Published online Oct 7, 2020. doi: 10.3748/wjg.v26.i37.5693
Peer-review started: July 29, 2020
First decision: August 8, 2020
Revised: August 21, 2020
Accepted: September 15, 2020
Article in press: September 15, 2020
Published online: October 7, 2020
Processing time: 60 Days and 14 Hours
The introduction of fine needle biopsies (FNB) to clinical practice presents a changing trend towards histology in the endoscopic ultrasound-guided tissue acquisition (EUS-TA).
To evaluate the clinical performance of a new FNB needle, the 22-gauge (22G) Franseen needle, when sampling pancreatic solid lesions.
Consecutive patients with an indication for EUS-TA for the assessment of pancreatic solid lesions were included in this prospective, single-center, single-arm trial. Each patient underwent a puncture of the lesion two times using the 22G Franseen needle and the obtained samples were directly placed into formalin for histological analysis. The primary study endpoint was the rate of high-quality obtained specimen. Secondary endpoints included the length and diameter of the core specimen, the diagnostic accuracy and the complication rate.
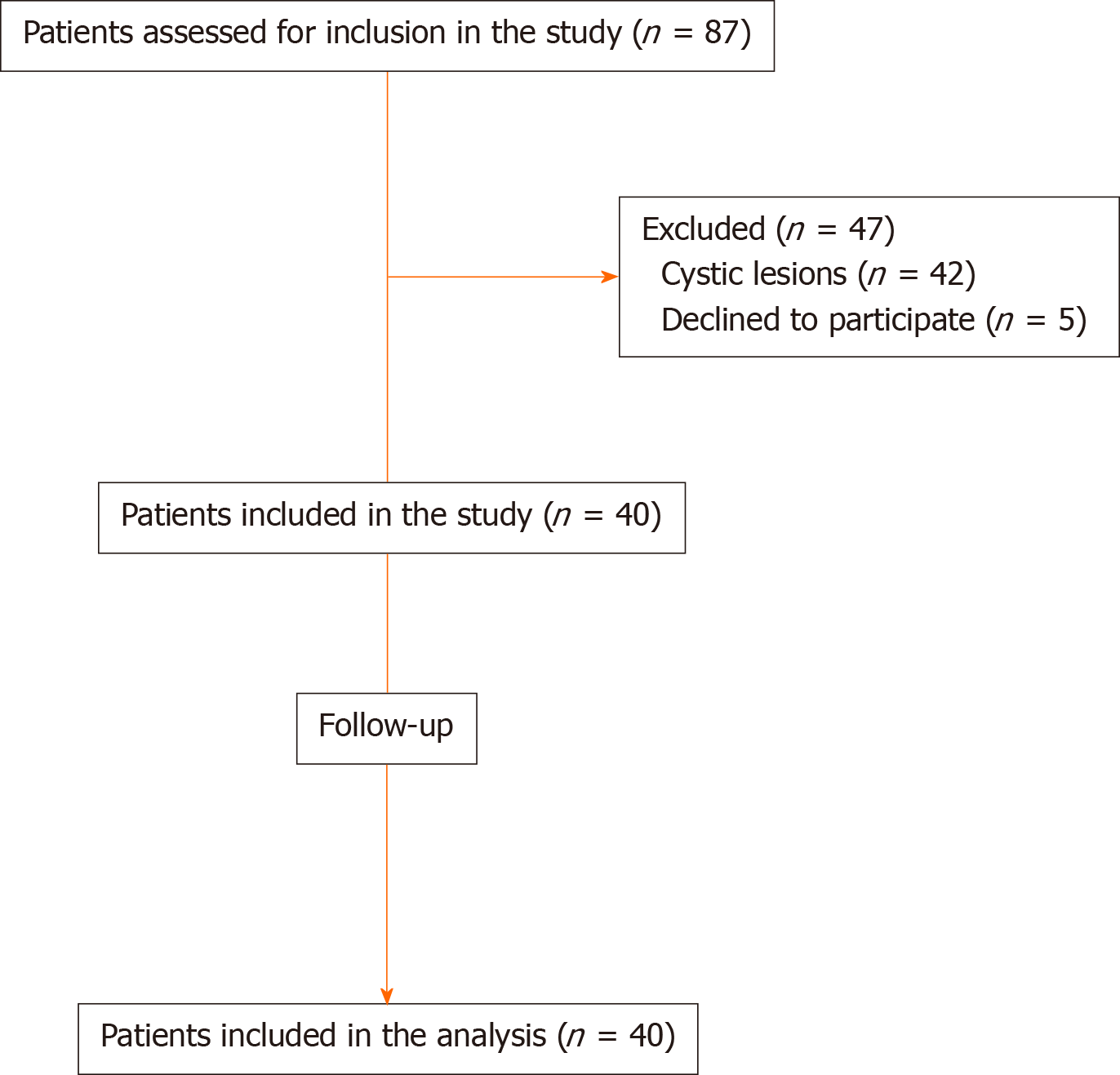
From June 2017 to December 2018, forty patients with pancreatic solid lesions (22 females; mean age 67.2 years) were enrolled. Tissue acquisition was achieved in all cases. High-quality histology, rated with Payne score 3, was obtained in 37/40 cases (92.5%) after two needle passes. The mean size of the acquired histological core tissue was 1.54 mm × 0.39 mm. The diagnostic accuracy for the correct diagnosis was 85% (34/40). Only one adverse event was occurred, consisting of a self-limiting bleeding in the puncture site.
The 22G Franseen needle achieved according to our standardized protocol a high rate of histological core procurement, and a high diagnostic accuracy, with one minor adverse event reported.
Core Tip: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) has been established in the evaluation of pancreatic masses and recently developed fine needle biopsy (FNB) needles improve the diagnostic yield of EUS-TA providing tissue blocks for performing immunohistochemistry and flow cytometry. We prospectively evaluated the Franseen needle when sampling pancreatic solid lesions. EUS-FNB with the 22-gauge Franseen needle achieved a high rate of histological core procurement and high diagnostic accuracy after only two passes and flushing out the acquired samples directly into formalin, without a rapid on-site evaluation by a cytopathologist or macroscopic on-site evaluation by the endoscopist.
- Citation: Stathopoulos P, Pehl A, Breitling LP, Bauer C, Grote T, Gress TM, Denkert C, Denzer UW. Endoscopic ultrasound-fine needle biopsies of pancreatic lesions: Prospective study of histology quality using Franseen needle. World J Gastroenterol 2020; 26(37): 5693-5704
- URL: https://www.wjgnet.com/1007-9327/full/v26/i37/5693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i37.5693
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) has been established and is being widely used for the evaluation of pancreatobiliary masses, metastases to the liver and adrenal gland, neoplastic subepithelial gastrointestinal lesions and peri-intestinal or mediastinal lymph nodes[1]. EUS-guided fine-needle aspiration (EUS-FNA) has been traditionally used for EUS-TA based mostly on cytological evaluation.
Although EUS-FNA is considered to be very safe and have a high diagnostic accuracy, its most important limitation is the poor capability to provide tissue blocks for performing immunohistochemistry, flow cytometry and molecular analysis. Nevertheless, retention of tissue architecture and morphological characterisation of certain neoplasms (e.g., presence of desmoplastic fibrosis in pancreatic cancer) may be required for individualized tumour therapy in the future[2-5]. Performing rapid on-site evaluation (ROSE) may improve the adequacy of FNA specimens and reduces the number of passes required[6-9]. Nevertheless, the presence of an onsite cytopathologist makes the procedure more time consuming and costly and is therefore not very common in European countries.
In recent years, fine needle biopsy (FNB) has been introduced in order to obtain samples with preserved tissue architecture. The FNB needle designs include reverse bevel needle (Echotip ProCore, Cook Medical), fork-tip needle (SharkCore, Medtronic) and Franseen-type needle (Acquire, Boston Scientific)[1,10].
One of the most recent introduced FNB needles mentioned above, the Franseen needle (Acquire, Boston Scientific) with three-pronged cutting edges (Franseen geometry), has been reported to acquire histological core tissue with a rate from 89.8% to 97.2%[5,11-28]. Therefore, we conducted this prospective single arm study to evaluate the quality of histologic tissue obtained with a predetermined biopsy protocol during EUS-guided sampling of pancreatic solid masses with the 22-gauge (22G) Franseen needle.
We performed a prospective, single-center, single-arm study of patients undergoing EUS-FNB with the 22G Acquire needle for pancreatic solid lesions at the Department of Interdisciplinary Endoscopy of the University Hospital in Marburg, Germany from June 2017 to December 2018. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. The study was approved by the local ethical review board (Philipps-Universität Marburg, study number 174/16) and was registered at the ClinicalTrials.gov database (ID: NCT03621852). Each patient provided written, informed consent for the procedure and study participation.
The primary endpoint of our study was the percentage of cases in which the obtained specimen was regarded by the pathologist as representative (score 3). The quality of the histological specimens was rated with scores from 0 to 3, [0, non-representative; 1, representation questionable (poorly preserved, crush artefacts, overlapping cell groups); 2, representation limited (scant amount of diagnostic cells); 3, representative], as modified from Payne et al[29].
Secondary endpoints included: (1) The length and diameter of the core specimen; (2) The rate of correct diagnosis of the obtained material (diagnostic accuracy for malignancy vs non-malignancy); and (3) The rate of procedure-related complications. The gold standard criterion for definite diagnosis was considered as one or more of the following: (a) Definite cytopathological analysis based on EUS-FNB; (b) Surgical resection; and (c) Clinical follow-up up to 12 mo. The procedure-related complications included bleeding, perforation, acute pancreatitis or death up to 24 h after the intervention.
All the patients, between 18 and 85 years old and with an indication for EUS-TA for the assessment of pancreatic solid lesions were included. Patients were excluded if they were undergoing EUS-TA of a cystic lesion without solid tissue or had a coagulopathy (Quick time < 40% or platelets < 40 G/L) or poor performance status (American Society of Anesthesiologists physical status classification – ASA IV).
For all patients, the following parameters were recorded: Basic characteristics (age, gender, body mass index), symptoms (pain, jaundice, inappetence, weight loss), laboratory data (complete blood count, liver enzymes, international normalised ratio) and available imaging studies prior to EUS. Following procedural or lesion-related characteristics were also recorded: Size, location and echogenicity of the lesion, dose of propofol administered for sedation, size of core specimen of each needle pass, cytological/histological quality of the material, cytohistological analysis result, final diagnosis and postinterventional complications. Follow-up was performed by telephone interviews and hospital visits.
All procedures were performed under conscious sedation by means of intravenous propofol administration with a linear array echoendoscope (GF-UCT180, Olympus Europa, Hamburg, Germany) connected to a processor featuring the colour Doppler function (EU-ME2, Olympus Europa, Hamburg, Germany) by two experienced endoscopists.
After careful evaluation of the lesion with EUS and exclusion of vessel interposition along the puncture route, using the colour Doppler function, the targeted lesion was punctured by the study needle. After the needle had successfully entered the lesion, its stylet was removed and suction was applied using a 10 mL syringe, followed by 5 to 10 needle movements back and forth. The needle was withdrawn from the lesion, after suction has been released. The puncture was repeated two times according to the standardized study protocol. The obtained samples from the two passes were directly placed into formalin for histological analysis using air flushing as well as by reinsertion of the stylet into the needle. No further passes were undertaken. The acquired material was completely paraffin embedded and cut to obtain haematoxylin-eosin stained sections. If necessary, further special stainings were performed.
Previous studies evaluating the Franseen needle reported a probability of obtaining high-quality histologic material of approximately 90%. The required sample size of 40 patients was calculated to provide power > 95% (type II error < 5%) for detecting the probability of obtaining high-quality histological material, which exceeds 70%.
Datasets were compiled by using Microsoft Excel and statistical analysis was performed using R version 3.4.1 software [R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.]. Continuous variables are presented as means with standard deviation or medians with range. Categorical variables are presented as absolute values and percentages. For proportions, 95% confidence intervals were calculated using exact methods as implemented in the Hmisc add-on package [Frank E Harrell Jr, with contributions from Charles Dupont and many others. (2019). Hmisc: Harrell Miscellaneous. R package version 4.2-0]. Test-performance measures (sensitivity, specificity, predictive values) were calculated for the diagnosis of malignancy in needle biopsy compared to our gold standard for definite diagnosis. Of note, a definite diagnosis of malignancy in needle biopsy was counted as a definite final diagnosis in our definition of gold standard -motivated by clinical practice and pragmatism-, which means that false positive results were not possible, resulting in perfect specificity and positive predictive value per definitionem. The statistical methods of this study were reviewed by Lutz P Breitling from Philipps-Universität Marburg.
From June 2017 to December 2018, 40 patients (18 males and 22 females) with a mean age of 67.2 years were enrolled in our study (Figure 1). Patients’ demographics and baseline characteristics are presented in detail in Table 1.
| Parameter | Value |
| No. of patients | 40 |
| Age (yr), mean (SD) | 67.2 (13.8) |
| Sex, n (%) | |
| Male | 18 (45) |
| Female | 22 (55) |
| BMI (kg/m2) | 24.3 (5.1) |
| Presenting symtom(s), n (%) | |
| Pain | 15 (37.5) |
| Weight loss | 23 (57.5) |
| Jaundice | 9 (22.5) |
| Anorexia | 16 (40) |
| Propofol sedation dose (mg)1, mean (SD) | 436 (201) |
| Examination prior to EUS | |
| Abdominal ultrasound | 3 (7.5) |
| CT | 25 (62.5) |
| MRI | 9 (22.5) |
| PET/CT | 3 (7.5) |
About 2/3 (67.5%) of the pancreatic lesions were located in the head of the gland, 8 (20%) were located in the body, and 5 (12.5%) were located in the tail. The mean lesion size was 31.5 mm. As shown in Table 2, a final diagnosis of pancreatic adenocarcinoma was made in 23 patients (57.5%), pancreatic metastases of other malignancies in 6 patients (15%), chronic pancreatitis in 3 patients (7.5%), and serous cystic neoplasm/serous cystadenoma in two patients (5%). Six patients (15%) had a variety of other diagnoses. The final diagnosis was confirmed based on definite EUS-FNB histological evaluation only in 21 patients (52.5%), surgery in 8 patients (20%), on both definite EUS-FNB and surgery in 5 patients (12.5%) and based on clinical follow-up up to 12 mo in 6 patients (15%).
| Parameter | Value |
| Location in pancreas, n (%) | |
| Head | 27 (67.5) |
| Body | 8 (20) |
| Tail | 5 (12.5) |
| Size max (mm), mean (SD) | 31.5 (12.3) |
| Echogenicity on EUS, n (%) | |
| Hypoechoic | 34 (85) |
| Isoechoic | 3 (7.5) |
| Hyperechoic | 0 |
| Non-homogeneous | 3 (7.5) |
| Final diagnosis, n (%) | |
| Pancreatic adenocarcinoma | 23 (57.5) |
| Pancreatic metastases1 | 6 (15) |
| Chronic pancreatitis | 3 (7.5) |
| Serous cystic neoplasm/serous cystadenoma | 2 (5) |
| Pancreatic NET | 1 (2.5) |
| Lymphoma | 1 (2.5) |
| Solid pseudopapillary neoplasm | 1 (2.5) |
| Acinar cell carcinoma | 1 (2.5) |
| Mucinous cystic neoplasm | 1 (2.5) |
| Intrapancreatic accessory spleen | 1 (2.5) |
| Gold standard method, n (%) | |
| Definite EUS-FNB | 21 (52.5) |
| Definite EUS-FNB + surgery | 5 (12.5) |
| Surgery | 8 (20) |
| Clinical follow up | 6 (15) |
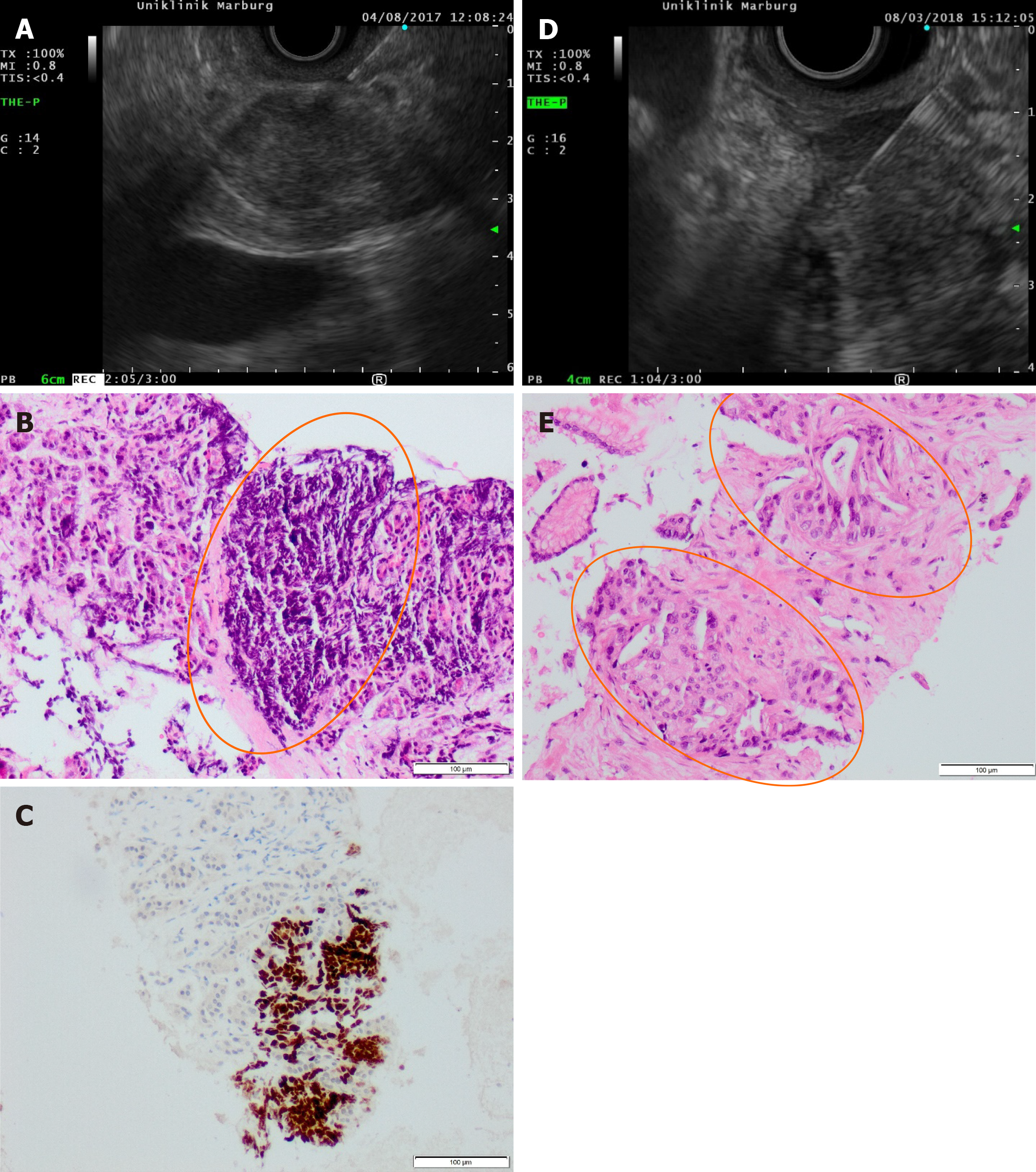
Technically successful advancement of the study needle into the target lesion and tissue acquisition was achieved in 36/40 cases (90%). As shown in Table 3, material for histology was obtained in all 40 cases (100%). High-quality histology, rated with score 3, was obtained in 37/40 cases (92.5%) after two needle passes. The mean size of the acquired histological core tissue was 1.54 mm × 0.39 mm. Immunhistochemistry staining was performed in 12/40 cases (30%) to confirm the diagnosis in a lymphoma, in suspected metastatic lesions and in indeterminate cases (Figure 2).
| Parameter | Value |
| Needle passes/patient, n (%) | 2 (100) |
| Acquired histology, n (%) | 40 (100) |
| Immunhistochemistry, n (%) | 12 (30) |
| Histologic quality (Payne score), n (%) | |
| Score 0 (needle pass 1/needle pass 2/overall) | 1 (2.5)/1 (2.5)/1 (2.5) |
| Score 1 (needle pass 1/needle pass 2/overall) | 2 (5)/3 (7.5)/1 (2.5) |
| Score 2 (needle pass 1/needle pass 2/overall) | 3 (7.5)/3 (7.5)/1 (2,5) |
| Score 3 (needle pass 1/needle pass 2/overall) | 34 (85)/33 (82.5)/37 (92.5) |
| Histologic quality, median (range) | 2.85 (0-3) |
| Acquired high quality histology1, n (%) | |
| Needle pass 1/needle pass 2/overall | 34 (85)/33 (82.5)/37 (92.5) |
| Length of the biopsy cylinder (mm), mean (SD) | |
| Needle pass 1/needle pass 2/overall | 1.56 (0.9)/1.5 (0.9)/1.54 (0.9) |
| Diameter of the biopsy cylinder (mm), mean (SD) | |
| Needle pass 1/needle pass 2/overall | 0.41 (0.1)/0.38 (0.1)/0.39 (0.1) |
| Diagnostic accuracy | 85%2/89.2%3 |
| Sensitivity | 78% |
| Specificity | 100% |
| Positive predictive value | 100% |
| Negative predictive value | 53% |
| Complications, n (%) | 1 (2.5)4 |
A correct diagnosis, compared to the gold standard, was achieved in 34/40 patients (85%). Missed cases included 5 pancreatic adenocarcinomas -3 of them were misinterpreted as chronic pancreatitis in EUS-FNB- and a pancreatic metastasis of a neuroendocrine tumour originating from the ileum. In two out of six missed cases the obtained sample was not of high quality (Payne score 0 and 1) and in four cases the needle did not obviously enter the targeted lesion. In four out of six missed cases the correct diagnosis was confirmed by surgical resection. In another one was confirmed by follow-up interval imaging because the patient used best supportive care measures. The last one was confirmed by follow-up interval imaging and an interval EUS-guided FNB once again.
Only one early-adverse event was occurred, consisting of a self-limiting bleeding in the puncture site in a patient with recurrence of a pancreatic adenocarcinoma. There were no late adverse events reported in any of our patients.
The introduction of the following three FNB needles to clinical practice in recent years presents a changing trend in EUS-TA. The reverse bevel needle (Echotip ProCore, Cook Medical) has a laterally placed, reverse facing bevel. In contrast, the Fork-tip needle (SharkCore, Medtronic) and the Franseen-type needle (Acquire, Boston Scientific) both have an opposing bevel design; the Fork-tip needle has two opposing bevels and the Franseen-type needle has three opposing bevels.
According to a meta-analysis by Bang et al[30] there is no evidence to support the superiority of ProCore over the standard FNA needle in the subgroup analysis of pancreatic mass lesions with regards to diagnostic accuracy (87% vs 85.3%) and histological core tissue procurement (79.2% vs 83.1%), except for establishing a diagnosis with fewer passes (standardized mean difference -1.03, P < 0.001). In contrast to the previous meta-analysis there is data supporting the superiority of the second-generation FNB needles (Franseen and Fork-tip) to FNA regarding the sample adequacy, even without ROSE. Facciorusso et al[21] reported in their meta-analysis a rate of histological core procurement and diagnostic accuracy of 92.5% and 95%, using the Franseen and the Fork-tip needle respectively, with no difference between the two needles. Sample adequacy in targeting pancreatic masses was superior with the two FNB needles over FNA and number of passes was significantly lower in comparison to FNA. Another recently published meta-analysis[31] reported no difference in the diagnostic yield rate between the Franseen and the Fork-tip needle (92.8% vs 92.7%, P = 0.98), giving similar results in the subgroup analysis of studies with and without ROSE (95.9% without ROSE vs 93.7% with ROSE), though there was no subgroup analysis of the results when targeting pancreatic lesions. Furthermore, the number of needle passes performed to obtain a successful sample was comparable between both needles.
Our study showed 90% technical success rate of EUS-FNB using the 22G Franseen needle and produced a 92.5% rate of high-quality histological tissue procurement (defined as Payne Score 3) according to a standardised protocol, namely only two needle passes, placing the obtained material directly into formalin without assessment of an on-site cytopathologist, as well as without macroscopic on-site evaluation (MOSE) by the endoscopist, taking into consideration that a visible core tissue does not always correlate with a true histologic core (tissue distortion, blood clot or necrosis). A correct diagnosis was rendered in 34/40 (85%) patients. Immunhistochemistry staining was performed in 12/40 cases (30%), where indicated.
Among prospective studies evaluating the Franseen needle alone[22,23], prospective comparative studies evaluating the Franseen needle vs FNA[5,27,28] and a prospective comparative study evaluating the Franseen needle vs Fork-tip needle[12], three studies[5,12,27] included only pancreatic lesions. In the other three studies[22,23,28] pancreatic lesions represented between 52% and 81% of the targeted lesions. Sugiura et al[23] prospectively assessed the 25G Franseen needle, whereas all other trials assessed the 22G Franseen needle.
Like our standardised protocol of only two passes, a predetermined number of passes was performed by the following trials. Mita et al[22] performed three passes, reporting an acquisition rate of adequate specimen for histological assessment (cellularity score ≥ 4) of 90.7% on the first pass and 98.7% on the best of three passes. Sugiura et al[23] performed one pass with the 25G Franseen needle, resulting in an acquisition rate of adequate specimen for histological assessment (cellularity score ≥ 4) of 81.5% when targeting pancreatic lesions. Leung et al[19] performed after one pass MOSE and if no macroscopic core was visualized a second pass was performed. In 93% of the targeted lesions a core histology was obtained and the final correlation of MOSE and histologic core was 94%, suggesting that MOSE could be interestingly a potential practical alternative to ROSE. In the comparative study (Franseen vs FNA) of Matsuno et al[27] one pass was performed with the Franseen needle and one pass with the FNA, resulting in a rate of adequate tissue obtained of 89.3% with the Franseen needle and 62.5% with the standard needle. Finally, Asokkumar et al[28] performed three passes for pancreatic lesions using each needle (Franseen and FNA), reporting that FNB obtained histological core tissue more frequent than FNA (97% vs 77%, P = 0.03). In spite of the different definitions of an adequate tissue sample in the previously mentioned studies, Franseen needle achieved high rates (> 80%) of acquisition of high-quality histological samples on the basis of a predetermined low number of passes (1-3). These data are comparable to our study resulting in a high-quality histological tissue procurement of 92.5% performing two passes and flushing out the acquired samples directly into formalin. However, we consider that further prospective studies with a standardised protocol of a predetermined low number of needle passes are required to draw a conclusion about the need of rapid on site evaluation.
We showed in our study a diagnostic accuracy of 85%, whereas the diagnostic accuracy for pancreatic lesions in other series was reported between 90% and 97.9%[5,11,14,16,18,19,24,26,28]. Bang et al[5] reported a significantly higher diagnostic accuracy of Franseen needle over FNA needle (97.8% vs 82.6%, P = 0.03), whereas three other series demonstrated an equal to higher diagnostic accuracy, though not significantly[14,26,28].
EUS-FNB is a safe diagnostic tool. Consistently with the frequency of adverse events reported in other series[11-28] and recently published meta-analyses[21,31], between 0%-4%, we experienced only one self-limiting bleeding as adverse event in our study (2.5%).
The strengths of the present study are its prospective design, the enrolment of patients with only pancreatic solid lesions and the fact that all procedures were performed by three experienced endoscopists. Nevertheless, it bears the limitation of only one participating centre.
In conclusion, our study showed a high rate of histological core procurement, adequate for interpretation, as well as high diagnostic accuracy, with only one minor adverse event reported. This study may suggest that two needle passes with the Franseen needle, submitting the obtained material directly into formalin and without visible assessment by the endoscopist or access to on-site cytopathologist are adequate for establishing a correct diagnosis during the evaluation of pancreatic solid lesions.
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is an established modality for the evaluation of pancreatic lesions. Recently fine needle biopsy (FNB) has been introduced in order to obtain samples with preserved tissue architecture. Studies evaluating one of the most recent introduced FNB needles, the Franseen needle, have shown very promising results of high histological tissue acquisition rate.
So far there is little evidence about the performance of the Franseen needle in the evaluation of pancreatic lesions, when applying a standardised protocol of a predetermined low number of needle passes, without access to an on-site cytopathologist and flushing out the obtained material direct into formalin.
We performed a prospective, single-arm study to evaluate the clinical performance of the 22-gauge (22G) Franseen needle, when sampling pancreatic lesions according to a standardised protocol, focusing on the quality of the acquired histological specimens.
Consecutive patients with an indication for EUS-FNB for the assessment of pancreatic lesions from June 2017 to December 2018 underwent a puncture of the lesion two times using the 22G Franseen needle in our department. The obtained material was treated like a biopsy specimen, placed directly into formalin, completely embedded in paraffin and cut to provide heamatoxylin-eosin stained sections for histological analysis. Our primary endpoint was the acquisition rate of high-quality histological specimen, regarded by the pathologist as representative (Payne score 3). Secondary endpoints were size of the core specimen, the diagnostic accuracy and the complication rate. The gold standard for definite diagnosis was considered one or more of the following: A definite histology obtained from EUS-FNB, a surgical resection or clinical follow-up up to twelve months.
Forty patients with a mean age of 67.2 years were included in this study. Tissue acquisition was achieved in all cases, whereas high-quality histology, rated with Payne score 3, was obtained in 37 out of 40 cases (92.5%). A correct diagnosis (diagnostic accuracy) was made in 34 out of 40 cases (85%). The final diagnosis was confirmed based on definite EUS-FNB histology in 21 patients, on surgical resection in 8 patients, on both definite EUS-FNB and surgery in 5 further patients and on interval follow-up in 6 patients. The only complication occurred was a self-limiting bleeding in the puncture site.
We demonstrated that EUS-FNB of pancreatic lesions with the 22G Franseen needle following a standardised simplified protocol achieved a high acquisition rate of representative histological specimen and a high diagnostic accuracy.
We consider that further prospective studies with a standardised protocol of a predetermined low number of needle passes are required to draw a conclusion about the need of ROSE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chin YK S-Editor: Gong ZM L-Editor: A P-Editor: Wang LL
| 1. | Wani S, Muthusamy VR, McGrath CM, Sepulveda AR, Das A, Messersmith W, Kochman ML, Shah J. AGA White Paper: Optimizing Endoscopic Ultrasound-Guided Tissue Acquisition and Future Directions. Clin Gastroenterol Hepatol. 2018;16:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Kopelman Y, Marmor S, Ashkenazi I, Fireman Z. Value of EUS-FNA cytological preparations compared with cell block sections in the diagnosis of pancreatic solid tumours. Cytopathology. 2011;22:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Varadarajulu S, Bang JY, Holt BA, Hasan MK, Logue A, Hawes RH, Hebert-Magee S. The 25-gauge EUS-FNA needle: Good for on-site but poor for off-site evaluation? Results of a randomized trial. Gastrointest Endosc. 2014;80:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 7. | Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Kong F, Zhu J, Kong X, Sun T, Deng X, Du Y, Li Z. Rapid On-Site Evaluation Does Not Improve Endoscopic Ultrasound-Guided Fine Needle Aspiration Adequacy in Pancreatic Masses: A Meta-Analysis and Systematic Review. PLoS One. 2016;11:e0163056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 10. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 11. | Bang JY, Hebert-Magee S, Hasan MK, Navaneethan U, Hawes R, Varadarajulu S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Adler DG, Muthusamy VR, Ehrlich DS, Parasher G, Thosani NC, Chen A, Buscaglia JM, Appannagari A, Quintero E, Aslanian H, Taylor LJ, Siddiqui A. A multicenter evaluation of a new EUS core biopsy needle: Experience in 200 patients. Endosc Ultrasound. 2019;8:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 14. | Mukai S, Itoi T, Yamaguchi H, Sofuni A, Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Fujita M, Yamamoto K, Matsunami Y, Asai Y, Kurosawa T, Nagakawa Y. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019;8:50-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Abdelfatah MM, Grimm IS, Gangarosa LM, Baron TH. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest Endosc. 2018;87:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Bang JY, Kirtane S, Krall K, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig Endosc. 2019;31:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | El Hajj II, Wu H, Reuss S, Randolph M, Harris A, Gromski MA, Al-Haddad M. Prospective Assessment of the Performance of a New Fine Needle Biopsy Device for EUS-Guided Sampling of Solid Lesions. Clin Endosc. 2018;51:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Haseeb A, Taylor LJ, Adler DG. Comparing endoscopic ultrasound-guided core biopsies of solid pancreatic and extrapancreatic lesions: a large single-operator experience with a new fine-needle biopsy needle. Ann Gastroenterol. 2018;31:742-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Leung Ki EL, Lemaistre AI, Fumex F, Gincul R, Lefort C, Lepilliez V, Pujol B, Napoléon B. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019;7:E189-E194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Ishikawa T, Kawashima H, Ohno E, Tanaka H, Sakai D, Iida T, Nishio R, Yamamura T, Furukawa K, Nakamura M, Miyahara R, Hashimoto S, Ishigami M, Hirooka Y. Clinical Impact of EUS-Guided Fine Needle Biopsy Using a Novel Franseen Needle for Histological Assessment of Pancreatic Diseases. Can J Gastroenterol Hepatol. 2019;2019:8581743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Facciorusso A, Del Prete V, Buccino VR, Purohit P, Setia P, Muscatiello N. Diagnostic yield of Franseen and Fork-Tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: a meta-analysis. Endosc Int Open. 2019;7:E1221-E1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 22. | Mita N, Iwashita T, Uemura S, Iwasa Y, Toda K, Mukai T, Miyazaki T, Yasuda I, Shimizu M. Endoscopic Ultrasound-Guided Fine Needle Biopsy Using 22-Gauge Franseen Needle for the Histological Diagnosis of Solid Lesions: A Multicenter Prospective Pilot Study. Dig Dis Sci. 2020;65:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Sugiura R, Kuwatani M, Yane K, Taya Y, Ihara H, Onodera M, Eto K, Sano I, Kudo T, Mitsuhashi T, Katanuma A, Sakamoto N; Hokkaido Interventional EUS/ERCP study (HONEST) group. Prospective, multicenter, observational study of tissue acquisition through EUS-guided fine-needle biopsy using a 25G Franseen needle. Endosc Ultrasound. 2019;8:321-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Mitri RD, Rimbaş M, Attili F, Fabbri C, Carrara S, Di Maurizio L, Inzani F, Repici A, Gasbarrini A, Costamagna G, Larghi A. Performance of a new needle for endoscopic ultrasound-guided fine-needle biopsy in patients with pancreatic solid lesions: A retrospective multicenter study. Endosc Ultrasound. 2018;7:329-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Alkhateeb K, Lee BB, Alatassi H, Sanders MA, Omer EM, McClave SA, Fraig M. Comparison between two types of needles for Endoscopic Ultrasound (EUS)-guided fine aspiration biopsy of pancreatic and upper gastrointestinal masses. Diagn Cytopathol. 2020;48:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Fujita A, Ryozawa S, Mizuide M, Araki R, Nagata K, Tanisaka Y, Harada M, Ogawa T, Tashima T, Nonaka K. Does endoscopic ultrasound-guided fine needle biopsy using a Franseen needle really offer high diagnostic accuracy? A propensity-matched analysis. Endosc Int Open. 2019;7:E1327-E1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Matsuno J, Ogura T, Kurisu Y, Miyano A, Imanishi M, Onda S, Okuda A, Nishioka N, Higuchi K. Prospective comparison study of franseen needle and standard needle use for pancreatic lesions under EUS guidance. Endosc Ultrasound. 2019;8:412-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Asokkumar R, Yung Ka C, Loh T, Kah Ling L, Gek San T, Ying H, Tan D, Khor C, Lim T, Soetikno R. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): a randomized study. Endosc Int Open. 2019;7:E955-E963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Payne M, Staerkel G, Gong Y. Indeterminate diagnosis in fine-needle aspiration of the pancreas: reasons and clinical implications. Diagn Cytopathol. 2009;37:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Mohan BP, Shakhatreh M, Garg R, Asokkumar R, Jayaraj M, Ponnada S, Navaneethan U, Adler DG. Comparison of Franseen and fork-tip needles for EUS-guided fine-needle biopsy of solid mass lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2019;8:382-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |