Published online Nov 28, 2020. doi: 10.3748/wjg.v26.i44.6909
Peer-review started: July 28, 2020
First decision: September 30, 2020
Revised: October 14, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: November 28, 2020
Pituitary stalk interruption syndrome (PSIS) is a rare congenital abnormality characterized by thinning or disappearance of the pituitary stalk, hypoplasia of the anterior pituitary and an ectopic posterior pituitary. Although the etiology of PSIS is still unclear, gene changes and perinatal adverse events such as breech delivery may play important roles in the pathogenesis of PSIS. PSIS can cause multiple hormone deficiencies, such as growth hormone, which then cause a series of changes in the human body. On the one hand, hormone changes affect growth and development, and on the other hand, they could affect human metabolism and subsequently the liver resulting in nonalcoholic fatty liver disease (NAFLD). Under the synergistic effect of multiple mechanisms, the progression of NAFLD caused by PSIS is faster than that due to other causes. Therefore, in addition to early identification of PSIS, timely hormone replacement therapy and monitoring of relevant hormone levels, clinicians should routinely assess the liver function while managing PSIS.
Core Tip: Pituitary stalk interruption syndrome (PSIS) characterized by thinning or disappearance of the pituitary stalk, hypoplasia of the anterior pituitary and an ectopic posterior pituitary, could cause isolated pituitary hormone deficiency or combined pituitary hormone deficiency. In addition to influencing the growth and development of humans, PSIS could also lead to liver changes such as nonalcoholic fatty liver disease through many mechanisms. As a result, it is important to assess liver function of patients with PSIS.
- Citation: Wu ZY, Li YL, Chang B. Pituitary stalk interruption syndrome and liver changes: From clinical features to mechanisms. World J Gastroenterol 2020; 26(44): 6909-6922
- URL: https://www.wjgnet.com/1007-9327/full/v26/i44/6909.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i44.6909
Pituitary stalk interruption syndrome (PSIS) is a rare congenital abnormality characterized by thinning or disappearance of the pituitary stalk, anterior pituitary hypoplasia and an ectopic posterior pituitary[1]. Fujisawa et al[2] first reported PSIS in 1987. The incidence of PSIS is not very clear. However, it was found that 6.8% of nonacquired growth hormone (GH) deficiency cases were due to PSIS[3]. This disease is mainly sporadic, and only 5% of the cases are familial[4]. The ratio of males to females is 2.3:1[4]. The age at diagnosis of PSIS ranges from newborn to adult. The diagnosis mainly depends on the deficiency of hormones and a typical abnormality of the pituitary gland as revealed by magnetic resonance imaging (MRI).
PSIS mainly causes changes in pituitary hormones, which can be manifested as an isolated GH deficiency or multiple pituitary hormone deficiencies. However, patients with PSIS could also have hyperprolactinemia[4,5]. A study reported growth hormone deficiency (100%), gonadotropins deficiency (97.2%), corticotrophin deficiency (88.2%) and thyrotropin deficiency (70.3%) in patients with PSIS[6]. Many patients with PSIS have more than three kinds of pituitary hormone deficiencies[5].
Changes in pituitary hormones have an important impact on the human body. The prevalence of obesity and hyperlipidemia in patients with GH deficiency is high[7]. In addition, studies have found that the prevalence of nonalcoholic fatty liver disease (NAFLD) is high in patients with hypopituitarism, and the severity of GH deficiency is positively correlated with the severity of hepatic steatosis in NAFLD[8,9]. Interestingly, NAFLD develops quickly in patients with hypothalamic dysfunction or hypopituitarism[10]. It has been suggested that pituitary dysfunction is one of the causes of NAFLD. In this review, the research progress into PSIS and the changes in the liver caused by PSIS are analyzed.
The etiology of PSIS is still unclear. Perinatal adverse events may play a role in the occurrence and development of PSIS. A large number of reports have shown that there are many perinatal adverse events in patients with PSIS, such as breech delivery, hypoxia, dystocia, etc. It has been reported that 26.9% of patients with pituitary stalk dysgenesis have a traumatic birth or perinatal complications[11]. Another study found that half of the patients had a cesarean section or breech delivery and/or neonatal hypoxemia[12]. Three cases of PSIS reported by Yoo[13] all had a history of breech delivery. Wang et al[14] studied 59 cases of children with PSIS, among which 54 cases had a breech delivery. A case reported in China involved a boy with a breech delivery who had PSIS, while his brother who had a normal delivery did not, although they had the same genotype[15]. Another case also reported that breech delivery is a risk factor for PSIS[16].
Some studies have pointed out that PSIS is more likely to be caused by gene mutations. One interesting hypothesis is that breech delivery and neonatal hypoxemia may be the results of a pituitary abnormality rather than the cause[17,18]. PSIS can occur in patients with Fanconi’s anemia, a rare autosomal recessive hematological disease, suggesting that gene mutations may play a role in the pathogenesis of PSIS[19]. In addition, 48% of patients with PSIS had extrapituitary malformations[20], which also suggests a role for genetic mutations in the pathogenesis of PSIS.
Multiple genes play an important role in the development of the pituitary such as GLI2, SOX2, SOX3, HESX1, LHX3 and LHX4, which are expressed in the early stage of pituitary development and PROP1 and POU1F1, which are expressed in the late stage[21]. Among these genes, many PSIS related gene mutations have been reported, including in HESX1[4,22], OTX2[23], SOX3[24], LHX4[4,25,26], PROP1[11], PROKR2[27,28],
| Ref. | Gene/chromosome |
| Liu et al[39], 2020 | ROBO1 |
| Wang et al[40], 2020 | NBPF9 |
| Bashamboo et al[29], 2016 | CDON |
| Guo et al[35], 2017 | NCOR2, NKD2, ZIC2, MAML3 |
| Bashamboo et al[32], 2017 | ROBO1 |
| Zwaveling-Soonawala et al[33], 2018 | DCHS1, ROBO2, CCDC88C, KIF14, KAT6A, GLI2, PROK2, NR0B1, DCHR7, CCD2DA |
| Yang et al[26], 2013 | HESX1, LHX4, SOX3 |
| Tatsi et al[30], 2013 | TGIF, SHH |
| Wang et al[41], 2019 | MUC4, NBPF10 |
| El Chehadeh-Djebbar et al[38], 2011 | 17q21.31 microdeletion |
| Reynaud et al[27], 2012 | PROKR2, HESX1 |
| Fang et al[42], 2020 | PTCH1, PTCH2, GLI2, TCTN1, ATR, GLI1, CDON, CREBBP, KIF7, LHX4, HHAT, STK36, MAPK3, SMO, PRKAR2A, PRKAR2B, EGR4, SPG11, AHI1, CHD7, CAD, CEP152, CEP290, DHCR24, DMXL2, FREM1, GPSM2, ISPD, NIN, ROBO2, SIX4, SLIT2, WDR11, ASPM, CENPJ, CEP41, DIS3L2, DISC1, DSC2, GH1, GNAS, LRP2, MARCKS, MYH10, NPHP1, NSD1, OTUD4, PCSK1, POMGNT1,PSEN1, RNF111, STIL, TACR3, TBC1D32, VIPR2, WNT5A, ZEB2, ZNF423 |
| Reynaud et al[43], 2006 | LHX4 |
| Dateki et al[44], 2019 | ROBO1 |
| McCormack et al[34], 2017 | PROKR2, WDR11 |
| Demiral et al[45], 2020 | GLI2 |
| Reynaud et al[4], 2011 | HESX1, LHX4 |
| Diaczok et al[23], 2008 | OTX2 |
| Han et al[28], 2016 | PROKR2 |
| Coutinho et al[46], 2019 | HESX1 |
| Vetro et al[37], 2014 | Chromosome 2p25 and 2q37 |
| Woods et al[24], 2005 | SOX3 |
| Castinetti et al[25], 2008 | LHX4 |
| Karaca et al[31], 2015 | GPR161 |
| Carvalhom et al[22], 2003 | HESX1 |
| Fernandez-Rodriguez et al[11], 2011 | PROP1 |
| Yang et al[36], 2019 | De novo 18p deletion |
Generally, PSIS is a disease with a complex pathogenesis. Multiple genes may be related to it, and perinatal adverse events may also play a promoting role in its pathogenesis. Hypophyseal dysfunction will have serious consequences. Therefore, for newborns with perinatal adverse events such as an abnormal birth position, neonatal hypoxia and other high-risk factors, we should be alert to the possibility of PSIS.
The initial manifestations of PSIS are diverse, and it can progress from isolated growth hormone deficiency to multiple hormone deficiencies[47], causing multiple system symptoms. The main complaint of patients with PSIS is growth delay[20]. Therefore, PSIS should be included in the differential diagnosis of retarded growth and delayed puberty in children[48]. However, Lee et al[49] reported a case of PSIS with multiple hormone deficiencies that did not affect growth, and the specific mechanism is still unclear. Some other atypical initial manifestations also need to be kept in mind. A patient with recurrent seizures due to hyponatremia was reported, and the final diagnosis was PSIS[50]. A case of PSIS with recurrent hyponatremia was also reported in Korea[51]. Therefore, children with severe hyponatremia need to be tested for multiple pituitary hormone deficiency[52], and PSIS should be considered. Neonatal jaundice, hypoglycemia and cryptorchidism or micropenis are also signs of possible hormone deficiency[20]. In addition, it was reported that the hormone deficiencies of PSIS patients with extrapituitary manifestations are more serious[4]. However, the authors of one study had a different opinion, namely, that there is no correlation between the degree of hormone deficiency and extrapituitary malformations[20]. This may be related to the size of the study sample.
PSIS patients may have extrapituitary malformations, such as septum pellucidum loss[53], central nervous system and/or craniofacial malformations[20]. Tatsi et al[30] also reported cases of PSIS with a single central incisor.
In addition, liver changes caused by pituitary hormone deficiency are of concern. As in our case, the liver lesions are very serious and progress rapidly, so a lack of pituitary hormones should also be considered in cases of unexplained liver diseases.
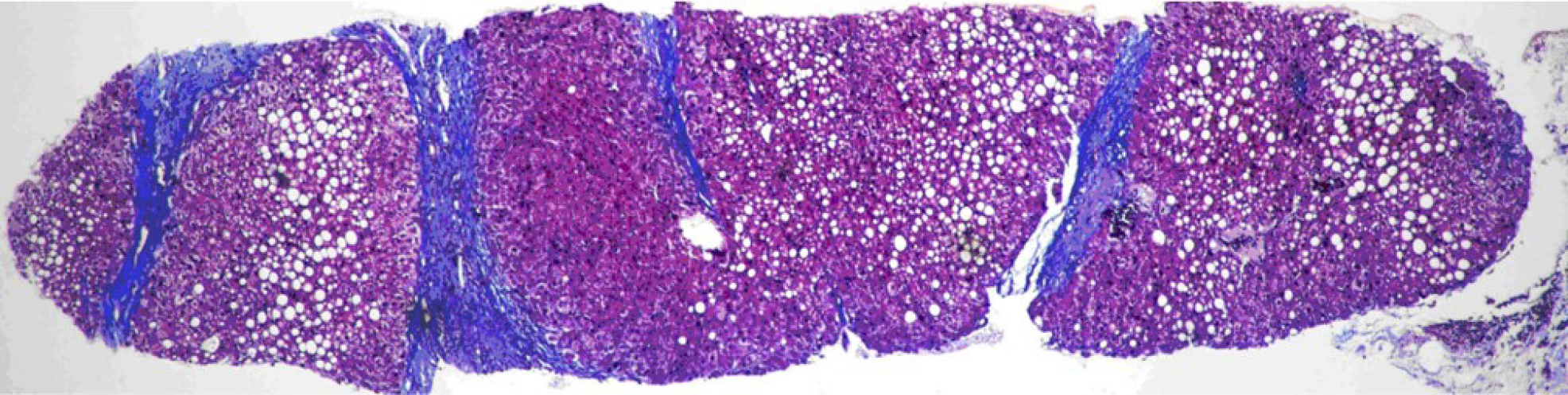
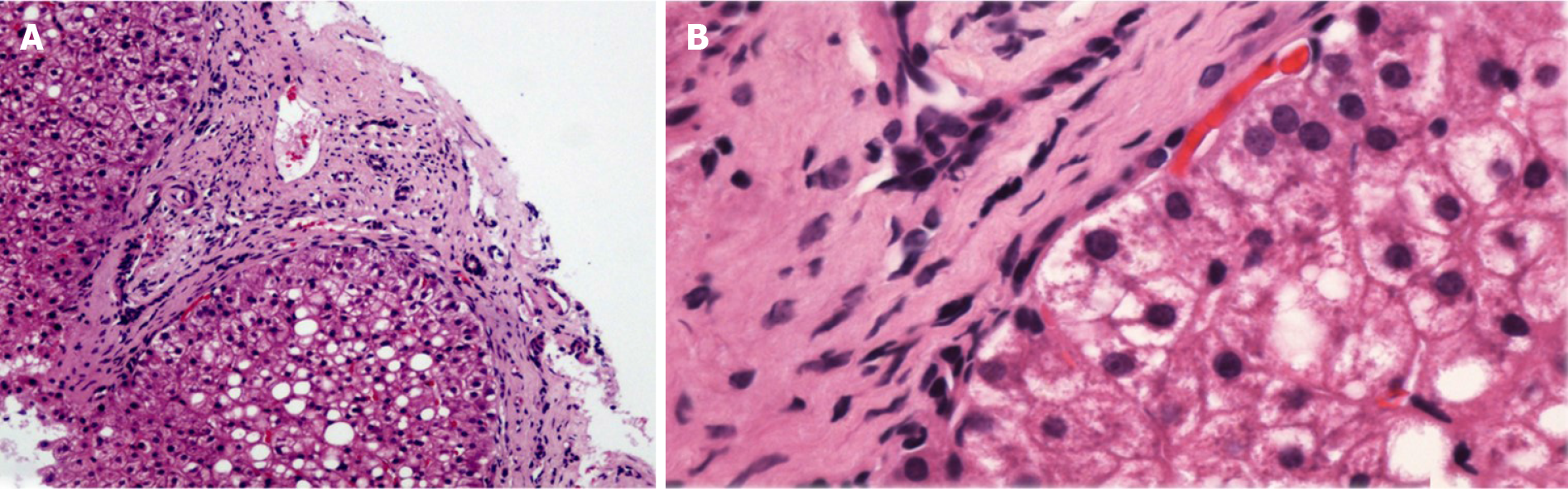
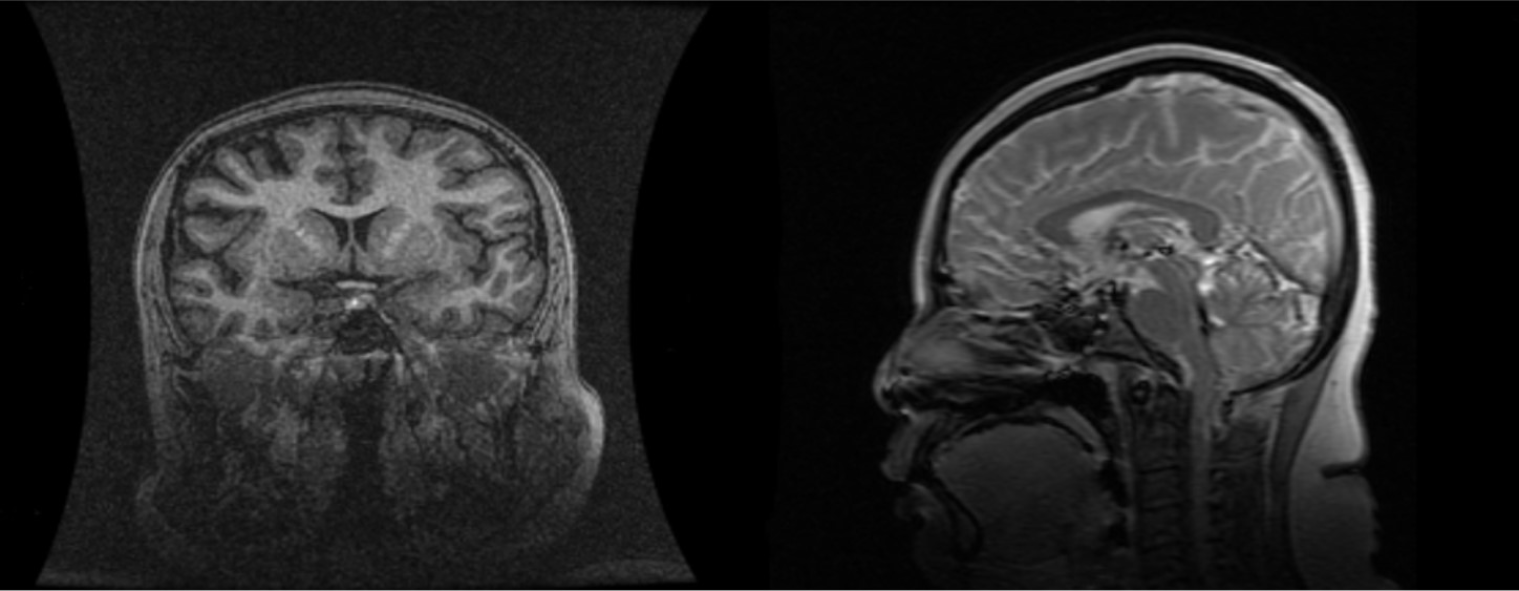
PSIS causes hormone deficiency, which not only causes growth and development problems but also causes liver lesions. We experienced a patient with PSIS complicated by cirrhosis. She was a 32-year-old woman with a height of 165 cm, weight of 73 kg, body mass index (BMI) of 26.81 kg/m2, dystocia with a foot presentation, delayed growth and development and no menstruation. Cirrhosis was found due to her abdominal distension. A liver biopsy revealed that the liver tissue was divided into nodules by fibrous septum with different widths. Most of the hepatocytes in the nodules showed bullous steatosis, as shown in Figure 1. Hepatocytes around the fibrous septum were edematous, a few of which showed balloon-like changes, and Mallory body could be seen (Figure 2). MRI showed that her pituitary stalk was truncated and the posterior pituitary was ectopic (Figure 3).
The relevant hormone assay results were: Adrenocorticotropic hormone (7.20-63.30) 8:00 1.00 pg/mL, 15:00 1.06 pg/mL, 24:00 1.00 pg/mL; cortisol (64.00-327.00 PM, 171.00–536.00 AM) 8:00 11.02 nmol/L, 15:00 9.66 nmol/L, 24:00 11.90 nmol/L; insulin-like growth factor-1 (IGF-1) < 25.00 ng/mL (115.00-358.00); free triiodothyronine 2.1500 pmol/L (2.6300-5.7000); free thyroxine 8.5700 pmol/L (9.0100-19.0500); thyroid stimulating hormone (TSH) 0.3372 mIU/L (0.3500–4.9400); follicle-stimulating hormone 0.56 mIU/mL; luteinizing hormone 0.14 mIU/mL; serum estradiol < 73.40 pmol/L; progesterone < 0.64 nmol/L; immunoglobulin A 1.25 g/L (0.70-3.80); immunoglobulin G 12.58 g/L (7.00-17.00); immunoglobulin M 2.73 g/L (0.60-2.50); γ globulin 26.1% (9.8-19.8); normal α and β globulin, C3 0.38 g/L (0.60-1.50); and C4 0.10 g/L (0.12-0.36). The related tests of hepatitis, autoimmune liver disease and rheumatic antibodies were negative. The serum ceruloplasmin was normal.
On the basis of her history and the examination results, we considered that the liver cirrhosis was caused by a hormone deficiency. The patient was finally diagnosed with PSIS and received hormone replacement therapy.
NAFLD is a disease spectrum, including nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), cirrhosis and related complications[54]. The prevalence of global NAFLD is estimated to be 24%[55]. Many factors such as genetic background, insulin resistance, hormones secreted by adipose tissue and intestinal microbiota play certain roles in the pathogenesis of NAFLD[56,57]. Significantly, many reports have pointed out that hypopituitarism is related to liver changes[58,59]. A Japanese study reported that the prevalence of NAFLD in hypopituitary patients with GH deficiency was higher (77%) compared with controls[60].
The progression of NAFLD is generally slower than that of other liver diseases. The progression from nonalcoholic fatty liver or NASH to cirrhosis or liver cancer generally takes 57 years and 28 years, respectively, and only 2.5% of NASH patients progress from NASH to cirrhosis or liver cancer[61,62]. Gonzalez Rozas et al[63] reported a patient with liver cirrhosis due to hypopituitarism. They believed that NAFLD in patients with hypopituitarism may develop rapidly into cirrhosis. Yang et al[64] reported that hypopituitarism could cause NAFLD and decompensated cirrhosis, and the average time from liver dysfunction to decompensated cirrhosis was only 6.9 yrs. Therefore, liver changes in patients with hypopituitarism are of concern.
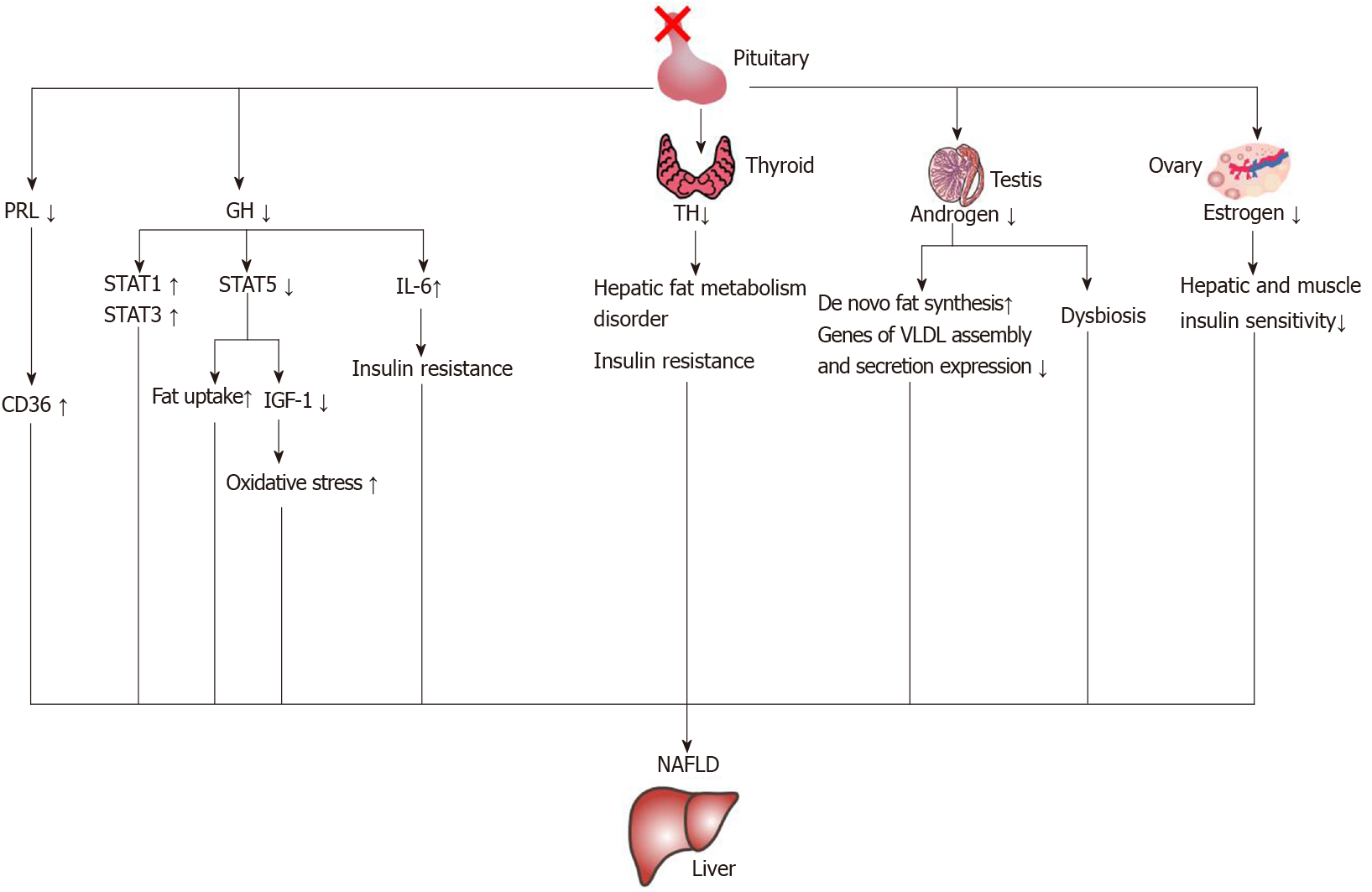
The characteristics of cholestasis caused by hypopituitarism are giant cell formation of hepatocytes with dysplasia of the bile duct and no or only a small amount of inflammatory cell infiltration. However, the giant cell formation of hepatocytes could be reversed after hormone therapy[65]. This suggests that early recognition of liver changes in patients with hypopituitarism could be reversed. In the current literature reports, growth hormone, thyroid hormone, gonadal hormones and prolactin are related to NAFLD. The mechanisms of hormone deficiency causing NAFLD are shown in Figure 4.
There have been many reports on the relationship between GH and the liver. Xu et al[66] reported that the prevalence of NAFLD increased when GH decreased. A decrease of GH level could independently predict the occurrence of NAFLD in male patients[67]. Moreover, a study reported that GH resistance can promote the development of liver cirrhosis[68], and GH replacement therapy can significantly improve the NASH in an adult patient with GH deficiency[69]. Therefore, GH deficiency in an adult may be a risk factor for hepatic steatosis and NASH[70].
It has been found that GH deficiency prevents the activation of signal transducer and activator of transcription-5 (STAT5), which leads to an increase of liver lipid uptake and an increase of phosphorylation of STAT1 and STAT3[71]. The activation of STAT1 and STAT3 can promote the development of NAFLD[72], and an increase of STAT1 would cause a decrease of the liver’s regeneration ability[73].
Oxidative stress plays an important role in the pathogenesis of NAFLD[74], and GH replacement therapy could reduce oxidative stress in the liver and serum in the GH deficiency patient with NASH[69]. In addition, Sesmilo et al[75] carried out a study on men with growth hormone deficiency and found that supplementing them with GH could reduce the levels of serum interleukin-6 and C-reactive protein, which may suggest that growth hormone can regulate the inflammatory response. The proinflammatory factor interleukin-6 can promote hepatic insulin resistance[74].
IGF-1 deficiency caused by GH deficiency may also play an important role in the development of NAFLD. The effects of IGF-1 and GH supplementation on hepatic steatosis and fibrosis in GH deficient rats were similar[76]. GH regulated the synthesis and secretion of IGF-1 through the GHR-STAT-5B signaling pathway[77,78]. The main site of IGF-1 synthesis and secretion was the liver[79]. IGF-1 was greatly reduced after knockout of the GH receptor in the mouse liver[80]. An imbalance of the GH/IGF-1 axis will cause NAFLD[81]. In addition, IGF-1 can improve mitochondrial function and reduce oxidative stress[82,83]. As a result, a decrease of IGF-1 may increase oxidative stress. The levels of GH and IGF-1 in NAFLD patients decreased[84], and IGF-1 levels further decreased with the development of NAFLD[85-87]. This may be one of the reasons for the rapid progression of liver lesions in patients with hypopituitarism.
However, another study found that GH deficiency had no significant effect on the liver. Meienberg et al[88] did not find any difference in liver fat content or the prevalence of liver steatosis between patients with GH deficiency and healthy controls after matching for age, sex, BMI and ethnicity. Moreover, GH replacement therapy had no effect on liver fat in patients with GH deficiency. We speculate that the possible reasons are as follows: First, the sample size of the study was relatively small, and thus the conclusion was biased; second, the treatment time of the study was 6 mo, which may not be long enough; and third, the dosage of growth hormone was not high enough.
It has been reported that the prevalence of central hypothyroidism in patients with PSIS is 79.8%, but one study found that only 5.6% of PSIS patients with hypothyroidism have low TSH levels. Therefore, the biological activity of TSH in patients with PSIS may be decreased[89]. Thyroid dysfunction might play an important role in the development of NAFLD[90]. Demir et al[91] carried out a histological study and found that rats with hypothyroidism had mild liver steatosis suggesting that hypothyroidism could cause NAFLD. Two systematic reviews found that patients with hypothyroidism had a higher risk of NAFLD[92,93].
Both in vitro and in vivo experiments confirmed that thyroid hormone can promote liver fat transformation and prevent hepatic steatosis through degradation of lipid droplets induced by hepatic autophagy, also known as lipophagy[94-96] In addition, in patients with hypothyroidism, low-density lipoprotein increased and liver triglyceride deposition increased[97]. Therefore, hypothyroidism can cause a liver fat metabolism disorder, which can lead to NAFLD. Hypothyroidism may also promote insulin resistance[98], which also plays a role in promoting the occurrence and development of NAFLD.
However, some studies have come to the opposite conclusions. One study reported that hypothyroidism was not associated with the occurrence of NAFLD[99]. However, there are some deficiencies in that study. The NAFLD was not confirmed by histology, only by ultrasound diagnosis, and the sample group was relatively young people[99]. Jaruvongvanich et al[100] carried out a meta-analysis and found no correlation between hypothyroidism and NAFLD, which may be related to multiple factors. First, the sample size of some included studies were small. Second, some studies did not use liver biopsies to diagnose NAFLD, just ultrasound for diagnosis. Therefore, early mild NAFLD may not have been correctly diagnosed. Third, the severity of hypothyroidism in the included population may not have been evenly distributed. In addition, an experiment conducted in mice fed a high-fat diet found that after thyroidectomy-induced hypothyroidism, the level of glucagon like peptide-1 (GLP-1) increased in mice, relieving hepatic steatosis[101]. A relationship between GLP-1 concentration and subclinical hypothyroidism was also found in humans. The serum GLP-1 concentration in patients with subclinical hypothyroidism increased[102]. The relationship between hypothyroidism and NAFLD needs to be further studied on a larger scale.
Estrogen plays an important role in liver lipid metabolism[103]. Studies have found that the prevalence of metabolic syndrome and NAFLD in postmenopausal women increased[104-106]. Animal experiments also found that ovariectomized mice could develop liver steatosis[107]. Estrogen can improve the liver and muscle insulin sensitivity of ovariectomized mice[108] and reduce liver fat deposition[109]. Another gonadal hormone, testosterone, is also associated with NAFLD, and a decrease of testosterone level indicates an increased risk of NAFLD[110,111]. Animal experiments showed that androgen receptor knockout mice were prone to develop insulin resistance and hepatic steatosis[112]. Testosterone could influence de novo fat synthesis through regulating expression of enzymes related to fatty acid synthesis[113]. It has also been found that in a high-fat fed mouse model of orchiectomy, a decrease in testosterone would cause changes in gene expression related to liver synthesis and secretion of very low-density lipoprotein, such as decreases in mRNA expression of microsomal triglyceride transporter, lipin-1, PGC-1α, etc., resulting in hepatic steatosis[114].
Androgen deficiency has an impact on the intestinal microbiota, such as an increase in the ratio of Firmicutes to Bacteroides and an increase of Lactobacillus species in the cecum[115]. A high fat diet could induce NAFLD through increasing the ratio of Firmicutes/Bacteroidetes, while reducing this ratio could relieve the inflammation of NAFLD[116]. The intestinal microbiota of Firmicutes and Bacteroides can regulate insulin resistance by regulating the secretion of GLP-1. A decrease of Firmicutes and Bacteroides could cause an increase of taurocholic acid, and an increase of taurocholic acid could promote the secretion of GLP-1 and improve insulin resistance[117]. Hypogonadism might influence the liver through the intestinal microbiota. The mechanisms require further study.
Zhang et al[118] found that a low level of prolactin (PRL) is a risk factor for the occurrence and development of NAFLD, and PRL can improve liver steatosis through PRL receptor mediated inhibition of fatty acid translocase/CD36. An increase of CD36 is associated with insulin resistance and hepatic steatosis[119]. It has been found that knockout of the PRL receptor in the mouse liver can increase the accumulation of triglycerides in the liver[120]. Therefore, a change of PRL may play a role in the development of NAFLD in patients with PSIS.
In conclusion, each hormone deficiency alone can cause liver metabolism changes and NAFLD, and NAFLD caused by multiple hormone deficiencies may be more serious and develop more rapidly.
The main treatment of PSIS is hormone replacement therapy. For PSIS, early diagnosis and monitoring are very important. It has been found that the shorter the baseline height, the better the response to GH treatment[20]. A retrospective analysis of 75 patients with PSIS of Han ethnicity in China found that GH supplement therapy was beneficial for adults[121]. Therefore, for PSIS hormone replacement therapy should be started as soon as possible, whether it is diagnosed in infants or adults. In view of the hypogonadism of PSIS patients, a study in China reported that after micropump pulse infusion of gonadorelin treatment for 12 wk, symptoms caused by androgen deficiency improved, and gonadal hormone levels increased[122].
PSIS can cause changes in pituitary hormones, and the changes in pituitary hormones can not only cause abnormal growth and development but also cause metabolic changes in the human body and lead to NAFLD. Moreover, NAFLD caused by pituitary hormone deficiency develops rapidly. For patients with a late onset, liver lesions may already exist at the time of onset. Therefore, in the clinical management of PSIS, besides the need for early identification of PSIS and timely hormone replacement therapy and monitoring of relevant hormone levels, routine assessments of the liver condition are necessary. At present, the etiology of PSIS is still unclear. Genes and adverse events during pregnancy and the perinatal period may be involved in its pathogenesis. The related genes of PSIS should be screened in the neonatal stage, and the possibility of PSIS should be kept in mind. Abnormalities of the pituitary gland should be excluded if any of these conditions, such as an abnormal birth position especially breech delivery, hypoxia, dystocia, recurrent hypoglycemia and/or prolonged jaundice, occur in the neonate. It should be noted that regardless of the age of the patient, it is necessary for patients with PSIS to actively take hormone replacement therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Protopapas A, Rakhshan V, Rodrigues AT S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Wang CZ, Guo LL, Han BY, Su X, Guo QH, Mu YM. Pituitary Stalk Interruption Syndrome: From Clinical Findings to Pathogenesis. J Neuroendocrinol. 2017;29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, Minami S, Sagoh T, Hiraoka T, Momoi T. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165:487-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 175] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Maghnie M, Lindberg A, Koltowska-Häggström M, Ranke MB. Magnetic resonance imaging of CNS in 15,043 children with GH deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinol. 2013;168:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Reynaud R, Albarel F, Saveanu A, Kaffel N, Castinetti F, Lecomte P, Brauner R, Simonin G, Gaudart J, Carmona E, Enjalbert A, Barlier A, Brue T. Pituitary stalk interruption syndrome in 83 patients: novel HESX1 mutation and severe hormonal prognosis in malformative forms. Eur J Endocrinol. 2011;164:457-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Han BY, Zhang Q, Li LL, Guo QH, Wang CZ, Cang L, Jin N, Chen F, Zhao L, Cui J, Gu XL, Ma FL, Zhang SC, Mu YM, Dou JT. Clinical Features of Pituitary Stalk Interruption Syndrome in 114 Cases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:534-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Wang W, Wang S, Jiang Y, Yan F, Su T, Zhou W, Jiang L, Zhang Y, Ning G. Relationship between pituitary stalk (PS) visibility and the severity of hormone deficiencies: PS interruption syndrome revisited. Clin Endocrinol (Oxf). 2015;83:369-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Irie M, Itoh Y, Miyashita Y, Tsushima T, Shirai K. Complications in adults with growth hormone deficiency--a survey study in Japan. Endocr J. 2004;51:479-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Hong JW, Kim JY, Kim YE, Lee EJ. Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm Metab Res. 2011;43:48-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yuan XX, Zhu HJ, Pan H, Chen S, Liu ZY, Li Y, Wang LJ, Lu L, Yang HB, Gong FY. Clinical characteristics of non-alcoholic fatty liver disease in Chinese adult hypopituitary patients. World J Gastroenterol. 2019;25:1741-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39:909-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Fernandez-Rodriguez E, Quinteiro C, Barreiro J, Marazuela M, Pereiro I, Peinó R, Cabezas-Agrícola JM, Dominguez F, Casanueva FF, Bernabeu I. Pituitary stalk dysgenesis-induced hypopituitarism in adult patients: prevalence, evolution of hormone dysfunction and genetic analysis. Neuroendocrinology. 2011;93:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Pinto G, Netchine I, Sobrier ML, Brunelle F, Souberbielle JC, Brauner R. Pituitary stalk interruption syndrome: a clinical-biological-genetic assessment of its pathogenesis. J Clin Endocrinol Metab. 1997;82:3450-3454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Yoo HW. Growth hormone deficiency associated with pituitary stalk interruption syndrome. Horm Res. 1998;49 Suppl 1:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Wang Q, Hu Y, Li G, Sun X. Pituitary stalk interruption syndrome in 59 children: the value of MRI in assessment of pituitary functions. Eur J Pediatr. 2014;173:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wang D, Zhang M, Guan H, Wang X. Osteogenesis Imperfecta Due to Combined Heterozygous Mutations in Both COL1A1 and COL1A2, Coexisting With Pituitary Stalk Interruption Syndrome. Front Endocrinol (Lausanne). 2019;10:193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Fukuta K, Hidaka T, Ono Y, Kochi K, Yasoshima K, Arai T. Case of pituitary stalk transection syndrome ascertained after breech delivery. J Obstet Gynaecol Res. 2016;42:202-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Maghnie M, Larizza D, Triulzi F, Sampaolo P, Scotti G, Severi F. Hypopituitarism and stalk agenesis: a congenital syndrome worsened by breech delivery? Horm Res. 1991;35:104-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kulkarni C, Moorthy S, Pullara SK, Rajeshkannan R, Unnikrishnan AG. Pituitary stalk transection syndrome: Comparison of clinico-radiological features in adults and children with review of literature. Indian J Radiol Imaging. 2012;22:182-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Dupuis-Girod S, Gluckman E, Souberbielle JC, Brauner R. Growth hormone deficiency caused by pituitary stalk interruption in Fanconi's anemia. J Pediatr. 2001;138:129-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Bar C, Zadro C, Diene G, Oliver I, Pienkowski C, Jouret B, Cartault A, Ajaltouni Z, Salles JP, Sevely A, Tauber M, Edouard T. Pituitary Stalk Interruption Syndrome from Infancy to Adulthood: Clinical, Hormonal, and Radiological Assessment According to the Initial Presentation. PLoS One. 2015;10:e0142354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonça BB, Brue T, Camper SA. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323:4-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Carvalho LR, Woods KS, Mendonca BB, Marcal N, Zamparini AL, Stifani S, Brickman JM, Arnhold IJ, Dattani MT. A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction. J Clin Invest. 2003;112:1192-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Diaczok D, Romero C, Zunich J, Marshall I, Radovick S. A novel dominant negative mutation of OTX2 associated with combined pituitary hormone deficiency. J Clin Endocrinol Metab. 2008;93:4351-4359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Woods KS, Cundall M, Turton J, Rizotti K, Mehta A, Palmer R, Wong J, Chong WK, Al-Zyoud M, El-Ali M, Otonkoski T, Martinez-Barbera JP, Thomas PQ, Robinson IC, Lovell-Badge R, Woodward KJ, Dattani MT. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet. 2005;76:833-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Castinetti F, Saveanu A, Reynaud R, Quentien MH, Buffin A, Brauner R, Kaffel N, Albarel F, Guedj AM, El Kholy M, Amin M, Enjalbert A, Barlier A, Brue T. A novel dysfunctional LHX4 mutation with high phenotypical variability in patients with hypopituitarism. J Clin Endocrinol Metab. 2008;93:2790-2799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Yang Y, Guo QH, Wang BA, Dou JT, Lv ZH, Ba JM, Lu JM, Pan CY, Mu YM. Pituitary stalk interruption syndrome in 58 Chinese patients: clinical features and genetic analysis. Clin Endocrinol (Oxf). 2013;79:86-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Reynaud R, Jayakody SA, Monnier C, Saveanu A, Bouligand J, Guedj AM, Simonin G, Lecomte P, Barlier A, Rondard P, Martinez-Barbera JP, Guiochon-Mantel A, Brue T. PROKR2 variants in multiple hypopituitarism with pituitary stalk interruption. J Clin Endocrinol Metab. 2012;97:E1068-E1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Han BY, Li LL, Wang CZ, Guo QH, Lv ZH, Mu YM, Dou JT. Correlation between Pituitary Stalk Interruption Syndrome and Prokineticin Receptor 2 and Prokineticin 2 Mutations. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Bashamboo A, Bignon-Topalovic J, Rouba H, McElreavey K, Brauner R. A Nonsense Mutation in the Hedgehog Receptor CDON Associated With Pituitary Stalk Interruption Syndrome. J Clin Endocrinol Metab. 2016;101:12-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Tatsi C, Sertedaki A, Voutetakis A, Valavani E, Magiakou MA, Kanaka-Gantenbein C, Chrousos GP, Dacou-Voutetakis C. Pituitary stalk interruption syndrome and isolated pituitary hypoplasia may be caused by mutations in holoprosencephaly-related genes. J Clin Endocrinol Metab. 2013;98:E779-E784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Karaca E, Buyukkaya R, Pehlivan D, Charng WL, Yaykasli KO, Bayram Y, Gambin T, Withers M, Atik MM, Arslanoglu I, Bolu S, Erdin S, Buyukkaya A, Yaykasli E, Jhangiani SN, Muzny DM, Gibbs RA, Lupski JR. Whole-exome sequencing identifies homozygous GPR161 mutation in a family with pituitary stalk interruption syndrome. J Clin Endocrinol Metab. 2015;100:E140-E147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Bashamboo A, Bignon-Topalovic J, Moussi N, McElreavey K, Brauner R. Mutations in the Human ROBO1 Gene in Pituitary Stalk Interruption Syndrome. J Clin Endocrinol Metab. 2017;102:2401-2406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Zwaveling-Soonawala N, Alders M, Jongejan A, Kovacic L, Duijkers FA, Maas SM, Fliers E, van Trotsenburg ASP, Hennekam RC. Clues for Polygenic Inheritance of Pituitary Stalk Interruption Syndrome From Exome Sequencing in 20 Patients. J Clin Endocrinol Metab. 2018;103:415-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | McCormack SE, Li D, Kim YJ, Lee JY, Kim SH, Rapaport R, Levine MA. Digenic Inheritance of PROKR2 and WDR11 Mutations in Pituitary Stalk Interruption Syndrome. J Clin Endocrinol Metab. 2017;102:2501-2507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Guo QH, Wang CZ, Wu ZQ, Qin Y, Han BY, Wang AP, Wang BA, Dou JT, Wu XS, Mu YM. Multi-genic pattern found in rare type of hypopituitarism: a whole-exome sequencing study of Han Chinese with pituitary stalk interruption syndrome. J Cell Mol Med. 2017;21:3626-3632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Yang A, Kim J, Cho SY, Lee JE, Kim HJ, Jin DK. A case of de novo 18p deletion syndrome with panhypopituitarism. Ann Pediatr Endocrinol Metab. 2019;24:60-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Vetro A, Pagani S, Silengo M, Severino M, Bozzola E, Meazza C, Zuffardi O, Bozzola M. Severe growth hormone deficiency and pituitary malformation in a patient with chromosome 2p25 duplication and 2q37 deletion. Mol Cytogenet. 2014;7:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | El Chehadeh-Djebbar S, Callier P, Masurel-Paulet A, Bensignor C, Méjean N, Payet M, Ragon C, Durand C, Marle N, Mosca-Boidron AL, Huet F, Mugneret F, Faivre L, Thauvin-Robinet C. 17q21.31 microdeletion in a patient with pituitary stalk interruption syndrome. Eur J Med Genet. 2011;54:369-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. |
Liu Z, Chen X.
A Novel Missense Mutation in Human Receptor Roundabout-1 |
| 40. | Wang CZ, Guo LL, Guo QH, Mu YM. NBPF9 Gene May Be Involved in Congenital Hypopituitarism: A Whole-Genome Study of a Boy with Pituitary Stalk Interruption Syndrome and His Family. Int J Endocrinol. 2020;2020:5401738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Wang CZ, Wei Q, Guo LL, Liu HY, Guo QH. Normal height and novel mutations in growth hormone deficiency adults with pituitary stalk interruption syndrome. Neuro Endocrinol Lett. 2019;40:299-304. [PubMed] [Cited in This Article: ] |
| 42. | Fang X, Zhang Y, Cai J, Lu T, Hu J, Yuan F, Chen P. Identification of novel candidate pathogenic genes in pituitary stalk interruption syndrome by whole-exome sequencing. J Cell Mol Med. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Reynaud R, Gueydan M, Saveanu A, Vallette-Kasic S, Enjalbert A, Brue T, Barlier A. Genetic screening of combined pituitary hormone deficiency: experience in 195 patients. J Clin Endocrinol Metab. 2006;91:3329-3336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Dateki S, Watanabe S, Mishima H, Shirakawa T, Morikawa M, Kinoshita E, Yoshiura KI, Moriuchi H. A homozygous splice site ROBO1 mutation in a patient with a novel syndrome with combined pituitary hormone deficiency. J Hum Genet. 2019;64:341-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Demiral M, Demirbilek H, Unal E, Durmaz CD, Ceylaner S, Özbek MN. Ectopic Posterior Pituitary, Polydactyly, Midfacial Hypoplasia and Multiple Pituitary Hormone Deficiency due to a Novel Heterozygous IVS11-2A>C(c.1957-2A>C) Mutation in the GLI2 Gene. J Clin Res Pediatr Endocrinol. 2020;12:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Coutinho E, Brandão CM, Lemos MC. Combined Pituitary Hormone Deficiency Caused by a Synonymous HESX1 Gene Mutation. J Clin Endocrinol Metab. 2019;104:2851-2854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Otto AP, França MM, Correa FA, Costalonga EF, Leite CC, Mendonca BB, Arnhold IJ, Carvalho LR, Jorge AA. Frequent development of combined pituitary hormone deficiency in patients initially diagnosed as isolated growth hormone deficiency: a long term follow-up of patients from a single center. Pituitary. 2015;18:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Yılmaz G. Pituitary Stalk Interruption Syndrome Presenting With Growth Retardation. Pediatr Neurol. 2016;62:75-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Lee SS, Han AL, Ahn MB, Kim SH, Cho WK, Cho KS, Park SH, Jung MH, Suh BK. Growth without growth hormone in combined pituitary hormone deficiency caused by pituitary stalk interruption syndrome. Ann Pediatr Endocrinol Metab. 2017;22:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Li J, Jia H, Chakraborty A, Gao Z. A case of pituitary stalk interruption syndrome with intermittent seizures as the first presentation. Neuro Endocrinol Lett. 2016;37:469-472. [PubMed] [Cited in This Article: ] |
| 51. | Jang KM, Ko CW. Delayed diagnosis of pituitary stalk interruption syndrome with severe recurrent hyponatremia caused by adrenal insufficiency. Ann Pediatr Endocrinol Metab. 2017;22:208-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Wójcik M, Janus D, Herman-Sucharska I, Starzyk JB. Generalized seizures as the first manifestation of multihormonal pituitary hormone deficiency causing normovolemic hyponatremia. Am J Case Rep. 2013;14:507-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Olszewska M, Kiełbasa G, Wójcik M, Zygmunt-Górska A, Starzyk JB. A case report of severe panhypopituitarism in a newborn delivered by a women with Turner syndrome. Neuro Endocrinol Lett. 2015;36:734-736. [PubMed] [Cited in This Article: ] |
| 54. | Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019;103:22-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 55. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2383] [Cited by in F6Publishing: 3042] [Article Influence: 507.0] [Reference Citation Analysis (2)] |
| 56. | Marchisello S, Di Pino A, Scicali R, Urbano F, Piro S, Purrello F, Rabuazzo AM. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 57. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1490] [Cited by in F6Publishing: 1706] [Article Influence: 213.3] [Reference Citation Analysis (1)] |
| 58. | Spray CH, Mckiernan P, Waldron KE, Shaw N, Kirk J, Kelly DA. Investigation and outcome of neonatal hepatitis in infants with hypopituitarism. Acta Paediatr. 2000;89:951-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Nyunt A, Kochar N, Pilz DT, Kingham JG, Jones MK. Adult cirrhosis due to untreated congenital hypopituitarism. J R Soc Med. 2005;98:316-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Nishizawa H, Iguchi G, Murawaki A, Fukuoka H, Hayashi Y, Kaji H, Yamamoto M, Suda K, Takahashi M, Seo Y, Yano Y, Kitazawa R, Kitazawa S, Koga M, Okimura Y, Chihara K, Takahashi Y. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol. 2012;167:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81-84. [PubMed] [Cited in This Article: ] |
| 62. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643-54. quiz e39-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 932] [Cited by in F6Publishing: 1017] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 63. | Gonzalez Rozas M, Hernanz Roman L, Gonzalez DG, Pérez-Castrillón JL. Panhypopituitarism due to Absence of the Pituitary Stalk: A Rare Aetiology of Liver Cirrhosis. Case Rep Endocrinol. 2016;2016:9071097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Yang Y, Qi ZR, Zhang TT, Kang YJ, Wang X. Rapidly progressive non-alcoholic fatty liver disease due to hypopituitarism. Report of 5 cases. Neuro Endocrinol Lett. 2018;39:99-104. [PubMed] [Cited in This Article: ] |
| 65. | Wada K, Kobayashi H, Moriyama A, Haneda Y, Mushimoto Y, Hasegawa Y, Onigata K, Kumori K, Ishikawa N, Maruyama R, Sogo T, Murphy L, Taketani T. A case of an infant with congenital combined pituitary hormone deficiency and normalized liver histology of infantile cholestasis after hormone replacement therapy. Clin Pediatr Endocrinol. 2017;26:251-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Xu L, Xu C, Yu C, Miao M, Zhang X, Zhu Z, Ding X, Li Y. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One. 2012;7:e44136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Lonardo A, Loria P, Leonardi F, Ganazzi D, Carulli N. Growth hormone plasma levels in nonalcoholic fatty liver disease. Am J Gastroenterol. 2002;97:1071-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Stiedl P, McMahon R, Blaas L, Stanek V, Svinka J, Grabner B, Zollner G, Kessler SM, Claudel T, Müller M, Mikulits W, Bilban M, Esterbauer H, Eferl R, Haybaeck J, Trauner M, Casanova E. Growth hormone resistance exacerbates cholestasis-induced murine liver fibrosis. Hepatology. 2015;61:613-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Takahashi Y, Iida K, Takahashi K, Yoshioka S, Fukuoka H, Takeno R, Imanaka M, Nishizawa H, Takahashi M, Seo Y, Hayashi Y, Kondo T, Okimura Y, Kaji H, Kitazawa R, Kitazawa S, Chihara K. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132:938-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Ichikawa T, Hamasaki K, Ishikawa H, Ejima E, Eguchi K, Nakao K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52:914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Barclay JL, Nelson CN, Ishikawa M, Murray LA, Kerr LM, McPhee TR, Powell EE, Waters MJ. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, Rasmiena AA, Kaur S, Gulati T, Goh PK, Treloar AE, Archer S, Brown WA, Muller M, Watt MJ, Ohara O, McLean CA, Tiganis T. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell 2018; 175: 1289-1306. e20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 73. | Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 Leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 74. | Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49:197-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 75. | Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med. 2000;133:111-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Nishizawa H, Takahashi M, Fukuoka H, Iguchi G, Kitazawa R, Takahashi Y. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem Biophys Res Commun. 2012;423:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Takahashi Y. The Role of Growth Hormone and Insulin-Like Growth Factor-I in the Liver. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 78. | Hwa V. STAT5B deficiency: Impacts on human growth and immunity. Growth Horm IGF Res. 2016;28:16-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 79. | Daughaday WH. Growth hormone axis overview--somatomedin hypothesis. Pediatr Nephrol. 2000;14:537-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937-19944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 81. | Adamek A, Kasprzak A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 82. | Hao CN, Geng YJ, Li F, Yang T, Su DF, Duan JL, Li Y. Insulin-like growth factor-1 receptor activation prevents hydrogen peroxide-induced oxidative stress, mitochondrial dysfunction and apoptosis. Apoptosis. 2011;16:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Puche JE, García-Fernández M, Muntané J, Rioja J, González-Barón S, Castilla Cortazar I. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology. 2008;149:2620-2627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Fusco A, Miele L, D'Uonnolo A, Forgione A, Riccardi L, Cefalo C, Barini A, Bianchi A, Giampietro A, Cimino V, Landolfi R, Grieco A, De Marinis L. Nonalcoholic fatty liver disease is associated with increased GHBP and reduced GH/IGF-I levels. Clin Endocrinol (Oxf). 2012;77:531-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Sumida Y, Yonei Y, Tanaka S, Mori K, Kanemasa K, Imai S, Taketani H, Hara T, Seko Y, Ishiba H, Okajima A, Yamaguchi K, Moriguchi M, Mitsuyoshi H, Yasui K, Minami M, Itoh Y. Lower levels of insulin-like growth factor-1 standard deviation score are associated with histological severity of non-alcoholic fatty liver disease. Hepatol Res. 2015;45:771-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 86. | Dichtel LE, Corey KE, Misdraji J, Bredella MA, Schorr M, Osganian SA, Young BJ, Sung JC, Miller KK. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2017;8:e217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Mallea-Gil MS, Ballarino MC, Spiraquis A, Iriarte M, Kura M, Gimenez S, Oneto A, Guitelman M, Machado R, Miguel CM. IGF-1 Levels in different stages of liver steatosis and its association with metabolic syndrome. Acta Gastroenterol Latinoam. 2012;42:20-26. [PubMed] [Cited in This Article: ] |
| 88. | Meienberg F, Yee M, Johnston D, Cox J, Robinson S, Bell JD, Thomas EL, Taylor-Robinson SD, Godsland I. Liver fat in adults with GH deficiency: comparison to matched controls and the effect of GH replacement. Clin Endocrinol (Oxf). 2016;85:76-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Zhang Q, Zang L, Li YJ, Han BY, Gu WJ, Yan WH, Jin N, Chen K, Du J, Wang XL, Guo QH, Yang GQ, Yang LJ, Ba JM, Lv ZH, Dou JT, Lu JM, Mu YM. Thyrotrophic status in patients with pituitary stalk interruption syndrome. Medicine (Baltimore). 2018;97:e9084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Lee J, Ha J, Jo K, Lim DJ, Lee JM, Chang SA, Kang MI, Cha BY, Kim MH. Male-specific association between subclinical hypothyroidism and the risk of non-alcoholic fatty liver disease estimated by hepatic steatosis index: Korea National Health and Nutrition Examination Survey 2013 to 2015. Sci Rep. 2018;8:15145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Demir Ş, Ünübol M, Aypak SÜ, İpek E, Aktaş S, Ekren GS, Yılmaz M, Tunca R, Güney E. Histopathologic Evaluation of Nonalcoholic Fatty Liver Disease in Hypothyroidism-Induced Rats. Int J Endocrinol. 2016;2016:5083746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | He W, An X, Li L, Shao X, Li Q, Yao Q, Zhang JA. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front Endocrinol (Lausanne). 2017;8:335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 93. | Mantovani A, Nascimbeni F, Lonardo A, Zoppini G, Bonora E, Mantzoros CS, Targher G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid. 2018;28:1270-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 94. | Chi HC, Tsai CY, Tsai MM, Yeh CT, Lin KH. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J Biomed Sci. 2019;26:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 95. | Sinha RA, You SH, Zhou J, Siddique MM, Bay BH, Zhu X, Privalsky ML, Cheng SY, Stevens RD, Summers SA, Newgard CB, Lazar MA, Yen PM. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. 2012;122:2428-2438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 96. | Tseng YH, Ke PY, Liao CJ, Wu SM, Chi HC, Tsai CY, Chen CY, Lin YH, Lin KH. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy. 2014;10:20-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Huang YY, Gusdon AM, Qu S. Cross-talk between the thyroid and liver: a new target for nonalcoholic fatty liver disease treatment. World J Gastroenterol. 2013;19:8238-8246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Erdogan M, Canataroglu A, Ganidagli S, Kulaksızoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest. 2011;34:488-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 99. | Lee KW, Bang KB, Rhee EJ, Kwon HJ, Lee MY, Cho YK. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study. Clin Mol Hepatol. 2015;21:372-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Jaruvongvanich V, Sanguankeo A, Upala S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur Thyroid J. 2017;6:208-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 101. | Kang JY, Kim M, Kang Y, Lee W, Ha TK, Seo JH, Son YG, Ha E. Thyroidectomy stimulates glucagon-like peptide-1 secretion and attenuates hepatic steatosis in high-fat fed rats. Biochem Biophys Res Commun. 2017;493:548-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 102. | Jin Y, Liu H, Ma SG, Cheng JP, Zhang K. Serum levels of glucagon-like peptide (GLP)-1 and GLP-2 in patients with Hashimoto's thyroiditis. J Res Med Sci. 2015;20:174-177. [PubMed] [Cited in This Article: ] |
| 103. | Palmisano BT, Zhu L, Stafford JM. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv Exp Med Biol. 2017;1043:227-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 104. | Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 105. | Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond). 2009;5:191-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Völzke H, Schwarz S, Baumeister SE, Wallaschofski H, Schwahn C, Grabe HJ, Kohlmann T, John U, Dören M. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 107. | Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161-2168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 108. | Camporez JP, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, Shulman GI. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154:1021-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 109. | Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 110. | Kim S, Kwon H, Park JH, Cho B, Kim D, Oh SW, Lee CM, Choi HC. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol. 2012;12:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 111. | Yim JY, Kim J, Kim D, Ahmed A. Serum testosterone and non-alcoholic fatty liver disease in men and women in the US. Liver Int. 2018;38:2051-2059. [PubMed] [Cited in This Article: ] |
| 112. | Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, Yeh S, Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47:1924-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 113. | Kelly DM, Nettleship JE, Akhtar S, Muraleedharan V, Sellers DJ, Brooke JC, McLaren DS, Channer KS, Jones TH. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci. 2014;109:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Senmaru T, Fukui M, Okada H, Mineoka Y, Yamazaki M, Tsujikawa M, Hasegawa G, Kitawaki J, Obayashi H, Nakamura N. Testosterone deficiency induces markedly decreased serum triglycerides, increased small dense LDL, and hepatic steatosis mediated by dysregulation of lipid assembly and secretion in mice fed a high-fat diet. Metabolism. 2013;62:851-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 115. | Harada N, Hanaoka R, Hanada K, Izawa T, Inui H, Yamaji R. Hypogonadism alters cecal and fecal microbiota in male mice. Gut Microbes. 2016;7:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 116. | Wu S, Hu R, Nakano H, Chen K, Liu M, He X, Zhang H, He J, Hou DX. Modulation of Gut Microbiota by Lonicera caerulea L. Berry Polyphenols in a Mouse Model of Fatty Liver Induced by High Fat Diet. Molecules. 2018;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 117. | Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, Seong JK, Seok YJ, Kim JB. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015;29:2397-2411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 118. | Zhang P, Ge Z, Wang H, Feng W, Sun X, Chu X, Jiang C, Wang Y, Zhu D, Bi Y. Prolactin improves hepatic steatosis via CD36 pathway. J Hepatol. 2018;68:1247-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 119. | Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60:1394-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 120. | Shao S, Yao Z, Lu J, Song Y, He Z, Yu C, Zhou X, Zhao L, Zhao J, Gao L. Ablation of prolactin receptor increases hepatic triglyceride accumulation. Biochem Biophys Res Commun. 2018;498:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Wang CZ, Guo LL, Han BY, Wang AP, Liu HY, Su X, Guo QH, Mu YM. Growth Hormone Therapy Benefits Pituitary Stalk Interruption Syndrome Patients with Short Stature: A Retrospective Study of 75 Han Chinese. Int J Endocrinol. 2016;2016:1896285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 122. | Shao WM, Bai WJ, Chen YM, Liu L, Wang YJ. [Micropump infusion of gonadorelin in the treatment of hypogonadotropic hypogonadism in patients with pituitary stalk interruption syndrome: cases analysis and literature review]. Beijing Da Xue Xue Bao Yi Xue Ban. 2014;46:642-645. [PubMed] [Cited in This Article: ] |