Published online Dec 28, 2020. doi: 10.3748/wjg.v26.i48.7693
Peer-review started: October 21, 2020
First decision: November 13, 2020
Revised: November 26, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: December 28, 2020
Processing time: 65 Days and 7.6 Hours
Coronavirus disease 2019 (COVID-19) disease can frequently affect the liver. Data on hepatic histopathological findings in COVID-19 is scarce.
To characterize hepatic pathological findings in patients with COVID-19.
We conducted a systematic review with meta-analysis registered on PROSPERO (CRD42020192813), following PRISMA guidelines. Eligible trials were those including patients of any age and COVID-19 diagnosis based on a molecular test. Histopathological reports from deceased COVID-19 patients undergoing autopsy or liver biopsy were reviewed. Articles including less than ten patients were excluded. Proportions were pooled using random-effects models. Q statistic and I2 were used to assess heterogeneity and levels of evidence, respectively.
We identified 18 studies from 7 countries; all were case reports and case series from autopsies. All the patients were over 15 years old, and 67.2% were male. We performed a meta-analysis of 5 studies, including 116 patients. Pooled prevalence estimates of liver histopathological findings were hepatic steatosis 55.1% [95% confidence interval (CI): 46.2-63.8], congestion of hepatic sinuses 34.7% (95%CI: 7.9-68.4), vascular thrombosis 29.4% (95%CI: 0.4-87.2), fibrosis 20.5% (95%CI: 0.6-57.9), Kupffer cell hyperplasia 13.5% (95%CI: 0.6-54.3), portal inflammation 13.2% (95%CI: 0.1-48.8), and lobular inflammation 11.6% (95%CI: 0.3-35.7). We also identified the presence of venous outflow obstruction, phlebosclerosis of the portal vein, herniated portal vein, periportal abnormal vessels, hemophagocytosis, and necrosis.
We found a high prevalence of hepatic steatosis and vascular thrombosis as major histological liver features. Other frequent findings included portal and lobular inflammation and Kupffer cell hyperplasia or proliferation. Further studies are needed to establish the mechanisms and implications of these findings.
Core Tip: In this systematic review of 18 studies, we identified that hepatic steatosis, congestion of hepatic sinuses, and vascular thrombosis are the main histological findings in deceased coronavirus disease 2019 (COVID-19) patients. Fibrosis is a common histological finding in deceased COVID-19 patients. Kupffer cell hyperplasia, portal inflammation, and lobular inflammation are related to the severe acute respiratory syndrome coronavirus 2 inflammatory process and are frequent histological findings. Other vascular abnormalities (such as venous outflow obstruction, phlebosclerosis of the portal vein, herniated portal vein, periportal abnormal vessels) are frequent histological finding in deceased COVID-19 patients.
- Citation: Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol 2020; 26(48): 7693-7706
- URL: https://www.wjgnet.com/1007-9327/full/v26/i48/7693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i48.7693
The pandemic of the novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (also known as coronavirus disease 2019, COVID-19) has infected more than 59 million individuals worldwide and caused more than 1.4 million deaths. Although the main manifestations of COVID-19 are related to respiratory symptoms, the compromise of multiple organs has been described in the literature, including the digestive system[1]. There are several gastrointestinal manifestations, including anorexia, diarrhea, nausea/vomiting, and abdominal pain. Those symptoms have a pooled prevalence of 17.6% and are frequently observed in hospitalized patients[1,2].
The liver is also one of the most common organs affected in COVID-19. In fact, between 2%-11% of affected patients have liver comorbidities and 16%-53% of cases reported abnormal liver tests[3,4]. The most frequent abnormalities are mildly elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and are more common in hospitalized patients[4,5]. Elevation of alkaline phosphatase and gamma-glutamyl transferase (GGT) has also been reported[6]. Those alterations have been related to expression of angiotensin-converting enzyme 2 (ACE-2), the putative receptor of SARS-CoV-2, in endothelial cells of the liver and the biliary epithelium[7]. In addition, since the frequency of liver dysfunction increases as COVID-19 is more severe, liver damage might be directly caused by the infection of liver. Abnormal liver tests can also be partially explained by drug-induced liver injury (DILI), cytokine storm, and/or pneumonia-associated hypoxia[4].
Pathological studies in patients with SARS-CoV-2 infection have confirmed the presence of the virus in liver tissue[8]. This finding was also described in SARS and MERS infection[3]. Although histopathological information is scarce, previous reports from SARS and MERS showed steatosis, mild portal tract and lobular lymphocytic inflammation, as well as mild cellular hydropic degeneration in hepatic parenchyma[7,9-11]. Regarding SARS-CoV-2 infection, initial reports of autopsies performed in COVID-19 patients have described steatosis, mild lobular and portal activity, lymphocytic endotheliitis, and necrosis[12-14]. However, data about the main histopathological findings in COVID-19 is still scarce. Thus, aiming to provide a comprehensive synthesis of the pathological findings in liver injury due to SARS-CoV-2 reported so far, we performed a systematic review and meta-analysis of each histopathological finding in liver samples from autopsies and biopsies of COVID-19 patients.
This systematic review with meta-analysis was registered on PROSPERO (ID: CRD42020192813) and followed a prespecified analysis plan. This study is reported in accordance with the Preferred Reporting Items for a Review and Meta-analysis (PRISMA) guidelines[15].
Eligible trials had to include patients diagnosed with COVID-19, regardless of age and gender. The diagnosis of COVID-19 had to be based on a compatible clinical history and molecular evidence with a quantitative real-time polymerase chain reaction (qRT-PCR) for SARS-CoV-2. We included liver histopathological reports from deceased COVID-19 patients who subsequently were studied with autopsy or liver biopsies performed in alive COVID-19 patients.
We planned to include all the studies that report liver histopathological data, regardless of the design (case-reports, case-series, descriptive cases, cross-sectional studies, cohort studies, and randomized controlled trials). We excluded studies performed in vitro, animal models, or lacking evidence of SARS-CoV-2 infection from this systematic review. We did not include manuscripts performed before December 1, 2019.
We performed an electronic search from December 1, 2019, to June 3, 2020, in MEDLINE (via PubMed) and Embase databases. We used keywords and free-text words related to SARS-CoV-2 infection, autopsies, and liver biopsies. We reported the search strategy used in PubMed and Embase databases in the appendix. We hand searched (up to June 3, 2020) preprint servers (bioRxiv, medRxiv, and SSRN) and coronavirus resource centers of The Lancet, JAMA, and New England Journal of Medicine. We did not limit our search by language. Two investigators (Díaz LA and Idalsoaga F) independently screened the titles and abstracts to ascertain whether each study met the eligibility criteria. The full texts of the identified eligible articles were then evaluated to determine whether they should be included in the analysis. Disagreements between the two reviewers were resolved by consensus. In case of persistent disagreement, arbitration by a third reviewer (Arab JP) settled the discrepancy.
Two authors (Díaz LA and Cannistra M) independently extracted data from included studies using forms specially designed for this purpose. The following data were extracted describing the study, participants, source of sample (liver biopsy or autopsy), and the main liver histopathological findings. Discrepancies were resolved by a third reviewer (Arab JP). Two investigators (Díaz LA and Idalsoaga F) independently assessed the risk of bias of each included trial with the Appraisal tool for Cross-Sectional Studies (AXIS) checklist for cross-sectional studies, the Institute of Health Economics (IHE) checklist tool for Case Series, the Newcastle-Ottawa Scale (NOS) for case-control studies and cohort studies, and the Cochrane Risk of Bias Tool for randomized trials and quasi-experimental studies.
The outcomes were defined by consensus with an expert pathologist (Graham R). The main outcomes were the frequency of vascular thrombosis (presence or absence), venous outflow obstruction (presence or absence), frequency of portal and lobular Inflammation and severity (mild, moderate, or severe), Kupffer cell hyperplasia (presence or absence), steatosis (presence and fat percentage), fibrosis (based on METAVIR score, scores range from F0 to F4, with higher numbers reflecting greater fibrosis), and ductopenia (presence or absence). Secondary outcomes were the frequency phlebosclerosis of the portal vein, herniated portal vein, congestion of hepatic sinuses, and necrosis.
Data were synthesized per each histopathological finding of SARS-CoV-2 infection. We estimated the prevalence of event rates in the form of a proportion (with a confidence interval of 95%). Proportions were pooled using random-effects models. Only those studies with a sample size of at least ten patients were included in the meta-analysis. We used Q statistic and I2 to quantify heterogeneity. We planned a subgroup analysis according to the liver sample source (biopsy in alive patients or autopsy), ethnicity, and gender. We planned sensitivity analysis excluding the high risk of bias studies. A small study effect was evaluated with a funnel plot. As such, subgroup and sensitivity analyses should be considered exploratory. All statistical analyses were performed using MedCalc Statistical Software version 19.3.1 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
The quality of evidence for the outcomes was graded with the GRADE framework.
The funding source only provided support for the financing of paid manuscripts during the review process. The researchers did not receive payment or other incentives.
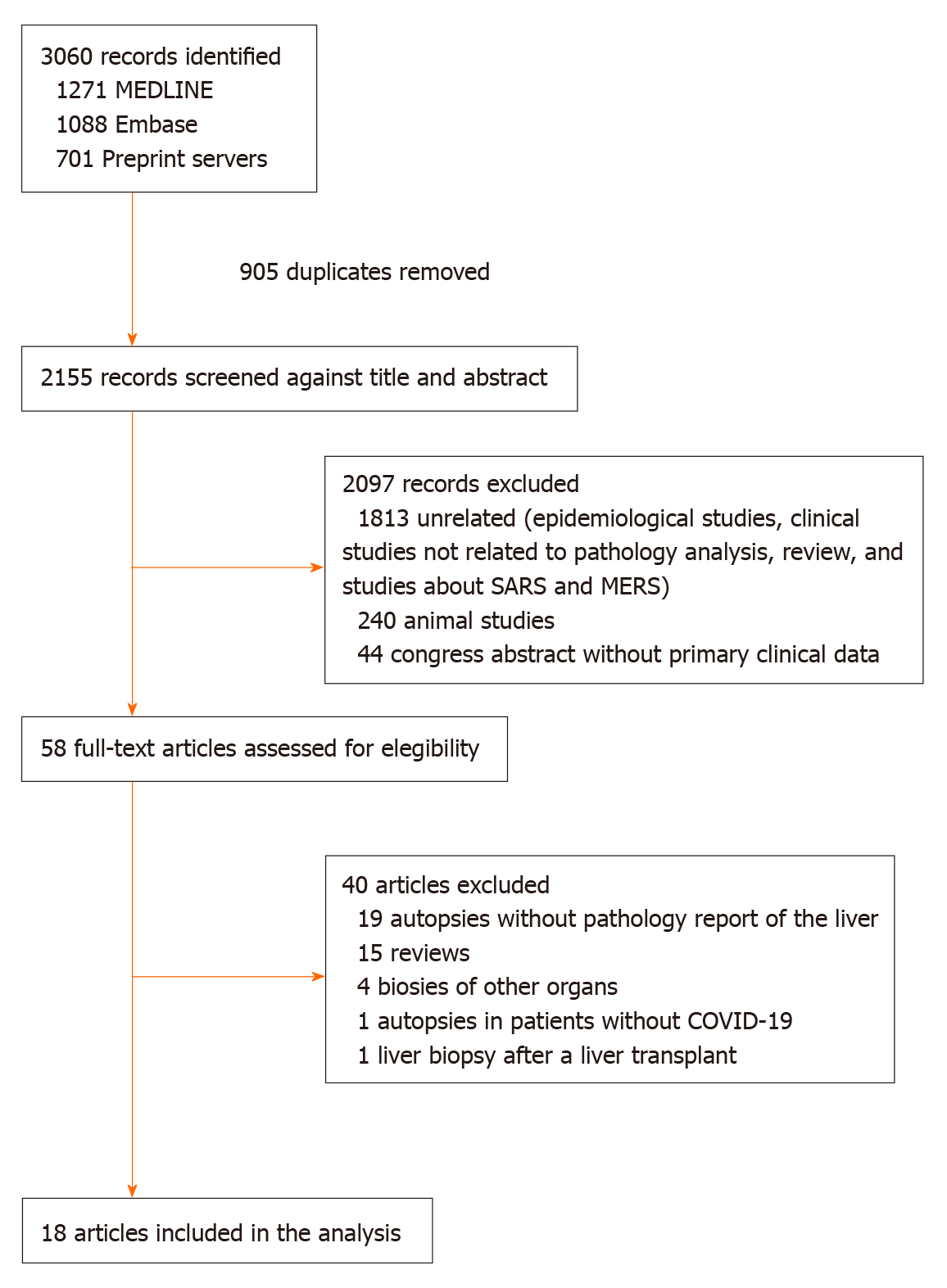
We identified 3060 records and 905 were duplicates. After a screening process against title and abstract, we obtained 58 full-text articles that were assessed for eligibility. We finally selected 18 studies for our systematic review from 7 countries (Austria, Belgium, China, Germany, Italy, Switzerland, and the United States). The selection process is described in Figure 1. All studies were observational in design; no randomized trials were identified. Among the 18 studies, 4 were case reports and 14 case series. The study with the largest sample size included a total of 48 cases[16]. Fifteen studies reported the proportion of each finding and the other three studies presented the pooled data without the prevalence of each finding. All the studies included autopsy data (i.e., no liver biopsies from living patients were identified). The risk of bias assessment was considered high.
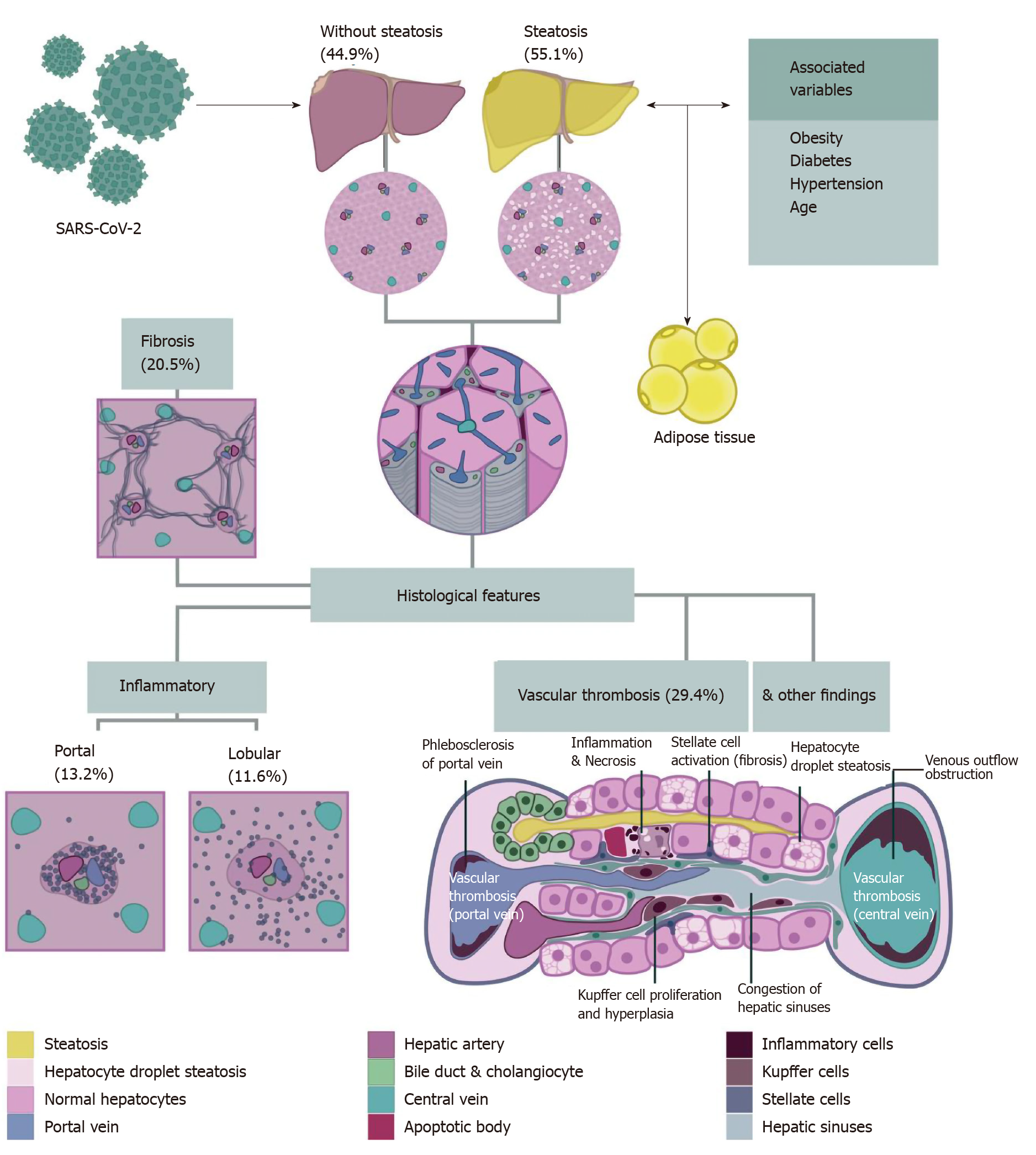
The pooled studies included a total of 167 patients. All the patients were over 15 years old and 67.2% were male. The grade of evidence was considered very low since all the information emerged from case reports and case series. For the meta-analysis of each finding, we included only five studies with at least ten patients. There was a maximum of 116 cases included in each meta-analysis. We did not perform subgroup analyses due to the low number of studies included. Table 1[17-30] summarizes the main baseline characteristics of each study. The main findings described in the selected studies were hepatic steatosis, congestion of hepatic sinuses, vascular thrombosis, fibrosis, Kupffer cell proliferation or hyperplasia, portal inflammation, lobular inflammation, venous outflow obstruction, phlebosclerosis of the portal vein, herniated portal vein, periportal abnormal vessels, hemophagocytosis, and necrosis. We did not find ductopenia in the selected studies. Figure 2 summarizes the major liver histological features. A detailed assessment of each feature is provided below. Funnel plot of the studies included in each meta-analysis are provided in Supplementary Figure 1 (Supplementary material).
| Ref. | Date | Country | Sources of samples | Nº of pts | Age (yr) | Gender | Ethnicity | Comorbidities |
| Barton et al[17] | April 2020 | United States | Autopsy | 2 | 77 and 42 | 2 Males | Not reported | Obesity (2/2), hypertension (1/2), splenectomy (1/2), myotonic dystrophy (1/2) |
| Bradley et al[18] | April 2020 | United States | Autopsy | 12 | 70.4 (42-84) | 5 Males and 7 females | Not reported | Not reported |
| Bryce et al[29] | May 2020 | United States | Autopsy | 22 | Not reported | Not reported | Not reported | Not reported |
| Buja et al[19] | May 2020 | United States | Autopsy | 3 | 62, 34 and 48 | 2 Males and 1 female | 2 Hispanic and 1 afro-american | Obesity (3/3), hypertension (1/3), heart failure (1/3), diabetes (1/3) and microcytic anemia (1/3) |
| Craver et al[20] | April 2020 | United States | Autopsy | 1 | 17 | Male | Afro-american | None |
| Lacy et al[21] | April 2020 | United States | Autopsy | 1 | 58 | Female | Not reported | Diabetes, obesity, hyperlipidemia, Asthma, and chronic lower extremity swelling with ulceration |
| Lax et al[27] | May 2020 | Austria | Autopsy | 11 | 80.5 (66-91) | 8 Males and 3 females | Not reported | Hypertension (9/11), diabetes mellitus (5/11), coronary artery disease (2/11), previous malignant disease (2/11), COPD (2/11), cerebrovascular disease (4/11), and dementia (4/11) |
| Martines et al[22] | May 2020 | United States | Autopsy | 8 | 73.5 | 4 Males and 4 females | 7 Caucasian and 1 hispanic | Arterial hypertension (6/8), chronic kidney disease (6/8) cardiovascular disease (6/8), obesity (5/8), diabetes (4/8), chronic lung disease (2/8), immunocompromised condition (3/8), among others |
| Menter et al[23] | May 2020 | Switzerland | Autopsy | 17 | Adult patients | Not specified | Not specified | Not specified |
| Prilutskiy et al[28] | March 2020 | United States | Autopsy | 4 | 64-91 | 3 Males and 1 female | 3 Afro-american and 1 caucasian | Not reported |
| Remmelink et al[24] | May 2020 | Belgium | Autopsy | 17 | 72 [62-77] | 12 Males and 5 females | Not reported | Cirrhosis (2/17), liver transplantation (1/17), hypertension (10/17), diabetes (9/17), cerebrovascular disease (4/17), coronary artery disease (4/17), and solid cancer (4/17) |
| Schaller et al[25] | May 2020 | Germany | Autopsy | 10 | 79 (64-90) | 7 Males and 3 females | Not reported | Fatty liver disease (1/10), hypertension (7/10), atrial fibrillation (4/10), chronic kidney failure (3/10), COPD (2/10), heart failure (2/10), obesity (2/10), diabetes (1/10), among others |
| Sonzogni et al[16] | May 2020 | Italy | Autopsy | 48 | 71.2 (32-87) | 35 Males and 13 females | Not reported | Not specified |
| Tian et al[14] | April 2020 | China | Autopsy | 4 | 78, 74, 81, 59 | 3 Males and 1 female | Asian | Chronic lymphocytic leukemia; Cirrhosis, DM2, HTA |
| Varga et al[13] | April 2020 | Switzerland | Autopsy | 1 | 58 | Female | Not reported | Diabetes, hypertension, and obesity |
| Wang et al[26] | May 2020 | China | Autopsy | 2 | 50 and 79 | 1 Male and 1 female | 2 Asian | Not reported |
| Xu et al[12] | February 2020 | China | Autopsy | 1 | 50 | Male | Asian | Not reported |
| Yao et al[30] | May 2020 | China | Autopsy | 3 | 63, 69, and 79 | 2 Males and 1 female | 3 Asian | Diabetes (1/3), hyperglycemia (1/3) bronchiectasis (1/3), solid cancer (1/3) |
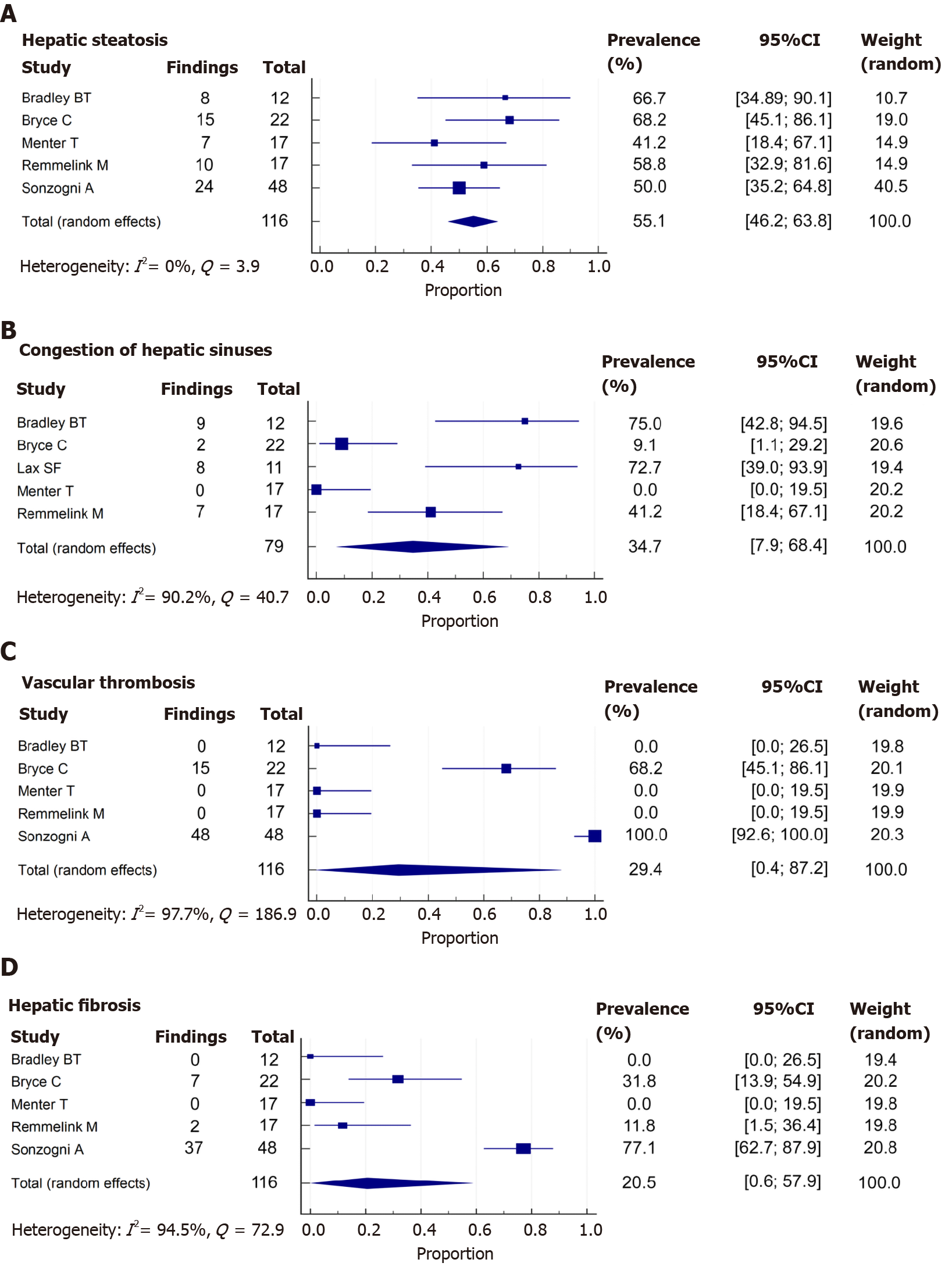
A total of 78 from 139 (65.1%) patients demonstrated hepatic steatosis. Lipid droplet size was described in only 7 cases (9%): 4 macrovesicular, 1 microvesicular, and 2 mixed (macrovesicular and microvesicular). The studies of Lax et al[27]and Prilutskiy et al[28] also described the presence of hepatic steatosis without the proportion of this finding. When meta-analyzed, data showed a pooled prevalence of hepatic steatosis of 55.1% [5 studies, 116 patients; 95% confidence interval (CI): 46.2-63.8], without significant heterogeneity among the studies (P = 0.411; I2 = 0%) (Figure 3A).
A total of 28 from 163 cases (17.2%) reported congestion of hepatic sinuses. Additionally, the study of Prilutskiy et al[28] described the presence of mild centrilobular congestion without the frequency of this finding. In the meta-analysis, the pooled prevalence of congestion of hepatic sinuses was 34.7% (5 studies, 79 patients; 95%CI: 7.9-68.4) (Figure 3B). The heterogeneity was significant among studies (P < 0.001; I2 = 90.2%). Regarding necrosis, 14 of 91 patients (15.4%) presented this finding. Necrosis was also described without a proportion in the study of Yao et al[30]. We could not perform a meta-analysis of this finding due to the low number of patients and studies that adequately described the presence or absence of necrosis.
A total of 63 from 139 (45.3%) cases presented vascular thrombosis. The type of vessel thrombosed was not specified in the studies. When meta-analyzed, data showed a pooled prevalence of vascular thrombosis of 29.4% (5 studies, 116 patients; 95%CI: 0.4-87.2). The heterogeneity was significant among studies (P < 0.001; I2 = 97.7%) (Figure 3C).
Other vascular alterations were identified in the studies. The presence of venous outflow obstruction was described exclusively by Bryce et al[29], with a prevalence of 36.4% (8 of 22 cases), and 5 of these cases (62.5%) were acute. The study by Sonzogni et al[16] reported additional vascular alterations in a serial of 48 cases: 29 patients demonstrated phlebosclerosis of the portal vein (60.4%), 36 had a herniated portal vein (75%), and 48 (100%) had abnormal periportal vessels (27 focal, 18 multifocal, and 3 diffuse).
A total of 51 from 139 (36.7%) patients had fibrosis. The grade of fibrosis was only described in 7 cases: 1 case was graded as F3 (METAVIR scale) and 6 cases were graded as F4. The study by Lax et al[27] and Prilutskiy et al[28] also described the presence of fibrosis without the proportion of this finding. In the meta-analysis, the pooled prevalence of fibrosis was 20.5% (5 studies, 116 patients; 95%CI: 0.6-57.9), with significant heterogeneity among the studies (P < 0.001; I2 = 94.5%) (Figure 3D).
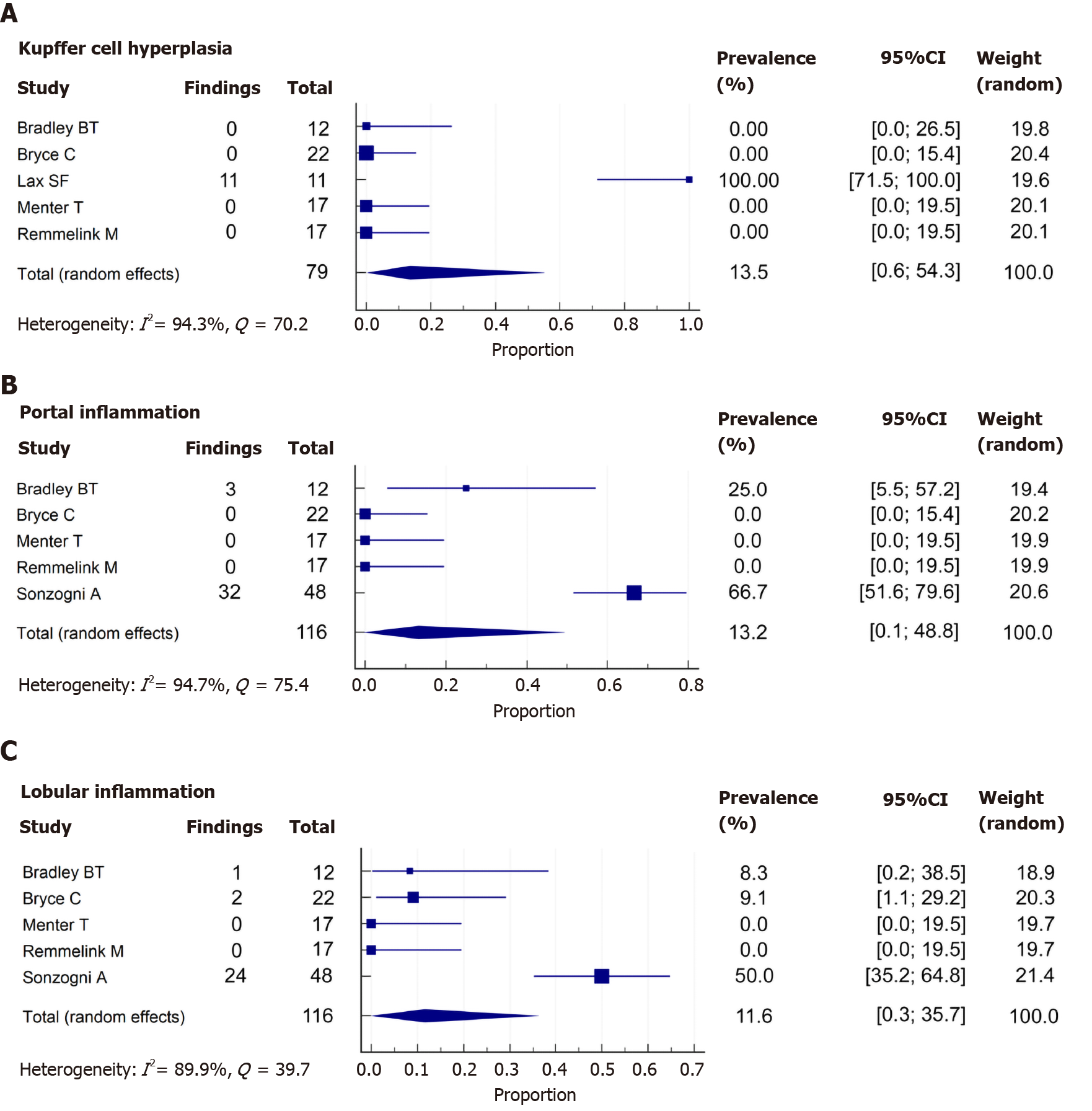
Only 12 of 115 cases (10.4%) reported Kupffer cell proliferation or hyperplasia. The studies by Sonzogni et al[16] and Prilutskiy et al[28] also described the presence of this phenomenon, but without a prevalence. Additionally, the study of Bryce et al[30] described the presence of hemophagocytosis. In the meta-analysis, the pooled prevalence of Kupffer cell hyperplasia was 13.5% (5 studies, 79 patients; 95%CI: 0.6-54.3%). The heterogeneity was significant among studies (P < 0.001; I2 = 94.3%) (Figure 4A).
Histologic evaluation in a total of 41 out of 139 (29.5%) patients showed portal inflammation. The grade of inflammation was reported in 37 of 41 cases (90.2%); all were graded as mild, and the cells were lymphocytes and plasma cells. When meta-analyzed, data showed a pooled prevalence of portal inflammation of 13.2% (5 studies, 116 patients; 95%CI: 0.1-48.8), with significant heterogeneity noted among studies (P < 0.001; I2 = 94.7%) (Figure 4B).
In the case of lobular inflammation, a total of 31 from 139 (22.3%) showed this finding. The grade of inflammation was reported in 28 of 31 cases (90.3%): 26 cases were mild (92.9%) and 2 were moderate (7.1%). The predominant cells were neutrophils and lymphocytes. In a meta-analysis, the pooled prevalence of lobular inflammation was 11.6% (5 studies, 116 patients; 95%CI: 0.3-35.7), with significant heterogeneity noted among studies (P < 0.001; I2 = 89.9%) (Figure 4C).
As previously reported for patients with SARS and MERS, the liver is frequently affected during a SARS-Cov-2-related disease (COVID-19)[3,5]. In this systematic review, we identified 18 studies from 7 countries (case reports and case series) that include data from autopsies of deceased patients with COVID-19. We identified multiple histopathological findings, including hepatic steatosis (55.1%), venous outflow obstruction (36.4%), congestion of hepatic sinuses (34.7%), vascular thrombosis (29.4%), fibrosis (20.5%), necrosis (15.4%), Kupffer cell hyperplasia (13.5%), portal inflammation (13.2%), and lobular inflammation (11.6%).
Several of the hepatic histopathological findings observed in this study have also been described in SARS and MERS patients. It may be related to the ongoing systemic inflammatory process and sepsis, affecting the liver rather than a direct manifestation of SARS-CoV-2[7,9-11]. There is still debate about whether SARS-CoV-2 directly causes liver injury or if the observed abnormalities in liver chemistries seen in COVID 19 are an indirect consequence of the disease reflecting severity, inflammation, and potentially confounding muscle injury that occurs in 19% of patients[31]. A recent study by Bloom et al[32] showed that AST-dominant aminotransferase elevation is common in COVID-19, is not associated with muscle damage markers and correlates with disease severity, probably reflecting true hepatic injury. In a series including 148 patients from a single-center in China, 55 (37.2%) had abnormal liver chemistries on admission. Patients with elevated admission liver chemistries were also more likely to have a high-grade fever and higher C-reactive protein[33]. In two large Chinese cohorts, 6.2% and 11.6% of patients with COVID-19 developed liver chemistries over 3 times the upper limit of normal, suggesting that a minority of patients experience significant biochemistry elevations[34,35].
The most frequent histopathological finding was steatosis. This prevalence is higher than the general population[36]. This can be partially explained by the baseline characteristics of the population. Admitted COVID-19 patients suffer from more severe disease and more frequently exhibit chronic diseases (i.e., diabetes mellitus, age, hypertension, obesity, and cardiovascular disease), which are also associated with hepatic steatosis[37]. However, existing data suggest that SARS-COV-2 may affect lipid metabolism[38]. In general, all viruses alter lipid synthesis and signaling in host cells as they highjack and utilize the cellular machinery to produce lipids for their envelope. Also, it has been shown that COVID-19 patients have elevated serum levels of fatty acids and infection with other SARS viruses determine long-lasting alterations in lipid metabolism[39]. It has been shown that metabolic-dysfunction associated fatty liver (MAFLD)[40] condition is independently associated with a higher risk of severe COVID-19 (odds ratio 2.67)[41]. Also, advanced MAFLD (i.e., fibrotic disease) is associated with a more severe disease irrespective of metabolic comorbidities. Since the liver hosts a significant mass of innate immune cells, hepatic release of proinflammatory cytokines may contribute to COVID-19 severity[42]. Also, some authors suggest NAFLD progression could be accelerated or exacerbated by COVID-19[42].
We observed an increased frequency of hepatic vascular alterations in patients with COVID-19. This may be due to endothelial dysfunction (endotheliitis), a pro-coagulant state, and direct vascular injury of the disease[13,43,44]. Congestion and necrosis are features of venous outflow obstruction and may also be explained by circulatory dysfunction, heart failure, and ischemia, which may complicate multiorgan failure.
Liver fibrosis was also frequently found in the published series of COVID-19 patients. Whether this is related to sustained liver injury or by prior history of chronic liver disease is unclear, though the latter is favored given the acute nature of COVID-19 disease. Of note, patients with cirrhosis have been shown to have a higher risk of mortality due to COVID-19 (relative risk of 4.6)[45].
It is important to notice that no specific histologic indicator of direct infection (i.e., viral cytopathic effect) in the liver tissue. Notably, there was a report from a pediatric living donor liver allograft recipient who, on a post-operative day 4, developed respiratory distress, fever, and an approximately 5-fold elevation of liver enzymes. The patient and donor were positive for SARS-CoV-2. Liver biopsy showed moderate acute hepatitis with prominent clusters of apoptotic hepatocytes, associated cellular debris, and lobular lymphohistiocytic inflammation. Typical portal features of mild to moderate acute cellular rejection were also noted[46]. This case report raises consideration for possible direct liver injury.
The possible mechanisms by which SARS-CoV-2 exerts its pathogenetic role in the liver have been speculated in the literature. There is a known tropism of SARS-CoV-2 for ACE-2 receptors, and they are abundantly expressed in cholangiocytes. Nevertheless, a cholestatic pattern of liver injury is not the most common finding on presentation, as one might expect[47]. A second proposed mechanism is the cytokine storm, which leads to a surge in inflammatory cytokines affecting the liver[48]. SARS-CoV-2 induced acute respiratory distress syndrome and systemic inflammatory response syndrome lead to hypoxemia and shock, which can cause ischemia-reperfusion injury[49]. It has been reported that SARS-CoV-2 can infect the endothelial cells directly and result in widespread endotheliitis[13]. Furthermore, administration of multiple drugs attempting to treat the disease have the potential to produce DILI, exacerbating the liver involvement of the disease[50].
The main limitation of our study is the inclusion of autopsies only, resulting in bias towards the most severe disease, with great influence by pre-existing co-morbid conditions. In our literature search, there were no reported liver biopsies from non-severe COVID-19 patients. A correlation between clinical condition, biochemistry, liver imaging, and autopsies would be desirable to explore. Another limitation of this systematic review is the high heterogeneity in the published articles. Finally, since this is a systematic review from autopsy data, relevant clinical information to interpret the causes of steatosis (such as alcohol consumption or body mass index) was not available.
In summary, in this systematic review and meta-analysis of autopsies from patients with COVID-19, we found a high prevalence of hepatic steatosis and the presence of vascular thrombosis as major histological liver features. Further studies are needed to establish the mechanism and implications of these findings.
The liver is frequently involved during severe acute respiratory syndrome coronavirus 2 infection and coronavirus disease 2019 (COVID-19). However, there is no consensus about the main histopathological findings in COVID-19.
Identifying the main histopathological findings could help understand the mechanism of liver injury frequently observed in COVID-19 patients.
The characterization of the liver histopathological findings will impact the interpretation of liver chemistries and liver biopsies in COVID-19 patients.
We conducted a systematic review and meta-analysis, including liver biopsies and autopsies of COVID-19 patients. Proportions were estimated using random-effects models.
We included 18 studies. The major histological findings are hepatic steatosis (55.1%), congestion of hepatic sinuses (34.7%), vascular thrombosis (29.4%), fibrosis (20.5%), Kupffer cell hyperplasia (13.5%), portal inflammation (13.2%), and lobular inflammation (11.6%). Other abnormalities can be identified, such as venous outflow obstruction, phlebosclerosis of the portal vein, herniated portal vein, periportal abnormal vessels, hemophagocytosis, and necrosis.
Steatosis, vascular thrombosis, fibrosis, and inflammatory abnormalities are the most frequent liver histopathological findings in COVID-19 patients.
The multiple liver histopathological findings observed in COVID-19 demonstrate the susceptibility to liver injury in risk populations, the inflammatory response, and thrombosis associated with this infection.
To Riquelme V, Faculty of Arts, Pontificia Universidad Católica de Chile, for her contribution with illustrations.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Y S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Díaz LA, García-Salum T, Fuentes-López E, Ferrés M, Medina RA, Riquelme A. Symptom Profiles and Risk Factors for Hospitalization in Patients With SARS-CoV-2 and COVID-19: A Large Cohort From South America. Gastroenterology. 2020;159:1148-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1133] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 3. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 4. | Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020;19:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB; AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020; 159: 320-334. e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 6. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 7. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 8. | Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, Nishioka H, Akita H, Sato Y, Kataoka M, Katano H, Tobiume M, Sekizuka T, Itokawa K, Kuroda M, Suzuki T. Clinicopathologic and Immunohistochemical Findings from Autopsy of Patient with COVID-19, Japan. Emerg Infect Dis. 2020;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 9. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 10. | Lang ZW, Zhang LJ, Zhang SJ, Meng X, Li JQ, Song CZ, Sun L, Zhou YS. [A clinicopathological study on 3 cases of severe acute respiratory syndrome]. Zhonghua Bingli Xue Zazhi. 2003;32:201-204. [PubMed] |
| 11. | Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 12. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5786] [Article Influence: 1157.2] [Reference Citation Analysis (2)] |
| 13. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4584] [Article Influence: 916.8] [Reference Citation Analysis (0)] |
| 14. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 15. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13355] [Article Influence: 834.7] [Reference Citation Analysis (0)] |
| 16. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 17. | Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 607] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 18. | Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 622] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 19. | Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 20. | Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. 2020;39:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 21. | Lacy JM, Brooks EG, Akers J, Armstrong D, Decker L, Gonzalez A, Humphrey W, Mayer R, Miller M, Perez C, Arango JAR, Sathyavagiswaran L, Stroh W, Utley S. COVID-19: Postmortem Diagnostic and Biosafety Considerations. Am J Forensic Med Pathol. 2020;41:143-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, Goldsmith CS, Silva-Flannery L, Seixas JN, Reagan-Steiner S, Uyeki T, Denison A, Bhatnagar J, Shieh WJ, Zaki SR; COVID-19 Pathology Working Group. Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States. Emerg Infect Dis. 2020;26:2005-2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 23. | Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 892] [Cited by in RCA: 912] [Article Influence: 182.4] [Reference Citation Analysis (0)] |
| 24. | Remmelink M, De Mendonça R, D'Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 25. | Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. Postmortem Examination of Patients With COVID-19. JAMA. 2020;323:2518-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 426] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 27. | Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 619] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 28. | Prilutskiy A, Kritselis M, Shevtsov A, Yambayev I, Vadlamudi C, Zhao Q, Kataria Y, Sarosiek SR, Lerner A, Sloan JM, Quillen K, Burks EJ. SARS-CoV-2 Infection-Associated Hemophagocytic Lymphohistiocytosis. Am J Clin Pathol. 2020;154:466-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 29. | Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, Al Rasheed M, Chen J, Li L, Wang D, Corben A, Haines K, Westra W, Umphlett M, Gordon RE, Reidy J, Petersen B, Salem F, Fiel M, El Jamal SM, Tsankova NM, Houldsworth J, Mussa Z, Liu W-C, Veremis B, Sordillo E, Gitman M, Nowak M, Brody R, Harpaz N, Merad M, Gnjatic S, Donnelly R, Seigler P, Keys C, Cameron J, Moultrie I, Washington K-L, Treatman J, Sebra R, Jhang J, Firpo A, Lednicky J, Paniz-Mondolfi A, Cordon-Cardo C, Fowkes M. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv 2020: 2020.2005.2018. 20099960;. [DOI] [Full Text] |
| 30. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bingli Xue Zazhi. 2020;49:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 31. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4761] [Cited by in RCA: 4704] [Article Influence: 940.8] [Reference Citation Analysis (0)] |
| 32. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2020;:. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 33. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 34. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 35. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 36. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7542] [Article Influence: 838.0] [Reference Citation Analysis (0)] |
| 37. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1466] [Article Influence: 293.2] [Reference Citation Analysis (0)] |
| 38. | Abu-Farha M, Thanaraj TA, Qaddoumi MG, Hashem A, Abubaker J, Al-Mulla F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 39. | Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, Xu L, Li X, Liu H, Yin P, Li K, Zhao J, Li Y, Wang X, Li Y, Zhang Q, Xu G, Chen H. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci Rep. 2017;7:9110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 40. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020; 158: 1999-2014. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 41. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 42. | Prins GH, Olinga P. Potential implications of COVID-19 in non-alcoholic fatty liver disease. Liver Int. 2020;40:2568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4313] [Cited by in RCA: 4066] [Article Influence: 813.2] [Reference Citation Analysis (0)] |
| 44. | Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 45. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 768-771. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 46. | Lagana SM, De Michele S, Lee MJ, Emond JC, Griesemer AD, Tulin-Silver SA, Verna EC, Martinez M, Lefkowitch JH. COVID-19 Associated Hepatitis Complicating Recent Living Donor Liver Transplantation. Arch Pathol Lab Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Bloom PP, Pasricha TS, Viveiros K. We Know Liver Biochemistries Are Elevated in COVID-19, But Should We Be Concerned? Clin Gastroenterol Hepatol. 2020;18:2384-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6752] [Article Influence: 1350.4] [Reference Citation Analysis (0)] |
| 49. | Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 50. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |