour is important in determining the scope of resection at the time of operation on IPNB (Figures 5 and 6).
Kubota et al[17] examined 119 patients with intrahepatic IPNB, and reported the 5-year survival: 84.6% in low or intermediate-grade neoplasia group, 90.9% in high-grade neoplasia group, and 79.2% in invasive carcinoma group. When complete resection was performed, no statistical significance was noted in overall survival between the group of infiltrating cancer and the other group of noninfiltrating cancers. As further examinations of infiltrating cancer, Onoe et al[18] reported the ratio of infiltrating site relative to the lesion and its prognosis on 184 patients with intrahepatic and extrahepatic bile duct cancer exhibiting intraductal papillary proliferation. The 5-year survival in four groups: Noninfiltrating cancer, infiltrating site < 10%, infiltrating site = 10%-50%, and infiltrating site > 50%, were 92%, 74%, 64%, and 33%, respectively. The former three groups showed no statistical between-group differences; however, the infiltrating site > 50% group showed significantly poor prognosis, almost equivalent to the 5-year survival of 35% in 460 patients with non-papillary bile duct cancer. Onoe et al[18], pointed out that there still was a moot point in the disease concept of IPNB but indicated that if bile duct cancer exhibiting intraductal papillary proliferation was an infiltrating cancer, with an infiltrating site accounting for not more than 50% of the entire cancer, its good prognosis was more significant than non-papillary bile duct cancer. Rocha et al[19] reported that 29 of 39 IPNB patients (74%) had infiltrating cancer. Significantly poor prognostic factors were infiltration exceeding 5 mm, and infiltration beyond the bile duct wall, exceeding 10% of the entire tumour. The ratio of infiltrating cancer in IPMN is 21%-48%[19,20] , while that in IPNB is 70%-80%, slightly higher[21,22]. It is reported that prognoses of IPMN-derived infiltrating cancer and infiltrating pancreatic ductal carcinoma are almost the same[23,24], or prognosis of IPMN-derived infiltrating cancer is slightly better[25,26]. Meanwhile, better prognosis is reported in IPNB as compared with normal bile duct cancer[27]. This is due to characteristics of IPNB tumour itself, and because of relatively early diagnosis that is possible with obstructive jaundice caused by its proliferation into the intraductal lumen.
Presence or absence of complete resection is an important prognostic factor, and reported in great numbers[18,19,28,29]. Luvira et al[28] reported that the bile duct resection stump tested positive for invasive carcinoma, and overall survival significantly decreased, while testing positive for dysplasia and carcinoma in situ did not impact prognosis. In contrast, Jung et al[29] reported that overall survival and recurrence-free survival of the group in which the bile duct resection stump tested positive for a low to intermediate grade dysplasia, significantly decreased to the extent similar to the overall survival of carcinoma in situ and invasive carcinoma, as compared with the group that tested negative. International consensus guidelines for the management of IPMN specifies that additional resection is indispensable if a clear high grade dysplasia or infiltrating cancer is present in the pancreatic resection stump while additional resection is not necessary even when a low grade dysplasia is present on the stump[30]. In IPNB, no guideline is available for a low grade dysplasia in the bile duct resection stump. Unlike IPMN where total pancreatectomy is therapeutic option depending on a patient, there is a resection limit for the bile duct, and thus, additional resection is performed as much as possible at each institution.
Presence or absence of metastases to lymph nodes is a major prognostic factor along with complete resection[18,28,29]. Luvira et al[28] reported that the 5-year survival of patients tested negative for metastases to lymph nodes was 51.2% while that of those tested positive was 11.1%, suggesting significantly poor prognosis. Of all 124 patients with IPNB, 98 patients underwent lymph node dissection. No difference in prognosis was noted between these 98 patients and 26 patients without lymph node dissection, and thus, the results failed to support the usefulness of lymph node dissection. Patients with infiltrating cancer include those patients with lymph node dissection, and lymph node dissection may seem necessary. However, metastases to lymph node rarely occur in an intraductal growth type of intrahepatic bile duct cancer. As there are type 1 and type 2 for IPNB, more meticulous consideration may be needed to discuss the necessity of lymph node dissection in the future.
IPNB is known to recur in the bile duct after surgical resection. Despite that the initial resection stump of the bile duct tested negative, intraductal papillary tumour presenting with tissue image similar to the initial resection recurred. This is the characteristics similar to IPMN. IPNB recurs after more than 10 years in some cases, appearing as if it were new carcinoma. This suggests a multicentric growth of IPMN[31]. Therefore, observation of clinical course is necessary, with postoperative intraductal recurrence in mind.
IPNB is classified into type 1 that is similar to IPMN and type 2 that is not similar to IPMN. Prognosis of IPNB is said to be better than normal bile duct cancer. Cholangioscopy is useful for diagnosis of localization and progression. It is important to determine an operative method based on information obtained through cholangioscopy and to observe clinical course.
The authors thanks Masaru Miyazaki for supporting this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karaca CA, Pan W S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Bosman FT, Camerio FC, Hurban RH. World Health Organization classification of tumours. Pathology and genetics of Tumours of the digestive system. 4th ed. IARC press, Lyon 2010. |
| 2. | Nakanuma Y, Sudo Y. Biliary tumors with pancreatic counterparts. Semin Diagn Pathol. 2017;34:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Nakanuma Y, Sasaki M, Ishikawa A, Tsui W, Chen TC, Huang SF. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002;17:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 4. | Nakanuma Y, Uesaka K, Miyayama S, Yamaguchi H, Ohtsuka M. Intraductal neoplasms of the bile duct. A new challenge to biliary tract tumor pathology. Histol Histopathol. 2017;32:1001-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 5. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E, Lee SG, Min YI. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Yeung YP, AhChong K, Chung CK, Chun AY. Biliary papillomatosis: report of seven cases and review of English literature. J Hepatobiliary Pancreat Surg. 2003;10:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K, Hamaguchi M, Ohno Y, Hsieh LL, Nimura Y. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Nakanuma Y, Curado M-P, Franceschi S, Gores G, Paradis V, Sripa B. Intrahepatic cholangiocarcinoma. Bosman FT, Carneiro F, Hruban RH, Theise ND. In: World Health Organization Classification of Tumours of the Digestive System (4th). WHO 2010: 217-224. |
| 10. | Albores-Saavedra J, Murakata L, Krueger JE, Henson DE. Noninvasive and minimally invasive papillary carcinomas of the extrahepatic bile ducts. Cancer. 2000;89:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Hoang MP, Murakata LA, Katabi N, Henson DE, Albores-Saavedra J. Invasive papillary carcinomas of the extrahepatic bile ducts: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 2002;15:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K, Takano S, Kondo Y, Miyazaki M. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Kim JE, Lee JM, Kim SH, Baek JH, Moon SK, Yu IS, Lee JY, Han JK, Choi BI. Differentiation of intraductal growing-type cholangiocarcinomas from nodular-type cholangiocarcinomas at biliary MR imaging with MR cholangiography. Radiology. 2010;257:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Sakai Y, Tsuyuguchi T, Tsuchiya S, Fukuda Y, Miyakawa K, Sugiyama H, Asano K, Ohtsuka M, Miyazaki M, Yokosuka O. Clinical utility of peroral cholangioscopy for mucin-producing bile duct tumor. Hepatogastroenterology. 2008;55:1509-1512. [PubMed] |
| 15. | Sakamoto E, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Uesaka K, Nimura Y. Treatment strategy for mucin-producing intrahepatic cholangiocarcinoma: value of percutaneous transhepatic biliary drainage and cholangioscopy. World J Surg. 1999;23:1038-43; discussion 1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Moriyasu F, Gotoda T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest Endosc. 2007;66:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H, Kamisawa T, Inui K. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2014;21:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Onoe S, Shimoyama Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nakamura S, Nagino M. Prognostic delineation of papillary cholangiocarcinoma based on the invasive proportion: a single-institution study with 184 patients. Surgery. 2014;155:280-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D'Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 20. | D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Nakanuma Y, Zen Y, Harada K, Ikeda H, Sato Y, Uehara T, Sasaki M. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci. 2010;17:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Paik KY, Heo JS, Choi SH, Choi DW. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632-6; discussion 637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, Chari ST, Farnell MB. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639-46; discussion 646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 25. | Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, Barthet M, O'Toole D, Rufat P, Partensky C, Cuillerier E, Lévy P, Belghiti J, Ruszniewski P. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Yeh CN, Jan YY, Yeh TS, Hwang TL, Chen MF. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004;11:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Luvira V, Pugkhem A, Bhudhisawasdi V, Pairojkul C, Sathitkarnmanee E, Luvira V, Kamsa-Ard S. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J Gastroenterol Hepatol. 2017;32:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 31. | Nakanishi Y, Kondo S, Hirano S, Ambo Y, Tanaka E, Morikawa T, Itoh T. Recurrence of mucosal carcinoma of the bile duct, with superficial flat spread, 12 years after operation. J Hepatobiliary Pancreat Surg. 2006;13:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |