Published online Sep 28, 2020. doi: 10.3748/wjg.v26.i36.5474
Peer-review started: April 1, 2020
First decision: May 29, 2020
Revised: June 2, 2020
Accepted: September 4, 2020
Article in press: September 4, 2020
Published online: September 28, 2020
Processing time: 175 Days and 7.5 Hours
Growing evidence supports a genetic link between non-alcoholic fatty liver disease (NAFLD) and chronic kidney disease (CKD). Interesting data demonstrated that both the major NAFLD risk polymorphisms such as the I148M polymorphism in the patatin like phospholipase containing domain 3 (PNPLA3) and the E167K allele in the transmembrane 6 superfamily member 2 gene (TM6SF2) affect renal function. Recently the hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) gene has been recognized as a novel genetic variant involved in NAFLD pathophysiology. In particular, it has been showed the protective effect of the rs72613567:TA variant of this gene against liver damage both in adults and children.
To investigate the impact of the rs72613567:TA variant of the HSD17B13 gene on estimated glomerular filtration rate (eGFR) in obese children.
We enrolled 684 obese children (mean age 10.56 ± 2.94 years; mean BMI-SDS 2.98 ± 0.78) consecutively attending our Obesity Clinic. All the patients underwent a careful clinical assessment and a comprehensive biochemical evaluation. To detect hepatic steatosis, a liver ultrasound was performed. NAFLD was defined by ultrasound detected liver steatosis and/or alanine aminotransferase (ALT) levels > 40 IU/L. The study population was divided on the basis of the NAFLD presence. Genotyping for the rs72613567:TA variant of the HSD17B13 gene in all the enrolled subjects was also made.
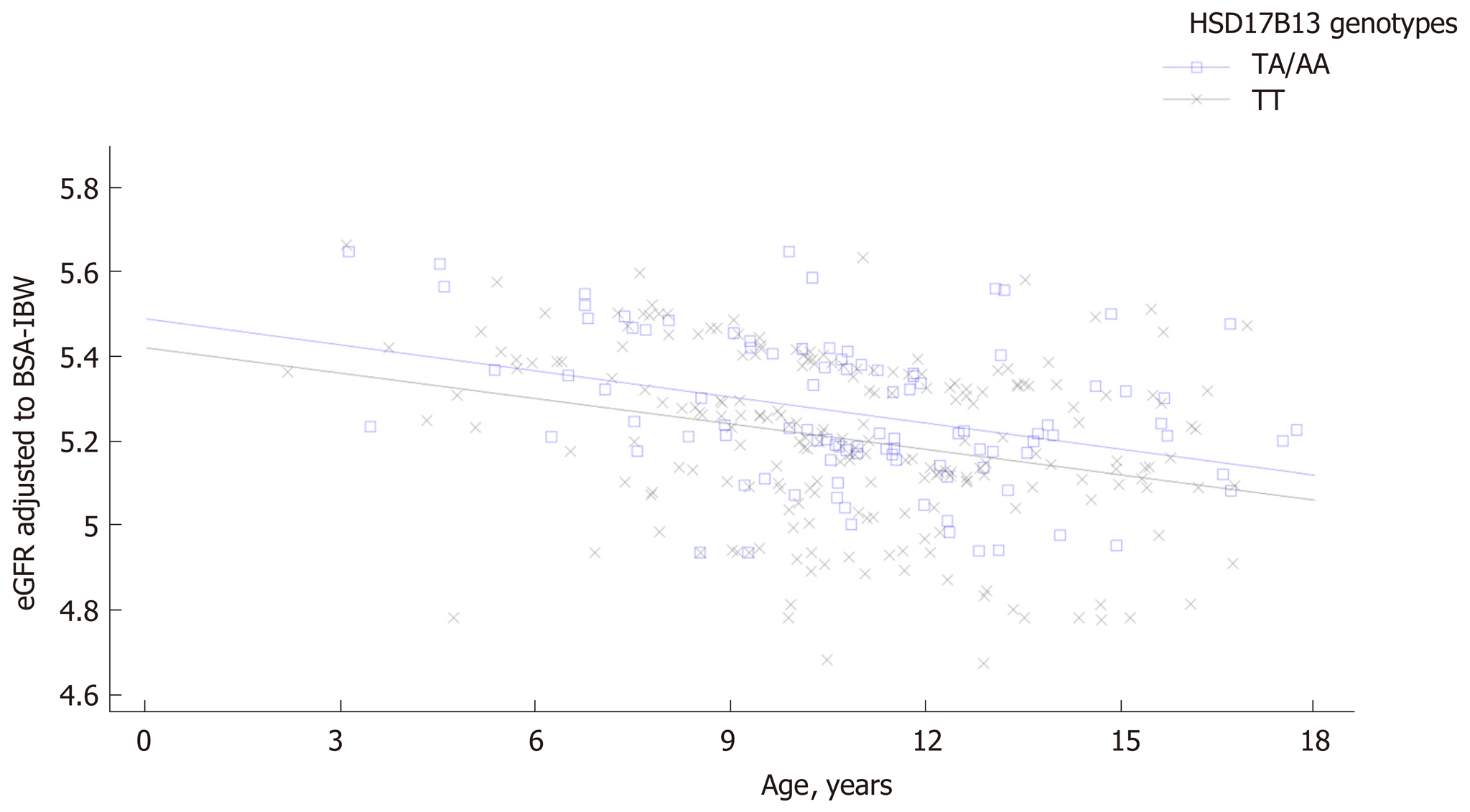
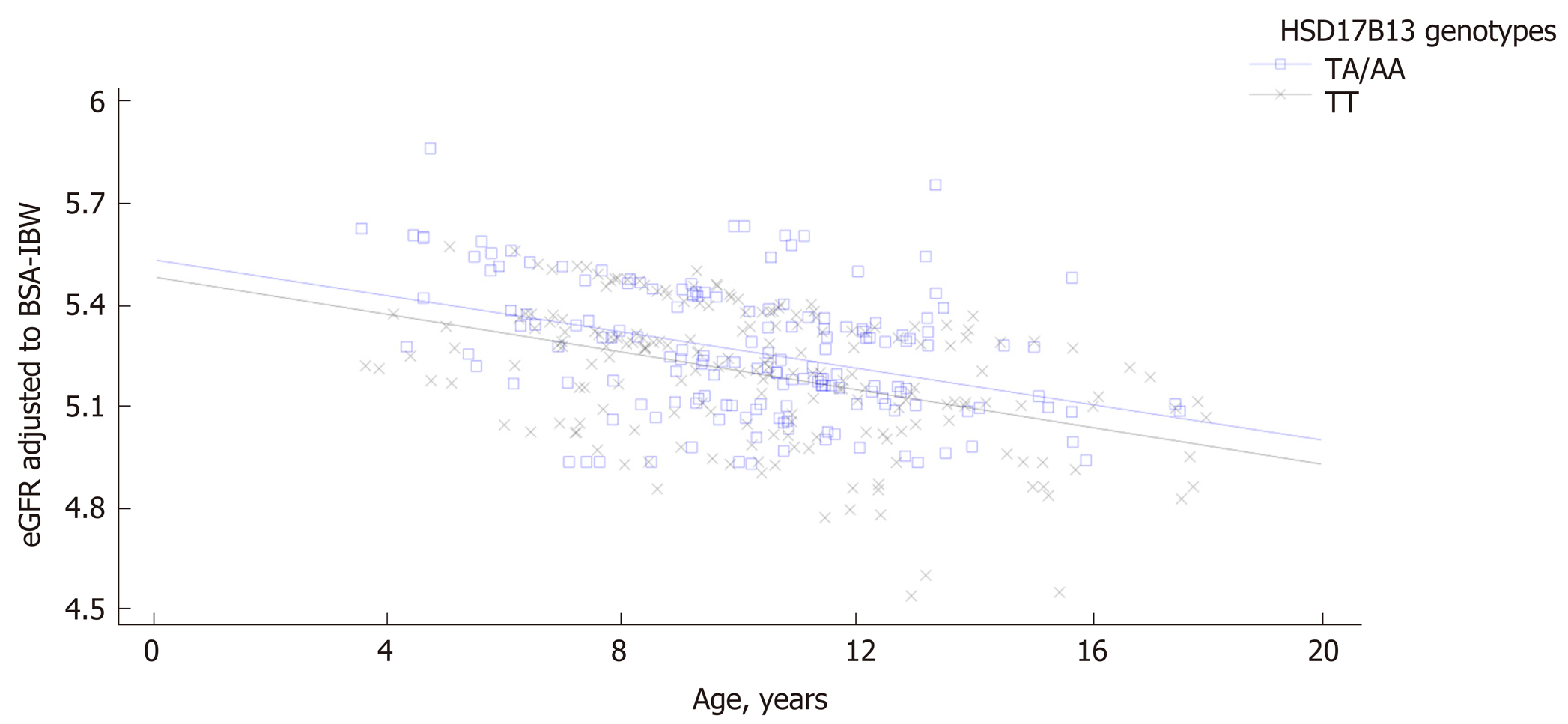
Patients carrying the HSD17B13 rare A allele showed higher eGFR levels compared with homozygous patients both among subjects with and without NAFLD. A general linear model confirmed a direct and significant association of eGFR values with HSD17B13 genotype independently of PNPLA3 and TM6SF2 polymorphisms both in patients with and without NAFLD. A comparison of regression line confirmed the influence of HSD17B13 genotype on the relationship between eGFR and age both among patients with and without NAFLD. Homozygous patients for HSD17B13 genotype with NAFLD showed a significantly higher decline of eGFR with the increase of the age compared with the patients with NAFLD carrying the HSD17B13 rare A allele (P value for intercepts = 0.005; P value for slopes = 0.94). The same effect was observed among patients without NAFLD (P value for intercepts = 0.0012; P value for slopes = 0.87).
Carriers of the HSD17B13 rare A allele showed higher eGFR levels than homozygous subjects both among subjects with and without NAFLD and independently of PNPLA3 I148M and TM6SF6 E167K polymorphisms.
Core Tip: Compelling evidence supports a genetic link between non-alcoholic fatty liver disease (NAFLD) and chronic kidney disease. In particular, both the major NAFLD risk polymorphisms such as the I148M polymorphism in the PNPLA3 and the E167K allele in the TM6SF2 gene affect renal function. Recently the rs72613567 variant in the HSD17B13 gene has been recognized as a novel genetic protective variant in the NAFLD scenario. We aimed to evaluate the effect of this variant on renal function in obese children.
- Citation: Di Sessa A, Umano GR, Cirillo G, Passaro AP, Verde V, Cozzolino D, Guarino S, Marzuillo P, Miraglia del Giudice E. Pediatric non-alcoholic fatty liver disease and kidney function: Effect of HSD17B13 variant. World J Gastroenterol 2020; 26(36): 5474-5483
- URL: https://www.wjgnet.com/1007-9327/full/v26/i36/5474.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i36.5474
Non-alcoholic fatty liver disease (NAFLD) and chronic kidney disease (CKD) represent worldwide public health concerns affecting up to 25%-30% and up to 10%-15% of the general population, respectively[1]. Both diseases are progressive chronic conditions representing a spectrum of diseases ranging from mild to severe disease with end-stage organ injury and sharing several pathogenic factors[1,2].
In childhood, NAFLD has been recognized as the most common chronic liver disease, with a prevalence of 8% in the general pediatric population and up to 34.2% among obese children[3].
To date, several studies demonstrated that NAFLD has been associated with increased prevalence of chronic kidney disease CKD (defined as sustained reduction in estimated glomerular filtration rate (eGFR) or evidence of structural or functional abnormalities of the kidneys on urinalysis, imaging or biopsy)[1,2,4,5]. In addition, it has been also showed that renal impairment obesity-related might occur already in childhood with a potential genetic influence[6-8].
Recent data added novel pieces in the knowledge of the intriguing link between NAFLD and renal function[1,9,10]. Compelling evidence showed that the major genetic NAFLD risk factors such as the I148M polymorphism in the patatin like phospholipase containing domain 3 (PNPLA3) and the E167K allele in the transmembrane 6 superfamily member 2 (TM6SF2) gene exert an effect on estimated glomerular filtration rate (eGFR) both in adults and children[1,5,9-12]. In particular, our group previously showed that children with obesity and homozygous for PNPLA3 rare allele had lower eGFR levels compared to other genotypes both among patients with and without NAFLD, with a major effect of this polymorphism in NAFLD context[10]. In addition, we demonstrated that obese children carrying the TM6SF2 E167K allele showed higher eGFR levels compared with the homozygous subjects for the TM6SF2 E167 allele, irrespective of presence of NAFLD[9].
In the NAFLD susceptibility gene variants scenario, the role of the hepatic lipid droplet protein hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) gene in NAFLD has been recently studied[3,13,14]. A growing body of evidence shows the role of the rs72613567 HSD17B13 polymorphism in protecting against liver damage both in adults and children[3,13-15]. This variant represents a splice region characterized by an adenine (A) insertion in the coding region adjacent to the donor splice site of exon 6, leading to a frame-shift and premature truncation of HSD17B13 protein[3].
To date, there are no studies evaluating the effect of the rs72613567 HSD17B13 polymorphism on eGFR in obese children and adolescents. Considering the evidence of the data both in adulthood and childhood supporting a genetic link between NAFLD and CKD[5,8-11], we hypothesized that this variant, as demonstrated on NAFLD, could show the same protective effect on renal function in obese children and adolescents. We aimed to investigate the role of the rs72613567 HSD17B13 polymorphism on eGFR levels in obese children with and without NAFLD and without known primary kidney disease.
We recruited 684 obese children consecutively attending our Obesity Clinic from February 1, 2017 to January 31, 2019. Both Body Mass Index (BMI) > 95th percentile according to reference values and normal renal function (eGFR > 90 mL/min/
Biochemical parameters were assayed and homeostasis model assessment (HOMA-IR) calculated[18]. Alanine transaminase (ALT) levels greater than 40 IU/L were considered as elevated. The Jaffè method was used to serum creatinine (mg/dL) measurement and the eGFR was calculated through the Schwartz equation[6,19]. The eGFR to the ideal body weight-derived body surface area was normalized.
A trained radiologist performed liver ultrasound imaging in order to detect the presence of steatosis. Liver steatosis was assessed as present or absent given the abnormally intense, high-level echoes arising from the hepatic parenchyma and liver–kidney differences in echo amplitude. NAFLD was defined by the presence of ultrasound detected liver steatosis and/or ALT levels > 40 IU/L.
At diagnosis, informed consent was collected for DNA extraction from peripheral whole blood.
Genomic DNA was extracted from peripheral whole blood with a DNA extraction kit (Promega, Madison WI, United States). All recruited subjects were genotyped for the single nucleotide polymorphism (SNP) rs72613567: TA allele of the HSD17B13 gene using a TaqMan allelic discrimination custom assay (ID: ANNKVTJ) (Applied Biosystems, United States) on ABI 7900HT Real Time PCR system. Patients were also genotyped for PNPLA3 I48M and TM6SF2 E167K polymorphisms[3].
A Chi Square test was used to verify whether the genotypes were in Hardy-Weinberg equilibrium and to compare categorical variables. We classified the population according to the presence/absence of NAFLD and we compared eGFR levels in these two groups (with and without NAFLD) on the basis of HSD17B13 polymorphism.
Differences for continuous variables were analyzed using ANOVA if normally distributed, or the Kruskal-Wallis test if non-normally distributed. Not-normally distributed variables were log-transformed before the analysis, but raw means are shown.
The genotype was coded with an additive model of inheritance. It was coded 0 or 1 corresponding to the subjects homozygous for the wild type allele and carrying the A rare allele, respectively.
A general linear model (GLM) for eGFR variance including gender, duration of obesity, HSD17B13, PNPLA3, and TM6SF2 genotypes, BMI-SDS, HOMA, LDL, and triglycerides was made both in patients with and without NAFLD. The non-normally distributed variables were log transformed for this analysis.
A comparison of regression lines by ANOVA was performed to examine the influence of HSD17B13 genotype on the relationship between eGFR and age both among patients with and without NAFLD.
The IBM SPSS Statistics software, Version 24 (IBM, Armonk, NY, United States) was used for all statistical analyses with the exception of the comparison of regression lines made by Stat-Graph XVII software for Windows. Data were expressed as means ± SD. P-values less than 0.05 were considered statistically significant.
We enrolled 684 obese patients with mean age of 10.56 ± 2.94 years and mean BMI-SDS of 2.98 ± 0.78. The frequency of the HSD17B13 polymorphism distribution was in Hardy Weinberg equilibrium (P > 0.05).
Patients carrying the HSD17B13 rare A allele showed higher eGFR levels compared with homozygous patients both among subjects with and without NAFLD (Table 1 and 2). As expected, ALT and aspartate transaminase (AST) levels were significantly lower in subjects carrying the HSD17B13 rare A allele in both groups (Table 1 and 2). A general linear model for eGFR variance including gender, duration of obesity, HSD17B13, PNPLA3, and TM6SF2 genotypes, BMI-SDS, HOMA, ALT and triglycerides confirmed a direct and significant association of eGFR values with HSD17B13 genotype both in patients with and without NAFLD (Table 3).
| HSD17B13 TT (n = 218) | HSD17B13 TA/AA (n = 100) | P value | |
| Age, yr | 10.72 ± 2.88 | 10.60 ± 2.80 | 0.67 |
| BMI-SDS | 2.98 ± 0.73 | 3.00 ± 0.75 | 0.85 |
| Duration of obesity, yr | 4.65 ± 1.93 | 4.64 ± 2.17 | 0.60 |
| Sex (male), % | 53.3 | 51.2 | 0.82 |
| SBP-SDS | 1.00 ± 1.15 | 1.12 ± 1.14 | 0.33 |
| DBP-SDS | 0.25 ± 0.89 | 0.30 ± 0.91 | 0.54 |
| W/Hr | 0.62 ± 0.05 | 0.63 ± 0.06 | 0.04 |
| ALT, U/L | 35.45 ± 21.69 | 30.60 ± 21.52 | 0.02 |
| AST, U/L | 26.86 ± 10.18 | 24.49 ± 8.86 | 0.01 |
| GGT, U/L | 20.42 ± 9.73 | 19.70 ± 9.49 | 0.45 |
| Total-Cholesterol, mg/dL | 160.13 ± 33.85 | 158.19 ± 30.80 | 0.55 |
| LDL, mg/dL | 95.84 ± 29.31 | 93.38 ± 23.93 | 0.40 |
| HDL, mg/dL | 44.07 ± 10.68 | 45.30 ± 11.24 | 0.67 |
| Triglycerides, mg/dL | 101.21 ± 51.36 | 99.92 ± 47.62 | 0.79 |
| Glycaemia, mg/dL | 83.05 ± 9.10 | 81.80 ± 8.44 | 0.14 |
| HOMA-IR | 6.19 ± 5.35 | 5.94 ± 3.86 | 0.62 |
| eGFR, mL/min/1.73m2 | 186.71 ± 36.55 | 197.45 ± 34.92 | 0.01 |
| HSD17B13 TT (n = 199) | HSD17B13 TA/AA (n = 167) | P value | |
| Age, yr | 10.36 ± 2.98 | 10.36 ± 3.20 | 0.89 |
| Duration of obesity | 2.96 ± 0.79 | 2.95 ± 0.96 | 0.94 |
| BMI -SDS | 4.41 ± 1.84 | 4.88 ± 2.51 | 0.24 |
| Sex (male), % | 50.5 | 55.5 | 0.68 |
| SBP-SDS | 1.08 ± 1.24 | 0.79 ± 1.20 | 0.09 |
| DBP-SDS | 0.18 ± 0.75 | 1.03 ± 1.15 | 0.64 |
| W/Hr | 0.61 ± 0.05 | 0.60 ± 0.05 | 0.21 |
| ALT, U/L | 22.95 ± 6.90 | 20.30 ± 6.56 | 0.003 |
| AST, U/L | 23.03 ± 4.92 | 21.35 ± 6.07 | 0.004 |
| GGT, U/L | 16.70 ± 5.80 | 17.38 ± 4.34 | 0.33 |
| Total-Cholesterol, mg/dL | 160.66 ± 27.11 | 161.84 ± 33.11 | 0.76 |
| LDL-C, mg/dL | 93.74 ± 24.59 | 98.10 ± 28.27 | 0.36 |
| HDL-C, mg/dL | 48.18 ± 15.28 | 45.96 ± 8.97 | 0.50 |
| Triglycerides, mg/dL | 96.90 ± 59.74 | 95.77 ± 38.79 | 0.87 |
| Glycaemia, mg/dL | 79.95 ± 7.34 | 80.52 ± 7.66 | 0.08 |
| HOMA-IR | 4.63 ± 3.16 | 5.16 ± 3.75 | 0.27 |
| eGFR, mL/min/1.73 m2 | 187.34 ± 37.76 | 197.82 ± 39.78 | 0.007 |
| Source | Patients with NAFLD | Patients without NAFLD | ||||
| Coefficient | F-ratio | P value | Coefficient | F-ratio | P value | |
| Model | 6.24 | < 0.0001 | 3.30 | 0.001 | ||
| Gender | -0.78 | 0.62 | 0.43 | 0.31 | 0.09 | 0.75 |
| Duration of obesity | -2.48 | 6.17 | 0.01 | -0.47 | 0.22 | 0.63 |
| HSD17B13 genotype | 1.89 | 42.58 | < 0.0001 | 4.63 | 21.49 | < 0.0001 |
| PNPLA3 genotype | -3.80 | 11.19 | 0.001 | -0.90 | 0.81 | 0.36 |
| TM6SF2 genotype | 1.13 | 11.82 | 0.001 | 1.40 | 1.97 | 0.16 |
| BMI-SDS | 0.62 | 0.38 | 0.53 | 0.51 | 0.26 | 0.61 |
| HOMA-IR | -1.20 | 1.44 | 0.23 | 0.63 | 0.40 | 0.52 |
| Triglycerides | -0.94 | 0.89 | 0.34 | 0.95 | 0.90 | 0.34 |
| LDL-Cholesterol | 0.69 | 0.47 | 0.49 | 0.23 | 0.05 | 0.81 |
A comparison of regression line slopes was performed to examine the influence of HSD17B13 genotype on the relationship between eGFR and age both among patients with and without NAFLD. Homozygous patients for HSD17B13 genotype with NAFLD showed a significantly higher decline of eGFR with the increase of the age compared with the patients with NAFLD carrying the HSD17B13 rare A allele (P value for intercepts = 0.005; P value for slopes = 0.94) (Figure 1). We also confirmed this effect among patients without NAFLD (P value for intercepts = 0.0012; P value for slopes = 0.87) (Figure 2).
The novel finding of our study was the association between the rs72613567 HSD17B13 polymorphism and higher eGFR levels in obese children and adolescents without evidence of primary kidney disease. We confirmed these results both in patients with and without NAFLD and independently of rs738409 PNPLA3 and TM6SF6 E167K polymorphisms. Notably, carriers of the HSD17B13 rare A allele both with and without NAFLD maintained significant higher eGFR levels increasing the age. Taken together, these results corroborate the protective role of the HSD17B13 gene in both NAFLD and CKD.
Emerging evidence suggests a potential influence of the major NAFLD susceptibility gene variants such as PNPLA3 I148M and TM6SF2 167K on CKD[9,10]. In fact, previous studies showed that both in adults and children with and without NAFLD the PNPLA3 I148M variant was associated with lower eGFR levels[1,5,10,11]. Interestingly, the PNPLA3 GG genotype was associated not only with higher risk of early glomerular injury but also tubular damage in NAFLD patients with persistently normal ALT levels[12]. Further researches investigating the role of the TM6SF2 167K allele on renal function showed a protective role of this variant on eGFR both in adults and children also regardless the presence of NAFLD[5,8,9].
The HSD17B13 gene encodes the hepatic lipid droplet protein hydroxyl-steroid 17-beta dehydrogenase 13, an uncharacterized member of the hydroxysteroid 17-beta dehydrogenase family involved in sex hormone and mainly in fatty acid metabolism[13]. The rs72613567 HSD17B13 splice variant alters m-RNA splicing resulting in a truncated protein with reduced enzymatic activity against several proinflammatory lipid species[13,14]. The mechanism linking HSD17B13 to liver disease is not referred to hepatic fat accumulation, but directly involves modulation of inflammation and fibrogenesis through an involvement in retinol metabolism[20]. Taken together, these findings support the beneficial influence of this loss-of-function against risk of chronic liver disease and hepatocellular carcinoma as well[14,20].
To date, the exact pathophysiologic function remains still poorly elucidated, but the protective role of the rs72613567 HSD17B13 variant in NAFLD development and progression has been confirmed both in adults and children[3,13,15]. As observed in the liver context, we could speculate that the rs72613567 HSD17B13 variant might exert a “protective” role on renal function through its role in retinol metabolism by modulating both inflammation and fibrogenesis
Recent data showed a significant association between NAFLD and the long-term risk of incident CKD[1,2]. Moreover, it should be noted that NAFLD is closely linked to an increased risk of cardiovascular disease (CVD), independent of the coexistence of common cardiometabolic risk factors[21]. In addition, it has been well established that CKD represents a major risk factor for end-stage renal disease, CVD and premature death[2]. Despite several newer hypotheses (including gut microbiota, platelet activation, and dietary changes) the major pathogenic link underlying these chronic diseases is represented by the activation of the shared proinflammatory and profibrotic factors[1]. In this context, the role of the HSD17B13 gene in retinol metabolism leading to lack of hepatic stellate cells activation might contribute to explain its protective effect on renal function[3]. Given that, this intriguing “vicious circle” among NAFLD, CVD and CKD represents a challenging field for clinicians that should be carefully evaluated. In fact, these findings emphasize the importance of an early detection of NAFLD diagnosis in order to potentially counteract the risk of both CKD and CVD. Beyond the established contribute in identifying subjects with greater susceptibility to NAFLD, a better NAFLD genetic characterization might be also helpful to individuate patients with NAFLD who may be at higher risk of chronic diseases such as CKD and CVD.
Our study has some limitations that deserve mention. First, there is a lack of a more accurate method to measure glomerular filtration rate (i.e., by cystatin C). NAFLD diagnosis has been performed by ultrasound and ALT levels and not by liver biopsy or magnetic resonance. Moreover, this is only a pathophysiological study evaluating only children with normal renal function, and as future perspective could be interesting to evaluate the effect of the rs72613567 HSD17B13 polymorphism on proteinuria.
In conclusion, for the first time in literature, we showed that the rs72613567 HSD17B13 polymorphism is associated with higher eGFR levels in obese children. This effect on renal function is confirmed in patients with and without NAFLD and independently of PNPLA3 I148M and TM6SF6 E167K polymorphisms. Of note, this variant exerts a protective role against renal function decline increasing the age both in patients with and without NAFLD. Given these results, we added to the existing knowledge of the role of genetics on the association between NAFLD and renal function already in childhood. Collectively, these findings might also have significant clinical implications in improving both prevention and treatment strategies of obese patients with NAFLD.
Accumulating data supports a genetic link between non-alcoholic fatty liver disease (NAFLD) and chronic kidney disease (CKD), mostly sustained by both the major NAFLD risk polymorphisms such as the I148M polymorphism in the patatin like phospholipase containing domain 3 (PNPLA3) and the E167K allele in the transmembrane 6 superfamily member 2 gene (TM6SF2). Recently the hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) gene has been recognized as a novel genetic variant involved in NAFLD pathophysiology with a protective role against liver damage both in adults and children.
Despite a growing interest regarding the potential genetic link between NAFLD and CKD, available literature data showed no studies investigating the effect of the rs72613567:TA variant of the HSD17B13 gene on estimated glomerular filtration rate (eGFR) in obese children.
In this study we aimed to evaluate the impact of the rs72613567:TA variant of the HSD17B13 gene on estimated glomerular filtration rate (eGFR) in obese children.
Anthropometric, laboratory, and instrumental evaluations were conducted in all the enrolled 684 obese children. NAFLD was defined by ultrasound detected liver steatosis and/or alanine aminotransferase (ALT) levels > 40 IU/L. Genotyping for the rs72613567:TA variant of the HSD17B13 gene in all the enrolled subjects was also performed.
Patients carrying the HSD17B13 rare A allele had higher eGFR levels than homozygous patients both among subjects with and without NAFLD. This association was independent of PNPLA3 and TM6SF2 polymorphisms both in patients with and without NAFLD. The eGFR decline in homozygous subjects for HSD17B13 genotype with and without NAFLD was more markedly with the increase of the age than in carriers the HSD17B13 rare A allele.
In line with the beneficial effect against NAFLD risk, the rs72613567:TA variant of the HSD17B13 gene exerts a protective role also on renal function in obese children with and without NAFLD and independently of PNPLA3 I148M and TM6SF6 E167K polymorphisms.
Findings from this study highlight the importance of a better NAFLD genetic assessment as possible clinical tool for improved strategies to identify patients at higher cardiometabolic risk already in childhood.
The authors are grateful to the patients and their families.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inamo Y, Yong D S-Editor: Gong ZM L-Editor: A P-Editor: Zhang YL
| 1. | Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72:785-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 2. | Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, Targher G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2018;79:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 3. | Di Sessa A, Umano GR, Cirillo G, Marzuillo P, Arienzo MR, Pedullà M, Miraglia Del Giudice E. The rs72613567: TA Variant in the Hydroxysteroid 17-beta Dehydrogenase 13 Gene Reduces Liver Damage in Obese Children. J Pediatr Gastroenterol Nutr. 2020;70:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Musso G, Cassader M, Cohney S, De Michieli F, Pinach S, Saba F, Gambino R. Fatty Liver and Chronic Kidney Disease: Novel Mechanistic Insights and Therapeutic Opportunities. Diabetes Care. 2016;39:1830-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Musso G, Cassader M, Gambino R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology. 2015;62:658-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Marzuillo P, Grandone A, Di Sessa A, Guarino S, Diplomatico M, Umano GR, Polito C, La Manna A, Perrone L, Miraglia Del Giudice E. Anthropometric and Biochemical Determinants of Estimated Glomerular Filtration Rate in a Large Cohort of Obese Children. J Ren Nutr. 2018;28:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Di Bonito P, Valerio G, Licenziati MR, Miraglia Del Giudice E, Baroni MG, Morandi A, Maffeis C, Campana G, Spreghini MR, Di Sessa A, Morino G, Crinò A, Chiesa C, Pacifico L, Manco M. High uric acid, reduced glomerular filtration rate and non-alcoholic fatty liver in young people with obesity. J Endocrinol Invest. 2020;43:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Targher G, Mantovani A, Alisi A, Mosca A, Panera N, Byrne CD, Nobili V. Relationship Between PNPLA3 rs738409 Polymorphism and Decreased Kidney Function in Children With NAFLD. Hepatology. 2019;70:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Marzuillo P, Di Sessa A, Cirillo G, Umano GR, Pedullà M, La Manna A, Guarino S, Miraglia Del Giudice E. Transmembrane 6 superfamily member 2 167K allele improves renal function in children with obesity. Pediatr Res. 2020;88:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Marzuillo P, Di Sessa A, Guarino S, Capalbo D, Umano GR, Pedullà M, La Manna A, Cirillo G, Miraglia Del Giudice E. Nonalcoholic fatty liver disease and eGFR levels could be linked by the PNPLA3 I148M polymorphism in children with obesity. Pediatr Obes. 2019;14:e12539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Mantovani A, Zusi C, Sani E, Colecchia A, Lippi G, Zaza GL, Valenti L, Byrne CD, Maffeis C, Bonora E, Targher G. Association between PNPLA3rs738409 polymorphism decreased kidney function in postmenopausal type 2 diabetic women with or without non-alcoholic fatty liver disease. Diabetes Metab. 2019;45:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Sun DQ, Zheng KI, Xu G, Ma HL, Zhang HY, Pan XY, Zhu PW, Wang XD, Targher G, Byrne CD, Chen YP, Yuan WJ, Zheng MH. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020;40:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, Liu Y, Kozlitina J, Stender S, Wood GC, Stepanchick AN, Still MD, McCarthy S, O'Dushlaine C, Packer JS, Balasubramanian S, Gosalia N, Esopi D, Kim SY, Mukherjee S, Lopez AE, Fuller ED, Penn J, Chu X, Luo JZ, Mirshahi UL, Carey DJ, Still CD, Feldman MD, Small A, Damrauer SM, Rader DJ, Zambrowicz B, Olson W, Murphy AJ, Borecki IB, Shuldiner AR, Reid JG, Overton JD, Yancopoulos GD, Hobbs HH, Cohen JC, Gottesman O, Teslovich TM, Baras A, Mirshahi T, Gromada J, Dewey FE. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med. 2018;378:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 610] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 14. | Pirola CJ, Garaycoechea M, Flichman D, Arrese M, San Martino J, Gazzi C, Castaño GO, Sookoian S. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J Lipid Res. 2019;60:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Gellert-Kristensen H, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. High Risk of Fatty Liver Disease Amplifies the Alanine Transaminase-Lowering Effect of a HSD17B13 Variant. Hepatology. 2020;71:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G, Cicognani A. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest. 2006;29:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 651] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 17. | Marzuillo P, Guarino S, Furlan D, Pecoraro A, Pedullà M, Miraglia Del Giudice E, La Manna A. Cleaning the genitalia with plain water improves accuracy of urine dipstick in childhood. Eur J Pediatr. 2018;177:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Di Sessa A, Umano GR, Cirillo G, Del Prete A, Iacomino R, Marzuillo P, Del Giudice EM. The Membrane-bound O-Acyltransferase7 rs641738 Variant in Pediatric Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2018;67:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1242] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 20. | Trépo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. J Hepatol. 2020;72:1196-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 21. | Di Sessa A, Umano GR, Miraglia Del Giudice E, Santoro N. From the liver to the heart: Cardiac dysfunction in obese children with non-alcoholic fatty liver disease. World J Hepatol. 2017;9:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |