Published online Feb 7, 2020. doi: 10.3748/wjg.v26.i5.499
Peer-review started: November 5, 2019
First decision: December 7, 2019
Revised: December 13, 2019
Accepted: January 2, 2020
Article in press: January 3, 2020
Published online: February 7, 2020
Processing time: 93 Days and 5.6 Hours
MicroRNA 34c (miR-34c) has been reported to be associated with malignant types of cancer, however, it remains unknown whether miR-34c is involved in chemoresistance in gastric cancer (GC).
To investigate the effect of miR-34c and its upstream transcription factor E2F1 on paclitaxel combined with cisplatin resistance in GC cells.
Paired GC tissues and adjacent normal tissues were randomly sampled from 74 GC patients. miR-34c and E2F1 were detected by real-time quantitative PCR (qPCR) and Western blot. In addition, the drug resistance of GC cells to paclitaxel and cisplatin was induced by concentration gradient increasing methods, and changes in miR-34c and E2F1 during this process were measured. Furthermore, E2F1 and miR-34c overexpression or underexpression vectors were constructed and transfected into drug-resistant GC cells. MTT was employed to test the sensitivity of cells to paclitaxel combined with cisplatin, qPCR was adopted to detect the expression of miR-34c, Western blot was applied to detect the expression levels of E2F1, drug resistance-related proteins and apoptosis-related proteins, and flow cytometry was used for the determination of cell apoptosis and cell cycle status.
E2F1 was overexpressed while miR-34c was underexpressed in GC. After inducing GC cells to be resistant to paclitaxel and cisplatin, E2F1 expression increased while miR-34c expression decreased. Both silencing E2F1 and over-expressing miR-34c could increase the sensitivity of drug-resistant GC cells to paclitaxel combined with cisplatin, promote cell apoptosis and inhibit cell proliferation. Among which, silencing E2F1 could reduce the expression of drug resistance-related proteins and apoptosis-related proteins, while over-expression of miR-34c could upregulate the expression of apoptosis-related proteins without affecting the expression of MDR-1, MRP and other drug resistance-related proteins. Rescue experiments demonstrated that inhibiting miR-34c could significantly weaken the sensitization of drug resistant cells, and Si E2F1 to paclitaxel combined with cisplatin.
E2F1 inhibits miR-34c to promote the proliferation of GC cells and enhance the resistance to paclitaxel combined with cisplatin, and silencing E2F1 is conducive to improving the efficacy of paclitaxel combined with cisplatin in GC cells.
Core tip: Gastric cancer (GC) is one of the most common types of cancer. The molecular mechanism of GC is intricate, and understanding the molecular mechanism of drug resistance is helpful for improving the efficacy of chemotherapy. The mutual binding of miRNAs and transcription factors is closely related to cancer occurrence. In this study, we found that E2F transcription factor 1 inhibited miR-34c transcription to both promote GC cell proliferation as well as enhance the resistance of paclitaxel combined with cisplatin. Therefore, silencing the expression of E2F transcription factor 1 in GC cells could improve the efficacy of paclitaxel combined with cisplatin.
- Citation: Zheng H, Wang JJ, Yang XR, Yu YL. Upregulation of miR-34c after silencing E2F transcription factor 1 inhibits paclitaxel combined with cisplatin resistance in gastric cancer cells. World J Gastroenterol 2020; 26(5): 499-513
- URL: https://www.wjgnet.com/1007-9327/full/v26/i5/499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i5.499
Gastric cancer (GC) ranks among the top four cancer fatality rates worldwide[1], and remains one of the four most prevalent types of cancer in China[2]. In GC, 90% of the cases are attributed to adenocarcinoma, which can be histologically divided into the following two types: Median-differentiated or intestinal type, and undifferentiated or diffuse type[3]. Dietary imbalance, excessive drinking, smoking, abnormal weight and Helicobacter pylori are all risk factors for GC, hence intervention of the above factors will help reduce the incidence of the disease[4]. At present, chemotherapy is one of the mainstream treatments for GC, whose effect, however, is reduced by the development of cell resistance[5]. Therefore, understanding the molecular mechanism of drug resistance is conducive to improving chemotherapy efficacy.
The molecular mechanism of GC remains complex and unknown[6]. Evidence has shown that E2F transcription factor 1 (E2F1) is highly expressed in GC, and that it may promote GC tumorigenesis. For example, Yan et al[5] revealed that the E2F1 overexpression could inhibit GC cell apoptosis, while enhancing both cell proliferation and multidrug resistance. The follow-up studies conducted by Yan et al[7] demonstrated that miR-34a monitored the down-regulation of E2F1 to promote the anti-tumor immunity of dendritic cells in GC. Besides, the regulatory relationship between E2F1 and miR-106b-25 clusters can affect the TGF-β pathway, leading to the formation of GC[8]. Moreover, the cooperation between E2F1 and lncRNA also has an impact on the occurrence of GC. Qi et al[9] validated that E2F1 promoted epithelial mesenchymal transformation by inducing LSINCT5 transcriptional activity, leading to GC progression. Furthermore, Guo et al[10] revealed that the apparent silencing effect of lncRNA HAGLR on E2F1 could inhibit the growth of non-small cell lung cancer (NSCLC).
miR-34c, an miRNA approximately 77 bp in length, is located on chromosome 11. It is lowly expressed in many cancers and is associated with biological functions such as apoptosis and proliferation[11-13]. The low expression of miR-34c is not only related to methylation silencing[14], but is also implicated in the regulation of upstream transcription factors[15,16].
In this study, E2F1 and miR-34c were found to be abnormally expressed in GC samples. In addition, it is hypothesized that E2F1 may mediate the transcriptional level of miR-34c and exert an influence on GC, as there are binding sites between E2F1 and miR-34c predicted by PROMO. However, no research has been carried out on the expression of E2F1 mediating miR-34c in GC at present. Therefore, by regulating the expression of E2F1 and miR-34c in GC, this study sets out to explore the related molecular mechanisms of E2F1 and miR-34c, so as to understand the relationship between the two and their effects on GC.
Paired GC tissues and adjacent normal tissues were obtained from 74 diagnosed GC patients (46 males and 28 females). The inclusion criteria was patients diagnosed with GC. In contrast, patients with psychiatric disorders, previous treatment (surgery, chemotherapy, radiotherapy or antibiotic treatment), complicated with other tumors, or those who did not cooperate with the treatment were excluded. This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Zunyi Medical University, and all sampling was obtained after patient consent. Tissue samples were pathologically sliced and stored in liquid nitrogen at -80 °C for detection.
GC cells (BGC-823, MGC-803, SGC-7901) and human gastric mucosal epithelial cells (GES-1) were purchased from the Conservation Genetics CAS Shanghai Cell Bank. The above-mentioned cells were cultured at 37 °C and 5% CO2 in an animal cell incubator, whose culture medium contained RPMI-1640 medium, 10% fetal bovine serum solution and 1% penicillin/streptomycin solution. Follow-up experiments were carried out until cells reached logarithmic phase.
Medium was replaced with fetal bovine serum medium 1 d before transfection, and on the day of transfection, cells were inoculated into 6-well plates at 1×105/well. E2F1-siRNA (si-E2F1), NC siRNA, E2F1-shRNA (sh-E2F1), NC shRNA, Mi-34c mimcs, NC mimcs, Mi-34c inhibitor and NC inhibitor vectors were purchased from Sangon Biotech (Shanghai) Co., Ltd. A Lipofectamine 2000 transfection kit (Invitrogen, United States) was employed to transfect the cell line in accordance with the kit instructions. After transfection for 8 h, the fresh medium was replaced, and the animal cell incubator was incubated at 37 °C and 5% CO2 for 48 h before subsequent experiments.
Tissue samples were ground to prepare the cell suspension. Then, 1 mL Trizol was added into the cell suspension, and repeatedly pipetted until the cells fully lysed to extract the total RNA from the tissue samples. Then, an ultraviolet spectrophotometer was adopted to detect the concentration and purity of total RNA at 260-280 nm, and those with OD260/OD280 > 1.8 were selected for the next experiment. RNA was reverse transcribed, amplified and quantified by reverse transcription and PCR using a FastKing one-step reverse transcription-fluorescence quantitative kit (Tiangen Biotech (Beijing) Co., Ltd., Catalog No.: FP314) and ABI PRISM 7000 (Applied Biosystems, United States). miR-34c primer was designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd., whose primer sequence was as follows: Upstream primer (5'→3'): GCA GCC AGG CAG TGT AGT TAG C, downstream primer (5'→3'): GTG CAG GGT CCG AGG T. The reaction system was conducted by referring to the kit instructions (50 μL): upstream primer: 1.25 μL, downstream primer: 1.25 μL, probe: 1.0 μL, RNA template: 10 pg/μg, 50×ROX Reference Dye ROX: 5 μL, and RNase-FreeddH_2 was added to reach the overall reaction system of 50 μL. Reaction process: Reverse transcription at 50 °C for 30 min, 1 cycle; pre-denaturation at 95 °C for 3 min, 1 cycle; PRC amplification, denaturation at 95 °C for 15 s, annealing at 60 for 30 s, 40 cycles. The results were analyzed by ABI PRISM 7000 instrument, and the internal reference gene U6 (upstream primer 5'→3': CTC GCT TCG GCA GCA, downstream primer 5'→3': AAC GCT TCA CGA ATT TGC GT) was standardized by the 2-△△ct method.
Then, 1 mL Trizol was added to 6-well plates in which the cell lines were cultured, and the solution was repeatedly pipetted until cell lysis. Finally, the total RNA of the cell lines was extracted, whose procedures were the same as those of the total RNA extraction of tissue samples.
Cell protein extract (1 mL, cell lysate: Protease inhibitor: Phosphatase inhibitor = 98:1:1, v/v/v) was added to the culture plate with the cell lines, and the solution was repeatedly pipetted until the cell was completely lysed, and then centrifuged at 1.2 × 104 r/min for 15 min to collect the supernatant. Next, the protein was isolated by SDS-PAGE electrophoresis, transferred to an NC membrane, sealed with 5% skim milk-PBS solution and placed at room temperature for 1 h. Then, MDR-1, MRP, Caspase-9, Bax and Bcl-2 primary antibody were added and stored overnight at 4 °C. After that, the NC membrane was washed three times with PBS solution, followed by the addition of goat anti-rabbit secondary antibody (HRP cross-linked), then continued to stand at room temperature for 1 h. Finally, the NC membrane was rinsed with PBS solution and visualized using the enhanced chemiluminescence method. With β-actin as the internal reference protein, the relative expression level of the protein to be measured was equal to the gray value of the band to be measured/the gray value of the β-actin band.
MDR-1 and MRP were both purchased from Santa Cruz, while Caspase 3, Caspase 9, Bax, Bcl-2, β-actin I and II anti-rabbit (HRP cross-linking) were all obtained from Shanghai Abcam.
Well-cultured transfected cells were digested for 2 min before the enzyme solution was absorbed and added to the fresh culture medium to prepare the cell suspension. Then, four 96-well plates were inoculated into the plates with 5 × 103 cells/100 μL per well, with three wells in each group. At an interval of 24 h, one plate was removed and 5 mg/ml MTT solution was added to the plate according to 10 μL/well, then cultured for 1 h, and then the OD value at 570 nm was measured by a microplate reader. The experiment was repeated three times, and the cell activity-time curve was plotted.
The drug resistance of GC cells to paclitaxel combined with cisplatin was induced by the low concentration gradient increasing method. After transfection, 8 × 103 drug resistant cells/per well were inoculated into 96-well plates and cultured at 37 °C and 5% CO2 in an animal cell incubator for 12 h, followed by further culture with the addition of paclitaxel and cisplatin (mixed concentration 1-1000 μmol/L, concentration gradient increased). After 48 h, 10 μL MTT was added to each well for 1 h of dark culture, then the culture medium was absorbed. Lastly, OD values at 570 nm were measured by a microplate reader, cell activity-concentration curves were drawn, and IC50 values of semi-inhibitory concentrations were determined by SPSS analysis curves. The experiment was repeated three times.
Cell suspension was prepared by the enzymatic hydrolysis of cells, and the number of cells was maintained at 1 × 106. At 4 °C, the cells were fixed in cold 70% ethanol solution for 30 min. Then the ethanol solution was removed and the cell particles were incubated in Annexin V-FITC/7-AAD mixed solution. Cell apoptosis was analyzed by FACScan flow cytometry (Becton Dickinson Company, United States). The cell cycle operation process was similar to that of apoptosis, except that Annexin V-FITC/7-AAD mixed solution should be replaced by propylene sodium iodide (50 ng/mL)/RNase (0.2 mg/mL)/0.1% Triton X-100 mixed solution after cell fixation, and treated at room temperature for 30 min.
The promoter fragment of miR-34c was amplified by PCR and cloned into the pmirGLO vector. GeneArt TM Site-Directed Mutagenesis PLUS System (Thermo Fisher Science) was employed to construct the pmirGLO-miR34c promoter-wt vector, pmirGLO-miR34c promoter-mut1, pmirGLO-miR34c promoter-mut2, and pmirGLO-miR34c promoter-mut3 mutation vectors, which were then co-transfected into cells with sh-E2F1 and NCsh, respectively. After 48 h of transfection, the luciferase intensity was detected by the double luciferase reporter gene system (Promega) strictly according to the instructions.
Statistical analysis and picture rendering of the collected data of indicators mentioned above were performed using SPSS20.0 (Asia Analytics Formerly SPSS, China) and GraphPad Prism 6.0, respectively. The measurement data were expressed as mean ± SD, and the multi-group comparisons were analyzed by one-way ANOVA. The LSD-t-test was applied for post-hoc pairwise comparison, and an independent sample t-test was adopted for intra-group comparison. All data were tested by a two-tailed test, with 95% as its confidence interval. A statistically significant difference was assumed at P < 0.05.
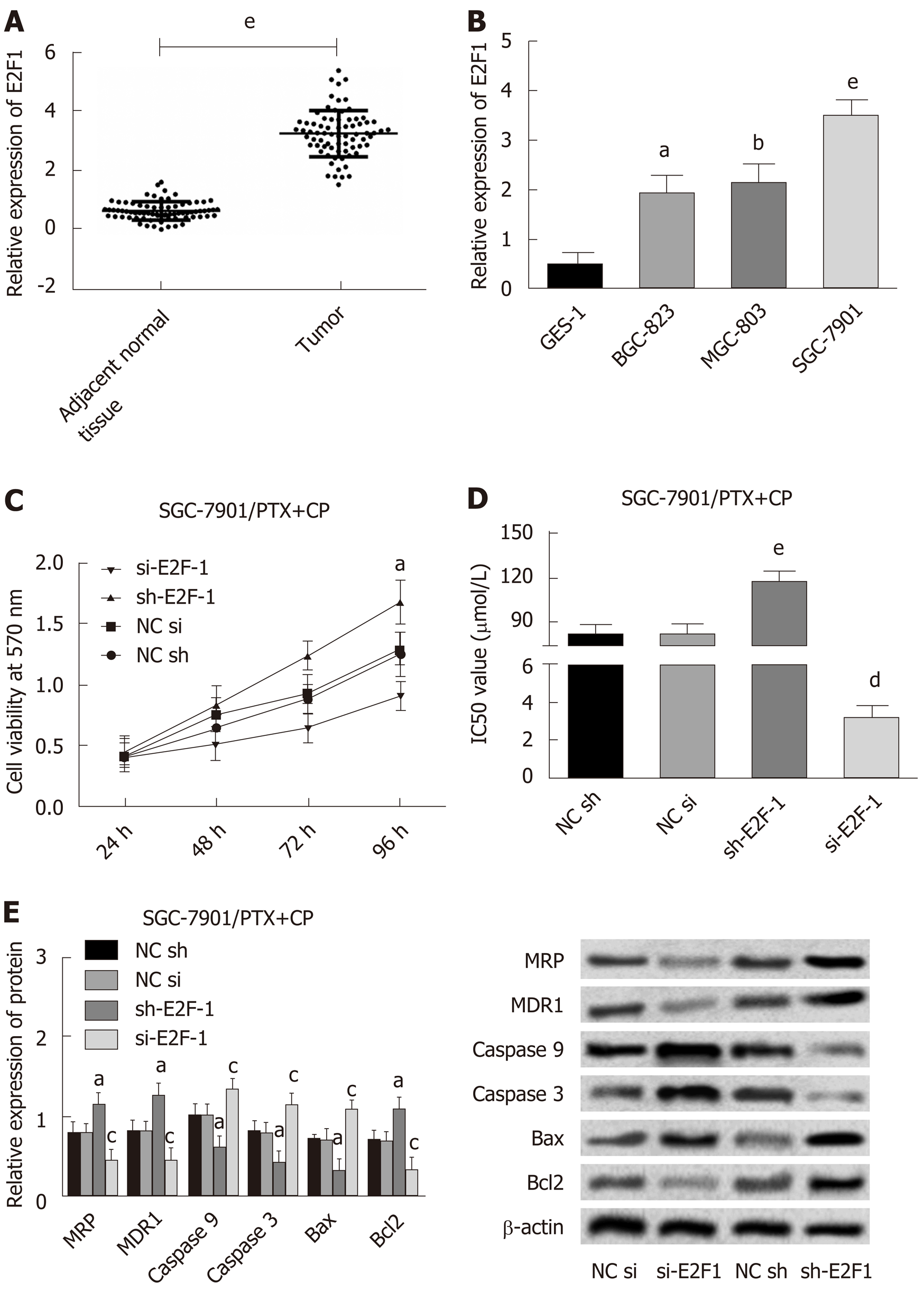
In this study, 74 cases of paired GC tissues and adjacent normal tissues were randomly selected as the study subjects, and the expression level of E2F1 in GC was determined by Western blot. Compared with normal adjacent tissues, E2F1 presented upregulated expression in GC tissues (Figure 1A). The subsequent detection of the expression levels of E2F1 in three GC cells (BGC-823, MGC-803, SGC-7901) and human normal gastric epithelial cells (GES-1) indicated that, compared with normal gastric epithelial cells, the expression of E2F1 was upregulated in GC cells, and the highest expression of E2F1 was found in SGC-7901 cells (Figure 1B). Owing to its marked expression of E2F1, SGC-7901 was selected as the research subject to induce the resistance of GC cells to paclitaxel combined with cisplatin by the concentration gradient increasing method, while the expression of E2F1 was detected by Western blot, and cell activity by MTT. Results revealed that the expression of E2F1 and cell activity in the induction group were higher than those in the non-induction group, which indicated that the induction of drug resistance was successful (Figure 1A and B).
After transfection of E2F1 overexpression/inhibition expression vectors into SGC-7901 drug resistant strains, the expression levels of MRP, MDR-1, Caspase 9, Caspase 3, Bax and Bcl2 were detected by Western blot, and changes in cell activity at different times and concentrations were measured by MTT, while the IC50 values were obtained according to cell activity curves. The detailed results can be found in Figure 1C-E. Compared with the NC sh group, the cell viability and IC50 values of the sh-E2F1 group increased, accompanied by the upregulation of MRP, MDR-1, Bcl2 and the down-regulation of Caspase 9, Caspase 3 and Bax. While the cell viability and IC50 values of the si-E2F1 group decreased, MRP, MDR-1, Bcl2 were down-regulated and Caspase 9, Caspase 3 and Bax were upregulated in comparison with the NC Si group.
These results suggested that the high expression of E2F1 promoted the increase of cell activity and the formation of multidrug resistance, and was related to the inhibition of cell apoptosis.
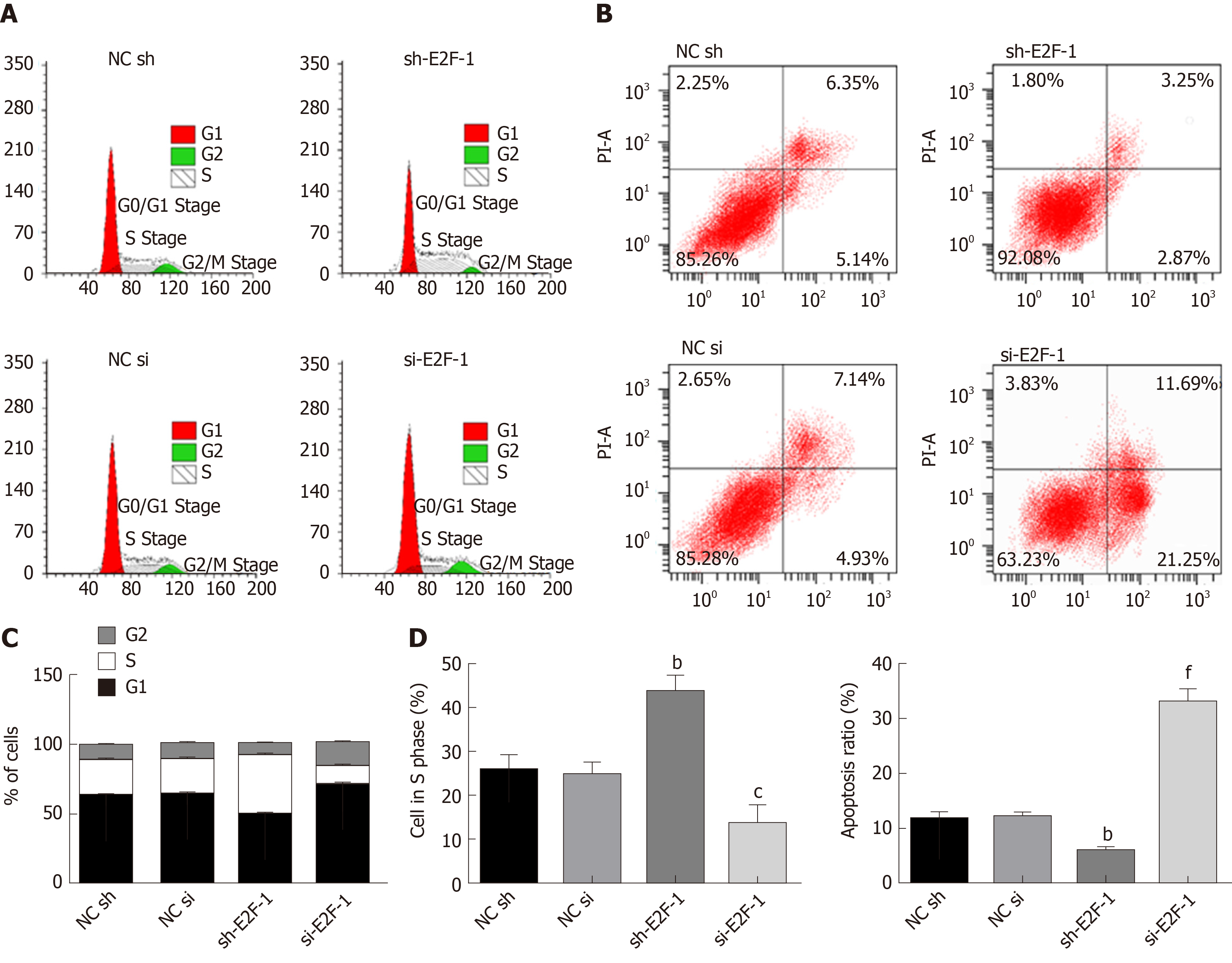
As shown in Supplementary Figure 1, E2F1 was related to cell proliferation and apoptosis. Flow cytometry was used to assess the effect of E2F1 on the proliferation and apoptosis of drug resistant cells. Detailed results are shown in Figure 2. Compared with the NC sh group, S-phase cells in the sh-E2F1 group significantly increased, and the apoptosis rate was notably reduced. Meanwhile, the sh-E2F1 group presented significantly reduced S-phase cells and markedly increased apoptosis rates when compared with the NC si group. Combined with Figure 1 and Figure 2, it can be concluded that E2F1 inhibited apoptosis by inhibiting Caspase 9, Caspase 3 and Bax, and promoted cell proliferation by upregulating Bcl2 expression and thus accelerating S-phase cell growth.
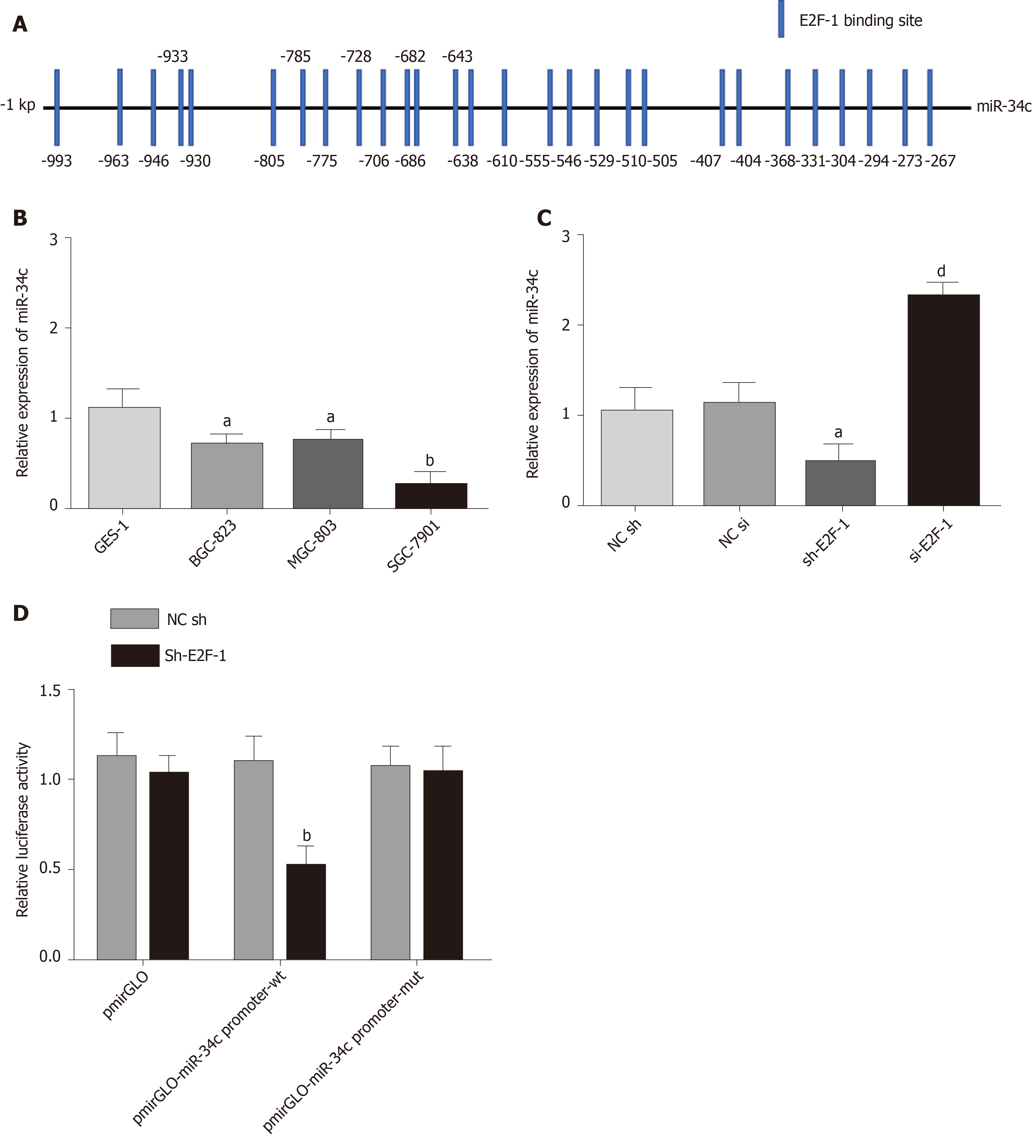
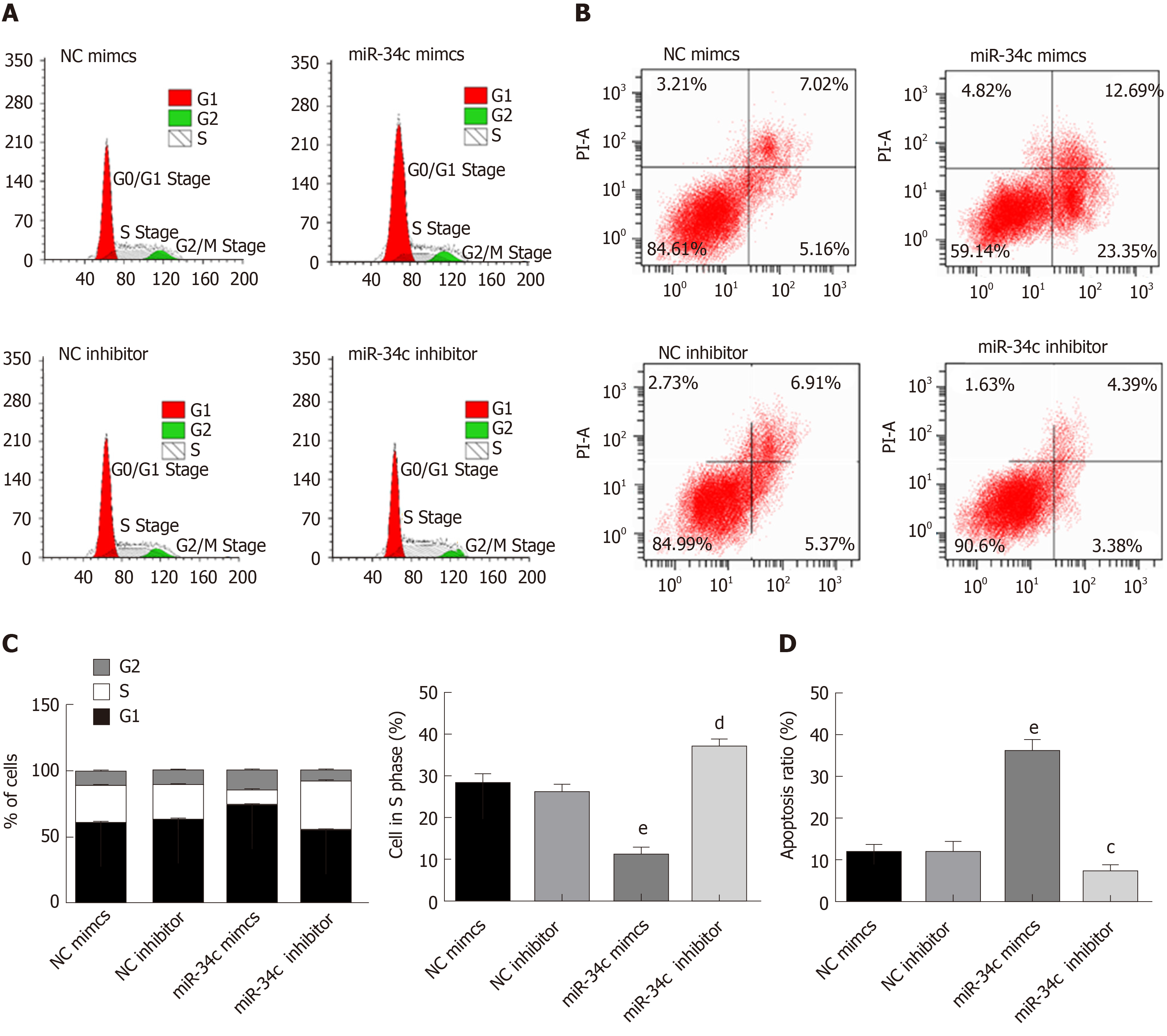
During the induction of paclitaxel combined with cisplatin resistance by GC cells, miR-34c was observed to be abnormally expressed (Figure 1D). The promoter region of miR-34c was predicted to have a binding site in E2F1 by the PROMO database (Figure 3A). QPCR showed that miR-34c was lowly expressed in GC cells, and miR-34c showed a corresponding down-regulation/upregulation when E2F1 was upregulated/down-regulated (Figure 3B). These results suggested that E2F1 might have a targeted regulatory relationship with miR-34c. To verify their targeting relationship, a double luciferase reporter gene was employed, whose results exhibited that when the miR-34c promoter was co-transfected with sh E2F1, luciferase activity was significantly reduced, while the luciferase activity in other co-transfected groups was not significantly affected (Figure 3C and D). These results suggested that E2F1 could inhibit the transcription and expression of miR-34c in GC cells.
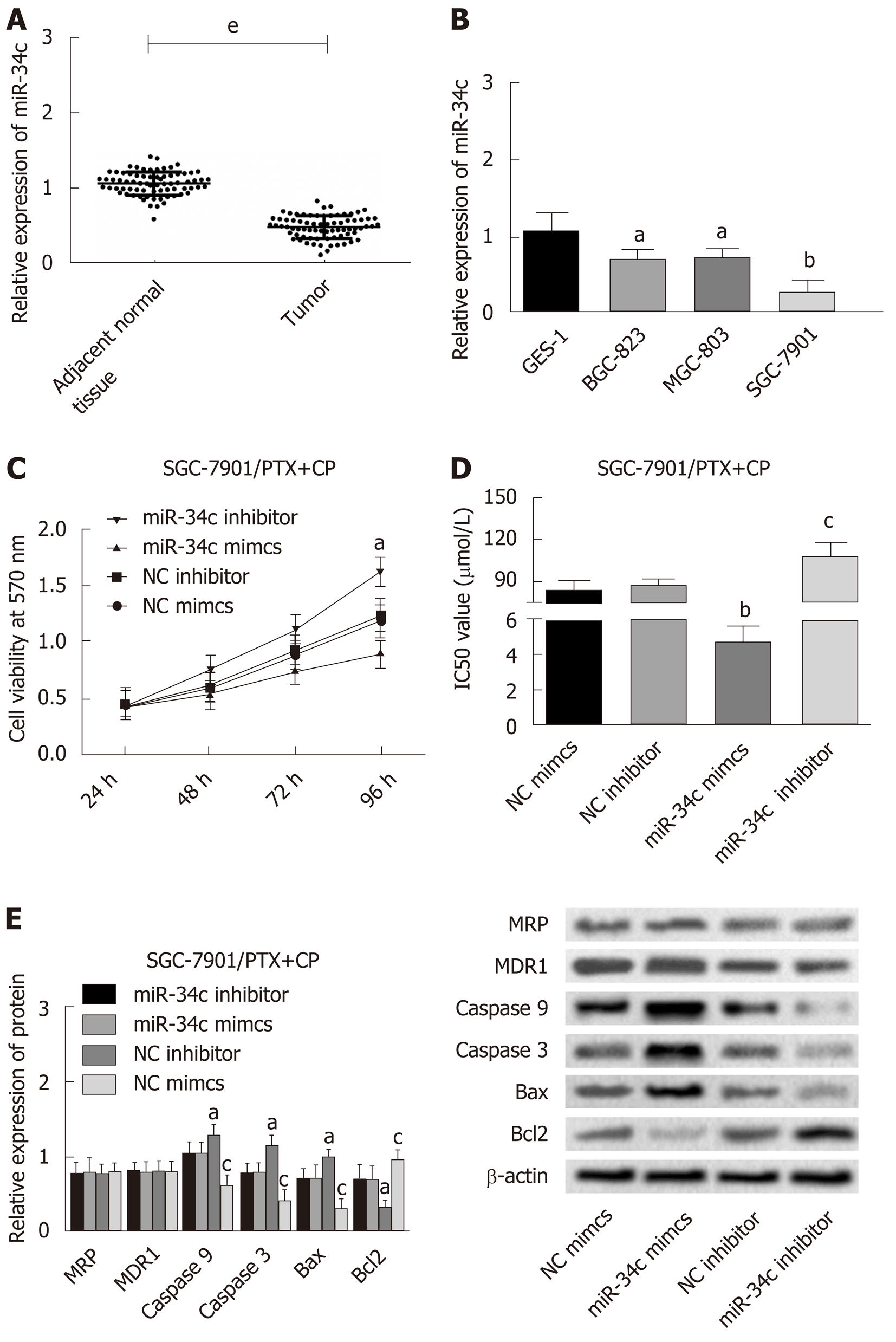
The following experiments were designed to explore the action mechanism of miR-34 to get a view of its role in GC cells. The expression levels of MRP, MDR-1, Caspase 9, Caspase 3, Bax and Bcl2 were detected by Western blot after transfection of the miR-34c overexpressed and inhibited expression vectors into the drug resistant strain of SGC-7901. The changes in cell activity were detected by MTT at different times and concentrations, and the IC50 values were obtained according to the cell activity curve, as shown in Figure 4. Compared with the NC inhibitor group, the cell viability and IC50 values of the miR-34c inhibitor group increased, accompanied by the upregulation of Bcl2, and the down-regulation of Caspase 9, Caspase 3 and Bax. While the cell viability and IC50 values of the miR-34c mimcs group decreased, Bcl2 was down-regulated and Caspase 9, Caspase 3 and Bax were upregulated in comparison with the NC mimcs group. It is worth mentioning that the expression levels of MRP and MDR-1 were not affected by miR-34c.
The effect of miR-34c on the proliferation and apoptosis of GC cells was detected by flow cytometry, as shown in Figure 5. Compared with the NC inhibitor group, the miR-34c inhibitor group exhibited an increase of S-phase cells, with a decreased apoptosis rate. Meanwhile, S-phase cells in the si-E2F1 group decreased and the apoptosis rate increased in contrast with the NC si group. These results suggested that miR-34c could promote apoptosis and inhibit drug resistance and proliferation.
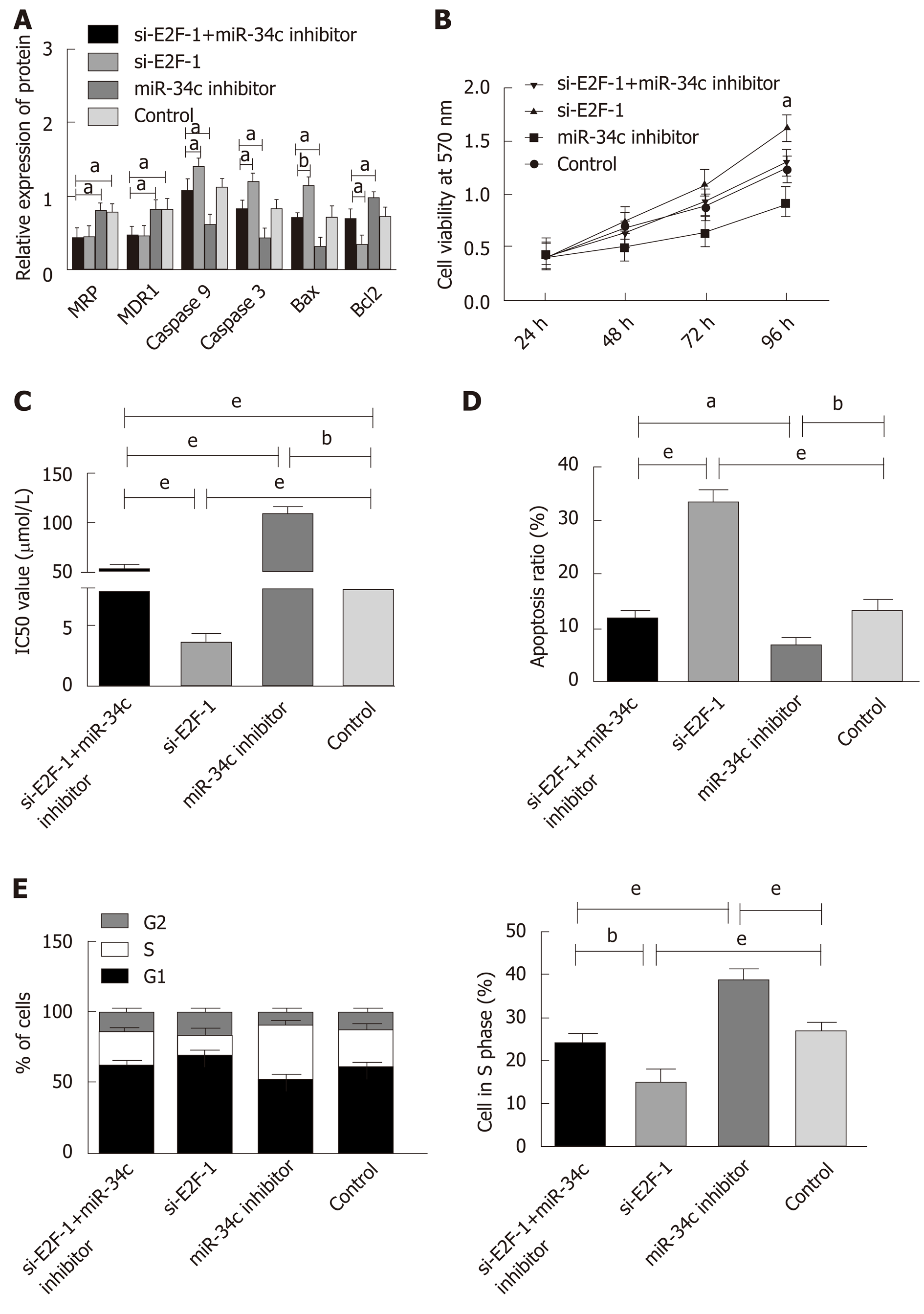
Drug resistant cells were transfected with si E2F1and miR-34c inhibitor, si E2F1, and miR-34c inhibitor, respectively, and the detection was performed using MTT, Western blot, and flow cytometry correspondingly, as shown in Figure 6. Compared with the si E2F1 group, the si E2F1 and miR-34c inhibitor groups presented enhanced cell activity, S-phase cells and IC50 values, upregulated Bcl2 expression, with decreased apoptotic rates and down-regulated Caspase 3, Caspase 9 and Bax expression levels, while MDR-1 and MRP did not significantly change. In contrast with the miR-34c inhibitor group, the cell activity, S-phase cells and IC50 values of the si E2F1 and miR-34c inhibitor groups decreased, Bcl2 expression was down-regulated, apoptotic rates were increased, and Caspase 3, Caspase 9 and Bax expression levels were down-regulated, while MDR-1 and MRP were not significantly changed. Whereas, irrespective of the downregulation of MDR-1 and MRP expression levels, no significant differences were observed in other indicators in the si E2F1 and miR-34c inhibitor groups when compared with the control group. These results indicated that inhibition of miR-34c expression could offset the sensitization effect of si-E2F1 to paclitaxel combined with cisplatin, and counteract the promotion of apoptosis and the inhibition of cell proliferation.
Tumor genomics is regulated by transcription factors, coding genes and miRNAs, which can form feedback networks to influence the formation and development of tumors. Numerous studies have demonstrated that the interaction between miRNAs and transcription factors can affect cell proliferation and growth. For example, Li et al[17] found that negative feedback cycles of E2F1 and miR-17-92 could promote the proliferation and cell cycle of palatal mesenchymal cells. In addition, Chen et al[18] revealed that TP53-miR125b-ID2 and JUN-miR30a-IL1A were related to the occurrence of esophageal adenocarcinoma, and believed that these transcription factor-miRNA-mRNA networks would be helpful for the diagnosis and treatment of esophageal adenocarcinoma. Luo et al[19] confirmed that the automatic regulation between E2F1 and miR-20a-5p/20b-5p was associated with the proliferation and differentiation of myoblasts. Reports have also identified that a complex network composed of E2F3 and various miRNAs monitors the balance of cell proliferation, apoptosis, metastasis and drug resistance[20]. For instance, the E2F3/miR-125a/DKK3 axis was found to be capable of promoting the development and grading of GC[21].
Many a study has exhibited that E2F1 is bound up with the formation of cell resistance. Rosenfeldt et al[22] proved that the regulatory relationship between E2F1 and ABCG2 could lead to the efflux of chemotherapy drugs for lung cancer and induce drug resistance. Alla et al[23] suggested that E2F1 mediated the regulation of the p73/miR-205 pathway on the ABC protein family and Bcl2, resulting in the formation of cell resistance. Loria et al[24] also found that E2F1 was involved in liposarcoma resistance. Therefore, studying the molecular mechanism of E2F1 and miRNA is not only beneficial to understanding the occurrence of cancer, but also to promoting the development of cancer treatment.
Previous studies[8] have shown that high expression of E2F1 promotes the proliferation and apoptosis of GC cells. Here, after transfection of E2F1 overexpression and silencing expression vectors into GC cells, it was found that E2F1 could promote cell proliferation and inhibit apoptosis. Meanwhile, inhibited E2F1 brought decreased IC50 values, and upregulated MDR-1 and MRP multidrug resistance protein expression levels, while E2F1 overexpression could upregulate MDR-1 and MRP expression levels, and increase IC50 values. In the study by Yan et al[5], E2F1 promoted the formation of drug resistance by regulating P-gp (MDR-1 mediated) and MRP1 molecular pumps. Combined with the results of previous studies, E2F1 may have the following effects on the drug resistance of paclitaxel combined with cisplatin in GC cells: (1) Directly or indirectly regulating the expression levels of MDR-1 and MRP to mediate drug resistance pathways; and (2) Promoting cell proliferation and inhibiting apoptosis can enhance the viability of GC cells, and ultimately contribute to their resistance to paclitaxel with cisplatin.
To investigate the specific molecular mechanism of E2F1 in GC, this study used the PROMO database to predict the binding site between E2F1 and the miR-34c promoter. Double lucigenase assays further confirmed that E2F1 could bind to the miR-34c promoter in a targeted way. When E2F1 was overexpressed or inhibited, the expression of miR-34c displayed a corresponding down-regulation or upregulation, suggesting that E2F1 could inhibit the expression of miR-34c at the transcriptional level, and might affect downstream genes and cell biological functions. In addition, we transfected GC cells with miR-34c overexpressed and silenced expression vectors, and found that miR-34c can promote cell apoptosis, inhibit cell proliferation and combine cisplatin resistance with taxol in GC cells. It is well-established that miR-34c exerts inhibitory effects on cancer cells[11-14]. In this study, the effect of E2F1 on cell proliferation and apoptosis may be realized through miR-34c-E2F1 inhibiting the expression of miR-34c, thus destroying the inhibitory effect of the latter, and ultimately promoting cell proliferation and inhibiting apoptosis. Remarkably, irrespective of the inhibitory effect of miR-34c on cell resistance, it does not affect the expression levels of MDR-1 and MRP, so other molecular mechanisms are implicated in the impact of E2F1 on MDR-1 and MRP.
Through a series of experiments, the current study found that E2F1 not only mediates miR-34c and regulates cell proliferation and apoptosis, but may directly or indirectly regulate the expression levels of MRP and MDR-1, which lead to changes in paclitaxel-cisplatin resistance in GC cells. To advance research on the effect of E2F1/miR-34c on GC cell resistance, further discussion will be conducted in future studies: (1) Whether E2F1 direct targeting regulates MRP and MDR-1; (2) Which downstream target genes are implicated in the regulation of E2F1/miR-34c on the proliferation and apoptosis of GC cells; and (3) Although miR-34c does not regulate MDR-1 and MRP to achieve the effect on drug resistance of GC cells, miR-34c still has an effect on the drug resistance of GC cells. Studies have confirmed that miR-34c mediates the expression of MAPT and other proteins, and determines the drug resistance of GC cells[2], so the relationship between miR-34c and the drug resistance of GC cells requires further exploration.
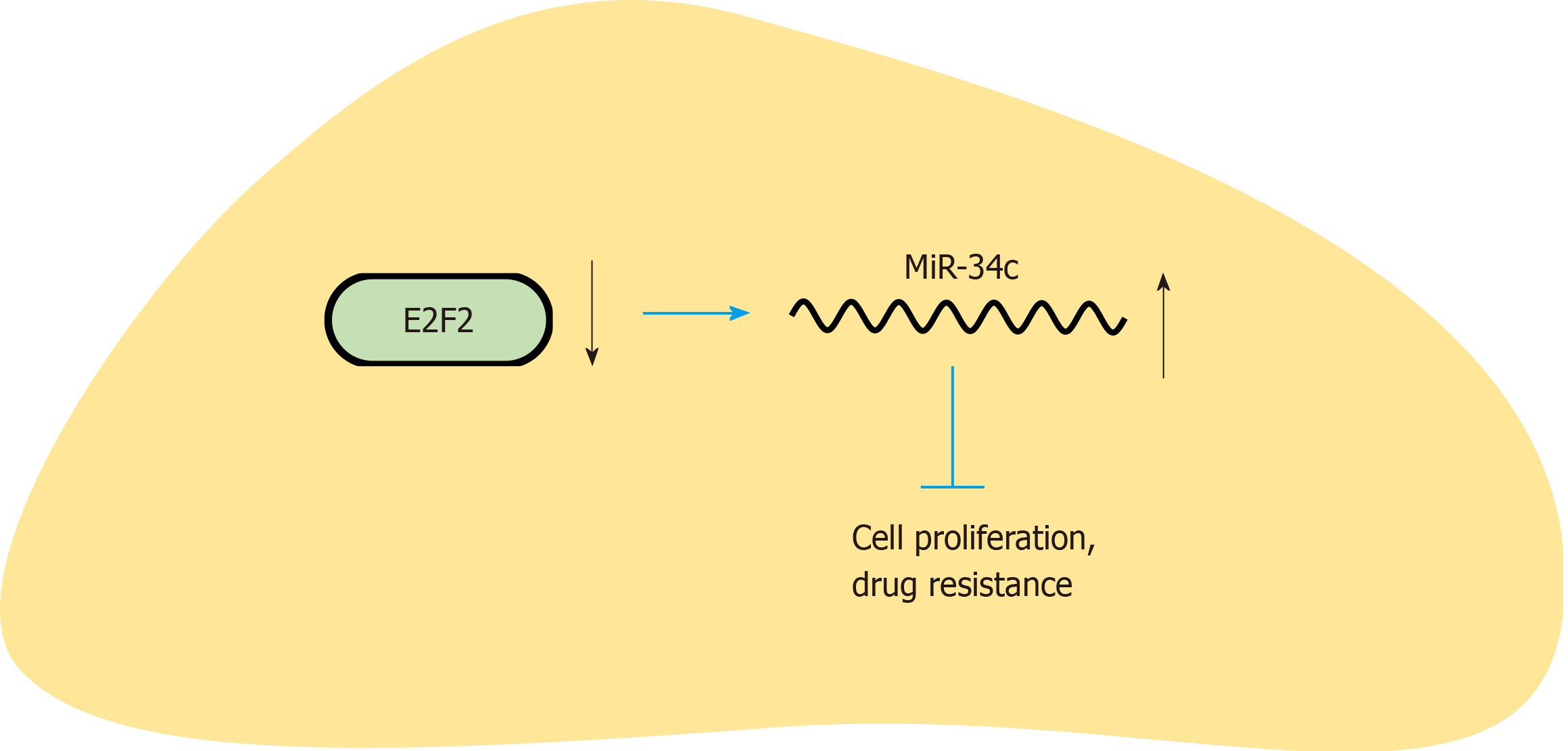
In summary, this study discussed the effect of E2F1/miR-34c on paclitaxel combined with cisplatin resistance in GC cells by studying the expression mechanism of E2F1 and miR-34c (Figure 7). E2F1 can regulate the expression of miR-34c and promote the apoptosis, proliferation, invasion and migration of cells. Meanwhile, E2F1 will promote the expression levels of MDR-1 and MRP, which ultimately leads to the enhancement of GC cell resistance to paclitaxel combined with cisplatin. Therefore, in clinical treatment, regulating the expression levels of E2F1/miR34c will help improve the sensitivity of GC cells to paclitaxel combined with cisplatin and enhance the therapeutic effect of drugs.
Gastric cancer (GC) is one of the most common types of cancer. The detailed roles of miR-34c and E2F transcription factor 1 (E2F1) have been proven in various cancer types. However, the relationship between them and GC has not been understood.
The promoter region of miR-34c was predicted to have a binding site in E2F1 by PROMO database. E2F1 was increased but miR-34c was decreased in GC. The relationship between them in GC has not been understood. Thus, it is required to study the function of E2F1/miR-34c in GC.
To investigate the effect of miR-34c and its upstream transcription factor E2F1 on paclitaxel combined with cisplatin resistance in GC cells.
Paired GC tissues and adjacent normal tissues were randomly sampled from 74 GC patients. MiR-34c and E2F1 were detected by qPCR and Western blot. In addition, the drug resistance of GC cells to paclitaxel and cisplatin was induced by the concentration gradient increasing method, and changes in miR-34c and E2F1 during this process were detected. Furthermore, E2F1 and miR-34c overexpression or low expression vectors were constructed and transfected into drug resistant GC cells. Moreover, MTT was employed to detect the sensitivity of cells to paclitaxel combined with cisplatin, qPCR was adopted to detect the expression of miR-34c, Western blot was applied to detect the expression of E2F1, drug resistance-related protein and apoptosis-related protein, and flow cytometry was used to detect cell apoptosis and cell cycle status.
E2F1 was overexpressed while miR-34c was underexpressed in GC. After inducing GC cells to be resistant to paclitaxel and cisplatin, E2F1 expression increased while miR-34c expression decreased. Both silencing E2F1 and over-expressing miR-34c could increase the sensitivity of drug-resistant GC cells to paclitaxel combined with cisplatin, promote cell apoptosis and inhibit cell proliferation. Among which, silencing E2F1 could reduce the expression of drug resistance-related proteins and apoptosis-related proteins, while over-expression of miR-34c could upregulate the expression of apoptosis-related proteins without affecting the expression of MDR-1, MRP and other drug resistance-related proteins. Rescue experiments demonstrated that inhibiting miR-34c could significantly weaken the sensitization of drug resistant cells and Si E2F1 to paclitaxel combined with cisplatin.
E2F1 inhibits miR-34c to promote the proliferation of GC cells and enhance the resistance to paclitaxel combined with cisplatin, while silencing E2F1 is conducive to improving the efficacy of paclitaxel combined with cisplatin in GC cells.
Slicing E2F1 or increasing miR-34c improves the sensitivity of GC cells to paclitaxel combined with cisplatin, and enhances the therapeutic effect of drugs. To advance research on the effect of E2F1/miR-34c on GC cell resistance, further discussion will be conducted in future studies: (1) Whether E2F1 directly targeting regulates MRP and MDR-1; (2) Which downstream target genes are implicated in the regulation of E2F1/miR-34c on the proliferation and apoptosis of GC cells; and (3) The relationship between miR-34c and the drug resistance of GC cells, which all require further study.
We thank all medical staff and technicians of dialysis centers who agreed to participate in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lazăr DC, Tarnawski AS S-Editor: Zhou JJ L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, Zhao R, Duan Y, Zeng Z, Li X, Li G, Xiong W, Zhou M. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 2. | Wu H, Huang M, Lu M, Zhu W, Shu Y, Cao P, Liu P. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother Pharmacol. 2013;71:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1252] [Article Influence: 65.9] [Reference Citation Analysis (8)] |
| 4. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 723] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 5. | Yan LH, Wei WY, Cao WL, Zhang XS, Xie YB, Xiao Q. Overexpression of E2F1 in human gastric carcinoma is involved in anti-cancer drug resistance. BMC Cancer. 2014;14:904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Ma J, Hong L, Chen Z, Nie Y, Fan D. Epigenetic regulation of microRNAs in gastric cancer. Dig Dis Sci. 2014;59:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Yan LH, Chen ZN, Li L, Chen J, Mo XW, Qin YZ, Wei WE, Qin HQ, Lin Y, Chen JS. E2F-1 promotes DAPK2-induced anti-tumor immunity of gastric cancer cells by targeting miR-34a. Tumour Biol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 9. | Qi P, Lin WR, Zhang M, Huang D, Ni SJ, Zhu XL, Bai QM, Sheng WQ, Du X, Zhou XY. E2F1 induces LSINCT5 transcriptional activity and promotes gastric cancer progression by affecting the epithelial-mesenchymal transition. Cancer Manag Res. 2018;10:2563-2571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Guo X, Chen Z, Zhao L, Cheng D, Song W, Zhang X. Long non-coding RNA-HAGLR suppressed tumor growth of lung adenocarcinoma through epigenetically silencing E2F1. Exp Cell Res. 2019;382:111461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Hagman Z, Larne O, Edsjö A, Bjartell A, Ehrnström RA, Ulmert D, Lilja H, Ceder Y. miR-34c is downregulated in prostate cancer and exerts tumor suppressive functions. Int J Cancer. 2010;127:2768-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Tu L, Long X, Song W, Lv Z, Zeng H, Wang T, Liu X, Dong J, Xu P. MiR-34c acts as a tumor suppressor in non-small cell lung cancer by inducing endoplasmic reticulum stress through targeting HMGB1. Onco Targets Ther. 2019;12:5729-5739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Li YQ, Ren XY, He QM, Xu YF, Tang XR, Sun Y, Zeng MS, Kang TB, Liu N, Ma J. MiR-34c suppresses tumor growth and metastasis in nasopharyngeal carcinoma by targeting MET. Cell Death Dis. 2015;6:e1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Suzuki H, Yamamoto E, Nojima M, Kai M, Yamano HO, Yoshikawa K, Kimura T, Kudo T, Harada E, Sugai T, Takamaru H, Niinuma T, Maruyama R, Yamamoto H, Tokino T, Imai K, Toyota M, Shinomura Y. Methylation-associated silencing of microRNA-34b/c in gastric cancer and its involvement in an epigenetic field defect. Carcinogenesis. 2010;31:2066-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Yang S, Wu B, Sun H, Ji F, Sun T, Zhao Y, Zhou D. Interrupted E2F1-miR-34c-SCF negative feedback loop by hyper-methylation promotes colorectal cancer cell proliferation. Biosci Rep. 2015;36:e00293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Alidadiani N, Ghaderi S, Dilaver N, Bakhshamin S, Bayat M. Epithelial mesenchymal transition Transcription Factor (TF): The structure, function and microRNA feedback loop. Gene. 2018;674:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Li L, Shi B, Chen J, Li C, Wang S, Wang Z, Zhu G. An E2F1/MiR-17-92 Negative Feedback Loop mediates proliferation of Mouse Palatal Mesenchymal Cells. Sci Rep. 2017;7:5148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Chen D, Lu T, Tan J, Zhao K, Li Y, Zhao W, Li H, Wang Q, Wang Y, Wei L. Identification of a transcription factor-microRNA network in esophageal adenocarcinoma through bioinformatics analysis and validation through qRT-PCR. Cancer Manag Res. 2019;11:3315-3326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Luo W, Li G, Yi Z, Nie Q, Zhang X. E2F1-miR-20a-5p/20b-5p auto-regulatory feedback loop involved in myoblast proliferation and differentiation. Sci Rep. 2016;6:27904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Gao Y, Feng B, Lu L, Han S, Chu X, Chen L, Wang R. MiRNAs and E2F3: a complex network of reciprocal regulations in human cancers. Oncotarget. 2017;8:60624-60639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Pei Y, Tang Z, Cai M, Yao Q, Xie B, Zhang X. The E2F3/miR-125a/DKK3 regulatory axis promotes the development and progression of gastric cancer. Cancer Cell Int. 2019;19:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Rosenfeldt MT, Bell LA, Long JS, O'Prey J, Nixon C, Roberts F, Dufès C, Ryan KM. E2F1 drives chemotherapeutic drug resistance via ABCG2. Oncogene. 2014;33:4164-4172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Alla V, Kowtharapu BS, Engelmann D, Emmrich S, Schmitz U, Steder M, Pützer BM. E2F1 confers anticancer drug resistance by targeting ABC transporter family members and Bcl-2 via the p73/DNp73-miR-205 circuitry. Cell Cycle. 2012;11:3067-3078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Loria R, Laquintana V, Bon G, Trisciuoglio D, Frapolli R, Covello R, Amoreo CA, Ferraresi V, Zoccali C, Novello M, Del Bufalo D, Milella M, Biagini R, D'Incalci M, Falcioni R. HMGA1/E2F1 axis and NFkB pathways regulate LPS progression and trabectedin resistance. Oncogene. 2018;37:5926-5938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |