Published online Dec 7, 2020. doi: 10.3748/wjg.v26.i45.7232
Peer-review started: August 4, 2020
First decision: September 30, 2020
Revised: October 9, 2020
Accepted: November 13, 2020
Article in press: November 13, 2020
Published online: December 7, 2020
Processing time: 122 Days and 0.2 Hours
Prediction of HAIC treatment response is important for improving the prognosis in patients with hepatocellular carcinoma (HCC). The progression of HCC is related to hypercoagulability and angiogenesis. It is known that ADAMTS13 and von Willebrand factor (VWF) are related to hypercoagulability. In addition, previous study reported that the association between ADAMTS13 and VWF, and angiogenesis via vascular endothelial growth factor (VEGF). Recently, ADAMTS13 and VWF have been associated with the prognosis in patients with various kinds of cancer undergoing chemotherapy.
To investigate whether ADAMTS13 and VWF become useful biomarkers of treatment response in HCC patients before the initiation of HAIC treatment.
Seventy-two patients were enrolled in this study. ADAMTS13 activity (ADAMTS13:AC), VWF antigen (VWF:Ag) and VEGF levels were determined via enzyme-linked immunosorbent assay. Univariable and multivariable analyses were performed to determine the predictive factors of treatment response in patients with HCC undergoing HAIC treatment.
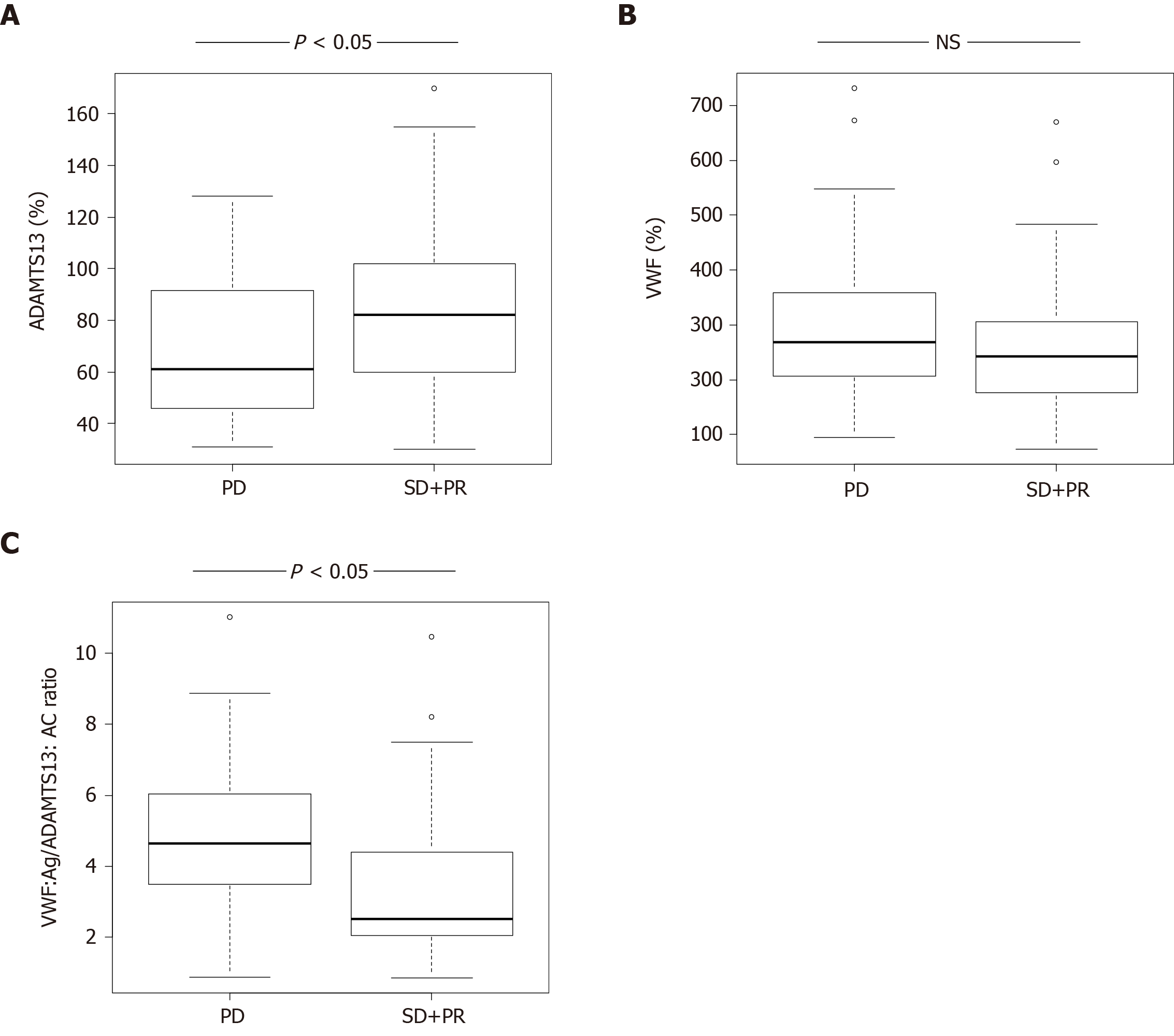
ADAMTS13:AC levels in HCC patients with stable disease (SD) + partial response (PR) of HAIC treatment were significantly higher than those with progressive disease (PD) (P < 0.05). In contrast, VWF:Ag/ADAMTS13:AC ratio and VEGF levels in HCC patients with SD + PR were significantly lower than those with PD (both P < 0.05). Patients with high VWF:Ag/ADAMTS13:AC ratio (> 2.7) had higher VEGF levels than those with low ratio (≤ 2.7). Multivariable analysis revealed that VWF:Ag/ADAMTS13:AC ratio was a predictive factor of HAIC treatment response.
VWF:Ag/ADAMTS13:AC ratio may become a useful biomarker of treatment response in HCC patients before the initiation of HAIC treatment.
Core Tip: The prediction of HAIC treatment response is needed to improve the prognosis in patients with hepatocellular carcinoma (HCC). Von Willebrand factor antigen (VWF:Ag)/ADAMTS13 activity (ADAMTS13:AC) ratio was significantly lower in HCC patients with stable disease + partial response than those with progressive disease. VWF:Ag/ADAMTS13:AC ratio become a useful biomarker to predict HAIC treatment response.
- Citation: Takaya H, Namisaki T, Moriya K, Shimozato N, Kaji K, Ogawa H, Ishida K, Tsuji Y, Kaya D, Takagi H, Fujinaga Y, Nishimura N, Sawada Y, Kawaratani H, Akahane T, Matsumoto M, Yoshiji H. Association between ADAMTS13 activity–VWF antigen imbalance and the therapeutic effect of HAIC in patients with hepatocellular carcinoma. World J Gastroenterol 2020; 26(45): 7232-7241
- URL: https://www.wjgnet.com/1007-9327/full/v26/i45/7232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i45.7232
Hepatocellular carcinoma (HCC) has one of the highest mortality rates of any cancer[1,2]. In Japan, HCC management follows the consensus-based clinical practice guidelines of the Japan Society of Hepatology (JSH)[3]. JSH recommends that advanced HCC patients with vascular invasion or more than four tumors should undergo chemotherapy such as HAIC or molecular-targeted drugs[3]. However, it is important to predict HAIC response before deciding on the appropriate chemotherapy protocol for improving the prognosis in patients with HCC.
ADAMTS13 is a metalloproteinase that is exclusively produced from hepatic stellate cells adjacent to endothelial cells (ECs)[4-8]. It specifically cleaves multimeric von Willebrand factor (VWF) between the Tyr1605 and Met1606 residues in the A2 domain[4-7]. VWF is synthesized in vascular ECs and released into the plasma as unusually large multimers[9]. During ADAMTS13 enzyme–VWF substrate imbalance, VWF is improperly cleaved, resulting in the accumulation of multimers and the induction of platelet thrombi formation in the microvasculature under high shear-stress conditions[10]. In other words, ADAMTS13 enzyme–VWF substrate balance is related to hypercoagulability. Furthermore, the blood coagulation cascade is related to cancer progression[11,12], and our previous study has reported that ADAMTS13 enzyme–VWF substrate imbalance becomes worse based on HCC progression[13,14].
Angiogenesis plays an important role in HCC progression[15]. A recent study has reported that ADAMTS13 enzyme–VWF substrate imbalance is related to angiogenesis[16] as well as hypercoagulability and is associated with the prognosis in patients with various kinds of cancer undergoing chemotherapy[14,17].
In the present study, we investigated the relationship between ADAMTS13 enzyme–VWF substrate balance and HCC in patients undergoing HAIC treatment. In addition, we sought to determine whether ADAMTS13 and VWF may become predictive biomarkers of treatment response in HCC patients before starting HAIC treatment.
This retrospective observational study included patients with HCC who underwent HAIC treatment from December 2009 to March 2019. Patients with HCC had no vascular invasion or less than four tumors were excluded. A total of 72 patients with HCC were included in this study. HAIC treatment was performed according to the Moriya method[18,19], which features a bi-monthly protocol that is simple and easy to manage. The patients underwent dynamic computed tomographic scanning or dynamic magnetic resonance imaging at various points, namely before starting HAIC treatment, 1 mo after commencement of the treatment, and every 2 mo thereafter. HAIC treatment responses were evaluated according to modified response evaluation criteria in solid tumors. This study had no patient with infection, uncontrolled hepatic encephalopathy, ascites, or gastroesophageal varices. This study was approved by the local ethics committee in Nara Medical University and was performed according to the ethical standards laid down in the Declaration of Helsinki. Informed consent was obtained from all patients included in the study.
We collected blood samples from each patient at the time of admission, during their hospital stay, or during regular outpatient treatment before starting HAIC treatment. The plastic tubes with 0.38% sodium citrate was used to store these samples. We centrifuged these samples at 3000 × g at 4 ℃ for 15 min to prepare the plasma and stored the plasma at -80 ℃ until analysis. Plasma ADAMTS13 activity (ADAMTS13:AC) was determined using a sensitive chromogenic enzyme-linked immunosorbent assay (ELISA) (Kainos Laboratories Inc., Tokyo, Japan)[20] to show a normal value of 99% ± 22%. Plasma VWF antigen (VWF:Ag) levels were measured via sandwich ELISA using a rabbit anti-human VWF polyclonal antiserum (Dako, Glostrup, Denmark). The normal VWF:Ag value is 102% ± 33%[21].
VEGF levels were determined using a commercially available kit (Immunoassay Kits, RayBiotech Inc., United States). The detection limit of VEGF was < 10 pg/mL.
The Mann–Whitney U-test and the Fisher’s exact test were performed to analyze differences between study groups and categorical data, respectively. Univariable and multivariable analysis were performed to evaluate HAIC response for HCC. Logistic regression analysis was performed to determine independent response factors, and data were expressed as median (interquartile range). A two-tailed P value of < 0.05 was considered significant. Analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Japan), a graphical user interface of R version 2.13.0 (The R Foundation for Statistical Computing, Vienna, Austria), and a modified version of R commander (version 1.6-3) that includes statistical functions that are frequently used in biostatistics[22].
Table 1 showed the clinical characteristics of HCC patients. The median period of HAIC treatment was 121 (range 41-218) d and the median age of HCC patients was 70.5 (range 64.2-76.1) years. Of the study population (57 males, 15 females), 17 patients had hepatitis B virus infection, 36 had hepatitis C virus infection, 10 had alcohol abuse, 4 had non-alcoholic steatohepatitis, and 5 had others. The median maximum tumor size was 3.3 (range 2.2-5.0) cm. Tumors numbering 1, 2, 3, 4, or > 4 were 9, 5, 6, 2, and 50, respectively. Thirty-one patients had vascular invasion and no patient had distant metastasis. Serum alpha-fetoprotein (AFP), des-γ-carboxy prothrombin, and lens culinaris agglutinin-reactive fraction of AFP levels were 95.3 (17.9–1162.5) ng/mL, 359.5 (58.0–5277.5) mAU/mL, and 33.7% (7.7%–73.4%), respectively. We investigated the HAIC treatment response between stable disease (SD) + partial response (PR) and progressive disease (PD). No significant differences were observed in HCC patients’ characteristics between SD + PR and PD, except for treatment periods.
| Variable | Total (n = 72) | SD + PR (n = 41) | PD (n = 31) | P value |
| Age (yr) | 70.5 (64.2–76.1) | 72.0 (66.4–76.4) | 67.8 (58.9–75.1) | NS |
| Sex (male/female) | 57/15 | 32/9 | 25/6 | NS |
| Etiology (HBV/HCV/alcohol/NASH/others) | 17/36/10/4/5 | 6/23/5/4/3 | 11/13/5/0/2 | NS |
| Albumin (g/dL) | 3.2 (3.0–3.6) | 3.3 (3.0–3.6) | 3.2 (3.0–3.7) | NS |
| Prothrombin time (%) | 76.0 (60.5–85.3) | 78.0 (57.0–86.0) | 73.0 (63.5–83.0) | NS |
| Total bilirubin (mg/dL) | 1.2 (0.8–2.1) | 1.2 (0.7–2.1) | 1.2 (0.8–2.2) | NS |
| Platelet count (× 104/mm3) | 10.4 (7.1–14.9) | 10.8 (7.0–13.2) | 10.1 (7.6–17.1) | NS |
| AFP (ng/mL) | 95.3 (17.9–1162.5) | 101.0 (21.2–931.5) | 87.1 (13.2–1349.2) | NS |
| DCP (mAU/mL) | 359.5 (58.0–5277.5) | 348 (42.3–1542.8) | 609.0 (88.2–279.4) | NS |
| AFP-L3% (%) | 33.7 (7.7–73.4) | 34.3 (6.9–73.4) | 22.2 (8.1–68.8) | NS |
| Maximum tumor size (cm) | 3.3 (2.2–5.0) | 3.0 (2.0–5.0) | 3.5 (2.9–3.5) | NS |
| Tumor number (1/2/3/4/> 4) | 9/5/6/2/50 | 5/4/3/2/27 | 4/1/3/0/23 | NS |
| Vascular invasion (present/absent) | 31/41 | 20/21 | 11/20 | NS |
| Treatment period (days) | 121 (41–218) | 191 (120–311) | 40.0 (26–61) | < 0.05 |
ADAMTS13:AC levels in HCC patients with SD + PR were significantly higher than those with PD (P < 0.05) (Figure 1A). VWF:Ag levels were no different between patients with SD + PR and PD (Figure 1B). The ratio of VWF:Ag to ADAMTS13:AC (VWF:Ag/ADAMTS13:AC ratio) in patients with SD + PR was significantly lower than those with PD (P < 0.05) (Figure 1C).
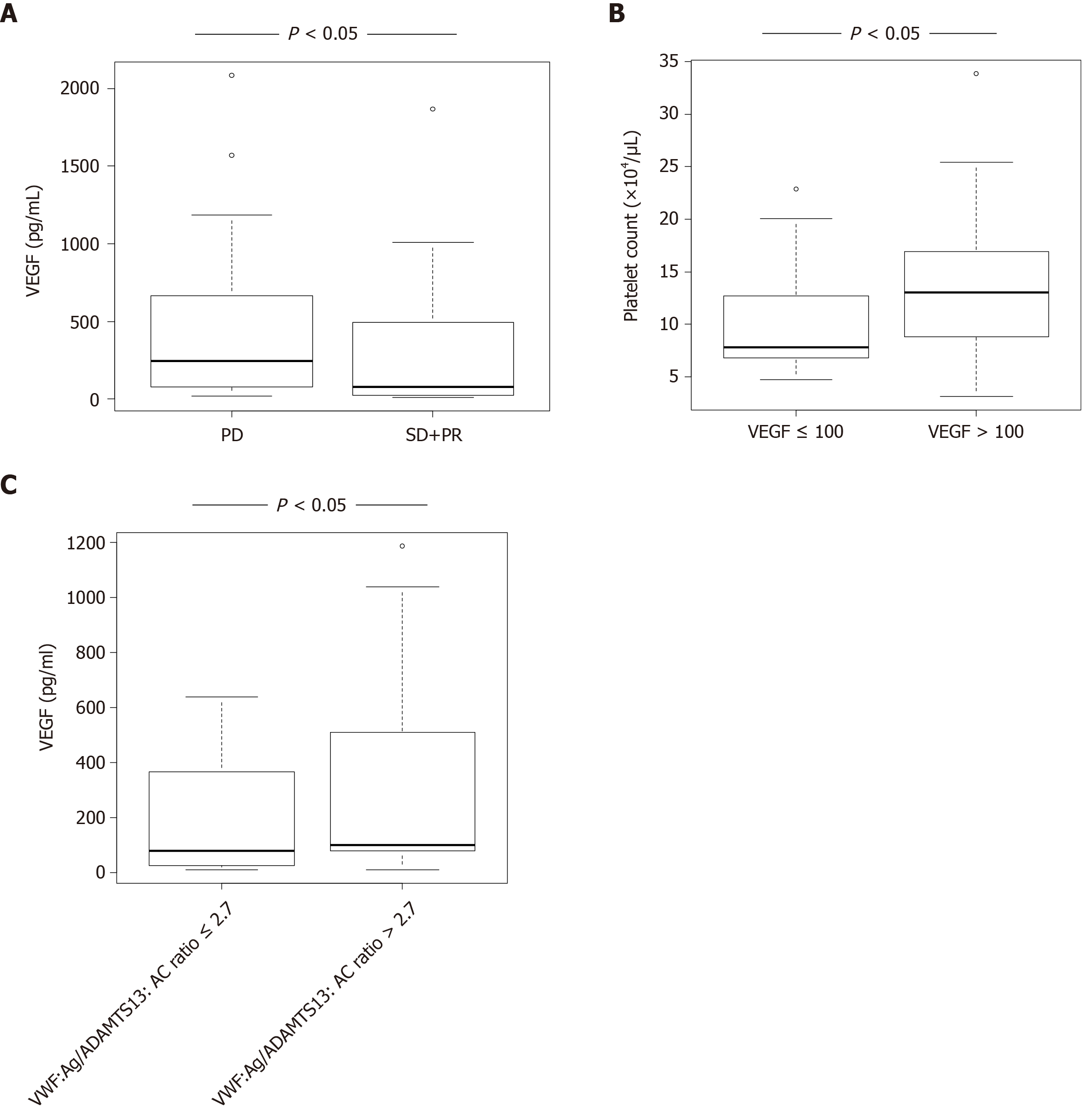
VEGF levels in HCC patients with SD + PR were significantly lower than those with PD (P < 0.05) (Figure 2A). Patients were categorized into two groups according to receiver operating characteristic (ROC) cut-off VEGF: Low, ≤ 100 and high, > 100. Patients with high VEGF levels also had higher platelet levels than those with low VEGF (Figure 2B). Patients were categorized into two groups according to ROC cut-off VWF:Ag/ADAMTS13:AC ratio: Low, ≤ 2.7 and high, > 2.7. Patients with high VWF:Ag/ADAMTS13:AC ratio had higher VEGF levels than those with low ratio (Figure 2C).
Patients were categorized into two groups according to ROC cut-off. Univariable analysis showed that HAIC treatment response is associated with prothrombin time (PT), VEGF, and VWF:Ag/ADAMTS13:AC ratio (Table 2). To determine the predictive factors of HAIC response, multivariable analysis was performed using PT, VEGF, and VWF:Ag/ADAMTS13:AC ratio, with these factors showing P < 0.05 in univariable analysis. VWF:Ag/ADAMTS13:AC ratio was significantly associated with HAIC treatment response via multivariable analysis (Table 2). ROC analysis showed that VWF:Ag/ADAMTS13:AC ratio is sensitivity of 53.7%, specificity of 87.1%, and area under the curve of 0.715.
| Variable | Univariable analysis | Multivariable analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age > 65 yr | 2.61 (0.905–7.5) | 0.076 | ||
| Sex (male vs female) | 0.853 (0.268–2.72) | 0.788 | ||
| Albumin > 2.8 g/dL | 1.40 (0.404–4.85) | 0.596 | ||
| Prothrombin time > 80% | 2.68 (1.010–7.10) | 0.0469 | 1.98 (0.654–5.96) | 0.227 |
| Total bilirubin > 2 mg/dL | 0.896 (0.317–2.53) | 0.836 | ||
| Platelet count > 20 × 104/µL | 1.57 (0.268–9.16) | 0.618 | ||
| AFP > 50 ng/mL | 0.523 (0.194–1.41) | 0.199 | ||
| DCP > 20 mAU/mL | 0.381 (0.0938–1.55) | 0.177 | ||
| AFP-L3% > 20% | 1.33 (0.491–3.62) | 0.572 | ||
| VEGF > 100 pg/mL | 0.223 (0.0802–0.618) | 0.0039 | 0.370 (0.127–1.07) | 0.0677 |
| Maximum tumor size > 2.3 cm | 0.414 (0.130–1.32) | 0.137 | ||
| Tumor number > 2 | 1.07 (0.261–4.35) | 0.928 | ||
| Vascular invasion (present/absent) | 1.73 (0.665–4.51) | 0.261 | ||
| ADAMTS13:AC > 75% | 1.25 (0.355–4.41) | 0.727 | ||
| VWF:Ag > 260% | 0.711 (0.279–1.81) | 0.476 | ||
| VWF:Ag/ADAMTS13:AC > 2.7 | 0.141 (0.0418–0.476) | 0.0016 | 0.176 (0.0493–0.631) | 0.00766 |
We suggests that VWF:Ag/ADAMTS13:AC ratio is a potential biomarker for HAIC treatment response in the present study. It is well-known that this ratio is related to the coagulation cascade[10], which in turn plays an important role in the cancer development, including HCC[11,12]. Previous studies have reported that ADAMTS13 enzyme–VWF substrate imbalance is associated with cancer progression, prognosis of patients with various kinds of cancer, and response to chemotherapy[17,23]. Our previous study reported that VWF:Ag[7] and VWF:Ag/ADAMTS13:AC ratio[13] are predictive and detective factors of HCC in patients with cirrhosis, respectively. Moreover, a study has reported that the association between ADAMTS13:AC and VWF:Ag, and the treatment efficiency of molecular-targeted drugs[14].
It is well-known that angiogenesis is related to the pathophysiology of HCC development[15] and that VEGF plays an important role in angiogenesis[15]. Recently, studies have reported that VWF reduces VEGF-dependent angiogenesis via multiple intracellular and extracellular pathways involving integrin avβ3 and angiopoietin-2[16,24,25] and that ADAMTS13 cleaves VWF and promotes VEGFR-2 phosphorylation, as the result, induces angiogenesis. This in turn results in enhancement of VEGF expression[26]. Xu have reported that the important role of ADAMTS13 enzyme–VWF substrate balance in the regulation of blood vessel formation[16]. A previous study has reported that HAIC treatment decreases VEGF levels in patients with advanced HCC[27]. Therefore, VWF:Ag/ADAMTS13:AC ratio may be associated with HAIC treatment response via VEGF and angiogenesis.
Furthermore, anti-platelet therapy inhibits VEGF that induces HCC development[28]. A recent study has reported that anti-platelet therapy for cirrhotic patients prevents HCC development[29] and prolongs survival time in hepatitis B virus mouse model of chronic liver disease[28]. ADAMTS13 enzyme–VWF substrate imbalance induces platelet thrombi formation[10]. In other words, ADAMTS13 enzyme–VWF substrate imbalance, VEGF, angiogenesis, and hypercoagulability are closely related to the cancer progression, including HCC. A previous study has found that VEGF is associated with HAIC treatment response and prognosis[30]. Our study reported the association between VWF:Ag/ADAMTS13:AC ratio and HAIC treatment response; however, our analysis indicated that VEGF is not a predictive factor of HAIC treatment response. VWF:Ag/ADAMTS13:AC ratio may become a more useful to predict HAIC treatment response than VEGF.
Our study has some limitations that include a small sample size and short observation. Cirrhotic patients with advanced HCC occasionally develop thrombosis or inflammation (e.g., portal thrombosis, and bacterial overgrowth and translocation). When VWF:Ag/ADAMTS13:AC ratio is used as a biomarker of HAIC treatment response, thrombosis and inflammation may affect the values[4,23,31]. In addition, VWF:Ag/ADAMTS13:AC ratio has high specificity but moderate sensitivity to predict HAIC treatment response. Therefore, we should continue to investigate high-sensitivity biomarkers.
In summary, VWF:Ag/ADAMTS13:AC ratio is an independent predictive factor for response in patients with HCC undergoing HAIC treatment.
Predicting HAIC treatment response is important for improving the prognosis of hepatocellular carcinoma (HCC) patients.
ADAMTS13 and von Willebrand factor (VWF) have been associated with the prognosis in patients with various kinds of cancer receiving chemotherapy.
The present study was investigated whether ADAMTS13 and VWF become useful biomarkers of treatment response in HCC patients before the initiation of HAIC treatment.
Multivariable analysis was performed to determine the predictive factors of HAIC treatment response in patients with HCC.
VWF antigen (VWF:Ag)/ADAMTS13 activity (ADAMTS13:AC) ratio predicted HAIC treatment response in multivariable analysis.
VWF:Ag/ADAMTS13:AC ratio may be a useful biomarker of treatment response in patients with HCC before HAIC treatment.
VWF:Ag/ADAMTS13:AC ratio has high specificity to predict HAIC treatment response. On the other hand, this biomarker has moderate sensitivity. Therefore, we should continue to investigate high-sensitivity biomarkers.
This work was helped by Ms. Yoshie Nakai, Prof. Masahito Uemura, and Professor Hiroshi Fukui.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ekpenyong CE, Srivastava M S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1874] [Article Influence: 208.2] [Reference Citation Analysis (4)] |
| 2. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 3. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 4. | Takaya H, Kawaratani H, Kubo T, Seki K, Sawada Y, Kaji K, Okura Y, Takeda K, Kitade M, Moriya K, Namisaki T, Mitoro A, Matsumoto M, Fukui H, Yoshiji H. Platelet hyperaggregability is associated with decreased ADAMTS13 activity and enhanced endotoxemia in patients with acute cholangitis. Hepatol Res. 2018;48:E52-E60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Takaya H, Uemura M, Fujimura Y, Matsumoto M, Matsuyama T, Kato S, Morioka C, Ishizashi H, Hori Y, Fujimoto M, Tsujimoto T, Kawaratani H, Toyohara M, Kurumatani N, Fukui H. ADAMTS13 activity may predict the cumulative survival of patients with liver cirrhosis in comparison with the Child-Turcotte-Pugh score and the Model for End-Stage Liver Disease score. Hepatol Res. 2012;42:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Takaya H, Yoshiji H, Kawaratani H, Sakai K, Matsumoto M, Fujimura Y, Fukui H. Decreased activity of plasma ADAMTS13 are related to enhanced cytokinemia and endotoxemia in patients with acute liver failure. Biomed Rep. 2017;7:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Takaya H, Kawaratani H, Tsuji Y, Nakanishi K, Saikawa S, Sato S, Sawada Y, Kaji K, Okura Y, Shimozato N, Kitade M, Akahane T, Moriya K, Namisaki T, Mitoro A, Matsumoto M, Fukui H, Yoshiji H. von Willebrand factor is a useful biomarker for liver fibrosis and prediction of hepatocellular carcinoma development in patients with hepatitis B and C. United European Gastroenterol J. 2018;6:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 422] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lämmle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1204] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 11. | Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52:3267-3272. [PubMed] |
| 12. | Snyder KM, Kessler CM. The pivotal role of thrombin in cancer biology and tumorigenesis. Semin Thromb Hemost. 2008;34:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Takaya H, Namisaki T, Kitade M, Kaji K, Nakanishi K, Tsuji Y, Shimozato N, Moriya K, Seki K, Sawada Y, Saikawa S, Sato S, Kawaratani H, Akahane T, Noguchi R, Matsumoto M, Yoshiji H. VWF/ADAMTS13 ratio as a potential biomarker for early detection of hepatocellular carcinoma. BMC Gastroenterol. 2019;19:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Takaya H, Namisaki T, Shimozato N, Kaji K, Kitade M, Moriya K, Sato S, Kawaratani H, Akahane T, Matsumoto M, Yoshiji H. ADAMTS13 and von Willebrand factor are useful biomarkers for sorafenib treatment efficiency in patients with hepatocellular carcinoma. World J Gastrointest Oncol. 2019;11:424-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Tsujinoue H, Masaki T, Fukui H. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Randi AM. Angiogenesis and the ADAMTS13-VWF balance. Blood. 2017;130:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Guo R, Yang J, Liu X, Wu J, Chen Y. Increased von Willebrand factor over decreased ADAMTS-13 activity is associated with poor prognosis in patients with advanced non-small-cell lung cancer. J Clin Lab Anal. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Moriya K, Namisaki T, Sato S, Furukawa M, Douhara A, Kawaratani H, Kaji K, Shimozato N, Sawada Y, Saikawa S, Takaya H, Kitagawa K, Akahane T, Mitoro A, Yamao J, Yoshiji H. Bi-monthly hepatic arterial infusion chemotherapy as a novel strategy for advanced hepatocellular carcinoma in decompensated cirrhotic patients. Clin Mol Hepatol. 2019;25:381-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Moriya K, Namisaki T, Sato S, Douhara A, Furukawa M, Kawaratani H, Kaji K, Kitade M, Shimozato N, Sawada Y, Seki K, Saikawa S, Takaya H, Akahane T, Mitoro A, Okura Y, Yamao J, Yoshiji H. Efficacy of bi-monthly hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. J Gastrointest Oncol. 2018;9:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Matsumoto M, Kawaguchi S, Ishizashi H, Yagi H, Iida J, Sakaki T, Fujimura Y. Platelets treated with ticlopidine are less reactive to unusually large von Willebrand factor multimers than are those treated with aspirin under high shear stress. Pathophysiol Haemost Thromb. 2005;34:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13341] [Article Influence: 1111.8] [Reference Citation Analysis (0)] |
| 23. | Pépin M, Kleinjan A, Hajage D, Büller HR, Di Nisio M, Kamphuisen PW, Salomon L, Veyradier A, Stepanian A, Mahé I. ADAMTS-13 and von Willebrand factor predict venous thromboembolism in patients with cancer. J Thromb Haemost. 2016;14:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD, Cutler DF, Laffan MA, Randi AM. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Lee M, Keener J, Xiao J, Long Zheng X, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci. 2015;72:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Matsui D, Nagai H, Mukozu T, Ogino YU, Sumino Y. VEGF in patients with advanced hepatocellular carcinoma receiving intra-arterial chemotherapy. Anticancer Res. 2015;35:2205-2210. [PubMed] |
| 28. | Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, Esposito A, Ruggeri ZM, Chisari FV, Iannacone M, Guidotti LG. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109:E2165-E2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 29. | Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, Cho H, Ahn H, Cho YY, Yoo JJ, Cho Y, Lee DH, Cho EJ, Yu SJ, Lee DH, Lee JM, Kim YJ, Yoon JH. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 30. | Niizeki T, Sumie S, Torimura T, Kurogi J, Kuromatsu R, Iwamoto H, Aino H, Nakano M, Kawaguchi A, Kakuma T, Sata M. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J Gastroenterol. 2012;47:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Samuelson Bannow BT, Konkle BA. Laboratory biomarkers for venous thromboembolism risk in patients with hematologic malignancies: A review. Thromb Res. 2018;163:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |