Published online Dec 7, 2020. doi: 10.3748/wjg.v26.i45.7222
Peer-review started: September 1, 2020
First decision: September 30, 2020
Revised: October 14, 2020
Accepted: October 27, 2020
Article in press: October 14, 2020
Published online: December 7, 2020
Processing time: 93 Days and 20.2 Hours
Ammonia is a normal constituent of body fluids and is found mainly through the formation of urea in the liver. Blood levels of ammonia must remain low as even slightly elevated concentrations (hyperammonemia) are toxic to the central nervous system.
To examine the relationship between the incidence of non-hepatic hype-rammonemia (NHH) and the prognosis of patients who were admitted to the intensive care unit (ICU).
This is a prospective, observational and single-center study. A total of 364 patients who were admitted to the ICU from November 2019 to February 2020 were initially enrolled. Changes in the levels of blood ammonia at the time of ICU admission and after ICU admission were continuously monitored. In addition, factors influencing the prognosis of NHH patients were analyzed.
A total of 204 patients who met the inclusion criteria were enrolled in this study, including 155 NHH patients and 44 severe-NHH patients. The incidence of NHH and severe-NHH was 75.98% and 21.57%, respectively. Patients with severe-NHH exhibited longer length of ICU stay and higher Acute Physiologic Assessment and Chronic Health Evaluation and Sequential Organ Failure Assessment scores compared to those with mild-NHH and non-NHH. Glasgow Coma Scale scores of patients with severe-NHH were than those of non-NHH patients. In addition, the mean and initial levels of ammonia in the blood might be helpful in predicting the prognosis of NHH.
High blood ammonia level is frequent among NHH patients admitted to the ICU, which is related to the clinical characteristics of patients. Furthermore, the level of blood ammonia may be helpful for prognosis prediction.
Core Tip: Ammonia is a normal constituent of body fluids, and the concentration of blood ammonia must remain low. Herein, a prospective and single-center study was conducted to investigate the relationship between the incidence of non-hepatic hyperammonemia and the prognosis of patients admitted to the intensive care unit. Furthermore, the level of blood ammonia may be helpful for prognosis prediction of critically ill patients.
- Citation: Yao ZP, Li Y, Liu Y, Wang HL. Relationship between the incidence of non-hepatic hyperammonemia and the prognosis of patients in the intensive care unit. World J Gastroenterol 2020; 26(45): 7222-7231
- URL: https://www.wjgnet.com/1007-9327/full/v26/i45/7222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i45.7222
The human body metabolizes three substances, including sugar, fat and protein. Ammonia in the human body is mainly produced as a byproduct of protein digestion and bacterial metabolism in the gut. The majority of ammonia is either reutilized for the biosynthesis of nitrogenous compounds (such as amino acids), or converted to urea by the urea cycle, with only a small amount of ammonia released into the blood[1-3]. As the main metabolite of amino acids, ammonia can enter the brain through the blood-brain barrier when blood ammonia increases. This may cause glial cell edema, changes in consciousness, increased intracranial pressure, and even hepatic encephalopathy, seriously threatening the life of patients. Generally, hyperam-monemia occurs due to a reduction in the metabolic capacity of the liver urea cycle. However, it may occur in the absence of hepatic diseases, namely non-hepatic hyperammonemia (NHH)[4-6]. In recent years, the importance of blood ammonia and NHH in the treatment and prognosis of patients is often neglected in clinical practice.
The occurrence of NHH in severe patients is easily ignored. As NHH patients have no history of liver diseases, this disorder is easily missed. In addition, critically ill patients often encounter various risk factors leading to high blood ammonia levels, including increased ammonia production and reduced clearance[7]. Increased ammonia production is common in microbial infections, such as urease production, pneumonia and fever, and other stress-related diseases, eventually resulting in high blood ammonia level. Catabolic states induced by seizures, extreme exercise, trauma, steroid use and gastrointestinal bleeding can also increase the production of ammonia[8-10]. Multiple studies have demonstrated that probiotics reduce inflammation and oxidative stress in liver cells, thereby leading to increased hepatic clearance of ammonia and reduced uptake of other toxins. Urea cycle disorders are inborn errors of ammonia detoxification/arginine synthesis caused by mutations in one of five core enzymes, one activating enzyme, or one of two mitochondrial anti-porters. Acute kidney injury may result in a large number of complications, including metabolic acidosis, high potassium level, uremia, and changes in fluid balance[11-13]. Therefore, blood ammonia level not only reflects the status of liver function and energy supply, but also affects the tricarboxylic acid cycle[14]. High blood ammonia mainly affects severe patients with insufficient energy supply or metabolism[15-17]. Therefore, monitoring blood ammonia is the first step of disease assessment in clinical practice. In addition, high blood ammonia is associated with the development and mortality of severe diseases.
The understanding of serum ammonia should not be limited to traditional liver diseases or hepatic encephalopathy, and NHH should also be given sufficient attention. In clinical practice, the first thing is to actively monitor blood ammonia and timely detect the increase of blood ammonia caused by various reasons. In addition, early intervention and treatment may play a key role in improving the prognosis of severe patients. Herein, a prospective and single-center study was conducted to investigate the relationship between the incidence of NHH and the prognosis of patients admitted to the intensive care unit (ICU).
This is a prospective, observational and single-center study. A total of 364 patients who were admitted to the ICU from November 2019 to February 2020 were initially enrolled. Inclusion criteria were as follows: (1) Patients who were admitted to the ICU during the study; and (2) Patients who were aged > 18 years. Exclusion criteria were as follows: (1) Patients with acute liver failure (ALF); (2) Patients with chronic liver disease (CLD); (3) Patients who were re-admitted to the ICU; or (4) Patients who did not sign the written informed consent form. Arterial blood was collected on ICU admission and at 09:00 a.m. each day after ICU admission.
HNN is defined as high blood ammonia level (> 35 μmol/L) in patients without liver diseases. According to the changes in blood ammonia level, the severity of NHH is classified into two stages: 36-99 μmol/L and ≥ 100 μmol/L representing mild-NHH and severe-NHH, respectively[18]. Blood samples were stored at 4 °C or wrapped in ice packs immediately after collection. All samples were analyzed within 30 min after collection. The study was approved by The Ethics Committee of The Second Affiliated Hospital of Harbin Medicine University (Nangang, China). Informed consent was obtained from patients or their families before the study.
Statistical data included patients’ demographic characteristics, history of other diseases, patients’ current conditions, and patients’ Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE-II) score at ICU admission[19]. Changes in blood ammonia levels, Glasgow Coma Scale (GCS) score, and Sequential Organ Failure Assessment (SOFA) score were recorded each day after ICU admission[20].
SPSS 20.0 software (IBM, Armonk, NY, United States) was used for all statistical analysis. Experimental data were expressed as mean ± SD. Continuous variables were analyzed using the Student’s t-test, and categorical variables were analyzed by the χ2 test. Mann-Whitney U test was used to compare the differences between two groups. One-way analysis of variance (ANOVA) was used to compare the differences among different groups. The correlation of blood ammonia level with length of ICU stay, APACHE-II, SOFA, and GCS scores was evaluated using the t-test. Pearson’s correlation coefficient was used to assess the relationship between blood ammonia level and related indicators. Logistic regression analysis revealed that the difference between mean blood ammonia level and initial blood ammonia level was statistically significant for predicting prognosis. A receiver operating characteristic (ROC) curve was plotted, and the area under curve (AUC) was calculated to evaluate the statistical significance of high-, mean-, and initial-levels of ammonia in the blood. P < 0.05 was considered statistically significant.
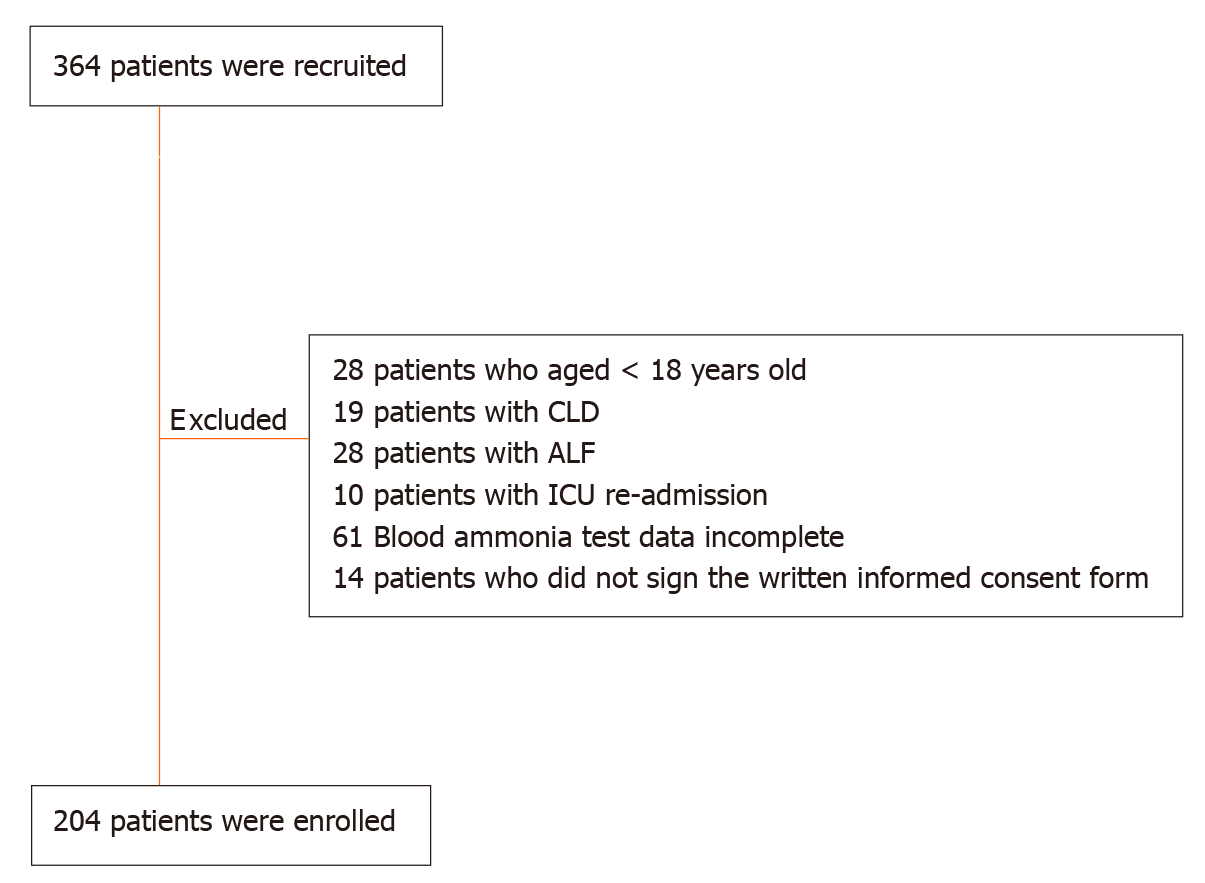
From May 2019 to August 2019, 364 patients were recruited, of whom 28 patients who were aged < 18 years, 19 patients with CLD, 28 patients with ALF, 10 patients with ICU re-admission, 61 patients with incomplete blood ammonia test data, and 14 patients who did not sign the written informed consent form were excluded (Figure 1). Finally, a total of 204 patients were enrolled, including 101 (49.51%) male patients and 103 (50.49%) female patients. The median age of the enrolled patients was 51.94 ± 18.34 years old. The mean length of ICU stay was 3.38 ± 2.96 d, and the mean APACHE-II score was 14.68 ± 9.29.
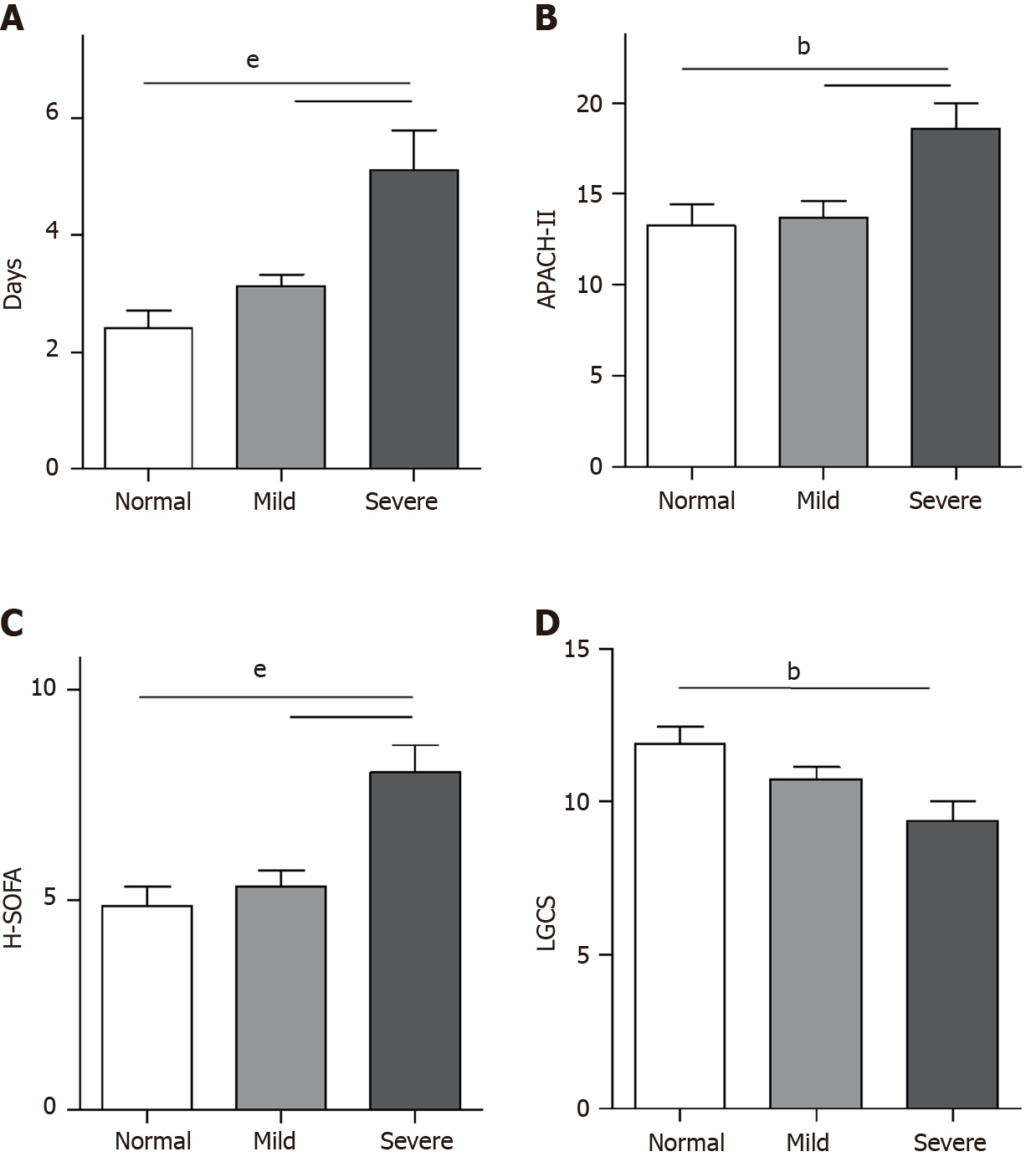
In this study, 155 NHH patients and 44 severe-NHH patients were enrolled. The incidence rate of NHH patients and severe-NHH patients was 75.98% and 21.57%, respectively. Patients with severe-NHH had a longer length of ICU stay and higher APACHE-II and SOFA scores compared to those with mild-NHH and non-NHH. Additionally, patients with severe-NHH had lower GCS scores than non-NHH patients. However, no statistically significant differences were observed in the length of ICU stay, APACHE-II, SOFA, and GCS scores between patients with mild-NHH and non-NHH (Figure 2).
Subsequent results demonstrated that the highest level of ammonia in the blood was closely correlated with the length of ICU stay, APACHE-II score, and the highest SOFA score, and was negatively correlated with the lowest GCS score and the median age of patients. Mean-level of ammonia (M-ammonia) and initial-level of ammonia (I-ammonia) in the blood showed no significant correlation with the median age of patients and the length of ICU stay (Table 1).
| H-Ammonia | M-Ammonia | I-Ammonia | ||||
| r | P value | r | P value | r | P value | |
| Age | -0.161 | 0.022 | 0.136 | 0.052 | 0.008 | 0.236 |
| Days in ICU | 0.314 | 0.000 | 0.004 | 0.572 | 0.004 | 0.573 |
| APACH-II | 0.240 | 0.000 | 0.221 | 0.002 | 0.263 | 0.000 |
| H-SOFA | 0.312 | 0.000 | - | - | - | - |
| L-GCS | -0.205 | 0.004 | - | - | - | - |
| M-SOFA | - | - | 0.279 | 0.000 | - | - |
| M-GCS | - | - | -0.183 | 0.009 | - | - |
| I-SOFA | - | - | - | - | 0.278 | 0.000 |
| I-GCS | - | - | - | - | -0.174 | 0.013 |
The patients were divided into the following two groups: good-prognosis group and poor-prognosis group, and their basic data and blood ammonia levels were assessed. The results showed that patients in the poor-prognosis group had higher APACHE-II scores and blood ammonia levels compared with those in the good-prognosis group (Table 2). Logistic regression analysis revealed that M-ammonia and I-ammonia in the blood were statistically significant for predicting prognosis (Table 3). The ROC curve showed that the AUC value of M-ammonia was 0.591 (P = 0.041). With consideration of M-ammonia equal to 69.5 μmol/L, critical values could be achieved to predict good-prognosis and poor-prognosis, with a sensitivity and specificity of 30.5% and 86.2%, respectively. In addition, the AUC value of I-ammonia was 0.612 (P = 0.012). With consideration of I-ammonia equal to 62.00 μmol/L, critical values could be attained to predict good-prognosis and poor-prognosis, with a sensitivity and specificity of 39% and 80%, respectively.
| Variables, mean ± SD | Good | Poor | t/Z | P value |
| Age (yr) | 51.58 ± 18.49 | 52.51 ± 18.24 | -0.434 | 0.665 |
| Sex (M/F) | 69/76 | 32/27 | 0.742 | 0.389 |
| Time in ICU (d) | 3.32 ± 2.57 | 3.53 ± 3.81 | -0.999 | 0.318 |
| APACHE-II | 12.36 ± 7.53 | 20.39 ± 10.77 | -4.933 | 0.000 |
| H-Ammonia, μmol/L | 67.06 ± 48.24 | 81.69 ± 59.63 | -1.776 | 0.076 |
| M-Ammonia, μmol/L | 47.70 ± 23.79 | 63.85 ± 46.35 | -2.039 | 0.041 |
| I-Ammonia, μmol/L | 48.43 ± 30.97 | 65.17 ± 46.26 | -2.516 | 0.012 |
| B | S.E. | Wald | P value | Exp (B) | 95%CI for Exp (B) | ||
| Lower | Upper | ||||||
| H-Ammonia | -0.0051 | 0.0028 | 3.2123 | 0.0731 | 0.9949 | 0.9894 | 1.0005 |
| M-Ammonia | -0.0144 | 0.0049 | 8.6664 | 0.0032 | 0.9857 | 0.9762 | 0.9952 |
| I-Ammonia | -0.0116 | 0.0041 | 7.8614 | 0.0051 | 0.9885 | 0.9805 | 0.9965 |
Similarly, we grouped patients according to central nervous system (CNS) disorders, circulatory system diseases, respiratory diseases, and multiple injuries. The results found that no statistically significant differences were observed in blood ammonia level among the groups. In addition, the patients were categorized into the drug poisoning group and non-drug poisoning group. Subsequent results revealed that blood ammonia level in the drug poisoning group was higher than that in the non-drug poisoning group (P < 0.05) (Table 4).
| Variables, mean ± SD | Drug poisoning n (20) | Non-drug poisoning n (184) | P value |
| H-Ammonia, μmol/L | 86.70 ± 46.54 | 69.61 ± 52.47 | 0.032 |
| M-Ammonia, μmol/L | 68.11 ± 36.88 | 50.66 ± 31.85 | 0.016 |
| I-Ammonia, μmol/L | 73.10 ± 46.37 | 51.12 ± 35.01 | 0.024 |
However, there was no statistically significant difference in the area under the ROC curve for H-, M- and I- between the drug poisoning group and the non-drug poisoning group.
In the present study, we investigated blood ammonia level in patients with NHH who were admitted to the ICU for the first time. The results showed that patients with severe-NHH had a longer length of ICU stay and a lower level of consciousness. The mean and initial levels of ammonia in the blood might be helpful in predicting prognosis. In addition, patients in the drug poisoning group had higher blood ammonia levels compared with those in the non-drug poisoning group.
Currently, it is well-known that hyperammonemia often occurs in patients with liver diseases. However, there is limited research on whether blood ammonia level increases in patients without liver diseases. A previous study reported that elevated blood ammonia level is common in critically ill patients (nearly 70%)[21]. When liver capacity is surpassed due to increased ammonia production and/or reduced ammonia degradation, kidneys, muscles and the CNS increase their participation in ammonia detoxification. In this study, our results confirmed this previously overlooked clinical phenomenon. This indicated that the majority of patients had excessive accumulation of ammonia in the blood, thereby resulting in hyperammonemia in the ICU.
Subsequently, we divided NHH patients into mild and severe disease, and found no statistically significant difference between mild-NHH and non-NHH patients. However, patients with severe-NHH had a longer length of ICU stay and a lower level of consciousness. These results suggested that patients with blood ammonia level > 100 μmol/L might be accompanied by severe diseases and physical conditions. A large number of studies have demonstrated that increased blood ammonia level is considered the most important factor in the pathogenesis of hepatic encephalopathy. The present study demonstrated that the higher the blood ammonia level, the worse the consciousness of patients. This may be due to the high blood ammonia level caused by cerebral edema and poor consciousness. In addition, poor consciousness may be related to the patient’s poor body condition. Thus, we suggest that further attention should be paid to patients with severe-NHH who are at high risk of infection. However, further experiments are required to explore the pathogenic mechanism.
Additionally, significant differences were observed in the APACHE-II score, mean blood ammonia level and initial blood ammonia level between the poor-prognosis and good-prognosis groups. Logistic regression analysis showed that high levels of mean blood ammonia and initial blood ammonia, but not high levels of ammonia, could reflect the poor prognosis of patients. Initial blood ammonia level reflects the untreated level of the disease. The duration of ammonia clearance is very short (about 7.7 h). Thus, the mean blood ammonia level reflects the continued level of the disease after treatment, which may have a greater impact on the prognosis of severely ill patients. Hyperammonia is only a transient metabolic abnormality, which, if treated in a timely manner, is reversible. It may be that hyperammonia is not statistically significant for disease prognosis. Therefore, real-time and continuous monitoring of blood ammonia level is highly essential for critically ill patients. Current studies have indicated that there are numerous factors influencing patients’ prognosis. However, whether increased blood ammonia level can be used as an independent prognostic indicator still requires large-scale experiments.
We further assessed the relationship between blood ammonia level with CNS disorders, circulatory system diseases, respiratory diseases and multiple injuries. However, no significant correlation was noted, which is consistent with the results of previous studies[21-31]. Our results also revealed that high-, mean-, and initial-level of ammonia in the blood increased markedly in the drug poisoning group compared with those in the non-drug poisoning group. However, there was no significant difference between the two groups due to the small number of patients in the poisoning group.
The limitation of our study is that it is a single-center study with a small sample size. Hence, further multi-center, large-scale, clinical studies should be conducted on NHH patients with elevated blood ammonia level admitted to the ICU.
At present, only guidelines released by the Middle East countries indicate that patients with blood ammonia level > 50 μmol/L require dietary treatment, and those with blood ammonia level > 100 μmol/L require medication. The current study revealed that patients with blood ammonia level > 100 μmol/L had more severe clinical symptoms and required urgent treatment. However, it is still unknown whether hyperammonia caused by severe non-hepatic diseases requires conventional ammonia-lowering treatments. In addition, the majority of ammonia-lowering drugs are only appropriate for patients with liver diseases. Hence, further research is needed to indicate whether these drugs can be applied in NHH patients with elevated blood ammonia levels. Besides, blood purification therapy may be an appropriate option for patients with increased blood ammonia level. Further studies are needed to confirm this hypothesis in the future.
High blood ammonia level is frequent among NHH patients admitted to the ICU, which is related to the clinical characteristics of patients. Furthermore, the level of blood ammonia may be helpful for prognosis prediction.
Ammonia is a normal constituent of body fluids, and the concentration of blood ammonia must remain low.
Ammonia is a normal constituent of body fluids and is treated mainly through the formation of urea in the liver. Blood levels of ammonia must remain low as even slightly elevated concentrations (hyperammonemia) are toxic to the central nervous system.
The aim of this study was to determine the relationship between the incidence of non-hepatic hyperammonemia (NHH) and the prognosis of patients who were admitted to the intensive care unit (ICU).
This is a prospective, observational and single-center study. A total of 204 patients who were admitted to ICU from November 2019 to February 2020 were finally enrolled. Changes in the levels of blood ammonia at the time of ICU admission and after ICU admission were continuously monitored. In addition, factors influencing the prognosis of NHH patients were analyzed.
A total of 204 patients who met the inclusion criteria were enrolled in this study, including 155 NHH patients and 44 severe-NHH patients. The incidence of NHH and severe-NHH was 75.98% and 21.57%, respectively. Patients with severe-NHH exhibited a longer length of ICU stay and higher Acute Physiologic Assessment and Chronic Health Evaluation and Sequential Organ Failure Assessment scores compared to those with mild-NHH and non-NHH. Glasgow Coma Scale scores of patients with severe-NHH were lower than those of non-NHH patients. In addition, the mean and initial levels of ammonia in the blood might be helpful in predicting the prognosis of NHH.
High blood ammonia level is frequent among NHH patients admitted to the ICU, which is related to the clinical characteristics of patients. Furthermore, the level of blood ammonia may be helpful for prognosis prediction.
It is necessary to explore the relationship between the incidence of NHH and the prognosis of patients in the ICU. Early intervention and treatment may be the key to improving the prognosis of critically ill patients, a hypothesis that needs to be confirmed by further studies in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koshy A, Lee M, Sharma M S-Editor: Huang P L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Babij P, Matthews SM, Rennie MJ. Changes in blood ammonia, lactate and amino acids in relation to workload during bicycle ergometer exercise in man. Eur J Appl Physiol Occup Physiol. 1983;50:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Ogino K, Osaki S, Kitamura H, Noguchi N, Hisatome I, Matsumoto T, Omodani H, Kato M, Kinugawa T, Miyakoda H, Kotake H, Mashiba H. Ammonia response to exercise in patients with congestive heart failure. Heart. 1996;75:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Dabrowska K, Skowronska K, Popek M, Obara-Michlewska M, Albrecht J, Zielinska M. Roles of Glutamate and Glutamine Transport in Ammonia Neurotoxicity: State of the Art and Question Marks. Endocr Metab Immune Disord Drug Targets. 2018;18:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012;49:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Hawkes ND, Thomas GA, Jurewicz A, Williams OM, Hillier CE, McQueen IN, Shortland G. Non-hepatic hyperammonaemia: an important, potentially reversible cause of encephalopathy. Postgrad Med J. 2001;77:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Sakusic A, Sabov M, McCambridge AJ, Rabinstein AA, Singh TD, Mukesh K, Kashani KB, Cook D, Gajic O. Features of Adult Hyperammonemia Not Due to Liver Failure in the ICU. Crit Care Med. 2018;46:e897-e903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22 Suppl 2:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Shin WK, Jang YE, Lee H, Min SH, Ryu HG. Sudden severe hyperammonemia and status epilepticus -a case report-. Korean J Anesthesiol. 2013;65:262-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Natesan V, Mani R, Arumugam R. Clinical aspects of urea cycle dysfunction and altered brain energy metabolism on modulation of glutamate receptors and transporters in acute and chronic hyperammonemia. Biomed Pharmacother. 2016;81:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Gropman A. Brain imaging in urea cycle disorders. Mol Genet Metab. 2010;100 Suppl 1:S20-S30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Kumar S, Asrani SK. Non-cirrhotic Hyperammonemia-when High Ammonia Is not Always from Cirrhosis. Curr Hepatology Rep. 2015;14:25-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Bessman AN, Evans JM. The blood ammonia in congestive heart failure. Am Heart J. 1955;50:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Tchan M. Hyperammonemia and lactic acidosis in adults: Differential diagnoses with a focus on inborn errors of metabolism. Rev Endocr Metab Disord. 2018;19:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Zimmerman JE, Wagner DP, Seneff MG, Becker RB, Sun X, Knaus WA. Intensive care unit admissions with cirrhosis: risk-stratifying patient groups and predicting individual survival. Hepatology. 1996;23:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Prado FA, Delfino VD, Grion CM, de Oliveira JA. Hyperammonemia in ICU patients: a frequent finding associated with high mortality. J Hepatol. 2015;62:1216-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Bing OHL. Hypothesis: role for ammonia neutralization in the prevention and reversal of heart failure. Am J Physiol Heart Circ Physiol. 2018;314:H1049-H1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 19. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 7769] [Article Influence: 267.9] [Reference Citation Analysis (1)] |
| 21. | Rose C. Effect of ammonia on astrocytic glutamate uptake/release mechanisms. J Neurochem. 2006;97 Suppl 1:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Olde Damink SW, Deutz NE, Dejong CH, Soeters PB, Jalan R. Interorgan ammonia metabolism in liver failure. Neurochem Int. 2002;41:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Pozefsky T, Tancredi RG, Moxley RT, Dupre J, Tobin JD. Effects of brief starvation on muscle amino acid metabolism in nonobese man. J Clin Invest. 1976;57:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Häberle J. Clinical practice: the management of hyperammonemia. Eur J Pediatr. 2011;170:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Görg B, Wettstein M, Metzger S, Schliess F, Häussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Venturini I, Corsi L, Avallone R, Farina F, Bedogni G, Baraldi C, Baraldi M, Zeneroli ML. Ammonia and endogenous benzodiazepine-like compounds in the pathogenesis of hepatic encephalopathy. Scand J Gastroenterol. 2001;36:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 28. | Brossier D, Goyer I, Ziani L, Marquis C, Mitchell G, Ozanne B, Jouvet P. Influence of implementing a protocol for an intravenously administered ammonia scavenger on the management of acute hyperammonemia in a pediatric intensive care unit. J Inherit Metab Dis. 2019;42:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ; US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018;67:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Hung TY, Chen CC, Wang TL, Su CF, Wang RF. Transient hyperammonemia in seizures: a prospective study. Epilepsia. 2011;52:2043-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Tseng YL, Huang CR, Lin CH, Lu YT, Lu CH, Chen NC, Chang CC, Chang WN, Chuang YC. Risk factors of hyperammonemia in patients with epilepsy under valproic acid therapy. Medicine (Baltimore). 2014;93:e66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |