Published online Mar 21, 2021. doi: 10.3748/wjg.v27.i11.1055
Peer-review started: January 26, 2021
First decision: February 8, 2021
Revised: February 14, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: March 21, 2021
Processing time: 49 Days and 20.6 Hours
Constipation is one of the most important nonmotor symptoms in Parkinson's disease (PD) patients, and constipation of different severities is closely related to the pathogenesis of PD. PD with constipation (PDC) is considered a unique type of constipation, but its mechanism of formation and factors affecting its severity have been less reported. Understanding the gastrointestinal motility characteristics and constipation classification of PDC patients is essential to guide the treatment of PDC. In this study, the colonic transit test and high-resolution anorectal manometry were used to identify the intestinal motility of PDC to provide a basis for the treatment of PDC.
To investigate the clinical classification of PDC, to clarify its characteristics of colonic motility and rectal anal canal pressure, and to provide a basis for further research on the pathogenesis of PDC.
Twenty PDC patients and 20 patients with functional constipation (FC) who were treated at Xuanwu Hospital of Capital Medical University from August 6, 2018 to December 2, 2019 were included. A colonic transit test and high-resolution anorectal manometry were performed to compare the differences in colonic transit time, rectal anal canal pressure, and constipation classification between the two groups.
There were no statistically significant differences in sex, age, body mass index, or duration of constipation between the two groups. It was found that more patients in the PDC group exhibited difficulty in defecating than in the FC group, and the difference was statistically significant. The rectal resting pressure, anal sphincter resting pressure, intrarectal pressure, and anal relaxation rate in the PDC group were significantly lower than those in the FC group. The proportion of paradoxical contractions in the PDC group was significantly higher than that in the FC group. There was a statistically significant difference in the type composition ratio of defecatory disorders between the two groups (P < 0.05). The left colonic transit time, rectosigmoid colonic transit time (RSCTT), and total colonic transit time were prolonged in PDC and FC patients compared to normal values. The patients with FC had a significantly longer right colonic transit time and a significantly shorter RSCTT than patients with PDC (P < 0.05). Mixed constipation predominated in PDC patients and FC patients, and no significant difference was observed.
Patients with PDC and FC have severe functional dysmotility of the colon and rectum, but there are certain differences in segmental colonic transit time and rectal anal canal pressure between the two groups.
Core Tip: In this study, we used the colonic transit test and rectal anal manometry to subtype constipation and detect corresponding indicators in patients with Parkinson's disease with constipation (PDC) and functional constipation, with the aim of clarifying the colonic and rectal motility characteristics of PDC and providing a basis for the treatment of PDC. It was found that the segmental colonic transit time and the constituent ratio of types of defecation disorders were statistically different between the two groups.
- Citation: Zhang M, Yang S, Li XC, Zhu HM, Peng D, Li BY, Jia TX, Tian C. Study on the characteristics of intestinal motility of constipation in patients with Parkinson's disease. World J Gastroenterol 2021; 27(11): 1055-1063
- URL: https://www.wjgnet.com/1007-9327/full/v27/i11/1055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i11.1055
Parkinson's disease (PD) is a common neurodegenerative disease, with a prevalence of 1% in the elderly and about 10% in patients before the age of 50[1]. The pathological signs of PD are abnormal deposition of α-synuclein and progressive degeneration and loss of dopamine neurons in the substantia nigra compacta[2]. The main clinical manifestations are resting tremor, myotonia, bradykinesia, and abnormal postural gait. Over the past 15 years, non-motor symptoms (NMS), mainly autonomic dysfunction, have attracted much attention in patients with PD, including gastrointestinal dysfunction (dysphagia, delayed gastric emptying, and constipation), hypo olfaction, and sleep disorders, which are caused by neuronal loss in other areas of the brain[3]. Among them, constipation is one of the most common NMS in patients with PD. Studies have shown that 20%-89% of PD patients experience constipation, and the incidence gradually increases with the progression of the disease[4]. Compared with non-PD subjects, the frequency of constipation in PD patients increased by 2 to 4 times[5], and the degree of constipation was positively correlated with PD. Autopsy of constipated patients revealed a significant decrease in neuronal density in the substantia nigra, which also provided pathological evidence that constipation increased the risk of PD. Constipation can occur more than 10 years earlier than motor symptoms, making it one of the earliest indicators in the process of PD formation; recently, constipation has been included in the predecessor diagnostic criteria for PD[6]. PD with constipation (PDC) is considered a unique type of constipation, but its mechanism of formation and factors affecting its severity have been less reported, and the conclusions are not uniform[7]. It is likely that there is a causal relationship between PD and constipation, such that they exacerbate one another and form a vicious cycle. To stop constipation, this cycle may be terminated to prevent or delay the occurrence of PD. However, PDC treatment is very difficult and prone to drug resistance[8,9]. Compared with functional constipation (FC), it is not clear whether the clinical characteristics and influencing factors of PDC are unique. Understanding the gastrointestinal motility characteristics and constipation classification of PDC patients is essential to guide the treatment of PDC.
A total of 20 patients with PDC and 20 patients with FC who visited Xuanwu Hospital of Capital Medical University between August 6, 2018 and December 2, 2019 were recruited as test and control groups, respectively. All patients underwent a colonic transit test (CTT) and high-resolution anorectal manometry (HRAM) to compare the differences in colonic transit time, rectal anal canal pressure, and constipation classification between the two groups. Inclusion criteria were: (1) Age older than 18 years; (2) Fulfilled the diagnostic criteria for PD and Rome IV functional constipation; and (3) Symptom onset of more than 6 mo. Exclusion criteria were: (1) Presence of chronic diseases such as hypertension, diabetes mellitus, and coronary heart disease; (2) Organic diseases of the colon; (3) Diseases that may affect gastrointestinal motility function, such as thyroid disease, renal dysfunction, connective tissue disease, mental disorders, etc.; (4) History of abdominal surgery; and (5) Taking drugs that affect gastrointestinal motility in the last 2 wk.
HRAM: The ManoScanTM High Resolution Anorectal Manometry System (given, United States) was used for all patients to measure rectal resting pressure, anal sphincter resting pressure, rectal maximum squeeze pressure, anal canal maximum squeeze pressure, intrarectal pressure, anal relaxation rate, residual anal pressure, and rectoanal pressure difference. According to the results, conditions can be divided into either defecatory impetus deficiency, or pelvic floor muscle synergism disorders.
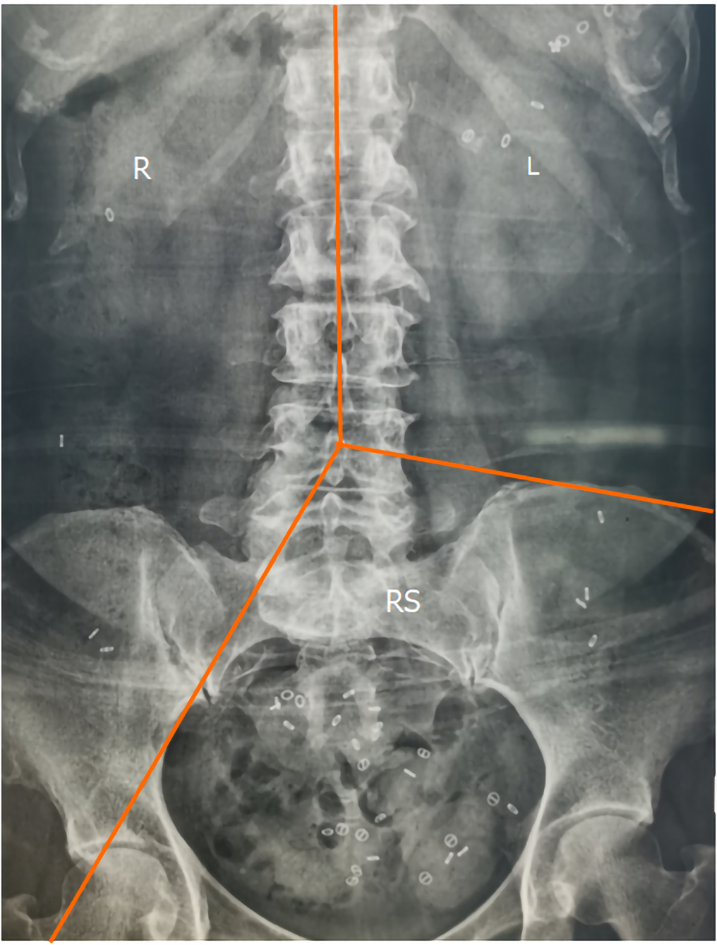
CTT: Radiopaque markers (Sitzmarks, Konsyl Pharmaceuticals, United States) were used for detection. Each capsule contained 24 radiopaque markers, measuring 1 mm × 4.5 mm, of three different shapes: The O-Ring shape, the Double D shape, and the Tri-chamber shape. On day 1, subjects took one capsule containing the Double D shape under supervision, and swallowed the second capsule containing the O-Ring shape and the third capsule containing the Tri-chamber shape after 24 h and 48 h, respectively. After 72 h, a kidney, ureter, and bladder examination of the abdomen was performed. If the transverse colon could not be fully displayed, an X-ray of the upper abdomen was taken. The transit time of the whole colon and segmented colon was then calculated according to Metcalfe's method[10] (Figure 1).
IBM SPSS 22.0 was used to analyze all data. Continuous variables were analyzed using a t-test (normally distributed) and expressed as mean ± SD. Data with a non-normal distribution were expressed as percentiles, and the Kruskal-Wallis test was used to compare groups. Categorical data were calculated using the Chi-square test and expressed as a ratio (%). A P < 0.05 was considered statistically significant.
Twenty patients with PDC and 20 patients with FC were included in the study, and there were no statistically significant differences in sex, age, body mass index (BMI), or duration of constipation between the two groups. The chief complaints of constipation across the two groups were compared, and it was found that more patients in the PDC group exhibited difficulty in defecating than in the FC group, and the difference was statistically significant (Table 1).
| FC | PDC | P value | |
| Male | 7 (35.0%) | 12 (60.0%) | 0.205 |
| Female | 13 (65.0%) | 8 (40.0%) | |
| Age, years | 70.80 (6.24) | 72.32 (4.80) | 0.402 |
| BMI | 23.32 (2.47) | 22.25 (4.32) | 0.343 |
| Decreased defecation frequency | 10 (50.0%) | 16 (80.0%) | 0.097 |
| Dry stools | 9 (45.0%) | 6 (30.0%) | 0.514 |
| Defecatory difficulties | 13 (65.0%) | 20 (100.0%) | 0.013 |
| Manual assisted defecation | 7 (35.0%) | 12 (60.0%) | 0.205 |
| Constipation duration, years | 10.00 (5.00, 20.00) | 5.00 (2.75, 6.50) | 0.051 |
The rectal resting pressure and anal sphincter resting pressure in the PDC group were significantly lower than those in the FC group. The intrarectal pressure and anal relaxation rate in the PDC group were significantly lower than those in the FC group. The proportion of paradoxical contractions in the PDC group was significantly higher than that in the FC group. These results are shown in Table 2. The study found that the proportion of defecatory impetus deficiency was 50% (10/20) in the PDC group and 40% (8/20) in the FC group; the proportion of pelvic floor muscle dyssynergia was 35% (7/20) in the PDC group and 20% (4/20) in the FC group. In addition, 10% (2/20) of patients in the PDC group demonstrated inadequate defecatory propulsion combined with pelvic floor muscle dyssynergia. Rectal anal manometry was normal in 40% (8/20) of the FC group and in only 5% (1/20) of the PDC group, with a statistically significant difference in the composition type ratio of defecatory disorders between the two groups (P < 0.05) (Table 3).
| FC | PDC | P value | |
| Rectal resting pressure (mmHg) | 82.36 (20.18) | 63.98 (30.94) | 0.032 |
| Anal sphincter resting pressure (mmHg) | 89.55 (16.25) | 66.50 (19.35) | < 0.001 |
| Rectal maximum squeeze pressure (mmHg) | 189.50 (159.95,250.68) | 188.95 (139.60,234.00) | 0.516 |
| Anal canal maximum squeeze pressure (mmHg) | 222.16 (86.26) | 195.25 (63.62) | 0.269 |
| Intrarectal pressure (mmHg) | 43.08 (20.74) | 21.73 (14.61) | 0.001 |
| Anal relaxation rate (%) | 23.00 (16.00, 42.58) | 0.16 (-0.07, 0.22) | < 0.001 |
| Residual anal pressure (mmHg) | 75.83 (33.55) | 69.70 (30.92) | 0.551 |
| Rectoanal pressure difference (mmHg) | -36.77 (36.56) | -47.98 (27.38) | 0.279 |
| Paradoxical contraction | 1 (5.0%) | 8 (40.0%) | 0.023 |
| FC | PDC | P value | |
| Defecatory impetus deficiency | 8 (40.0) | 10 (50.0) | 0.037 |
| Pelvic floor muscle dyssynergia | 4 (20.0) | 7 (35.0) | |
| Inadequate defecatory propulsion combined with pelvic floor muscle dyssynergia | 0 (0.0) | 2 (10.0) | |
| Normal | 8 (40.0) | 1 (5.0) |
Left colonic transit time (LCTT), rectosigmoid colonic transit time (RSCTT), and total colonic transit time (TCTT) were prolonged in PDC and FC patients when compared with normal values. A comparison of the segmented colonic transit time between the two groups revealed that the patients with FC had a significantly longer right colonic transit time (RCTT) and a significantly shorter RSCTT than the patients with PDC (P < 0.05), whereas there were no significant differences in the LCTT and TCTT between the two groups (Table 4).
| FC | PDC | P value | |
| RCTT (h) | 10.50 (3.12) | 8.40 (3.28) | 0.045 |
| LCTT (h) | 19.50 (6.07) | 20.15 (5.07) | 0.715 |
| RSCTT (h) | 24.45 (4.56) | 27.60 (3.68) | 0.021 |
| TCTT (h) | 55.10 (7.46) | 56.00 (8.31) | 0.721 |
Patients with PDC were divided into three subtypes based on CTT and HRAM: (1) Slow transit constipation: Prolonged CTT only (1, 5%); (2) Defecatory disorder: Altered HRAM only (4, 20.0%); and (3) Mixed constipation: Prolonged CTT and altered HRAM (15, 75.0%). Patients with FC were divided into four subtypes: (1) Slow transit constipation: (7, 35.0%); (2) Defecatory disorder: (2, 10.0%); (3) Mixed constipation: (10, 50.0%); and (4) Normal transit constipation: No functional abnormality (1, 5.0%). There were no statistically significant differences in constipation subtypes between the two groups, as seen in Table 5.
| FC | PDC | P value | |
| Slow transit constipation | 7 (35.0) | 1 (5.0) | 0.067 |
| Defecatory disorder | 2 (10.0) | 4 (20.0) | |
| Mixed constipation | 10 (50.0) | 15 (75.0) | |
| Normal transit constipation | 1 (5.0) | 0 (0.0) |
Constipation is one of the most important nonmotor symptoms in PD patients, and can occur several years or even decades before the onset of exercise symptoms[11,12]. Taiwanese scholars[5] conducted a follow-up survey of the relationship between the severity of constipation and the risk of PD over 5 years. The study showed that the incidence rate of PD was 1.57/100000 for those without constipation. The incidence rate of PD in patients with mild, moderate, and severe constipation increased 5 years later, to 4.04/100000, 5.28/100000, 12.67/100 000, and 12.67/100000, respectively. Therefore, constipation of different severities is closely related to the pathogenesis of PD. Although constipation may be the first symptom of PD, the incidence rate of constipation is high and is influenced by many factors. It is not specific for predicting PD[13]. Thus, this study compared PDC and FC and analyzed the constipation symptoms of the two groups of patients. At the same time, the colonic transit test and HRAM were used to classify constipation and detect the corresponding indicators, in order to find more sensitive and specific indicators to predict which constipation patients will eventually develop PD and to identify the gastrointestinal motility of PDC. It is expected to provide a basis for the treatment of PDC.
The rectal anal manometry classification in this study identified that the incidence of rectoanal dysfunction in the PDC group was 95%, suggesting that anal dysfunction plays an important role in the formation of PDC. In addition, this study compared the rectal and anal dynamics of patients with PDC and FC and found that the resting rectal and anal canal pressures of the PDC group were significantly lower than those of the FC group. This is mainly derived from the internal anal sphincter tension, accounting for about 70%-85% of resting pressure[14], suggesting that patients with PDC have internal sphincter dysfunction. Some previous[14,15] neuropathological studies have found Lewy bodies in the central nervous system, peripheral autonomic system, and enteric nervous system (ENS) of PD patients. The latter can cause neurodegenerative changes in the ENS. It is speculated that neuropathy may involve the autonomic nerves innervating the internal anal sphincter, causing low resting pressure in the rectum and anal canal. In simulated defecation, the rectal defecation pressure and anal relaxation rate of the PDC group were significantly lower than those of the FC group. Rectal defecation pressure is mainly produced by abdominal pressure. Fontana et al[16] has found that the increase in abdominal pressure in patients with PD is significantly lower than that in healthy controls. Fontana et al[16] believed that the mechanism of impaired abdominal pressure may be due to increased axial muscle tension and decreased contractility. In this study, patients with PDC had an insufficient rectal defecation driving force, such that the anus could not be effectively relaxed resulting in abnormal contraction. This is considered a local dystonia in patients with PD, where rectal contraction is reduced during defecation and the abdominal tension becomes weak. A coordinated movement disorder results due to reduced rectoanal muscle contraction and puborectal and pelvic smooth muscle dysfunction. The performance of this dystonia is aggravated during the "close" phase of PD and lessened during the "open" phase[17].
Our results showed that the LCTT, RSCTT, and TCTT were significantly prolonged in PDC patients, and the distribution of colonic transmission time in each segment of PDC patients was not uniform, especially in the left semicolon and rectum sigmoid colon, which was consistent with previous studies[18-20]. However, to our knowledge, no study has compared the segmental colon transmission time between PDC and FC patients. Our results suggest that the RSCTT of PDC patients was significantly longer than that of FC patients while the RCTT was significantly shorter than that of FC patients; this revealed the similarities and differences in the mechanism of slow colon transmission between patients with PDC and those with FC. The common mechanism may be explained as follows: (1) Compared with healthy individuals, vasoactive intestinal peptide (VIP) and VIP receptors in the colon mucosa of PDC and FC patients were downregulated[20]. VIP is a non-adrenergic non-cholinergic neuroinhibitory neurotransmitter, and its reduction could increase colon segment peristalsis, weakening the effective promotion of movement. At the same time, through abnormal colon secretion, VIP could reduce the fecal water content and lead to stool sclerosis, thus prolonging colon transmission time; and (2) ENS degeneration, a decrease in the number of intermuscular plexuses of the colonic wall, and the formation of ganglion cell vacuoles may all cause a decrease in the peristaltic function of the colon. The differences between PDC patients and FC patients are as follows: (1) PD leads to the loss of dopaminergic neurons between intestinal muscles, which may lead to decreased colonic transport function[21]. The ENS is rich in cholinergic neurons, including intestinal intermuscular motor neurons, primary transmission neurons, intermediate neurons, and other neurons projecting to the prevertebral sympathetic ganglion. Dopamine is a reactant of tyrosine hydroxylase in 4%-11% of enteric muscle neurons. Therefore, dopamine plays an important role in regulating gastrointestinal motility. Moreover, the innervation of the digestive tract gradually decreases from proximal to distal, and the motor function reduction caused by the loss of dopaminergic neurons is more serious in the distal colon segment[22]; and (2) PDC patients have more abnormal rectal-anal manometry results than FC patients. Some researchers believe that PD patients may have an independent "pelvic floor cooperative motion disorder" which is an element of extrapyramidal disease and a sequelae of neurodegeneration. These are also the reasons why the RSCTT of those with PDC was significantly longer than that of FC patients in this study.
In this study, mixed constipation was dominant in both PDC patients and FC patients, suggesting that both groups of patients had severe functional dyspraxia in the colon and rectum. There was no statistical difference between the two groups. According to the results of HRAM, the rectal sensorimotor dysfunction in patients with PDC was mainly caused by insufficient bowel movement impetus, which may in turn be caused by autonomic neuropathy innervating the rectum. A small number of patients with pelvic floor muscle synergy disorder may also have pelvic floor muscle dystonia caused by PD.
This study has several limitations. The overall sample size of this study was small. In addition, a sample of healthy individuals were not included in the study. Increasing the sample size of patients with PD and including healthy subjects will help us to better understand the pathophysiological mechanism of PDC.
In conclusion, cases of PDC and FC were associated with a prolonged CTT and abnormal HRAM. However, the rectal resting pressure, anal sphincter resting pressure, intrarectal pressure, and anal relaxation rate in the PDC group were significantly lower than those in the FC group. The proportion of paradoxical contractions in the PDC group was significantly higher than that in the FC group. The different segments of the CTT were also significantly different. The RSCTT of PDC patients was significantly longer than that of FC patients, and the RCTT was significantly shorter. The above indexes can be helpful for further studies of the mechanism of PDC and FC and for early diagnosis and treatment of patients with PD.
Patients with PDC and FC have severe functional dysmotility of the colon and rectum, but there are certain differences in segmental colonic transit time and rectal anal canal pressure between the two groups.
Parkinson's disease (PD) is a common neurodegenerative disease characterized clinically by typical motor symptoms such as tremor, bradykinesia and myotonia and non motor symptoms such as constipation, depression and dysmetria. Constipation is one of the most common clinical manifestations of PD patients. Investigations have shown that the incidence of constipation among PD patients is up to 88%, and constipation is regarded as one of the independent risk factors for PD. PD constipation (PDC) is considered a unique type of constipation that is clinically inadequately treated, severely affecting patient quality of life. Current studies on the characteristics of intestinal motility in patients with Parkinson's disease with constipation are less frequently reported, and the control groups are mostly healthy subjects, and the conclusions are not uniform.
It is likely that there is a causal relationship between PD and constipation, such that they exacerbate one another and form a vicious cycle. However, PDC treatment is very difficult and prone to drug resistance. Compared with functional constipation, it is not clear whether the clinical characteristics and influencing factors of PDC are unique. Understanding the gastrointestinal motility characteristics and constipation classification of PDC patients is essential to guide the treatment of PDC.
To identify the gastrointestinal motility of PDC and provide a basis for its treatment. Moreover, to find more sensitive and specific indicators to predict which constipation patients will eventually develop PD.
A colonic transit test and high-resolution anorectal manometry were performed to compare the differences in colonic transit time, rectal anal canal pressure, and constipation classification between Patients with PDC and functional constipation (FC).
The study found that the rectal resting pressure, anal sphincter resting pressure, intrarectal pressure, and anal relaxation rate in the PDC group were significantly lower than those in the FC group. The proportion of paradoxical contractions in the PDC group was significantly higher than that in the FC group. The different segments of the colonic transit test (CTT) were also significantly different. The rectosigmoid colonic transit time of PDC patients was significantly longer than that of FC patients, and the right colonic transit time was significantly shorter.
Cases of PDC and FC were associated with a prolonged CTT and abnormal high-resolution anorectal manometry. There are certain differences in segmental colonic transit time, rectal anal canal pressure and composition type ratio of defecatory disorders between the two groups.
This study can be helpful for further studies of the mechanism of PDC and FC and for early diagnosis and treatment of patients with PD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thiam A, Ward Z, Yao B S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, Manubens-Bertran JM, Alpérovitch A, Rocca WA. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 518] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol. 2010;120:131-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 441] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Pfeiffer RF. Non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2016;22 Suppl 1:S119-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 4. | Pfeiffer RF. Gastrointestinal Dysfunction in Parkinson's Disease. Curr Treat Options Neurol. 2018;20:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Lin CH, Lin JW, Liu YC, Chang CH, Wu RM. Risk of Parkinson's disease following severe constipation: a nationwide population-based cohort study. Parkinsonism Relat Disord. 2014;20:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30:1600-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 914] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 7. | Sharma A, Kurek J, Morgan JC, Wakade C, Rao SSC. Constipation in Parkinson's Disease: a Nuisance or Nuanced Answer to the Pathophysiological Puzzle? Curr Gastroenterol Rep. 2018;20:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Knowles CH, Lindberg G, Panza E, De Giorgio R. New perspectives in the diagnosis and management of enteric neuropathies. Nat Rev Gastroenterol Hepatol. 2013;10:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Tateno F, Sakakibara R, Kishi M, Ogawa E, Yoshimatsu Y, Takada N, Suzuki Y, Mouri T, Uchiyama T, Yamamoto T. Incidence of emergency intestinal pseudo-obstruction in Parkinson's disease. J Am Geriatr Soc. 2011;59:2373-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 641] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Knudsen K, Krogh K, Østergaard K, Borghammer P. Constipation in parkinson's disease: Subjective symptoms, objective markers, and new perspectives. Mov Disord. 2017;32:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Stirpe P, Hoffman M, Badiali D, Colosimo C. Constipation: an emerging risk factor for Parkinson's disease? Eur J Neurol. 2016;23:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Mahlknecht P, Seppi K, Poewe W. The Concept of Prodromal Parkinson's Disease. J Parkinsons Dis. 2015;5:681-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Lubomski M, Davis RL, Sue CM. Gastrointestinal dysfunction in Parkinson's disease. J Neurol. 2020;267:1377-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Uyar GÖ, Yildiran H. A nutritional approach to microbiota in Parkinson's disease. Biosci Microbiota Food Health. 2019;38:115-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson's disease. Am J Respir Crit Care Med. 1998;158:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Yuan ZH, Wang K, Duan LP, Fan DS, Xu ZJ, Xia ZW, Ge Y. [The characteristics of anorectal manometry in Parkinson's disease with constipation and functional constipation]. Zhonghua Nei Ke Za Zhi. 2013;52:562-566. [PubMed] |
| 18. | Jost WH, Schrank B. Defecatory disorders in de novo Parkinsonians--colonic transit and electromyogram of the external anal sphincter. Wien Klin Wochenschr. 1998;110:535-537. [PubMed] |
| 19. | Sakakibara R, Odaka T, Uchiyama T, Asahina M, Yamaguchi K, Yamaguchi T, Yamanishi T, Hattori T. Colonic transit time and rectoanal videomanometry in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Knudsen K, Fedorova TD, Bekker AC, Iversen P, Østergaard K, Krogh K, Borghammer P. Objective Colonic Dysfunction is Far more Prevalent than Subjective Constipation in Parkinson's Disease: A Colon Transit and Volume Study. J Parkinsons Dis. 2017;7:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Blandini F, Balestra B, Levandis G, Cervio M, Greco R, Tassorelli C, Colucci M, Faniglione M, Bazzini E, Nappi G, Clavenzani P, Vigneri S, De Giorgio R, Tonini M. Functional and neurochemical changes of the gastrointestinal tract in a rodent model of Parkinson's disease. Neurosci Lett. 2009;467:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Knudsen K, Haase AM, Fedorova TD, Bekker AC, Østergaard K, Krogh K, Borghammer P. Gastrointestinal Transit Time in Parkinson's Disease Using a Magnetic Tracking System. J Parkinsons Dis. 2017;7:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |