Published online Feb 7, 2020. doi: 10.3748/wjg.v26.i5.514
Peer-review started: December 3, 2019
First decision: December 23, 2019
Revised: January 7, 2020
Accepted: January 15, 2020
Article in press: February 7, 2020
Published online: February 7, 2020
Processing time: 66 Days and 8.8 Hours
Gastrointestinal (GI) dysfunction is a common and important complication of acute pancreatitis (AP), especially in patients with severe AP. Despite this, there is no consensus means of obtaining a precise assessment of GI function.
To determine the association between acute gastrointestinal injury (AGI) grade and clinical outcomes in critically ill patients with AP.
Patients with AP admitted to our pancreatic intensive care unit from May 2017 to May 2019 were enrolled. GI function was assessed according to the AGI grade proposed by the European Society of Intensive Care Medicine in 2012, which is mainly based on GI symptoms, intra-abdominal pressure, and feeding intolerance in the first week of admission to the intensive care unit. Multivariate logistic regression analysis was performed to assess the association between AGI grade and clinical outcomes in critically ill patients with AP.
Among the 286 patients included, the distribution of patients with various AGI grades was 34.62% with grade I, 22.03% with grade II, 32.52% with grade III, and 10.84% with grade IV. The distribution of mortality was 0% among those with grade I, 6.35% among those with grade II, 30.11% among those with grade III, and 61.29% among those with grade IV, and AGI grade was positively correlated with mortality (χ2 = 31.511, P < 0.0001). Multivariate logistic regression analysis showed that age, serum calcium level, AGI grade, persistent renal failure, and persistent circulatory failure were independently associated with mortality. Compared with the Acute Physiology and Chronic Health Evaluation II score (area under the curve: 0.739 vs 0.854; P < 0.05) and Ranson score (area under the curve: 0.72 vs 0.854; P < 0.01), the AGI grade was more useful for predicting mortality.
AGI grade is useful for identifying the severity of GI dysfunction and can be used as a predictor of mortality in critically ill patients with AP.
Core tip: Gastrointestinal (GI) dysfunction is a common and important complication of acute pancreatitis, especially in patients with severe acute pancreatitis. Despite this, no consensus has been reached on a more precise assessment of GI function. In this manuscript, we studied the feasibility of using the acute GI injury grade to evaluate GI function, and concluded that the acute GI injury grade is helpful to identify the severity of GI dysfunction and can be used as a predictor of mortality in patients with acute pancreatitis.
- Citation: Ding L, Chen HY, Wang JY, Xiong HF, He WH, Xia L, Lu NH, Zhu Y. Severity of acute gastrointestinal injury grade is a good predictor of mortality in critically ill patients with acute pancreatitis. World J Gastroenterol 2020; 26(5): 514-523
- URL: https://www.wjgnet.com/1007-9327/full/v26/i5/514.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i5.514
The gut plays an important role in the pathogenesis and progression of acute pancreatitis (AP), especially in patients with severe AP (SAP), and is considered the “motor” of the systemic inflammatory response and multiple organ failure[1,2]. Gastrointestinal (GI) problems were reported to occur frequently in critically ill patients and usually associated with adverse outcomes, including those with SAP[3]. Despite this, no consensus has been reached on a more precise assessment of GI function. Furthermore, GI function is not included in the 2012 Atlanta classification of AP, which was widely used to assess the severity of AP. The importance of GI dysfunction in AP patients may be underestimated, which is obviously due to its lack of a precise definition.
In 2012, a more accurate and detailed definition of acute gastrointestinal injury (AGI) was proposed by the European Society of Intensive Care Medicine (ESICM), which was based on current medical evidence and expert opinions[4]. Several studies have shown that AGI grade is helpful to evaluate GI function, and it may be used as an early tool to predict the severity and adverse clinical outcomes in intensive care unit (ICU) patients[5,6]. However, the associations among AGI grade, the severity of GI dysfunction, and adverse outcomes in critically ill patients with AP remain to be elucidated.
Consequently, we performed this retrospective study focusing on the critically ill patients with AP in our pancreatic ICU (PICU). We aimed to evaluate the feasibility of using AGI grade to evaluate GI function, to investigate the association between AGI grades and clinical outcomes, and to evaluate the prognostic value of AGI grade alone and in combination with other severity scores in AP patients.
Records of patients with AP admitted to our PICU of the First Affiliated Hospital of Nanchang University from May 2017 to May 2019 were reviewed using the AP database, which is a data repository for the clinical data of all AP patients, including diagnostic, therapeutic, and follow-up data recorded by a special research assistant. The exclusion criteria for patients included the following: (1) Admission > 72 h after the onset of AP; (2) History of chronic pancreatitis or pancreatic malignancy; (3) History of GI tumors, inflammatory bowel disease, or abdominal surgery; (4) Pregnancy; (5) Hospital stay < 48 h; (6) No intra-abdominal pressure (IAP) measurement; and (7) Incomplete data. The Ethics Committee of the First Affiliated Hospital of Nanchang University approved the study (No. 2011001).
The detailed records of patients who met the inclusion criteria were further reviewed. The AGI grade was evaluated within the first week of admission according to the recommendation of the ESICM. In the first three days of ICU stay, the AGI grade is assessed mainly on GI symptoms and IAP, and it is combined with feeding intolerance (FI) in the following 4 d. The AGI grade was evaluated daily, and the worst AGI grade during the first week of ICU stay was recorded as the global AGI grade. In view of the features of population in our ICU, which is an AP treatment center, we needed to modify the AGI grade to assess the GI function specifically in AP patients[4,7]. For example, in the early stage, patients with AP do not experience diarrhea due to GI paralysis. The definition and examples of the AGI grades and the AGI grades specific to AP patients are shown in Supplementary Tables 1 and 2. Enteral nutrition was carried out in accordance with current clinical practice guidelines for AP[8,9]. If the goal of 20 kcal/kg BW/d cannot be achieved via enteral route, or if enteral feeding has to be stopped due to any clinical reason, the presence of FI should be considered. IAP was measured by the widely accepted methodology, which was the transvesical method. Intra-abdominal hypertension and abdominal compartment syndrome was defined according to the International Conference of Experts[10]. The high gastric residual volume was considered if a single volume exceeded 200 mL.
In addition, the following information was also collected: (1) Demographic characteristics including age, sex, body mass index, etiology of AP, history of alcohol and tobacco use, diabetes mellitus, and hypertension; (2) Laboratory examinations and severity scores (collected within the first 24 h of ICU admission) including serum albumin level, C-reactive protein level, procalcitonin level, D-dimer level, urea nitrogen level, glucose level, calcium level, Acute Physiology and Chronic Health Evaluation (APACHE) II score[11], Ranson score[12], and Modified Marshall score[13]; and (3) Clinical outcomes including infectious complications, persistent organ failure (> 48 h) defined as a score of two or more for one of these three organ systems (respiratory, circulatory, and renal systems) represented by a Modified Marshall score ≥ 2, ICU stay duration, and hospital stay duration[13,14].
Quantitative variables are described as medians and interquartile ranges and were analyzed by the Kruskal-Wallis test. Categorical variables are presented as absolute numbers and proportions and were tested by the chi-square test or Fisher’s exact test. Factors associated with mortality in univariate analysis (P < 0.2) were included in a multivariable model. Multivariate Logistic regression analysis (stepwise regression) was used to identify independent risk factors with odds ratios (ORs) and 95% confidence intervals (CIs). Because of the relatively small sample size in our study, the AGI grade was handled as a binary variable (I/II vs III/IV) in the multivariate analysis. A Kaplan-Meier survival analysis was used to estimate the cumulative survival, and the survival rates of different subgroups were compared using the log-rank test. Receiver operating characteristic curves were plotted to assess the ability of each scoring system to predict the mortality of AP. P < 0.05 was considered statistically significant. Data were analyzed using SPSS software (v17.0; SPSS Inc., Chicago, IL, United States).
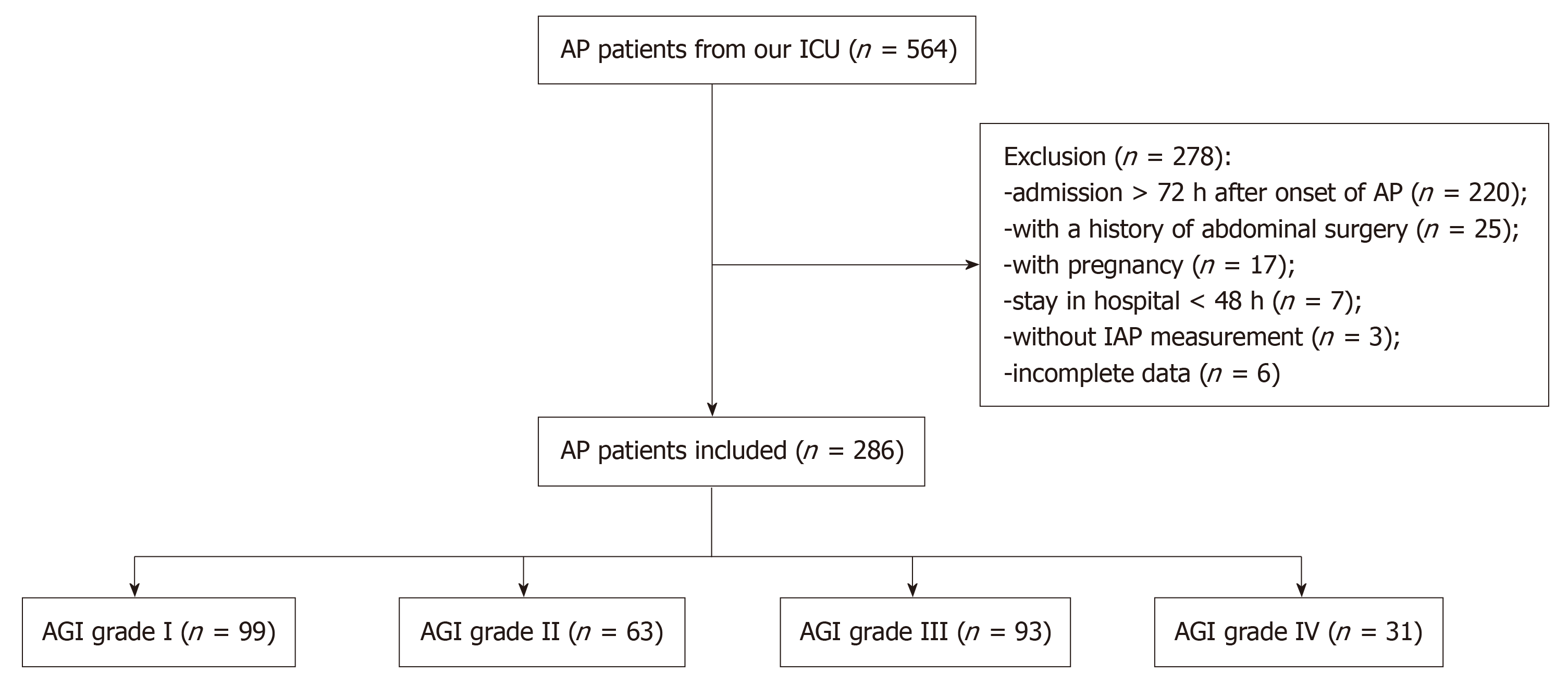
Between May 2017 and May 2019, 564 AP patients from our PICU were screened. Among them, 278 patients did not meet the inclusion criteria: 220 patients were admitted > 72 h after the onset of AP; 25 patients had a history of abdominal surgery; 17 patients were pregnant; 7 patients stayed in hospital < 48 h; IAP was not measured in 3 patients; and 6 patients had incomplete data. Finally, a total of 286 patients were evaluated in the first week of their admission, and the distribution of the global AGI grades was 34.62% with grade I (n = 99), 22.03% with grade II (n = 63), 32.52% with grade III (n = 93), and 10.84% with grade IV (n = 31). The flow chart is shown in Figure 1.
The average age of all patients was 49 (39, 64) years, and the study included 181 males (63.29%). The principal causes of AP were hyperlipidemic (42.31%), biliary (39.51%), and alcoholic origins (12.94%). Other values on admission and the clinical outcomes are presented in Table 1. There were no differences among the patients with different grades of AGI with regard to age, body mass index, history of tobacco use, diabetes mellitus, hypertension, and serum glucose levels (P > 0.05). There were significant differences in sex, etiology of AP, history of alcohol use, serum C-reactive protein levels, procalcitonin levels, urea nitrogen levels, calcium levels, D-dimer levels, and albumin levels among different grades of AGI (P < 0.05). With increasing AGI grade, the prevalence of infected pancreatic necrosis, extrapancreatic infection, persistent organ failure, and persistent multiple organ failure increased (P < 0.05) and the lengths of hospital and ICU stays also increased (P < 0.05).
| Characteristic | AGI grade I (n = 99) | AGI grade II (n = 63) | AGI grade III (n = 93) | AGI grade IV (n = 31) | P value |
| Male | 61 (61.62) | 29 (46.03) | 67 (72.04) | 24 (77.42) | 0.003 |
| Age, yr | 49 (40,65) | 49 (36,64) | 48 (40,64) | 51 (39,62) | 0.995 |
| BMI, kg/m2 | 24.34 (21.69, 26.42) | 23.97 (20.78, 27.43) | 24.63 (22.68, 27.7) | 25.23 (22.4, 28.54) | 0.251 |
| Etiology | 0.037 | ||||
| Biliary | 38 (38.38) | 31 (49.21) | 37 (39.78) | 7 (22.58) | |
| Alcoholic | 9 (9.09) | 4 (6.35) | 15 (16.13) | 9 (29.03) | |
| Hyperlipidemic | 45 (45.45) | 25 (39.68) | 39 (41.94) | 12 (38.71) | |
| Others | 7 (7.07) | 3 (4.76) | 2(2.15) | 3 (9.68) | |
| History of tobacco use | 28 (28.28) | 19 (30.16) | 25 (26.88) | 13 (41.94) | 0.444 |
| History of alcohol use | 28 (28.28) | 12 (19.05) | 35 (37.63) | 16 (51.61) | 0.006 |
| Diabetes mellitus | 17 (17.17) | 10 (15.87) | 11 (11.83) | 9 (29.03) | 0.167 |
| Hypertension | 18 (18.18) | 13 (20.63) | 19 (20.43) | 8 (25.81) | 0.836 |
| 1C-reactive protein, mg/L | 138 (91.7, 268.5) | 266 (149, 384) | 318 (225, 399) | 276.5 (240, 386.5) | < 0.001 |
| 1Procalcitonin, ng/mL | 0.94 (0.26, 2.84) | 1.05 (0.37, 3.44) | 7.59 (2.11, 23.44) | 7.8 (1.18, 20.87) | < 0.001 |
| 1Urea nitrogen, mmol/L | 5.7 (4.3, 7.5) | 7.5 (4.5, 9.4) | 10.5 (7.3, 16) | 12.3 (8.2, 16.8) | < 0.001 |
| 1Calcium, mmol/L | 2.12 (2.02, 2.26) | 1.99 (1.88, 2.18) | 1.87 (1.68, 2.13) | 1.77 (1.62, 1.92) | < 0.001 |
| 1D-dimer, mg/L FEU | 2.42 (1.23, 4.33) | 3.30 (2.02, 5.33) | 4.9 (2.84, 8.88) | 4.23 (2.4, 7.51) | < 0.001 |
| 1Glucose, mmol/L | 8.54 (5.91, 11.07) | 8.3 (6.93, 11.5) | 8.49 (7.05,11.78) | 7.79 (6.5, 10.55) | 0.737 |
| 1Albumin, g/L | 34.8 (32.4, 39.3) | 33.6 (30.8, 36.6) | 32.75 (30.45, 35.1) | 33 (28.7, 34.6) | < 0.001 |
| IPN | 4 (4.04) | 7 (11.11) | 26 (27.96) | 15 (48.39) | < 0.001 |
| 2Extrapancreatic infection | 3 (3.03) | 10 (15.87) | 31 (33.33) | 18 (58.06) | < 0.001 |
| 3Persistent OF | 13 (13.13) | 35 (55.56) | 69 (74.19) | 30 (96.77) | < 0.001 |
| 4Persistent multiple OF | 1 (1.01) | 6 (9.52) | 32 (34.41) | 25 (80.65) | < 0.001 |
| Length of hospital stay, d | 10 (8,14) | 15 (9,23) | 20 (12,33.5) | 23 (9,51) | < 0.001 |
| Length of ICU stay, d | 3 (2,5) | 7 (4,10) | 11 (6,17.5) | 19 (9,32) | < 0.001 |
| Death | 0 (0) | 4 (6.35) | 28 (30.11) | 19 (61.29) | < 0.001 |
| Variable | B | OR (95%CI) | P value |
| Male sex | 0.075 | 1.078 (0.573-2.028) | 0.817 |
| Age | 0.043 | 1.044 (1.024-1.064) | < 0.001 |
| BMI | -0.020 | 0.980 (0.913-1.052) | 0.574 |
| Etiology of AP | |||
| Biliary | Ref | Ref | Ref |
| Alcoholic | 0.630 | 1.878 (0.822-4.293) | 0.135 |
| Hyperlipidemic | -0.669 | 0.512 (0.249-1.053) | 0.069 |
| Others | -0.507 | 0.602 (0.127-2.858) | 0.523 |
| History of tobacco use | -0.256 | 0.774 (0.389-1.541) | 0.467 |
| History of alcohol use | 0.723 | 2.060 (1.108-3.829) | 0.022 |
| Diabetes mellitus | -0.067 | 0.935 (0.408-2.143) | 0.874 |
| Hypertension | 0.496 | 1.643 (0.818-3.297) | 0.163 |
| Urea nitrogen | 0.190 | 1.209 (1.140-1.283) | < 0.001 |
| Calcium | -2.444 | 0.087 (0.029-0.263) | < 0.001 |
| D-dimer | 0.090 | 1.094 (1.045-1.146) | < 0.001 |
| Glucose | -0.014 | 0.986 (0.924-1.053) | 0.671 |
| Albumin | -0.088 | 0.916 (0.850-0.986) | 0.020 |
| Global AGI grade (I/II vs III/IV) | 3.183 | 24.110 (8.382-69.352) | < 0.001 |
| Persistent respiratory failure | 2.834 | 17.013 (5.933-48.785) | < 0.001 |
| Persistent renal failure | 2.489 | 12.048 (5.634-25.764) | < 0.001 |
| Persistent circulatory failure | 3.731 | 41.719 (18.402-94.584) | < 0.001 |
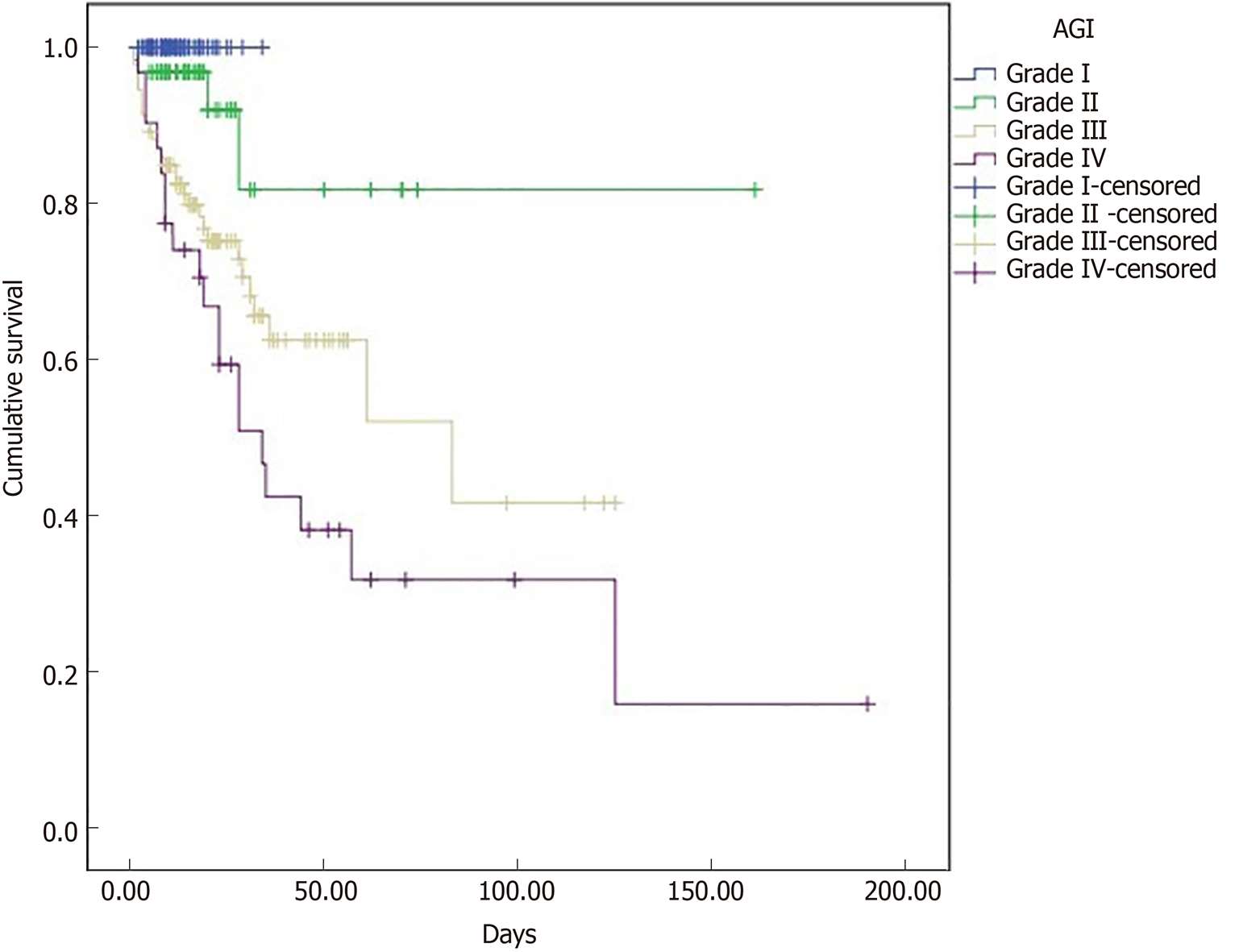
Figure 2 shows the Kaplan-Meier curves for mortality stratified by global AGI grade. Patients with AGI grades III and IV had mortality rates of 30.11% and 61.29%, respectively, which were significantly higher than those of the patients with AGI grades I and II (0% and 6.35%, respectively; χ2 = 31.511, P < 0.0001). There were no differences between AGI grades I and II (χ2 = 3.586, P = 0.058) or between AGI grades III and IV (χ2 = 2.966, P = 0.085) in mortality.
Univariate logistic regression analysis showed that age, alcohol use, serum urea nitrogen levels, calcium levels, D-dimer levels, albumin levels, AGI grade, persistent respiratory failure, persistent renal failure, and persistent circulatory failure were significantly (P < 0.05) associated with mortality (Table 2). In the multivariate analysis including these variables, age (OR = 1.096; 95%CI: 1.055-1.139; P < 0.001), serum calcium levels (OR = 0.117; 95%CI: 0.015-0.901; P < 0.05), AGI grade (OR = 3.487; 95%CI: 1.685-7.214; P = 0.001), persistent renal failure (OR = 4.538; 95%CI: 1.347-15.292; P < 0.05), and persistent circulatory failure (OR = 24.148; 95%CI: 7.919-73.638; P < 0.001) remained independent predictors of mortality (Table 3).
| Variables | B | OR (95%CI) | P value |
| Age | 0.092 | 1.096 (1.055-1.139) | < 0.001 |
| Calcium | -2.147 | 0.117 (0.015-0.901) | 0.039 |
| Global AGI grade (I/II vs III/IV) | 1.249 | 3.487 (1.685-7.214) | 0.001 |
| Persistent renal failure | 1.513 | 4.538 (1.347-15.292) | 0.015 |
| Persistent circulatory failure | 3.184 | 24.148 (7.919-73.638) | < 0.001 |
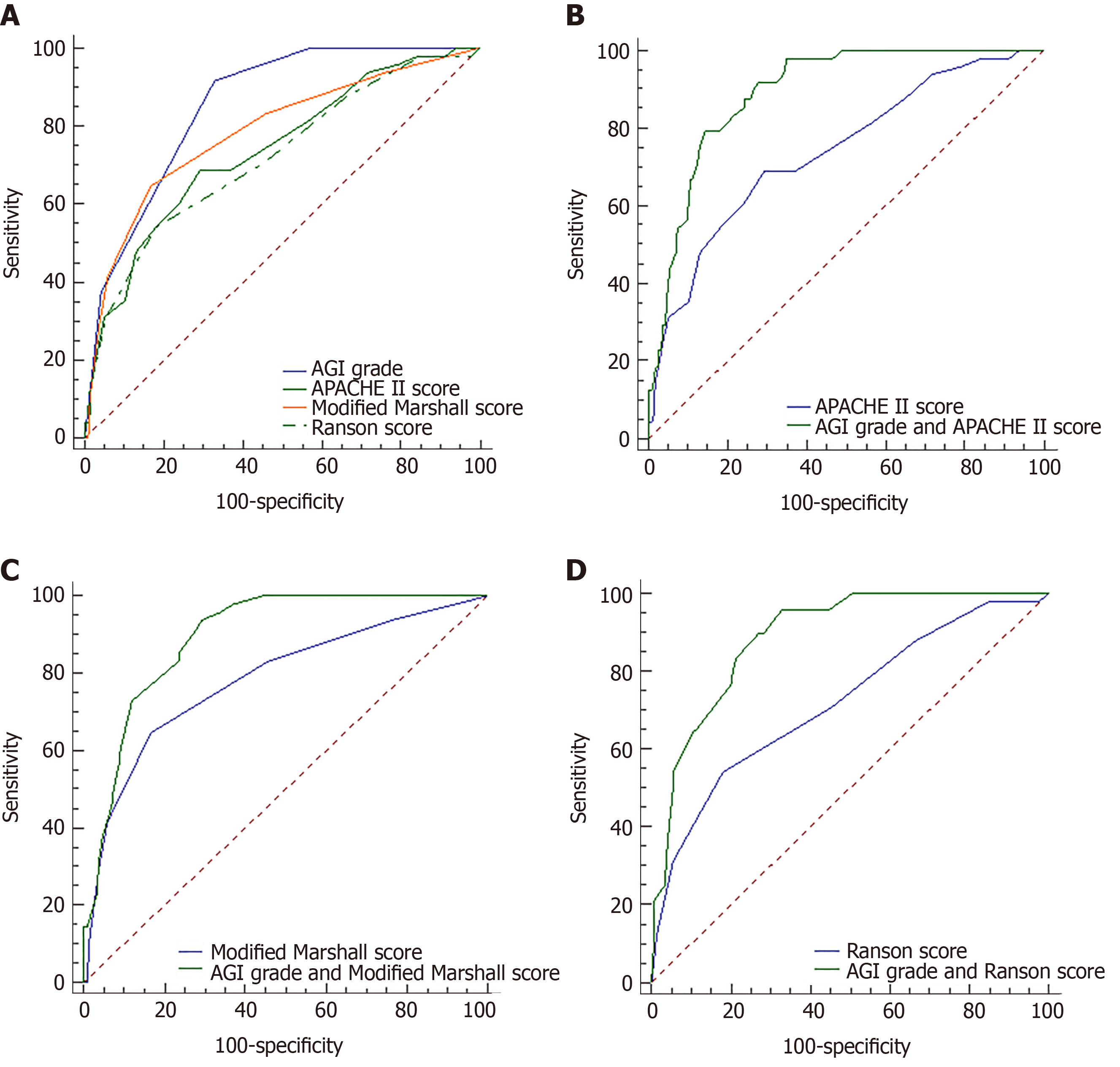
The area under the curve (AUC) for predicting mortality on the basis of AGI grade was 0.854 (95%CI: 0.806-0.895), the sensitivity was 91.67%, and the specificity was 67.13%, with a cutoff value of AGI grade II. Compared with the APACHE II score (AUC: 0.739 vs 0.854; P < 0.05) and Ranson score (AUC: 0.72 vs 0.854; P < 0.01), the AGI grade was more useful for predicting mortality. The Modified Marshall score was similar to the AGI grade with regard to the ability to predict mortality (AUC: 0.785 vs 0.854; P > 0.05). The combinations of AGI grade and APACHE II score (AUC: 0.893), AGI grade and Modified Marshall score (AUC: 0.895), or AGI grade and Ranson score (AUC: 0.89) exhibited greater predictive values that were superior to any of the scoring systems used alone (P < 0.01) (Table 4, Figure 3).
| Variables | Sensitivity | specificity | AUC (95%CI) | Cutoff value |
| AGI grade | 91.67% | 67.13% | 0.854 (0.806-0.895) | > grade II |
| APACHEII score | 68.75% | 70.83% | 0.739 (0.682-0.791) | > 12 |
| Modified Marshall score | 64.58% | 83.33% | 0.785 (0.730-0.833) | > 2 |
| Ranson score | 54.17% | 81.94% | 0.720 (0.662-0.774) | > 4 |
GI dysfunction was demonstrated to influence the AP patients’ outcome in previous studies[15]. However, in these studies, the absence of a widely accepted and scaled system for assessing GI function has been a major limiting factor. To the best of our knowledge, the present study is the first report to identify that the AGI grade recommended by the ESICM in 2012 is helpful to identify the severity of GI dysfunction and can be used to predict the mortality in critically ill patients with AP. Furthermore, the AGI grade combined with the APACHE II score, Modified Marshall score, or Ranson score allowed better prediction of mortality than did any of these scoring systems alone.
A meta-analysis including 18 studies found that the combined prevalence of gut barrier dysfunction was 59% (95%CI: 48-70)[16]. However, the prevalence of gut barrier dysfunction cannot represent the actual level of GI dysfunction because the definitions of gut barrier dysfunction were different in those studies, making it difficult to compare one study to another. In addition, the human GI tract has many functions, including not only barrier functions but also digestion, absorption, endocrine and immune functions. In our study, we used AGI grade to assess GI dysfunction and found that the prevalence of GI dysfunction in AP patients in the ICU was 34.62% with grade I, 22.03% with grade II, 32.52% with grade III, and 10.84% with grade IV. Compared with the distribution of AGI grades in ICU patients in the study by Hu et al[5], which were 24.5% with grade I, 49.4% with grade II, 20.6% with grade III, and 5.5% with grade IV, the prevalence of grades III + IV was higher. This may be due to the different main reasons for ICU admission. The main reasons to enter ICU were severe respiratory distress (45.6%), shock (32.8%), and acute kidney injury (18.5%) in the study by Hu et al[5]. However, we included all AP patients in our PICU, which indicates that patients with AP are more prone to developing severe GI dysfunction.
GI function was shown to influence the patients’ outcomes in previous studies in which GI dysfunction in ICU patients was assessed by GI symptoms or the gastrointestinal failure (GIF) score[17,18]. In a prospective study, Reintam et al[17] reported that three or more GI symptoms on the first day of ICU were independently associated with a threefold increase in the risk of death. In another prospective study, the mean GIF (based on the combination of FI with IAP for the first 3 d of ICU) score was identified as an independent risk factor for mortality (OR = 3.02; 95%CI: 1.63-5.59; P < 0.001)[18]. Several studies have investigated the association between GI dysfunction assessed by the AGI grade and adverse clinical outcomes in ICU patients. A recent study including 470 adult patients with AGI from 14 general ICUs showed that the AGI grade is helpful to identify the severity of GI dysfunction. In addition, the study also supported the finding that continuous FI in the first week after ICU hospitalization is an independent determinant of mortality[5]. Another study found that the AGI grade appeared to be more valid for predicting prognosis when it was differentiated into two grades (AGI I + II vs III + IV) than the AGI 4-grade system[19]. Thus, the AGI grade is helpful to identify the severity of GI dysfunction and can be used to predict mortality in ICU patients. However, the associations among AGI grade, the severity of GI dysfunction, and adverse outcomes in AP patients remain to be elucidated.
Studies on GI dysfunction in AP patients are limited. Sun et al[20] proposed a modified GIF score (based on the number of FI symptoms, IAP, endotoxin, and computed tomography findings) and concluded that the modified GIF score seemed to be valuable for predicting hospital mortality, multiple organ dysfunction syndrome, and pancreatic infection. Because the study included 52 SAP patients who had been present in the ICU for longer than 7 d, the modified GIF score could not assess GI function in the early stage of the disease[20]. In our study, we included patients who were admitted within 72 h after AP onset, and the AGI grade was assessed within the first week of the subject’s ICU admission. Thus, we assessed the GI function in the early stage of AP. Moreover, the present study is in line with previous studies on ICU patients and demonstrated that AGI grade is an independent risk factor for mortality and that AGI grade could add to the predictive value of the APACHE II, Modified Marshall, and Ranson scores.
There are some limitations to this study. First, FI was determined on the basis of failure to achieve enteral nutrition caloric targets, which depends more on subjective judgement and lacks objectivity. Second, even when following the ESICM criteria, the diagnosis and classifications of AGI grade were a little difficult due to its complicated manifestations, which potentially affected the applicability of our results and biased the outcome. Because of the small sample size and single-center design of this study, the representativeness might be limited, and the accuracy of AGI grade should be tested by further large-sample and multicenter studies.
In conclusion, the AGI grade is an independent predictor of mortality in critically ill patients with AP. The AGI grade combined with the APACHE II, Modified Marshall, and Ranson scores allows better prediction of mortality than does the use of any of these scoring systems alone.
Gastrointestinal (GI) dysfunction is a common complication of acute pancreatitis (AP), especially in severe AP. Due to a lack of a precise definition of GI dysfunction, there is little data regarding the prognostic value of GI dysfunction in AP patients.
We wanted to determine the feasibility of using acute gastrointestinal injury (AGI) grade to evaluate GI function in critically ill patients with AP, and investigate the association between AGI grades and clinical outcomes.
To evaluate the relationship between AGI grade and mortality in critically ill patients with AP, and to investigate the prognostic value of AGI grade alone and in combination with other severity scores in AP patients.
A retrospective cohort study was conducted, and 286 patients were included and divided to four groups according to AGI grades. A Kaplan-Meier survival analysis was performed to estimate the cumulative survival. Logistic regression analysis (stepwise regression) was used to identify independent risk factors.
The distribution of patients with various AGI grades was 34.62% with grade I, 22.03% with grade II, 32.52% with grade III, and 10.84% with grade IV. AGI grade was positively correlated with mortality, and was an independent risk factor for mortality. Compared with the APACHE II score and Ranson score, the AGI grade was more useful for predicting mortality. The combinations of AGI grade and APACHE II score [area under curve (AUC): 0.893], Modified Marshall score (AUC: 0.895), or Ranson score (AUC: 0.89) exhibited greater predictive values that were superior to any of these scoring systems used alone.
The AGI grade is feasible for evaluating GI function in critically ill patients with AP, and is an independent predictor of mortality. The AGI grade combined with the APACHE II, Modified Marshall, and Ranson scores allows better prediction of mortality than does the use of any of these scoring systems alone.
GI dysfunction has an adverse effect on the prognosis, and AGI grade may be an available evaluation tool. We hope that a future prospective study may focus on the development of new biochemical indicators and scoring systems for the evaluation of GI dysfunction.
Manuscript source: Unsolicited Manuscript
Corresponding Author's Membership in Professional Societies: Chinese Society of Gastroenterology (Fellow).
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ueda H, Wang HP, Krishna S, Inal V, Juneja D S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | de Jong PR, González-Navajas JM, Jansen NJ. The digestive tract as the origin of systemic inflammation. Crit Care. 2016;20:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 3. | Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The "undrained abscess" of multiple organ failure. Ann Surg. 1993;218:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 298] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 5. | Hu B, Sun R, Wu A, Ni Y, Liu J, Guo F, Ying L, Ge G, Ding A, Shi Y, Liu C, Xu L, Jiang R, Lu J, Lin R, Zhu Y, Wu W, Xie B. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit Care. 2017;21:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Zhang D, Fu R, Li Y, Li H, Li Y, Li H. Comparison of the clinical characteristics and prognosis of primary versus secondary acute gastrointestinal injury in critically ill patients. J Intensive Care. 2017;5:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Reintam Blaser A, Jakob SM, Starkopf J. Gastrointestinal failure in the ICU. Curr Opin Crit Care. 2016;22:128-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 561] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 9. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1385] [Article Influence: 115.4] [Reference Citation Analysis (3)] |
| 10. | Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D'Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 863] [Article Influence: 45.4] [Reference Citation Analysis (2)] |
| 11. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1949] [Cited by in RCA: 1746] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 14. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 15. | Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46 Suppl:S46-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101:1644-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Reintam Blaser A, Poeze M, Malbrain ML, Björck M, Oudemans-van Straaten HM, Starkopf J; Gastro-Intestinal Failure Trial Group. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39:899-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 18. | Reintam A, Parm P, Kitus R, Starkopf J, Kern H. Gastrointestinal failure score in critically ill patients: a prospective observational study. Crit Care. 2008;12:R90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Li H, Zhang D, Wang Y, Zhao S. Association between acute gastrointestinal injury grading system and disease severity and prognosis in critically ill patients: A multicenter, prospective, observational study in China. J Crit Care. 2016;36:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Sun JK, Li WQ, Ni HB, Ke L, Tong ZH, Li N, Li JS. Modified gastrointestinal failure score for patients with severe acute pancreatitis. Surg Today. 2013;43:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |