Published online Feb 21, 2020. doi: 10.3748/wjg.v26.i7.740
Peer-review started: November 15, 2019
First decision: December 4, 2019
Revised: December 5, 2019
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: February 21, 2020
Processing time: 97 Days and 11.8 Hours
The incidence of post-endoscopic retrograde cholangiopancreatography (ERCP) cholangitis (PEC) in patients who underwent mechanical lithotripsy (ML) for large stone removal is high (up to 13.3%). One of the main causes is remaining small fragments or sludge that can impair normal biliary drainage. Endoscopic placement of a nasobiliary tube or a conventional plastic biliary stent has been commonly used under such conditions, but the patient may suffer from significant discomfort after the placement of a nasobiliary tube, while additional endoscopy is required for stent removal. We developed a biliary spontaneous dislodgement spiral stent (BSDSS) to overcome those shortcomings.
To evaluate the feasibility, safety, and effectiveness of inserting a BSDSS for patients who underwent ML for large stone removal.
We conducted a single-center, retrospective, cohort study at West China Hospital, Sichuan University. A total of 91 consecutive patients with large biliary stones (≥ 10 mm) in the common bile duct who underwent ML between November 2017 and July 2018 were included. The 49 eligible patients were divided into the BSDSS group and the nasobiliary tube group. Technical success, post-ERCP adverse events (including PEC, post-ERCP pancreatitis, stone recurrence, BSDSS retention, self-extraction and dislocation of the nasobiliary tube), drainage time, and postoperative stay were measured and compared.
Twenty-one patients in the BSDSS group and 28 patients in the nasobiliary tube group were included in the analyses. The baseline characteristics and clinical information were similar in the two groups. Insertions of BSDSS and nasobiliary tube were technically successful in all 49 patients. There was no significant difference in the incidence of overall post-ERCP adverse events between the two groups (4.8% in the BSDSS group vs 17.9% in the nasobiliary tube group, P = 0.219). The median duration of drainage time (3 d in the BSDSS group vs 4 d in the nasobiliary tube group) and length of postoperative stay (4 d in the BSDSS group vs 5 d in the nasobiliary tube group) also did not differ (P = 0.934, and P = 0.223, respectively).
Endoscopic placement of a BSDSS appears to be feasible, safe and effective for patients who underwent ML for large stone removal.
Core tip: This retrospective cohort study describes the feasibility, safety, and effectiveness of inserting a biliary spontaneous dislodgement spiral stent (BSDSS) for patients who underwent mechanical lithotripsy for large stone removal. All BSDSSs were inserted successfully and evacuated spontaneously after a median duration of 3 d without additional injuries to the digestive tract. Comparable results of post-endoscopic retrograde cholangiopancreatography adverse events, drainage time, and length of postoperative stay were observed in the BSDSS group (n = 21) and the nasobiliary tube group (n = 28).
- Citation: Ye LS, Yuan XL, Wu CC, Liu W, Du J, Yao MH, Tan QH, Hu B. Biliary spontaneous dislodgement spiral stent for patients who underwent mechanical lithotripsy. World J Gastroenterol 2020; 26(7): 740-748
- URL: https://www.wjgnet.com/1007-9327/full/v26/i7/740.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i7.740
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the main methods of removing biliary stones in the common bile duct (CBD)[1-3]. Endoscopic sphincterotomy (EST) and endoscopic papillary balloon dilation (EPBD) are the most commonly used modalities for stone removal[1-3], but mechanical lithotripsy (ML) may also be required to remove large biliary stones. ML is the simplest method to fragment large CBD stones, but it carries the risk of remaining debris (including small fragments and sludge), even in patients with successful stone removal according to the judgment of the operating endoscopists. In general, placement of a nasobiliary tube or a conventional plastic biliary stent[4] can be performed in this setting, but the applications of both devices have obvious shortcomings. Patients with nasobiliary tubes may suffer from significant discomfort due to the transnasal placement[5,6], which could lead to self-extraction and dislocation of the tube; additionally, bile loss caused by external drainage can lead to electrolyte imbalance[5,6], which is especially risky for patients with arrhythmia. Patients with conventional plastic biliary stents have to undergo another endoscopy for stent removal[6], which incurs additional medical costs. We developed a biliary spontaneous dislodgement spiral stent (BSDSS) to overcome the above shortcomings of nasobiliary tubes and conventional biliary stents[7,8]. This retrospective cohort study assessed the feasibility, safety, and effectiveness of the placement of a BSDSS for patients who underwent ML for large stone (≥ 10 mm) removal by comparing the clinical outcomes of BSDSS patients with those of nasobiliary tube patients.
This single-center, retrospective cohort study was conducted at West China Hospital, Sichuan University, a tertiary hospital. The study protocol was approved by the Biomedical Research Ethics Committee, West China Hospital, Sichuan University.
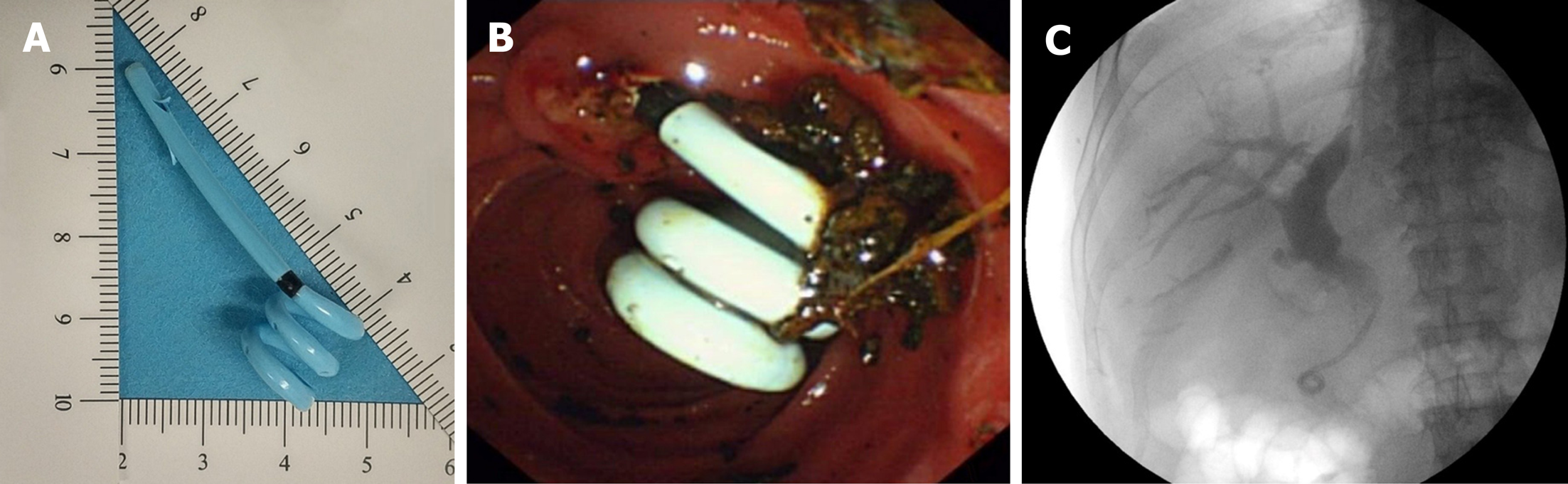
The BSDSS used in this study is made of soft and pliable thermoplastic polyurethane, which is different from the commonly used plastic biliary stent (polyte-trafluoroethylene). The main difference in shape between the BSDSS and the conventional plastic biliary stent is the duodenal end (Figure 1). The BSDSS has more than one spiral, whereas the conventional has no or only one spiral (straight type and pigtail type, respectively). There are several side holes in the spirals of the BSDSS, and the diameter of the spirals is 12 mm. Another difference lies in the shape of the flanges in the bile duct end. Unlike the long flanges in the conventional stents, the BSDSSs have two short, thin flanges. The outer diameter of the BSDSS is 7 Fr, and its length is 7 cm.
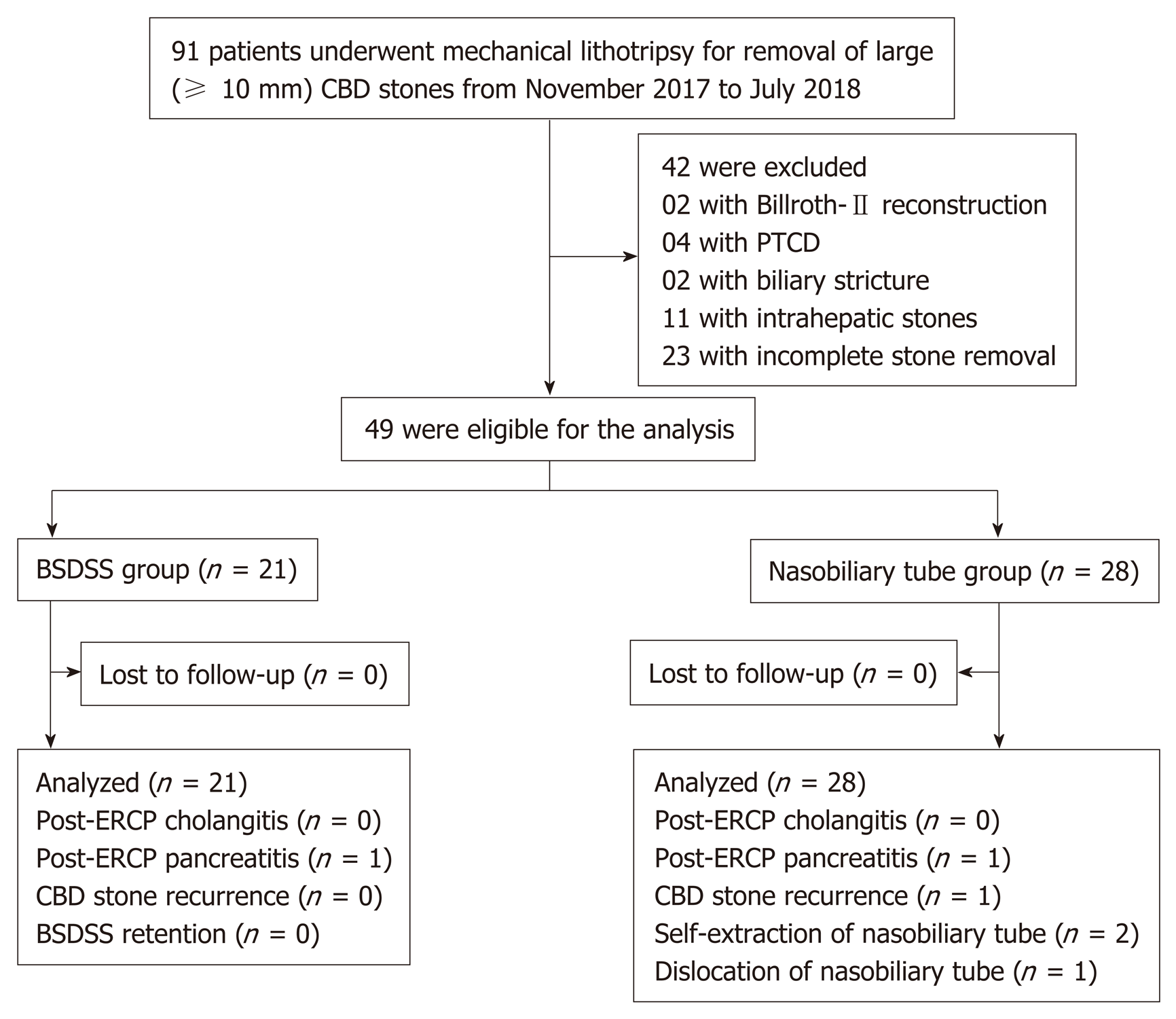
Consecutive patients with large biliary stones (≥ 10 mm) who underwent ML for stone removal between November 2017 and July 2018 were retrospectively collected from our prospectively collected database and the hospital medical records. The exclusion criteria were as follows: (1) Patients with altered anatomy; (2) Patients with percutaneous transhepatic cholangial drainage; (3) Patients with biliary stricture; (4) Patients with intrahepatic stones; and (5) Patients with incomplete stone removal (including failed ML and intolerance to repeated stone extraction).
All ERCP procedures were performed by an experienced endoscopist who underwent > 300 ERCP procedures per year. Patients were administered diazepam, pethidine, and anisodamine for conscious sedation, pain control, and bowel relaxation. ERCP was performed in the prone position using a standard duodenoscope (TJF-260V; Olympus, Tokyo, Japan). After selective cannulation of the CBD, a minimum of 25% omnipaque was injected to confirm the number and size of CBD stones. According to the endoscopist’s judgment, limited EST (3-5 mm), with or without small EPBD (8-10 mm), was performed to facilitate stone removal, followed by the application of ML. A trapezoid RX wire-guided retrieval basket (Boston Scientific Corporation; Marlborough, MA, USA) was used to fragment the stone, and then a grasping basket (FG-22Q-1; Olympus, Tokyo, Japan) was applied to extract fragments repeatedly.
For patients with nasobiliary tubes, a nasobiliary tube (7 Fr; Micro-Tech (Nanjing) Co., Ltd, Nanjing, China) was inserted into the intrahepatic duct using routine instruments and methods[5]. For patients with BSDSSs, a BSDSS [7 Fr × 7 cm; Micro-Tech (Nanjing) Co., Ltd, Nanjing, China] was advanced into the CBD using a guide wire (JagwireTM; Boston Scientific, Natick, MA, USA) aided by a plastic stent introduction device. The BSDSS was then released under fluoroscopic guidance, leaving the duodenal end with spirals outside the duodenal papilla. The BSDSS location was adjusted by the stent introduction device or endoscopic forceps according to the reference mark. Drainage of bile, small fragments or sludge, were confirmed before withdrawal of the duodenoscope.
After the procedure, the patients fasted for at least 24 h. Blood tests, including complete blood counts, liver function tests, and pancreatic enzymes, were performed 6-48 h after the procedure. Computed tomography (CT) or magnetic resonance cholangiopancreatography (MRCP) was performed when needed. Daily abdominal radiography was scheduled to determine the BSDSS location until the BSDSS was noted when the patient had a bowel movement. Postoperative cholangiography (for patients with nasobiliary tube and without self-extraction or dislocation of nasobiliary tube) or abdominal ultrasound (for patient with BSDSS, and for patients with nasobiliary tube but with self-extraction or dislocation of nasobiliary tube) was performed to detect residual debris, and additional ERCP was performed when needed. Cholecystectomy was recommended for patients with cystic stones.
Patients were followed via clinical visits every 3-6 mo, during which clinical symptoms and laboratory tests including liver function tests were recorded; abdominal ultrasound, CT, or MRCP were performed to identify CBD stone recurrence. Follow-up for each patient was discontinued till CBD stone recurrence or October 2019.
We evaluated the technical success, post-ERCP adverse events, drainage time, and postoperative stay.
Technical success was defined as the successful insertion of the BSDSS or nasobiliary tube into the bile duct in an appropriate position based on endoscopic and fluoroscopic confirmation.
Post-ERCP adverse events mainly included post-ERCP cholangitis (PEC), post-ERCP pancreatitis (PEP) and CBD stone recurrence. Other events relevant to the BSDSS (BSDSS retention and BSDSS-related injuries to the digestive tract) or nasobiliary tube (self-extraction and dislocation of the nasobiliary tube) were also recorded. PEC was defined as a fever (> 38°C), leukocytosis, and evidence of cholestasis[9]. PEP was defined as persistent pain associated with a serum amylase (or serum lipase) level ≥ 3 times the normal upper limit.[9] As a recent study[10] revealed that the revised Atlanta criteria[11] better reflect the severity of PEP than a previous consensus by Cotton et al[12], the revised Atlanta classification was used as a grading standard in this study. CBD stone recurrence was defined as the observation of CBD stones six months or more after ERCP[13].
Because BSDSS dislodgement cannot be precisely detected, the duration from BSDSS placement to evacuation (i.e., evacuation time) was measured as the drainage time for the BSDSS. For patients with nasobiliary tubes, the drainage time was defined as the duration from tube placement to tube extraction.
Postoperative stay was defined as the duration from the ERCP procedure to discharge. For patients who underwent additional ERCP to remove residual debris during the same hospitalization, the postoperative stay was calculated from the initial ERCP to discharge.
SPSS 25.0 was used for statistical analysis. Numerical variables are expressed as the means (standard deviations) or medians (interquartile ranges, IQRs) according to their distribution and were compared using Student’s t test or Mann-Whitney U-test, accordingly. Categorical variables are expressed as the numbers or proportions and were compared using χ2 tests or Fisher’s exact tests as appropriate. P values < 0.05 were considered significant.
From November 2017 to July 2018, a total of 91 patients with large biliary stones (≥ 10 mm) underwent ML for stone removal (Figure 2), and 49 patients met the criteria for inclusion in this study. Among these 49 patients, 21 underwent endoscopic placement of a BSDSS, while the other 28 patients underwent endoscopic placement of a nasobiliary tube. The baseline characteristics and clinical information in each group were similar (Table 1).
| BSDSS group (n = 21) | Nasobiliary tube group (n = 28) | P value | |
| Age, mean ± SD, yr | 64 ± 16 | 67 ± 19 | 0.5725 |
| Sex, male/female | 11/10 | 13/15 | 0.6806 |
| Diagnosis | 0.9517 | ||
| Biliary colic | 6 (28.6) | 10 (35.7) | |
| Obstructive jaundice | 7 (33.3) | 8 (28.6) | |
| Acute cholangitis1 | 6 (28.6) | 7 (25.0) | |
| Acute pancreatitis1 | 2 (9.5) | 3 (10.7) | |
| Comorbidity2 | 10 (47.6) | 18 (64.3) | 0.2436 |
| Gallbladder status | 0.4107 | ||
| Post cholecystectomy | 12 (57.1) | 13 (46.4) | |
| Cholecystectomy after ERCP | 2 (9.5) | 1 (3.6) | |
| Gallbladder stones in situ | 5 (23.8) | 6 (21.4) | |
| No gallbladder stones | 2 (9.5) | 8 (28.6) | |
| Previous EST | 2 (9.5) | 6 (21.4) | 0.4387 |
| Periampullary diverticulum | 11 (52.4) | 12 (42.9) | 0.5096 |
| Maximum CBD diameter, median (IQR), mm | 13 (12-16) | 15 (13-15) | 0.2148 |
| Maximum stone diameter, median (IQR), mm | 13 (11-16) | 12 (12-15) | 0.5818 |
| Minimum stone diameter, median (IQR), mm | 10 (9-12) | 12 (10-12) | 0.7618 |
| Stones number, < 3/≥ 3 | 14/7 | 25/3 | 0.0766 |
| ERCP modalities for stone removal | 0.5957 | ||
| ML3 | 1 (4.8) | 4 (14.3) | |
| EST + ML | 3 (14.3) | 6 (21.4) | |
| EPBD + ML3 | 1 (4.8) | 2 (7.1) | |
| EST + EPBD + ML | 16 (76.2) | 16 (57.1) | |
| Residual debris4 | 1 (4.8) | 5 (17.9) | 0.2197 |
The clinical outcomes in the two groups are shown in Table 2. Insertion of the BSDSS or nasobiliary tube was technically successful with a single attempt in all 49 patients. There was no need to use forceps to adjust the location of the BSDSS. There was no PEC in the two groups, but mild PEP was noted in one patient in the BSDSS group (4.8%, 1/21) and one in the nasobiliary tube group (3.6%, 1/28); which was controlled in both patients with conservative treatment. During a median follow-up of 18 mo (IQR, 15-21; range, 13-23), CBD stone recurrence was detected in one patient in the nasobiliary tube group (3.6%, 1/28), and additional ERCP was performed to remove the recurrent stone. In addition, all BSDSSs were dislodged and evacuated spontaneously after a median duration of 3 d (IQR, 3-5; range, 2-8), without additional injuries to the digestive tract; most patients (76.2%, 16/21) noticed the dislodged BSDSS when they had a bowel movement. Two patients in the nasobiliary tube (7.1%, 2/28) extracted the tube by themselves on postoperative day 1, due to intolerance of the transnasal placement of the tube; dislocation of the nasobiliary tube was also noted in 1 patient in the nasobiliary tube group (3.6%, 1/28). There were no significant differences in the incidence of overall post-ERCP adverse events (4.8% in the BSDSS group vs 17.9% in the nasobiliary tube group, P = 0.219).
| BSDSS group (n = 21) | Nasobiliary tube group (n = 28) | P value | |
| Technical success | 21 (100) | 28 (100) | - |
| Overall post-ERCP adverse events | 1 (4.8) | 5 (17.9) | 0.2194 |
| Cholangitis | 0 (0) | 0 (0) | - |
| Pancreatitis1 | 1 (4.8) | 1 (3.6) | 1.0004 |
| CBD stone recurrence | 0 (0) | 1 (3.6) | 1.0004 |
| Other events2 | 0 (0) | 3 (10.7) | 0.2504 |
| Follow-up duration, median (IQR), mo | 19 (17-22) | 18 (15-21) | 0.3655 |
| Drainage time3, median (IQR), d | 3 (3-5) | 4 (2-5) | 0.9345 |
| Postoperative stay, median (IQR), d | 4 (3-6) | 5 (3-7) | 0.2235 |
The median drainage time in the BSDSS group was 3 d and that in the nasobiliary tube group was 4 d, without a significant difference between the groups (P = 0.934). The median length of postoperative stay was also similar in the two groups (4 d in the BSDSS group vs 5 d in the nasobiliary tube group, P = 0.223).
This retrospective cohort study was conducted to evaluate the feasibility of the placement of a BSDSS for patients who underwent ML for large stone (≥ 10 mm) removal. All inserted BSDSSs were dislodged and evacuated spontaneously without additional injuries to the digestive tract, and the incidence of post-ERCP adverse events in the BSDSS group was low and comparable with that in the nasobiliary tube group. Although the duration from BSDSS placement to evacuation was uncontrollable, similar results in terms of the drainage time and postoperative stay were noted in the two groups. Our findings show the feasibility, safety, and effectiveness of BSDSS for patients who underwent ML for large stone removal.
Apart from EST and EPBD, ML is helpful for removing large stones due to its ability to fragment stones, but the rate of PEC after ML can be high (13.3%, 6/45)[4]. Residual small fragments or sludge, as well as injuries to the biliary tract and papillary edema caused by repeated manipulations, may be potential causes. The use of a nasobiliary tube in patients who underwent ML ensures the direct evaluation of the drainage characteristics and facilitates postoperative cholangiography to detect residual debris. However, external drainage from the nasobiliary tube could cause significant discomfort due to the transnasal placement and bile loss. Discomfort in the nostril and throat may lead to self-extraction and dislocation of the nasobiliary tube[14], which was noted in 3 patients with nasobiliary tubes in this study (10.7%, 3/28). Tube kinking, compression ulcers, and aspiration pneumonia can also occur[5,6,15]. Although external drainage-induced electrolyte imbalance, such as hypokalemia, is uncommon during short-term biliary drainage (0% in this study), it is risky for patients with arrhythmia once developed. In addition, abdominal ultrasound can be applied to detect residual debris as a substitute for postoperative cholangiography. As shown in Table 1, residual debris was detected by abdominal ultrasound in one patient in the BSDSS group and one patient in the nasobiliary tube group who underwent additional ERCP for debris removal. Given the above, the application of a BSDSS avoids the nasobiliary tube-related medical risks and improves the quality of life of patients.
Compared with conventional plastic biliary stents, the main strength of the BSDSS is its ability to dislodge and evacuate spontaneously after a short period of internal biliary drainage, which was noted in all patients in this study (100%, 21/21). We postulate that the BSDSS is dislodged after papillary edema abates, with the help of bowel movements and/or the passage of high-fiber chyme. In contrast, only 5%-10% of conventional plastic biliary stents can migrate distally[16]; thus, additional endoscopy is frequently required for stent removal. In addition, the BSDSS is soft and has several spirals on the duodenal side, which makes it less likely to lead to stent-related bowel perforation or fistula that have been reported previously in patients with conventional plastic biliary stents[17-21].
The main disadvantage of BSDSS is the lack of control over the timing of dislodgement and evacuation. The length of EST, as well as the size of EPBD, may seriously affect BSDSS dislodgement. For patients who underwent complete EST (and/or large EPBD), the BSDSS may be dislodged within a couple of hours because of the large opening of the papilla; thus, the application of a BSDSS in these patients seems unadvisable. However, as reported by previous studies[22-24], complete EST and large EPBD (12-20 mm) have been regarded to be associated with a higher rate of late adverse events; thus, our routines of performing limited (3-5 mm) EST, small (8-10 mm) EPBD and ML for large stone removal seem reasonable. In addition, we used daily radiography to identify the BSDSS location in this study, but BSDSS dislodgement still could not be detected accurately, and there may be a significant difference in the duration from BSDSS dislodgment to evacuation among patients. Because all BSDSSs were evacuated spontaneously after a median duration of 3 d (IQR, 3-5), daily radiography may not be necessary due to increased radiation exposure. We suggest that single radiography on postoperative day 5 may be preferable for patients with BSDSS if they ignore the evacuated BSDSS when they have a bowel movement. Further methods may be proposed for determining the real-time positioning of BSDSS and to clarify the real drainage time with the BSDSS.
The biodegradable stent reported by Anderloni et al[25] ensures different degradation times for distinct clinical demands using various polymeric mixtures, making it a promising stent for patients who underwent ML; however, the use of such biodegradable stents should be approached cautiously because partially degraded stents may impair normal drainage and affect the findings of follow-up abdominal imaging.
The present study had several limitations. First, it was a single-center, retrospective study with a small sample size, but consecutive patients who underwent ML for large stone removal were included, which helps reduce the selection bias. Prospective, multicenter, and large-scale studies are needed to further evaluate the role of BSDSSs in such patients. Second, a comparison with the conventional plastic biliary stent was absent. This is mainly due to the rare use of conventional plastic stents in patients who underwent successful stone extraction after ML in our endoscopy center; these patients routinely received nasobiliary tube before the introduction of BSDSS. Third, there was no comparative group without drainage, and thus, the necessity of placing a BSDSS needs to be further investigated. Although the three patients with tube self-extraction or dislocation in the nasobiliary tube group did not develop PEC, considering the reported high incidence of PEC in patients who underwent ML for stone removal (13.3%, 6/45)[4], a comparative study regarding the placement of BSDSS vs no BSDSS should be carefully conducted.
In conclusion, endoscopic placement of a BSDSS in patients who underwent ML for large stone removal appears to be feasible, safe and effective.
The incidence of post-endoscopic retrograde cholangiopancreatography (ERCP) cholangitis (PEC) in patients who underwent mechanical lithotripsy (ML) for large stone removal is high (up to 13.3%). One of the main causes is remaining small fragments or sludge that can impair normal biliary drainage. Endoscopic placement of a nasobiliary tube or a conventional plastic biliary stent was commonly used under such conditions, but the patient may suffer from significant discomfort after the placement of a nasobiliary tube, while additional endoscopy is required for stent removal.
We developed a biliary spontaneous dislodgement spiral stent (BSDSS) to overcome nasobiliary tube-related and conventional plastic biliary stent-related shortcomings. The duodenal end of the BSDSS is with several spirals, and its bile duct end has two short and thin flanges. We postulate that the BSDSS is dislodged after papillary edema abates, with the help of bowel movements and/or the passage of high-fiber chyme.
In this retrospective cohort study, we evaluated the feasibility, safety, and effectiveness of inserting a BSDSS for patients who underwent ML for large stone removal by comparing the clinical outcomes of BSDSS patients with those of nasobiliary tube patients.
From November 2017 to July 2018, a total of 91 consecutive patients underwent ML for large (≥ 10 mm) stone removal. Of these, 49 patients were eligible for this study, and they were divided into the BSDSS group and the nasobiliary tube group. Technical success, post-ERCP adverse events (including PEC, post-ERCP pancreatitis, stone recurrence, BSDSS retention, self-extraction and dislocation of the nasobiliary tube), drainage time, and postoperative stay were measured and compared.
Twenty-one patients in the BSDSS group and 28 patients in the nasobiliary tube group were included in the analyses. The baseline characteristics and clinical information were similar in the two groups. Insertions of BSDSS and nasobiliary tube were technically successful in all 49 patients. There was no significant difference in the incidence of overall post-ERCP adverse events between the two groups (4.8% in the BSDSS group vs 17.9% in the nasobiliary tube group, P = 0.219), as well as the median duration of drainage time (3 d in the BSDSS group vs 4 d in the nasobiliary tube group, P = 0.934) and the median length of postoperative stay (4 d in the BSDSS group vs 5 d in the nasobiliary tube group, P = 0.223).
Endoscopic placement of a BSDSS appears to be feasible, safe and effective for patients who underwent ML for large stone removal.
Multi-center studies with a large sample size are warranted to further confirm the safety and effectiveness of BSDSS. Comparative study regarding the placement of BSDSS vs no BSDSS is expected to clarify the necessity of routine application of BSDSS in patients who undergo ML for large stone removal.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Archibugi L, Langerth A S-Editor: Wang LY L-Editor: MedE- Ma JY E-Editor: Zhang YL
| 1. | Williams E, Beckingham I, El Sayed G, Gurusamy K, Sturgess R, Webster G, Young T. Updated guideline on the management of common bile duct stones (CBDS). Gut. 2017;66:765-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 2. | Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, Barthet M, Domagk D, Dumonceau JM, Gigot JF, Hritz I, Karamanolis G, Laghi A, Mariani A, Paraskeva K, Pohl J, Ponchon T, Swahn F, Ter Steege RWF, Tringali A, Vezakis A, Williams EJ, van Hooft JE. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 3. | ASGE Standards of Practice Committee; Buxbaum JL, Abbas Fehmi SM, Sultan S, Fishman DS, Qumseya BJ, Cortessis VK, Schilperoort H, Kysh L, Matsuoka L, Yachimski P, Agrawal D, Gurudu SR, Jamil LH, Jue TL, Khashab MA, Law JK, Lee JK, Naveed M, Sawhney MS, Thosani N, Yang J, Wani SB. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. 2019;89:1075-1105.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 4. | Stefanidis G, Viazis N, Pleskow D, Manolakopoulos S, Theocharis L, Christodoulou C, Kotsikoros N, Giannousis J, Sgouros S, Rodias M, Katsikani A, Chuttani R. Large balloon dilation vs. mechanical lithotripsy for the management of large bile duct stones: a prospective randomized study. Am J Gastroenterol. 2011;106:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Tsuyuguchi T, Takada T, Kawarada Y, Nimura Y, Wada K, Nagino M, Mayumi T, Yoshida M, Miura F, Tanaka A, Yamashita Y, Hirota M, Hirata K, Yasuda H, Kimura Y, Strasberg S, Pitt H, Büchler MW, Neuhaus H, Belghiti J, de Santibanes E, Fan ST, Liau KH, Sachakul V. Techniques of biliary drainage for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:35-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Lee JK, Lee SH, Kang BK, Kim JH, Koh MS, Yang CH, Lee JH. Is it necessary to insert a nasobiliary drainage tube routinely after endoscopic clearance of the common bile duct in patients with choledocholithiasis-induced cholangitis? A prospective, randomized trial. Gastrointest Endosc. 2010;71:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ye L, Hu B. Sa1366 Endoscopic Placement of A Plastic Spiral Stent for Short-Term Biliary Drainage in Patients with Cholelithiasis. Gastrointest Endosc. 2018;87:Ab232-Ab232. [DOI] [Full Text] |
| 8. | Ye L, Yuan X, Zeng X, Guo L, Yang W, Hu B. 1114 Placement of a Biliary Spontaneous Dislodgement Spiral Stent for Short-Term Biliary Drainage after Endoscopic Clearance of Common Bile Duct Stones: A Prospective Pilot Study. Gastrointest Endosc. 2019;89:AB137-AB138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1851] [Article Influence: 123.4] [Reference Citation Analysis (1)] |
| 10. | Smeets X, Bouhouch N, Buxbaum J, Zhang H, Cho J, Verdonk RC, Römkens T, Venneman NG, Kats I, Vrolijk JM, Hemmink G, Otten A, Tan A, Elmunzer BJ, Cotton PB, Drenth J, van Geenen E. The revised Atlanta criteria more accurately reflect severity of post-ERCP pancreatitis compared to the consensus criteria. United European Gastroenterol J. 2019;7:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 12. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2037] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 13. | Keizman D, Ish-Shalom M, Konikoff FM. The clinical significance of bile duct sludge: is it different from bile duct stones? Surg Endosc. 2007;21:769-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Xu XD, Dai JJ, Qian JQ, Wang WJ. Prevention of pancreatitis after papillary balloon dilatation by nasobiliary drainage: a randomized controlled trial. Dig Dis Sci. 2015;60:1087-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Isayama H, Nakai Y, Tsujino T, Kawabe T, Omata M. Which types of drainage tube should we select for endoscopic biliary drainage? Current status. Dig Endosc. 2006;18:S110-S111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | ASGE Technology Assessment Committee; Pfau PR, Pleskow DK, Banerjee S, Barth BA, Bhat YM, Desilets DJ, Gottlieb KT, Maple JT, Siddiqui UD, Tokar JL, Wang A, Song LM, Rodriguez SA. Pancreatic and biliary stents. Gastrointest Endosc. 2013;77:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Malhotra NR, Esparza Monzavi CA, Trepanier JS, Nordenstam J, Abern MR. Biliary Stent Migration: A Rare Cause of a Bladder Stone. Urology. 2017;104:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Kusters PJ, Keulen ET, Peters FP. Duodenal perforation following bile duct endoprosthesis placement. Endoscopy. 2014;46 Suppl 1 UCTN:E646-E647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Alcaide N, Lorenzo-Pelayo S, Herranz-Bachiller MT, de la Serna-Higuera C, Barrio J, Perez-Miranda M. Sigmoid perforation caused by a migrated biliary stent and closed with clips. Endoscopy. 2012;44 Suppl 2 UCTN:E274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Wilhelm A, Langer C, Zoeller G, Nustede R, Becker H. Complex colovesicular fistula: A severe complication caused by biliary stent migration. Gastrointest Endosc. 2003;57:124-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Størkson RH, Edwin B, Reiertsen O, Faerden AE, Sortland O, Rosseland AR. Gut perforation caused by biliary endoprosthesis. Endoscopy. 2000;32:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yasuda I, Tomita E, Enya M, Kato T, Moriwaki H. Can endoscopic papillary balloon dilation really preserve sphincter of Oddi function? Gut. 2001;49:686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Yasuda I, Fujita N, Maguchi H, Hasebe O, Igarashi Y, Murakami A, Mukai H, Fujii T, Yamao K, Maeshiro K, Tada T, Tsujino T, Komatsu Y. Long-term outcomes after endoscopic sphincterotomy versus endoscopic papillary balloon dilation for bile duct stones. Gastrointest Endosc. 2010;72:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Hakuta R, Kawahata S, Kogure H, Nakai Y, Saito K, Saito T, Hamada T, Takahara N, Uchino R, Mizuno S, Tsujino T, Tada M, Sakamoto N, Isayama H, Koike K. Endoscopic papillary large balloon dilation and endoscopic papillary balloon dilation both without sphincterotomy for removal of large bile duct stones: A propensity-matched analysis. Dig Endosc. 2019;31:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Anderloni AA, Fugazza A, luca m, Maselli R, Ormando VM, D'Amico F, Carrara S, Mangiavillano B, Omodei PD, Preatoni P, Lamonaca L, Cappello A, Pellegatta G, Repici A. 1111 Feasibility of New Biliary and Pancreatic Biodegradable Stent Placement: Interim Analysis of an Ongoing Single-Center, Prospective, Pilot Study. Gastrointest Endosc. 2019;89:Ab136-Ab136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |