Published online Nov 21, 2020. doi: 10.3748/wjg.v26.i43.6810
Peer-review started: May 27, 2020
First decision: July 29, 2020
Revised: August 7, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: November 21, 2020
Processing time: 176 Days and 23.1 Hours
The complications acute lung injury and acute kidney injury caused by severe inflammation are the main reasons of high mortality of severe acute pancreatitis (SAP). These two complications can both lead to water metabolism and acid-base balance disorders, which could act as additional critical factors affecting the disease trend. Aquaporins (AQPs), which can regulate the transmembrane water transport, have been proved to participate in the pathophysiological process of SAP and the associated complications, such as acute lung injury and acute kidney injury. Thus, exploring herbs that can effectively regulate the expression of AQP in SAP could benefit the prognosis of this disease.
To determine whether Yue-Bi-Tang (YBT) can regulate the water metabolism in rats with severe acute pancreatitis via regulating the expression of aquaporins.
Sprague-Dawley rats were randomly divided into three groups, sham operation group (SOG), model group (MG), and treatment group (TG). SAP was induced with 3.5% sodium taurocholate in the MG and TG. Rats in the TG were administered with YBT while SOG and MG rats were given the same volume of saline. Blood and tissue samples were harvested to detect serum inflammatory cytokines, histopathological changes, malondialdehyde and superoxide dismutase in the lung, and protein and mRNA expression of kidney injury molecule-1, α-smooth muscle actin, and vimentin in the kidney, and AQP1 and 4 in the lung, pancreas, and kidney.
The serum interleukin-10, tumor necrosis factor α, and creatinine levels were higher in the MG than in the SOG. Tumor necrosis factor α level in the TG was lower than that in the MG. Malondialdehyde level in lung tissues was higher than in the SOG. The pathological scores and edema scores of the pancreas, lung, and kidney tissues in the MG were all higher than those in the SOG and TG. The protein expression of AQP4 in lung tissues and AQP1 in kidney tissues in the MG were higher than those in the SOG and TG. The expression of vimentin was significantly higher in the MG than in the SOG. The expression of AQP1 mRNA in the lung and kidney, and AQP4 mRNA in the kidney was up-regulated in the MG compared to the SOG.
YBT might regulate water metabolism to reduce lung and kidney edema of SAP rats via decreasing AQP expression, and alleviate the tissue inflammatory injury.
Core Tip: This is the first study to verify the effects of Yue-Bi-Tang (YBT) in regulating water metabolism and reducing tissue injury in rats with severe acute pancreatitis complicated with acute lung injury and acute kidney injury. We demonstrated the protective effect of YBT on lung and renal injury associated with severe acute pancreatitis from two aspects, alleviation of inflammatory injury and reduction of edema. Furthermore, the edematous elimination effect of YBT is achieved by regulating water metabolism via decreasing the expression of aquaporins.
- Citation: Hu J, Zhang YM, Miao YF, Zhu L, Yi XL, Chen H, Yang XJ, Wan MH, Tang WF. Effects of Yue-Bi-Tang on water metabolism in severe acute pancreatitis rats with acute lung-kidney injury. World J Gastroenterol 2020; 26(43): 6810-6821
- URL: https://www.wjgnet.com/1007-9327/full/v26/i43/6810.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i43.6810
The mortality rate of severe acute pancreatitis (SAP) is as high as 35%-50% in the first week of SAP onset, due to acute lung injury (ALI) and acute kidney injury (AKI) caused by serious inflammation[1,2]. As is known, acute respiratory failure, which is the first organ failure from SAP that takes place after ALI, leads to hypoxia, and has a serious influence on the clinical development and prognosis of SAP patients[3]. Furthermore, ALI and AKI may cause water metabolism and acid-base balance disorders, which could act as additional critical factors affecting the disease trend[4]. As shown in some studies, hypoxemia caused by respiratory failure would lead to or aggravate renal injury[5]. On the other hand, the retention of water and sodium caused by kidney failure or aggressive fluid resuscitation can cause or worsen acute lung edema[6]. The interrelationship between ALI and AKI reveals that water circulation may be the key point in treating SAP. Therefore, finding suitable methods for regulating the water mechanism and electrolyte balance may alleviate the condition of patients with SAP and mitigate the associated complications.
Aquaporins (AQPs), which are proteins in the cell membrane that are part of family of major intrinsic proteins constituting water channels in the cell membrane, facilitate water transportation between cells[7] and are expressed in many organs including the pancreas, lung, and kidney[8-12]. It has been proved that AQPs could participate in the pathophysiological process of SAP and the associated complications, such as ALI and AKI[13]. In an ALI rat model, high expression of AQP4 has been found on alveolar type II cells[14]. The increase in the extravascular lung water volume was consistent with ascension of AQP5, and increasing AQP5 was detected in alveolar lavage fluid in rats with SAP[15]. Lung fluid transport also apparently decreased in AQP5 knockout mice, and the same association was found for AQP1 and AQP4[16]. In renal tissues, AQP1 and 4, which display very high water osmotic permeability, were also found[17]. AQP2 also plays a role in the membrane effector of antidiuretic hormones to promote the reabsorption of water from urine as it is removed from the blood by the kidney[18]. These results prove the effect of AQPs on the water metabolism of the lung and kidney. Some other studies showed that AQP4 can be up-regulated by tumor necrosis factor α (TNF-α), which is an inflammatory factor during SAP, via activation of TNF-receptor-1[19]. Based on these results, it can be inferred that the systemic water metabolic disorder caused by SAP may be related to the abnormal function of AQPs. Thus, exploring herbs that can effectively regulate the expression of AQPs in SAP could benefit the prognosis of this disease.
Yue-Bi-Tang (YBT) was first described in “Jin-Gui-Yao-Lue”, a classical traditional Chinese medicine work. It contains five herbal medicines: Mahuang (Ephedra), shigao (gypsum), shengjiang (ginger), dazao (Chinese dates), and gancao (liquorice). YBT has been widely used as a diaphoretic and edema-clearing decoction to treat edema generated from some respiratory diseases based on the theory of traditional Chinese medicine for ages. In modern Chinese medicine treatments, YBT is directly used to treat some edema conditions resulting from kidney injury, such as acute glomerulonephritis[20]. However, there is no study discussing the effect of YBT on ALI-AKI in rats with SAP. In this paper, we designed an experiment to verify whether YBT could treat lung and kidney injury simultaneously in SAP and whether the therapeutic mechanism functions by regulating the expression of AQPs in SAP.
Male specific pathogen free Sprague-Dawley rats (n = 36) weighing 220 ± 15 g were purchased from Chengdu Dossy Experimental Animals Co. Ltd. (Chengdu, China). All animals were fed and treated in accordance with the Guidelines described in previous articles of our group[21]. After one week of adaptive feeding, these animals were fasting for 24 h before SAP modelling and anesthesia during the experiment. All rats were handled according to the University Guidelines and the Animal Care Committee Guidelines of West China Hospital (Chengdu, China) (protocol number, 2018167A).
Particles obtained by spray drying of mahuang (NO: 17110037), shigao (NO: 18010165), shengjiang (NO: 17120189), dazao (NO: 17120063), and gancao (NO: 18010126) were purchased from Sichuan Hospital of Traditional Chinese Medicine (Chengdu, China). They were stored at 4 ℃ before use. YBT comes from Jin-Gui-Yao-Lue, in which the herb dosages of YBT are described as mahuang 18 g, shengjiang 9 g, shigao 24 g, dazao 9 g, and gancao 6 g. The method to mix the powders of YBT was as previously described[21]. Rats in YBT-treatment group (TG) were intragastrically administered with YBT at a dosage of 5.63 g/kg BW, calculated following the Method of Pharmacology that the equivalent dosage for rats is 6.3 times more than that for human being[22].
The rats were divided into three groups randomly (n = 12 each): Sham operation group (SOG), model group (MG), and TG. All the rats would receive 12 h of pre-operation fasting. After anesthetization with 2% sodium pentobarbital (intraperitoneal injection, 40 mg/kg BW) , SAP was induced in rats by retrograde injection of 3.5% sodium taurocholate (Sigma, St. Louis, MO, United States) into the biliopancreatic duct (1 mL/kg BW) at a velocity of 6 mL/h using a micro-infusion pump. The SOG received a saline injection replacing 3.5% sodium taurocholate. YBT was administered intragastrically to rats in the TG 12 h after operation. Meanwhile, the rats in the SOG and MG were given equal volume of saline. All the rats received subcutaneous injection of 1 mL/100 g weight body of saline q2h after operation. Twenty-four hours after YBT administration, all rats were euthanized. Serum samples were obtained and used to detect amylase (AMY) and inflammatory mediators. Pancreas, lung, and kidney tissues were harvested for histological analysis, Western blot analysis, and mRNA detection.
AMY, creatinine (Cr), and blood urea nitrogen (BUN) levels were detected in blood collected from the heart, with a spectrophotometer following the manufacturer’s instructions. Levels of inflammatory cytokines, such as TNF-α, interleukin (IL)-6, and IL-10, were measured with immunoassay kits (Millipore Corporation, Billerica, MA, United States) that we used formerly[21].
Pieces of pancreas, lung, and kidney tissues were fixed in 4% formaldehyde overnight, and then embedded in paraffin after dehydration. Sections (6 µm) were used for hematoxylin and eosin staining and then examined by light microscopy. The severity of pancreatitis was evaluated in the same way that our previous study used[22]. Lung injury was scored with a previous scoring system in a blinded manner, with thickening of the septum, edema, congestion, and intestinal leukocyte infiltration scored on a 0 (absent) to 4 (extensive) scale[23,24]. The grade of tubulointerstitial damage was scored semi-quantitatively on a 0 to 5+ scale, based on the percentage of the outer medulla area affected by necrosis and/or apoptosis of tubules, tubular atrophy, brush border loss, and tubular dilation (0 = none, + = < 25%, + + = > 25% to 50%, + + + = > 50% to 75%, + + + + = > 75% to < 100%, and + + + + + = 100%)[25].
One hundred milligrams of fresh lung tissues from rats were cut into small pieces, and mixed with 100 μL of saline. After 5 min of homogenization and 2 min of ultrasonic breakage, the suspensions were obtained and centrifuged at 10000 rpm for 10 min. Supernatants were collected and stored at -80 ℃ in a refrigerator first, and latter detected using malondialdehyde (MDA) (cargo number: A003-1, batch number: 20170916) and superoxide dismutase (SOD) kits (WST-1 method, item No: A001-3, batch number: 20170919) purchased from Nanjing Institute of Biotechnology following the instructions.
Western blot was used to detect the protein content of AQP1-4, kidney injury molecule-1 (KIM-1), α-smooth muscle actin (α-SMA), and vimentin. The steps were as previously described[26]. The primary antibodies used for Western blot were purchased from Abcam (KIM-1: ab190696; α-SMA: ab32575; vimentin: ab92547; AQP1: ab168387; AQP4: ab46182). Grey bands on the picture were semi-quantitatively analyzed with ImageJ, with GAPDH used as the internal control.
Trizol reagent (Cargo No. 15596026, Life Technologies) was used to isolate total RNA from tissues following the standard protocol. Total RNA (9 μg) was reverse transcribed using Revert Aid First Stand complementary deoxyribonucleic acid Synthesis Kit (No: K1622, Thermo scientific). Quantitative polymerase chain reaction was performed using SYBR Premix Ex Taq II (NO: RR820A) for detecting the mRNA expression of KIM-1 and AQP1 and 4. The internal control used was GAPDH. The primer sequences used are: AQP1 R: 5’- ACCTGCTGGCCATTGACTAC-3’ and F: 5’- CCAGGGCACTCCCAATGAAT-3’ (129 bp); AQP3 R: 5’- GAGTTGATG AACCGTTGCGG-3’ and F: 5’- TTGATGGTGAGGAAGCCACC-3’ (164 bp); AQP4 R: 5’- GGAAGGCATGAGTGACGGAG-3’ and F: 5’- CAGACGCCTTTGAAAGCCAC-3’ (95 bp); Kim-1 R: 5’- GTTAAACCAGAAATTCCCACAAG-3’ and F: 5’- TCTCATGGGCATAAAATGTAGTG-3’ (191 bp); GAPDH R: 5’-GGTGAAGGTCGGTGTGAACG-3’ and F: 5’- CTCGCTCCTGGAAGATGGTG-3’.
Statistical software SPSS 23.0 (IBM SPSS Statistics 23.0) was used to process the data. All the values are expressed as the mean ± SD. One-way ANOVA was used when data had homogeneity of variance and a normal distribution. The least-significance-difference method was used for pairwise comparison of independent samples. The Kruskal-Wallis H test with multiple independent samples was used when data did not have homogeneity of variance and a normal distribution. The significance level was set at P < 0.05 for comparison.
The rats in the MG had higher serum AMY, Cr, and BUN levels than those in the SOG (P < 0.05), and the AMY level in the TG was lower than those in the other two groups (P < 0.05) (Table 1). As seen from the above results, we have successfully built a model of SAP with AKI, and it can be treated with YBT. However, there was no statistical difference in Cr or BUN levels between the MG and TG. The serum levels of IL-10 and TNF-α in the TG were lower than those in the MG, but higher than those in the SOG (P < 0.05) (Table 2). This result showed an anti-inflammatory effect of YBT, but no effect on kidney function.
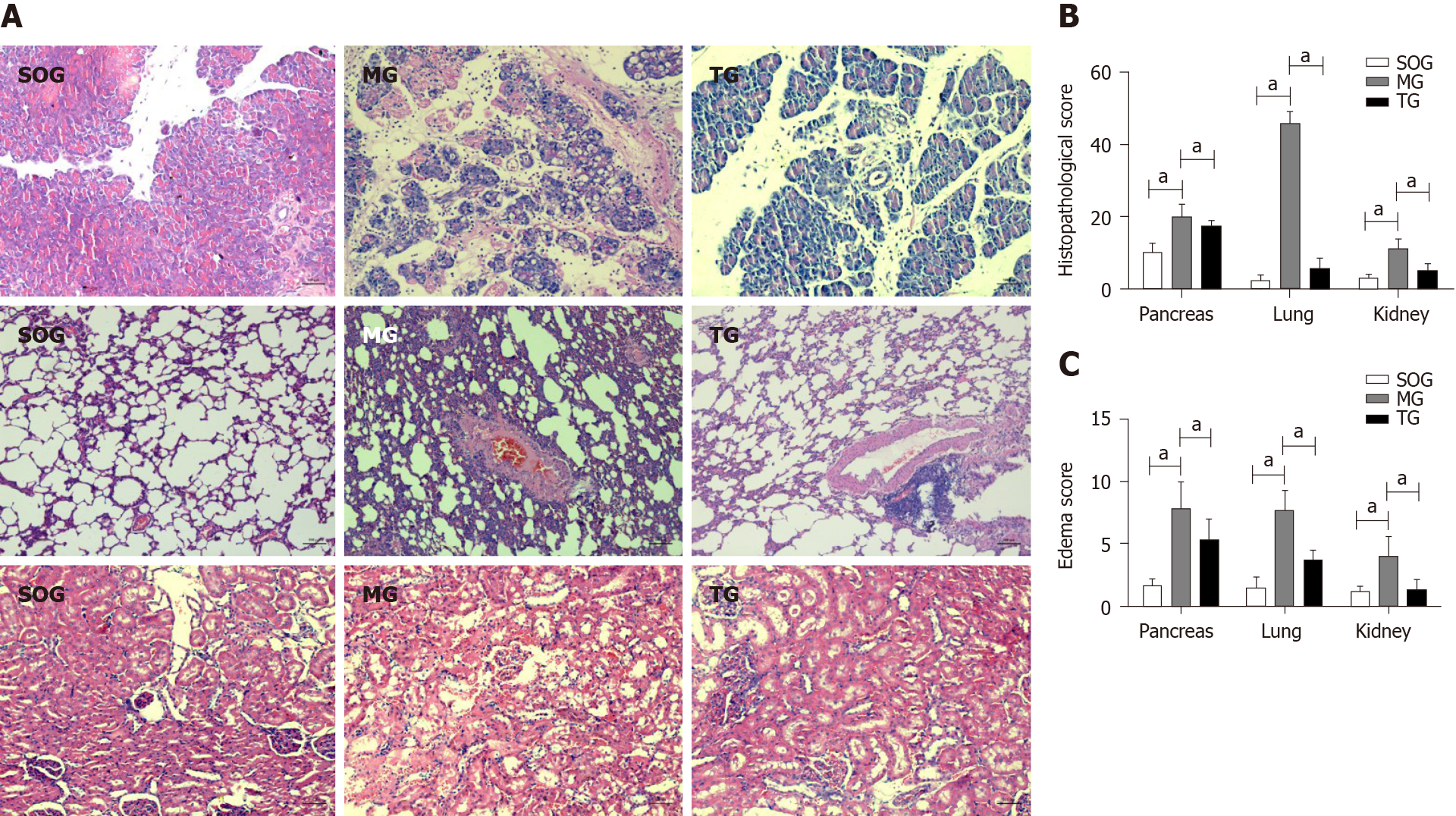
Pancreas: The SOG presented mild edema with few tissue hemorrhages and no necrosis; the MG group had serious edema, inflammatory cells infiltration, and obvious tissue hemorrhage and necrosis (MG = 19.83 ± 3.71 vs SOG = 10.17 ± 2.40, P < 0.05); the TG group had milder hemorrhage, necrosis, and interstitial edema in the tissues than the MG group, but it produced no difference in inflammatory infiltration (TG = 17.33 ± 1.63 vs MG = 19.83 ± 3.71, P > 0.05).
Lungs: The SOG showed some edema and infiltration of neutrophils and lymphocytes, but no congestion, hyaline membrane, or cystic dilatation; distinct interstitial edema, inflammatory cell infiltration, hemorrhage, and necrosis were found in the tissue of MG rats (MG = 46.00 ± 3.16 vs SOG = 2.33 ± 1.51, P < 0.05); the tissue injuries in the TG were milder (TG = 5.67 ± 2.80 vs MG = 46.00 ± 3.16, P < 0.05).
Kidneys: Loss of the brush border in a few areas, with no atrophy or dilation, was found in the SOG; vacuolar degeneration and loss of brush border were found in many regions, along with periodic tubular hemorrhage and necrosis, in the MG (MG = 11.17 ± 2.64 vs SOG = 3.00 ± 1.10, P < 0.05); mild injury was found in the TG (TG = 5.00 ± 2.10 vs MG = 11.17 ± 2.64, P < 0.05) (Figure 1A and B).
Tissue edema: The edema degree of the three organs in the MG was significantly higher than that in the SOG (pancreas: MG = 7.83 ± 2.41 vs SOG =1 .67 ± 0.52, P < 0.05; lung: MG = 7.67 ± 1.63 vs SOG=1.50 ± 0.84, P < 0.05; kidney: MG = 4.00 ± 1.55 vs SOG=1.17 ± 0.41, P < 0.05), while that in the TG was lower than the degree in the MG (Pancreas: TG = 5.33 ± 1.63 vs MG = 7.83 ± 2.41, P < 0.05; lung: TG = 3.67 ± 0.82 vs MG = 7.67 ± 1.63, P < 0.05; kidney: TG = 1.33 ± 0.82 vs MG = 4.00 ± 1.55, P < 0.05) (Figure 1C).
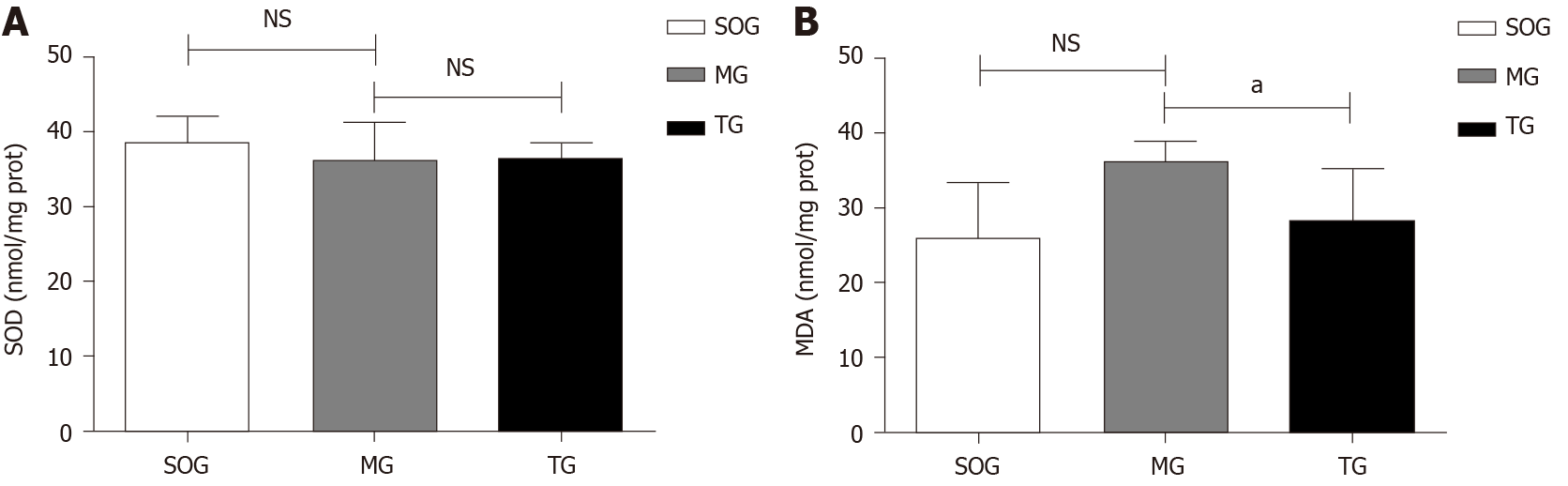
MDA content in the lung tissue in the MG was higher than that in the SOG (MG = 72.41 ± 5.52 vs SOG = 52.07 ± 14.96, P < 0.05), but there was no significant difference in the MDA and SOD contents between MG and TG (Figure 2). As is known, MDA is a marker for oxidative stress, so those result showed that the rat model of SAP combined with ALI-AKI was in a high oxidative stress state.
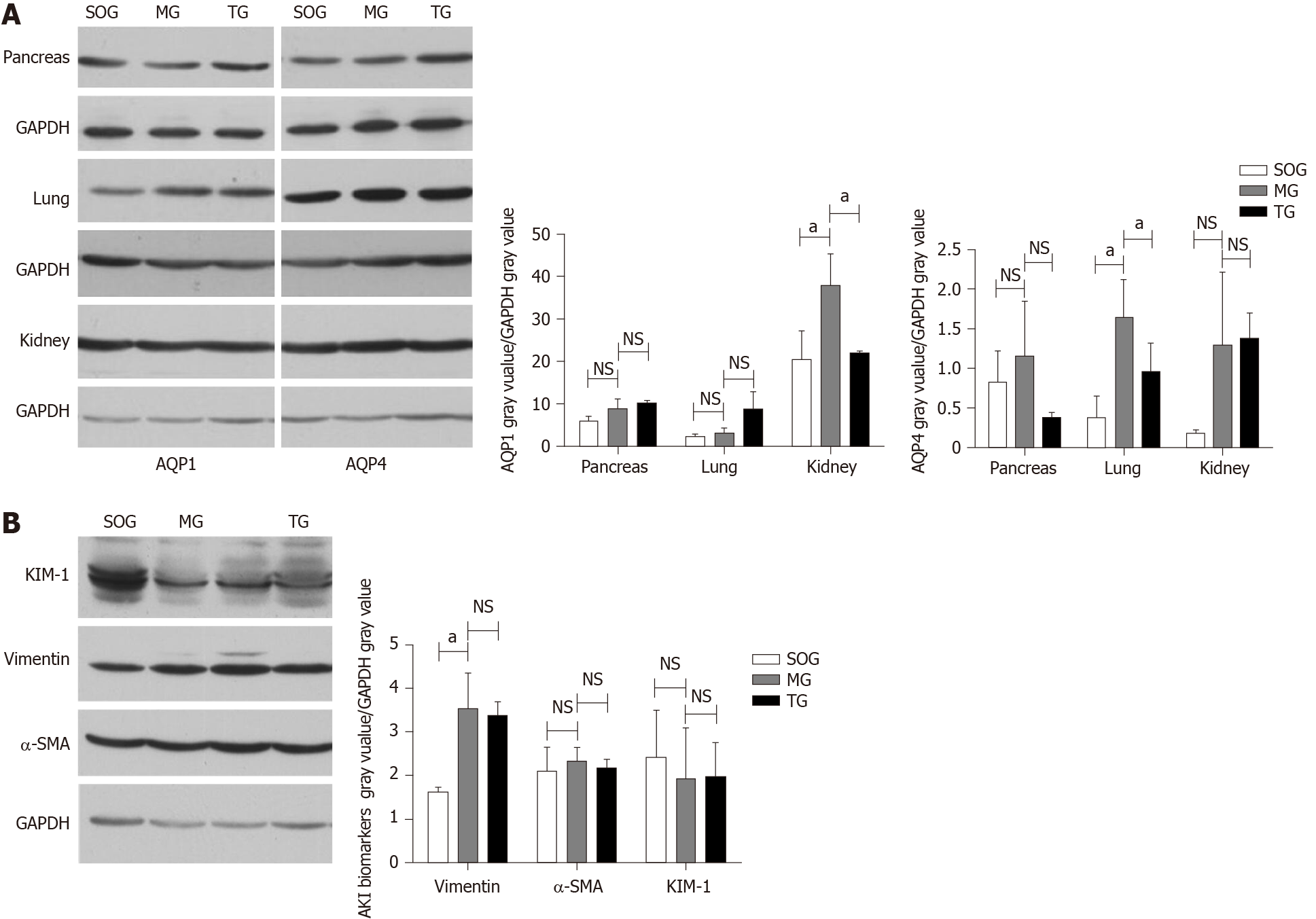
The expression of AQP4 in the lungs of MG rats was significantly higher than that in the SOG and TG (MG = 1.651 ± 0.471 vs SOG = 0.375 ± 0.271, P < 0.05; MG = 1.651 ± 0.471 vs TG = 0.958 ± 0.360, P < 0.05), but AQP4 in the pancreas and kidneys showed no significant differences among the three groups. The expression of AQP1 in the kidneys of MG rats (MG = 3.811 ± 0.714 vs SOG = 2.044 ± 0.677, MG = 3.811 ± 0.714 vs TG 2.221 ± 0.032, P < 0.05) was significantly higher than that in the other groups. In the lungs, AQP1 had no significant differences among the three groups. The expression of vimentin, one of the biomarkers of AKI, was significantly higher in the MG than in the SOG (MG = 3.549 ± 0.795 vs SOG = 1.643 ± 0.104, P < 0.05), but had no significant difference from that in the TG. Another two biomarkers of AKI, KIM-1 and α-SMA, showed no significant differences among the three groups (Figure 3).
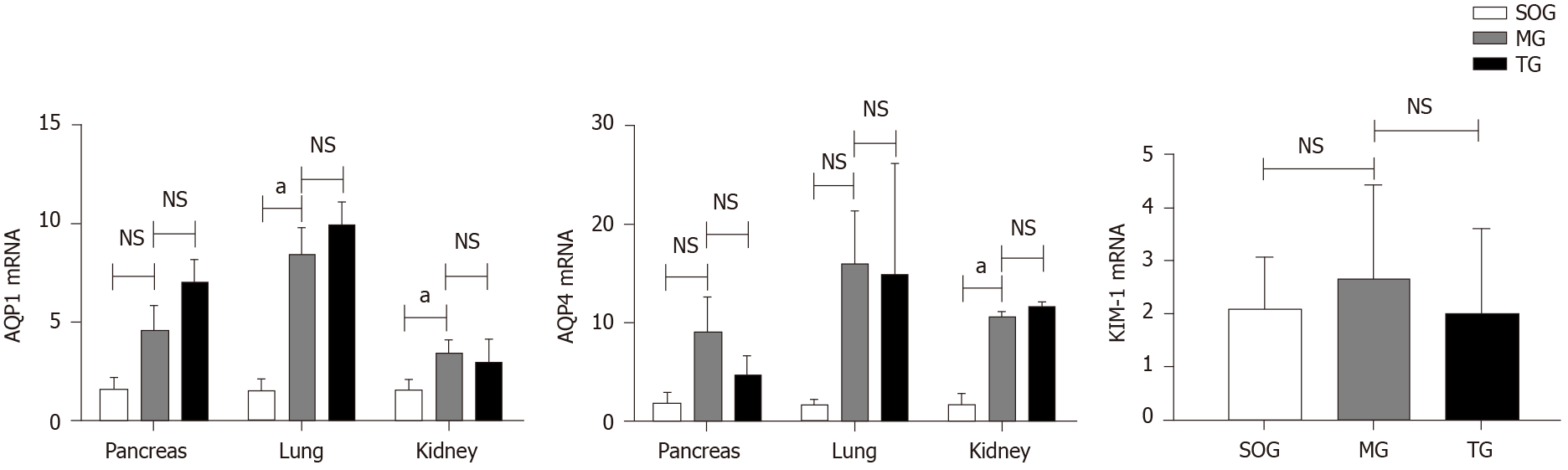
The AQP1 mRNA expression in the lungs and kidneys of MG rats was significantly higher than that in the SOG (P < 0.05), but had no significant differences from that in the TG. Meanwhile, the mRNA expression of AQP4 in the kidneys of MG rats was significantly higher than that in the SOG. No significant differences in the mRNA expression of pancreatic AQP1 and 4, or pulmonary AQP4 or KIM-1 were found among the three groups (Figure 4).
In this study, some molecular changes reflecting tissue inflammatory injury were found in rats with SAP, such as TNF-α in serum, the MDA contents and SOD activity in the lungs, both of which indicated high oxidative stress in this animal model. The expression of vimentin in renal tissue and creatinine level in serum also indicated kidney injury. As a key point of our study, AQPs in lung and kidney tissues of SAP rats changed considerably. The protein expression of AQP4 in the lungs and AQP1 in the kidneys of MG rats was obviously higher than that in SOG rats. Treatment with YBT could regulate AQP4 protein expression in the lungs and AQP1 protein expression in the kidney. Furthermore, YBT treatment can also alleviate the histopathological lesion and edema in the pancreas, lung, and kidney of SAP rats complicated with ALI-AKI.
In the development of SAP, inflammatory injury is the main and lethal factor that damages the organism[27]. Besides inflammatory injury of tissue and organs, fluid loss into the third space contributes to many of the early systemic complications in SAP[28], leading to pleural effusion, seroperitoneum and skin swelling, and hypovolemic shock, all of which are fatal complications of SAP[29]. Meanwhile, water metabolic disorder also exists in tissues when SAP occurs, the irritation of inflammatory substances and overload of the infused fluid while resuscitation result in edema of the cells within the tissue, which could cause organ dysfunction, particularly acute renal failure and respiratory failure[30]. Based on the results of our study, we can deduce that there were tissue inflammatory injury and water metabolic disorder caused by abnormal expression of AQPs in SAP. Studies have shown that both Chinese herb prescription Da-Cheng-Qi decoction and patent medicine Shenfu injection could treat SAP rats with AKI, but the mechanism of these formulas is to ameliorate inflammatory injury not to regulate water metabolism[31,32]. However, the present study found that the effect of YBT on SAP rats with ALI-AKI was achieved not only by decreasing inflammation but also by regulating water metabolism to reduce tissue edema.
The pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine IL-10 are usually regarded as early predictors of SAP severity and prognoses[33]. The results showed the serum TNF-α and IL-10 increased significantly when SAP occurred, while YBT intervention could reduce TNF-α but had no effect on IL-10 levels. From these results, we can deduce that YBT could decrease the production of TNF-α and inhibit inflammation, but did not interfere with anti-inflammatory ability of SAP rats. Ephedra, the herb component of YBT, and its extract ephedrine had been proven to reduce TNF-α in serum and lung tissue of mice with pneumococci induced lung injury[34]. The values of MDA and SOD can reflect the oxidative stress injury of organism, therefore they are often used to evaluate the body’s inflammatory levels[35]. The MDA in lung tissue rose after YBT treatment in SAP rats, which means that YBT could protect the lungs from inflammatory injury. Abnormal expression of vimentin and creatinine, which serve as biomarkers for human renal injury, revealed the existence of AKI in our model[36]. Thus, by combining these results with those of our study, we believe that the SAP rat model had complicated lung and kidney injury, and YBT could alleviate the tissue inflammatory injury in rats with SAP.
Recent studies showed that AQPs play a key role in liquid metabolism in organisms, because the AQP family, which is expressed on the endothelial cells of the capillaries and cell membranes of many tissues, can regulate transmembrane transport of water[37]. In lung tissue, AQP1, AQP3, AQP4, and AQP5 were found, among which AQP1 has high water permeability and is mainly expressed in pulmonary bronchial capillary endothelial cells and alveolar type I epithelial cells[38,39]. AQP4 is mainly distributed in airway epithelial of different sizes; it is partially expressed in the alveolar type I epithelial cells, ciliary tubes, and basal membranes of acinar cells. AQP1 and AQP4 play an important role in the airspace-to-capillary transport of water and elimination of lung interstitial edema[40]. Thus, they were employed to evaluate the disorder of lung water metabolism of SAP rats and the effect of YBT on lung edema in our study. The results showed the upregulation of AQP4 protein expression and more serious edema in the MG than in the SOG. After treatment with YBT, the AQP4 in the lungs was down-regulated, and the tissue edema was reduced significantly. It has been proven that AQP4 has a positive correlation with the tissue moisture content[41], and increasing the expression of AQP4 would exacerbate tissue edema. Thus, one important effect of YBT in SAP treatment is to decrease AQP4 to alleviate the lung edema.
In the kidneys, AQP1 is present in the brush border apical membrane and basal lateral membrane of proximal renal tubules in the wall segment of the descending branch of the medullary loop, and it undertakes the major role in water reabsorption in the kidneys. AQP4 is expressed at the basolateral membrane of renal collecting duct cells and provides entrance to the blood when water enters a renal collecting duct cell[37]. Those two proteins could illustrate the entire process of water translocation from the kidneys to vascular system, which is the pathway of edema elimination. Apparently increased expression of APQ1 in kidney tissue in the MG, which was the group with more serious edema, indicated that up-regulation of the protein could lead to tissue edema. After treatment with YBT, the expression of APQ1 declined, and the tissue edema of the kidneys in the TG decreased significantly. Studies also have found that suppression of AQP1 could facility water and sodium excretion[42]. Thus, we can deduce that YBT might reduce the urine reabsorption and produce diuretic effects via regulating AQP1 expression in the kidneys, thereby regulating water metabolism and reducing tissue edema. However, YBT seemed to have no effect on AQP1 and 4 in the pancreas, though there might be some other mechanisms responsible for the edema elimination function in the pancreas.
The mRNA expression of lung AQP1 and kidney AQP4 in the MG increased, but the protein expression was hardly changed. One reason for the inconsistent expression of mRNA and protein may be related to the changes in the factors affecting protein transcription caused by SAP-related pathophysiological changes[43]. Another reason is that the mRNA expression of AQPs in SAP rats can be increased by glucocorticoid, which would increase in the early stage of SAP, and the complications of SAP, such as intra-abdominal hypertension and abdominal compartment syndrome[44,45]. Therefore, there would be an increase in mRNA expression of AQPs in MG, but no significant increase in protein expression or functional changes.
Some studies report that AQP expression can be regulated by TNF-α signaling[46] through the TNF-receptor-1 pathway[47]. The TNF-α level in serum exhibited the same changes as AQP expression in the MG, and YBT could reduce the level. Based on these results, we can deduce that YBT could affect the expression of AQPs in the lungs and kidneys by reducing TNF-α, thereby reducing tissue edema.
Determining whether YBT could treat SAP-induced water metabolic disorders can provide some new insights for clinical treatments. This is the first time that YBT has been used in an animal model to treat SAP, though many methods are being tried and need further exploration and improvement. The still-unknown mechanisms of YBT in regulating AQPs also need to be verified in future research.
In conclusion, lung and kidney edema of SAP rats may relate to disorder of water metabolism caused by up-regulation of AQP expression. YBT might regulate water metabolism to reduce lung and kidney edema in SAP rats via decreasing AQP expression, and finally alleviate the tissue inflammatory injury.
The complications acute lung injury (ALI) and acute kidney injury (AKI) caused by severe inflammation are the main reasons for high mortality of severe acute pancreatitis (SAP). These two complications can both lead to water metabolism and acid-base balance disorders, which could act as additional critical factors affecting the disease trend. Aquaporins (AQPs), which can regulate the transmembrane water transport, have been proved to participate in the pathophysiological process of SAP and the associated complications, such as ALI and AKI. Thus, exploring herbs that can effectively regulate the expression of AQPs in SAP could benefit the prognosis of this disease.
Our previous studies have demonstrated that SAP rats would be complicated with ALI, AKI, and some other manifestations of fluid metabolism disorders, such as pulmonary edema, pleural effusion, and seroperitoneum. Yue-Bi-Tang (YBT) has been widely used as a diaphoretic decoction to treat edema generated from some respiratory diseases based on the theory of traditional Chinese medicine for ages. In modern Chinese medicine treatments, YBT is directly used to treat some edema diseases resulting from kidney injury, such as acute glomerulonephritis. The question is whether YBT could alleviate ALI and AKI by regulating AQP expression in SAP rats. Therefore, this study aimed to verify the effect of YBT treatment in SAP rats complicated with ALI-AKI and explore the underlying mechanism.
To determine whether YBT can regulate the water metabolism in rats with severe acute pancreatitis via regulating expression of aquaporins.
Healthy male Sprague-Dawley rats were randomly divided into a sham-operated group (SOG), model group (MG), and YBT-treatment group (TG), with 12 rats in each group. SAP was induced with 3.5% sodium taurocholate in the MG and TG. Rats in the TG were administered with YBT while SOG and MG rats were given the same volume of saline. Blood and tissue samples were harvested to detect serum inflammatory cytokines, histopathological changes, malondialdehyde and superoxide dismutase in the lung, protein and mRNA expression of kidney injury molecule-1, α-smooth muscle actin, and vimentin in the kidney, protein and mRNA expression of AQP1 and 4 in the lung, pancreas, and kidney.
The serum IL-10, TNF-α, and creatinine levels in the MG were higher than those in the SOG. Treatment with YBT could decrease TNF-α level. Malondialdehyde level in the lung was higher than that in SAP model rats. Rats of the MG had more serious pathological injury and edema in the pancreas, lung, and kidney, and higher protein expression of AQP4 in the lung and AQP1 in the kidney than those of the other two groups. The expression of vimentin was significantly higher in the MG than in the SOG. The expression of AQP1 mRNA in the lung and kidney, and AQP4 mRNA in the kidney in the MG were all up-regulated compared to that of the SOG.
YBT might regulate water metabolism to reduce lung and kidney edema in SAP rats via decreasing AQP expression, and alleviate the tissue inflammatory injury.
As we observed that YBT might regulate water metabolism to reduce lung and kidney edema in SAP rats via decreasing AQP expression, and alleviate the tissue inflammatory injury, further investigation of the underlying molecular mechanisms of YBT in regulating AQP is required to provide experimental evidence for wider clinical usage.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Herrera Quiñones G, Ljubicic N, Soares RLS S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Andersson R, Andersson B, Haraldsen P, Drewsen G, Eckerwall G. Incidence, management and recurrence rate of acute pancreatitis. Scand J Gastroenterol. 2004;39:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Lin HY, Lai JI, Lai YC, Lin PC, Chang SC, Tang GJ. Acute renal failure in severe pancreatitis: A population-based study. Ups J Med Sci. 2011;116:155-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Pastor CM, Matthay MA, Frossard JL. Pancreatitis-associated acute lung injury: new insights. Chest. 2003;124:2341-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Wen X, Peng Z, Kellum JA. Pathogenesis of acute kidney injury: effects of remote tissue damage on the kidney. Contrib Nephrol. 2011;174:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Koyner JL, Murray PT. Mechanical ventilation and the kidney. Blood Purif. 2010;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Malek M, Hassanshahi J, Fartootzadeh R, Azizi F, Shahidani S. Nephrogenic acute respiratory distress syndrome: A narrative review on pathophysiology and treatment. Chin J Traumatol. 2018;21:4-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Agre P. The aquaporin water channels. Proc Am Thorac Soc. 2006;3:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 296] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Cho SJ, Sattar AK, Jeong EH, Satchi M, Cho JA, Dash S, Mayes MS, Stromer MH, Jena BP. Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc Natl Acad Sci USA. 2002;99:4720-4724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Burghardt B, Nielsen S, Steward MC. The role of aquaporin water channels in fluid secretion by the exocrine pancreas. J Membr Biol. 2006;210:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Hanley MJ. Isolated nephron segments in a rabbit model of ischemic acute renal failure. Am J Physiol. 1980;239:F17-F23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Machida Y, Ueda Y, Shimasaki M, Sato K, Sagawa M, Katsuda S, Sakuma T. Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential. Hum Pathol. 2011;42:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Sands JM. Aquaporin 2: not just for moving water. J Am Soc Nephrol. 2012;23:1443-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Ohta E, Itoh T, Nemoto T, Kumagai J, Ko SB, Ishibashi K, Ohno M, Uchida K, Ohta A, Sohara E, Uchida S, Sasaki S, Rai T. Pancreas-specific aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am J Physiol Cell Physiol. 2009;297:C1368-C1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Chen CL, Li TP, Zhu LH. [Effect of MAPK signal transduction pathway inhibitor U0126 on aquaporin 4 expression in alveolar type II cells in rats with oleic acid-induced acute lung injury]. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:1525-1528. [PubMed] |
| 15. | Cai DL. Treatment with penehyclidine hydrochloride reduces the levels of MIF in serum and AQP-5 in bronchoalveolar lavage fluid in rats with acute necrotizing pancreatitis and acute lung injury. Shijie Huaren Xiaohua Zazhi. 2011;36:3672-3677. |
| 16. | Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Nielsen S, Agre P. The aquaporin family of water channels in kidney. Kidney Int. 1995;48:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Dibas AI, Mia AJ, Yorio T. Aquaporins (water channels): role in vasopressin-activated water transport. Proc Soc Exp Biol Med. 1998;219:183-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Wang LW, Chang YC, Chen SJ, Tseng CH, Tu YF, Liao NS, Huang CC, Ho CJ. TNFR1-JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS-sensitized hypoxic-ischemic injury in the immature brain. J Neuroinflammation. 2014;11:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Zhao P, Zhang BL. Zhang Bolin's experience in treating edema. Shandong Zhongyi Zazhi. 2016;7:2. [DOI] [Full Text] |
| 21. | Zhang YM, Ren HY, Zhao XL, Li J, Li JY, Wu FS, Su H, Tang WF. Pharmacokinetics and pharmacodynamics of Da-Cheng-Qi decoction in the liver of rats with severe acute pancreatitis. World J Gastroenterol. 2017;23:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Zhao X, Zhang Y, Li J, Wan M, Zhu S, Guo H, Xiang J, Thrower EC, Tang W. Tissue Pharmacology of Da-Cheng-Qi Decoction in Experimental Acute Pancreatitis in Rats. Evid Based Complement Alternat Med. 2015;2015:283175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Thandassery RB, Yadav TD, Dutta U, Appasani S, Singh K, Kochhar R. Dynamic nature of organ failure in severe acute pancreatitis: the impact of persistent and deteriorating organ failure. HPB (Oxford). 2013;15:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1041] [Article Influence: 86.8] [Reference Citation Analysis (6)] |
| 25. | Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, Bonventre JV. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125:1620-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Khan MA, Farahvash A, Douda DN, Licht JC, Grasemann H, Sweezey N, Palaniyar N. JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis. Sci Rep. 2017;7:3409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ, Foitzik T. Effect of endothelin and endothelin receptor blockade on capillary permeability in experimental pancreatitis. Gut. 2000;46:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 30. | Zhang XH, Li ML, Wang B, Guo MX, Zhu RM. Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J Gastroenterol. 2014;20:10457-10463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Yuan L, Zhu L, Zhang Y, Chen H, Kang H, Li J, Zhao X, Wan M, Miao Y, Tang W. Effect of Da-Cheng-Qi decoction for treatment of acute kidney injury in rats with severe acute pancreatitis. Chin Med. 2018;13:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Zheng CM, Wang ZJ, Dou HH. The influence of Shenfu injection on the expression of P38 MAPK protein and inflammatory factors in rats with severe acute pancreatitis complicated with acute renal injury. Qiqihaer Yixueyuan Xuebao. 2019;14. |
| 33. | Zhang Y, Liang D, Dong L, Ge X, Xu F, Chen W, Dai Y, Li H, Zou P, Yang S, Liang G. Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury. Respir Res. 2015;16:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Yang P, Jin SA, Che LJ, He SM, Fu SG, Yuan Y. Effects of Four Chinese Herbs Belong to Lung on Expression of TNF-α and IL-1β in Mice with Lung Heat Syndrome. Zhongguo Shiyan Fangjixue Zazhi. 2014;20:5. [DOI] [Full Text] |
| 35. | Gökçe Çokal B, Yurtdaş M, Keskin Güler S, Güneş HN, Ataç Uçar C, Aytaç B, Durak ZE, Yoldaş TK, Durak İ, Çubukçu HC. Serum glutathione peroxidase, xanthine oxidase, and superoxide dismutase activities and malondialdehyde levels in patients with Parkinson's disease. Neurol Sci. 2017;38:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Jiang S, Quan DV, Sung JH, Lee MY, Ha H. Cigarette smoke inhalation aggravates diabetic kidney injury in rats. Toxicol Res (Camb). 2019;8:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, Bill RM, Conner MT. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta. 2014;1840:1492-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 38. | Snuggs JW, Day RE, Bach FC, Conner MT, Bunning RAD, Tryfonidou MA, Le Maitre CL. Aquaporin expression in the human and canine intervertebral disc during maturation and degeneration. JOR Spine. 2019;2:e1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Benga G. The first discovered water channel protein, later called aquaporin 1: molecular characteristics, functions and medical implications. Mol Aspects Med. 2012;33:518-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Song Y, Ma T, Matthay MA, Verkman AS. Role of aquaporin-4 in airspace-to-capillary water permeability in intact mouse lung measured by a novel gravimetric method. J Gen Physiol. 2000;115:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Kong L, Bao XT, Hu JL, Li J, Li Y, Wu QH. Study on relationship between aquaporin-4 and cerebral injury in rats with acute necrotizing pancreatitis. Waike Lilun Yu Shijian. 2017;2:139-142. |
| 42. | Varela VA, Oliveira-Sales EB, Maquigussa E, Borges FT, Gattai PP, Novaes ADS, Shimoura CG, Campos RR, Boim MA. Treatment with Mesenchymal Stem Cells Improves Renovascular Hypertension and Preserves the Ability of the Contralateral Kidney to Excrete Sodium. Kidney Blood Press Res. 2019;44:1404-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Xu J, Huang B, Wang Y, Tong C, Xie P, Fan R, Gao Z. Emodin ameliorates acute lung injury induced by severe acute pancreatitis through the up-regulated expressions of AQP1 and AQP5 in lung. Clin Exp Pharmacol Physiol. 2016;43:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Zhang ZJ, Yang CQ. The Role of Intra-abdominal Hypertension on the Expression of Aquaporin1 and Aquaporin5 in the lung of Cirrhotic Mice. Huaxi Yixue Zazhi. 2011;26:165-169. |
| 45. | Li J, Li Y, Yi YG, Shi Z. Expression and significance of steroid receptor coactivator-3 in the acute pancreatitis. Chongqing Yixue Zazhi. 2010;39:1995-1998. [DOI] [Full Text] |
| 46. | Lu F, Wang F, Chen Z, Huang H. Effect of mesenchymal stem cells on small intestinal injury in a rat model of acute necrotizing pancreatitis. Stem Cell Res Ther. 2017;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |