Published online Sep 21, 2020. doi: 10.3748/wjg.v26.i35.5375
Peer-review started: June 28, 2020
First decision: July 28, 2020
Revised: August 11, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: September 21, 2020
Processing time: 80 Days and 16 Hours
Radiofrequency ablation (RFA) and microwave ablation (MWA) represent the standard of care for patients with early hepatocellular carcinoma (HCC) who are unfit for surgery. The incidence of reported adverse events is low, ranging from 2.4% to 13.1% for RFA and from 2.6% to 7.5% for MWA. Gastrointestinal tract (GIT) injury is even more infrequent (0.11%), but usually requires surgery with an unfavourable prognosis. Due to its low incidence and the retrospective nature of the studies, the literature reporting this feared complication is heterogeneous and in many cases lacks information on tumour characteristics, comorbidities and treatment approaches.
A 77-year-old man who had undergone extended right hepatectomy for HCC was diagnosed with early disease recurrence with a small nodule compatible with HCC in the Sg4b segment of the liver with a subcapsular location. He was treated with percutaneous RFA and a few week later he was urgently admitted to the Surgery ward for abdominal pain and fever. A subcutaneous abscess was diagnosed and treated by percutaneous drainage. A fistulous tract was then documented by the passage of contrast material from the gastric antrum to the abdominal wall. The oesophagogastroduodenoscopy confirmed a circular wall defect at the lesser curvature of gastric antrum, leading directly to the purulent abdominal collection. An over-the-scope clip (OTSC) was used to successfully close the defect
This is the first reported case of RFA-related GIT injury to have been successfully treated with an OTSC, which highlights the role of this endoscopic treatment for the management of this complication.
Core Tip: Thermal ablative therapies have a key role in the treatment algorithm for hepatocellular carcinoma, and besides their efficacy and tolerability, several studies have proven their overall safety. Nevertheless, albeit rarely, a number of complications have been reported and awareness is crucial to proposing the best treatment for each patient. We report the unusual case of a gastric perforation that was treated in our division and how it was managed with an endoscopic over-the-scope clip for the first time. The literature review aims to discuss the most relevant published data on gastrointestinal tract injuries after thermal ablation therapies.
- Citation: Rogger TM, Michielan A, Sferrazza S, Pravadelli C, Moser L, Agugiaro F, Vettori G, Seligmann S, Merola E, Maida M, Ciarleglio FA, Brolese A, de Pretis G. Gastrointestinal tract injuries after thermal ablative therapies for hepatocellular carcinoma: A case report and review of the literature. World J Gastroenterol 2020; 26(35): 5375-5386
- URL: https://www.wjgnet.com/1007-9327/full/v26/i35/5375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i35.5375
Hepatocellular carcinoma (HCC) represents about 90% of primary liver cancers and, with an ever-greater incidence, is the seventh most common malignant tumour and the fourth major cause of cancer death worldwide[1]. Approximately 70%-90% of HCC cases occur in cirrhotic liver and the choice of the most appropriate treatment must take into account not only cancer staging, but also liver function assessment, evaluation of extrahepatic disease, patient comorbidities and performance status[2-4]. All the available therapeutic options have drawbacks that can affect both safety and health-related quality of life. Clinicians have to choose those interventional procedures whose benefits outweigh the risks for each individual patient[5,6].
Although liver transplantation and surgical resection remain the gold standard for HCC, only a small proportion of patients are eligible, making locoregional therapies valuable options with a good survival benefit and safety profile[7,8]. Of these, according to the most widely accepted staging and treatment algorithm for HCC, the Barcelona Clinic Liver Cancer algorithm[9,10], ablative modalities have earned a pivotal role, representing the first-line option with curative intent for unresectable Stage 0 (very early) or Stage A (early) HCC. In addition, they can represent an alternative to resection in single tumours with favourable locations or for those tumours < 3 cm in size[11]. They can also be included in a multimodal approach for intermediate and advanced cases, or play a role as a bridging therapy prior to transplantation[12,13]. Radiofrequency ablation (RFA) and microwave ablation (MWA) are the most extensively clinically-validated thermal ablative therapies[14,15]. Using one or more electrodes that generate electrical current and electromagnetic energy respectively, they induce heat in the tumour tissue above a lethal threshold, leading to coagulative necrosis[15,16]. Although they are less invasive than surgical resection, the complication rate ranges from 2.4% to 13.1% for RFA and from 2.6% to 7.5% for MWA[8], with no significant difference between the two techniques[14]. Direct mechanical injury caused by the passage of the electrode through the vessels or biliary tree may lead to bleeding or bile leakage[17,18]. Heat damage represents the other mechanism that can complicate these procedures causing gastrointestinal tract (GIT), diaphragm or gallbladder injury, pleural effusion, bile duct strictures, biloma, vascular injury with consequent liver infarction and grounding pad burns. Other possible adverse events include tumour seeding along the tract, septic complications with hepatic abscess or cholangitis, and vasovagal reflex[12,19,20]. GIT haemorrhagic complications after thermal ablative therapies have also been reported and appear to be mostly associated with a worsening of pre-existing portal hypertension or portal vein thrombosis[19,21,22].
Thermal damage with GIT injury is an uncommon yet severe complication. At the current time, there are a few reviews analysing the complications of thermal ablation treatments, none specifically investigating GIT injuries[23-25].
We report the case of an RFA-related gastric perforation that was successfully managed using an over-the-scope clip (OTSC). To the best of our knowledge, this is the first reported endoscopic treatment for an RFA-related GIT complication. We also briefly review and discuss the most relevant published data on GIT injuries after thermal ablation therapies, with regard to their prevalence, risk factors and proposed treatment.
In March 2020, a 77-year-old man was urgently admitted to the Surgery ward for abdominal pain and fever.
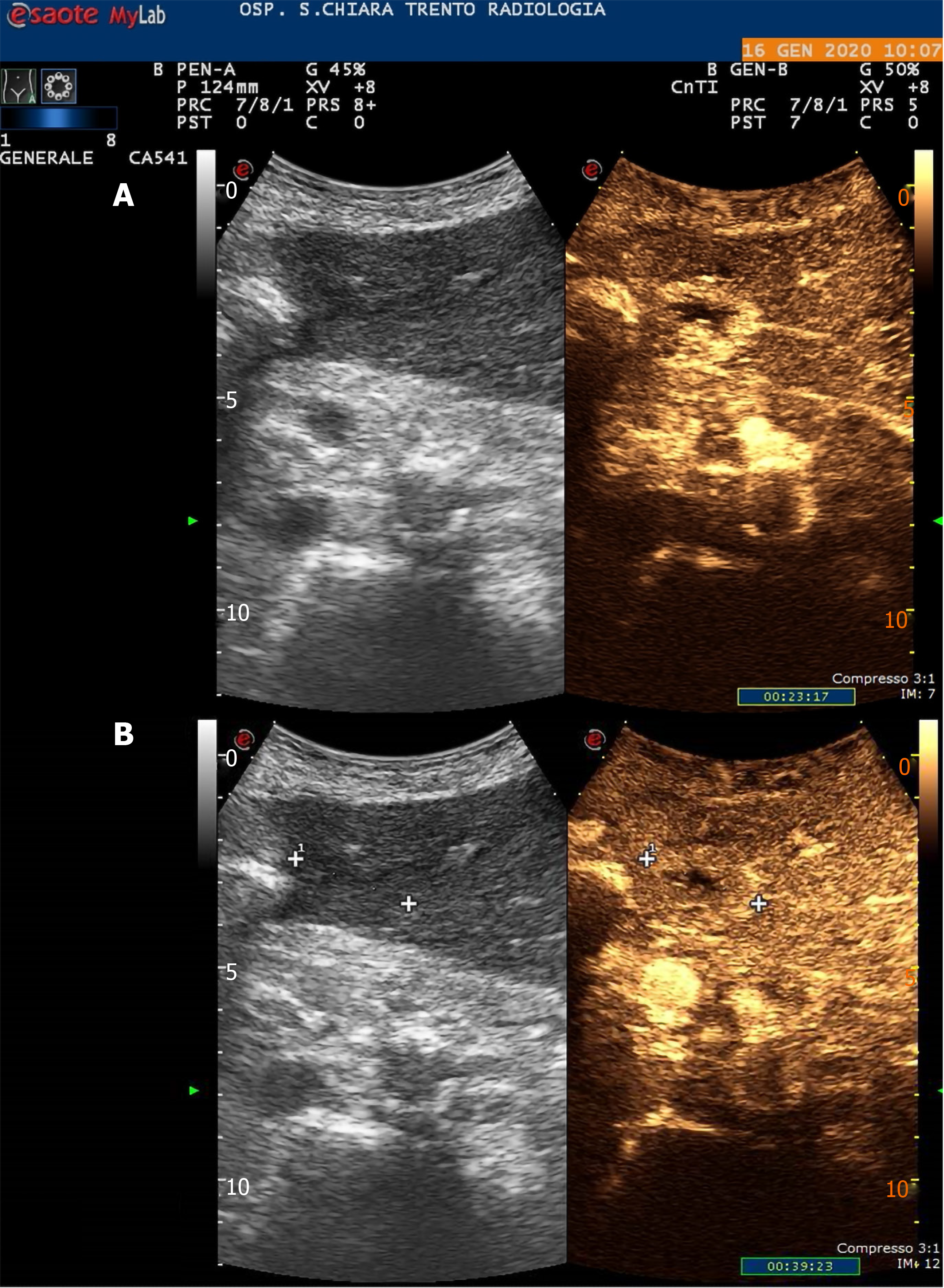
In June 2019, he was diagnosed with a bulky hepatic mass with a maximum diameter of 28 cm involving the whole right lobe, with no evidence of pre-existing liver disease. The condition was diagnosed at another hospital and the patient was subsequently treated with combined extended right hepatectomy, hepatic pedicle lymphadenectomy and cholecystectomy. The histopathological study showed a moderately differentiated hepatocellular carcinoma and confirmed a healthy liver parenchyma. No lymph nodes were involved and the tumour resection margins were clear (staging pT3N0G2 – R0). The 6-mo follow-up total-body computed tomography scan showed early disease recurrence and progression with a small 27 mm nodule compatible with HCC in the Sg4b segment of the liver with a subcapsular location, on the edge of the previous partial resection site, and a 5 cm adrenal metastasis. A subsequent contrast-enhanced ultrasound (US) scan of the liver confirmed the diagnosis (Figure 1). In January 2020, the patient had a multidisciplinary consultation at our hospital and was deemed fit for locoregional treatments. In February 2020, he was admitted in our Surgery ward and, once written informed consent had been obtained, he was treated with chemioembolisation of the adrenal metastasis and percutaneous RFA of the small HCC in Sg4b. The ablation was performed with anaesthesiological support, and under real-time US guidance. The device used to apply the radiofrequency current was a 20 cm long, 17-Gauge electrode with an uninsolated 3 cm tip (RF-AMICA probe, HS Hospital Service, Aprilia, Italy). No immediate complications were reported after the procedure.
The patient’s medical history included arterial hypertension, surgical resection of a parathyroid adenoma and radioactive iodine therapy for Plummer’s disease.
The clinical abdominal examination revealed epigastric tenderness. The patient’s temperature was 38.5 °C, blood pressure was 140/90 mmHg, heart rate was 100 bpm, respiratory rate was 15 breaths per min, and oxygen saturation in room air was 99%.
The patient’s biochemistry tests showed no clear evidence of systemic inflammation. White blood cells and serum C-reactive protein were at the upper limit of the normal range. Serum transaminases, liver function tests and routine blood biochemistry were normal.
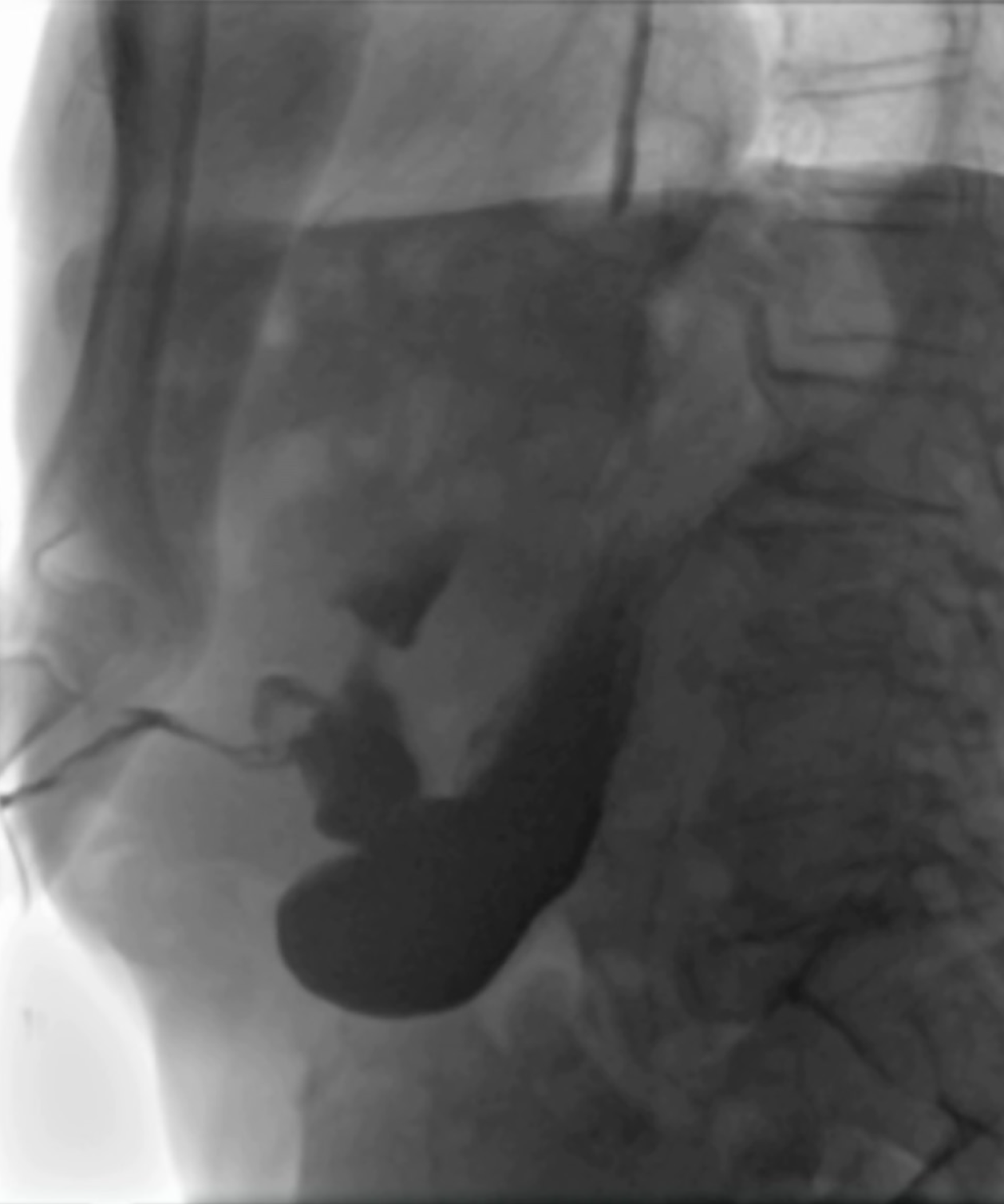
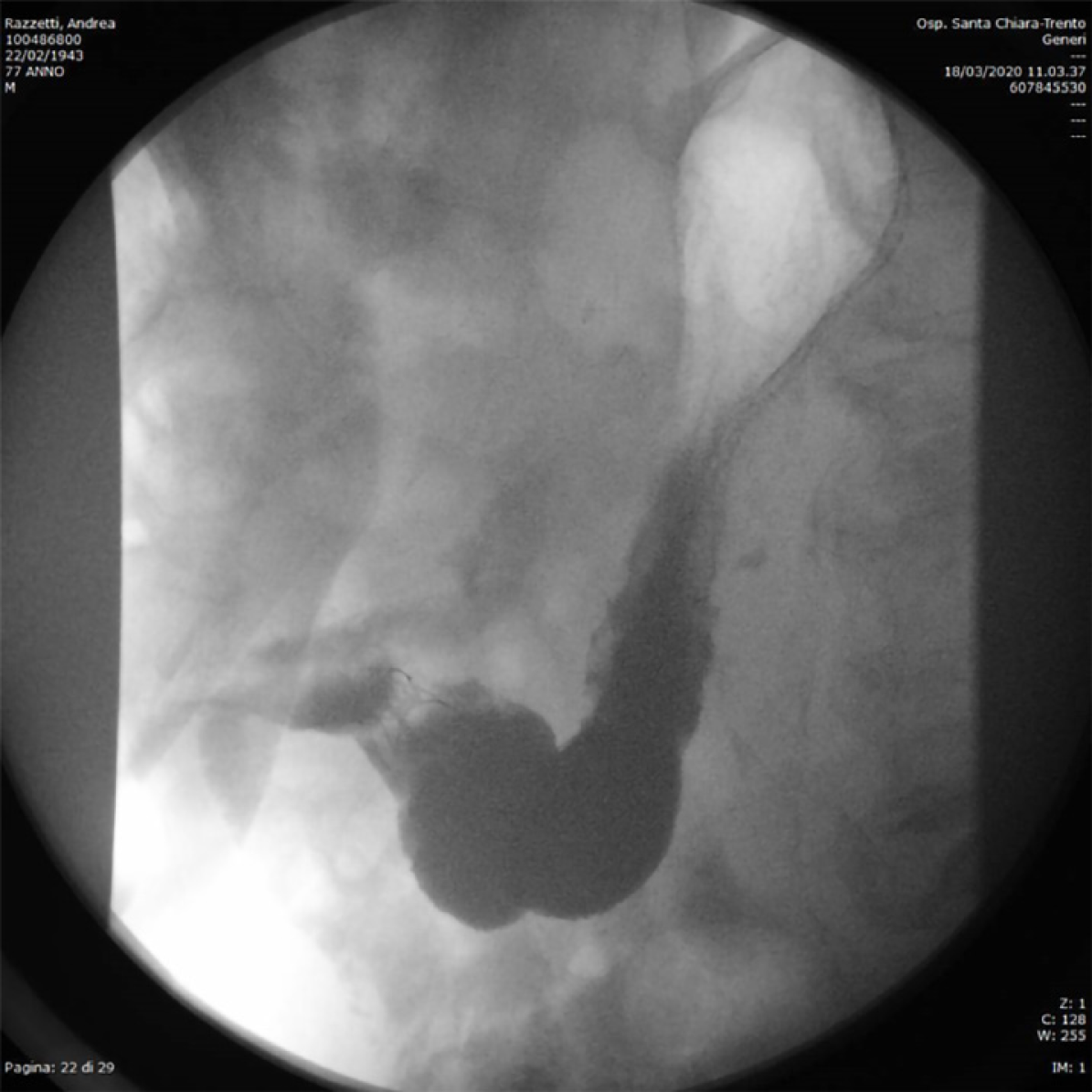
A subcutaneous abscess was diagnosed by abdominal ultrasound and treated with percutaneous drainage, observing necrotic and purulent secretion, together with air leak. Since no decrease in drain output was observed over the following d, an abdominal film with oral water-soluble contrast agent was performed. A fistulous tract was documented by the passage of contrast material from the gastric antrum to the abdominal wall (Figure 2).
The final diagnosis of the presented case was RFA-related gastric perforation.
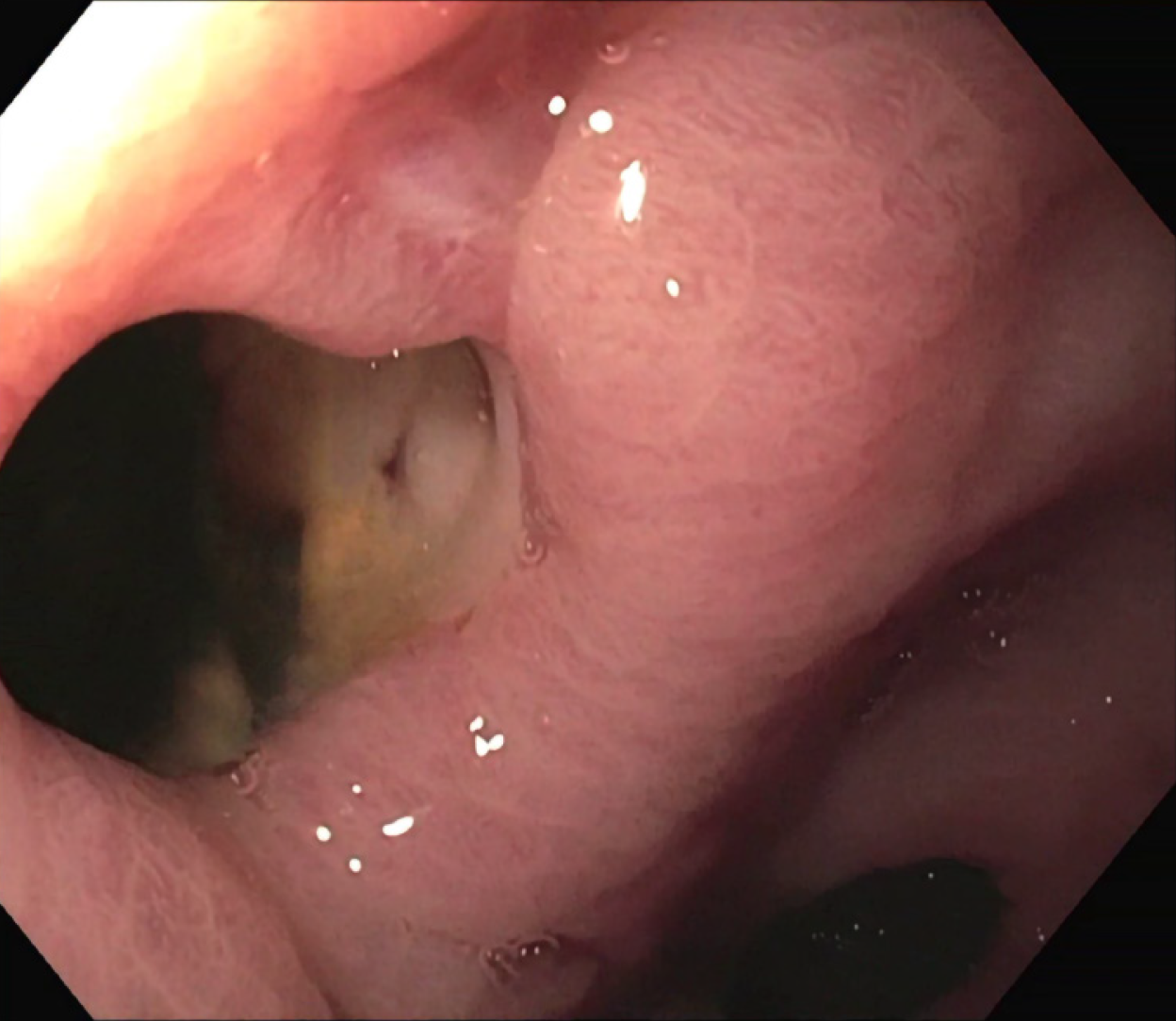
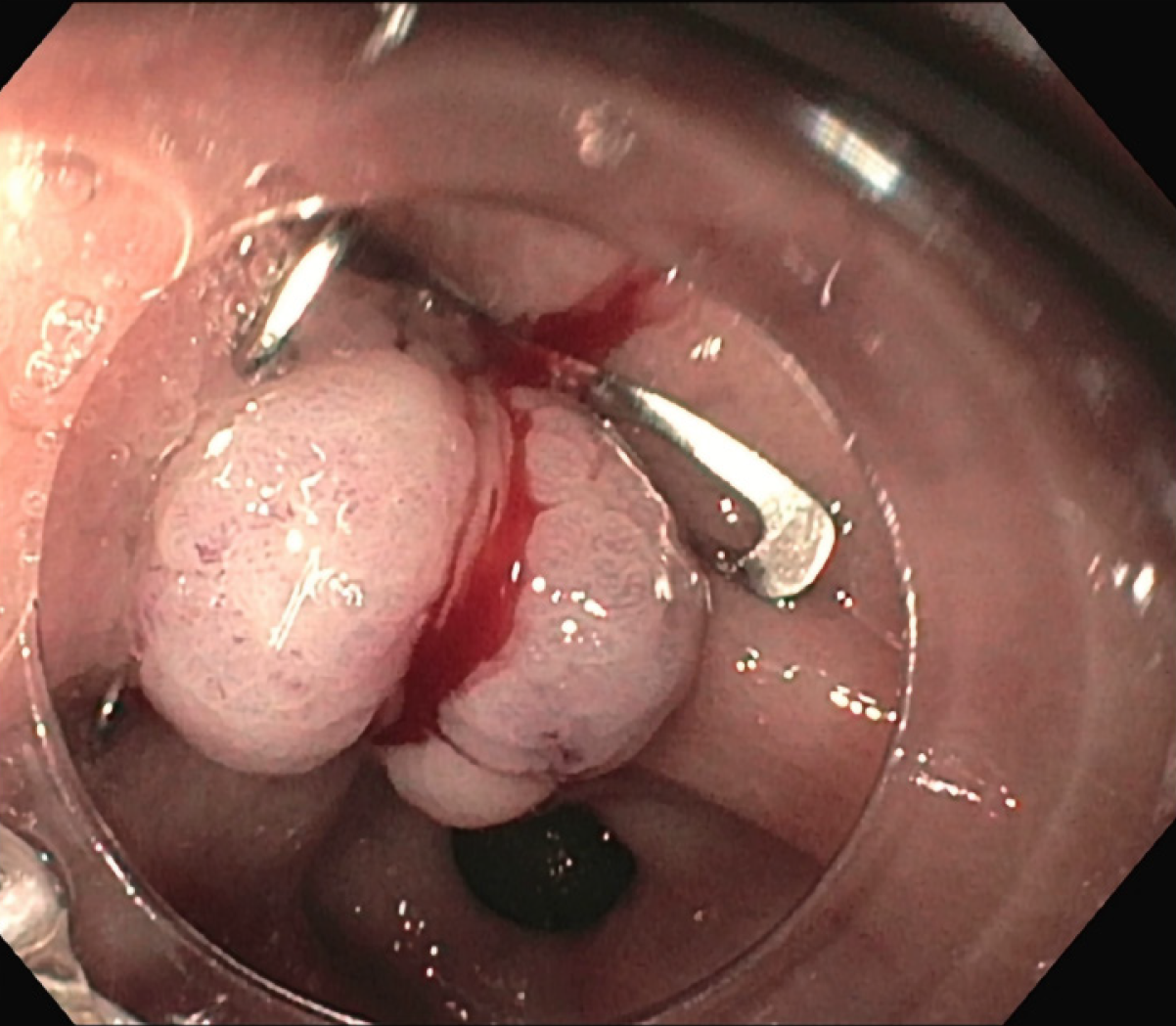
The patient was treated conservatively with fasting and broad-spectrum antibiotics; a prompt endoscopic assessment was planned. The oesophagogastroduodenoscopy confirmed a circular wall defect, approximately 15 mm in size, at the lesser curvature of gastric antrum, leading directly to the purulent abdominal collection (Figure 3). An OTSC was used to successfully close the defect (Figure 4) and there were no immediate complications. Technical success was confirmed after one week by an abdominal film with oral water-soluble contrast agent (Figure 5). The patient gradually resumed oral feeding and was finally discharged in good conditions.
A short time after the complete closure of the external fistula, recurrence of a mild cutaneous leakage (less than 40 mL/d) was observed for 40 d, followed by spontaneous closure. In June 2020, no signs of perforation recurrence were detected and the HCC was in remission in the remaining liver, according to mRECIST criteria[26]. The case report timeline is showed in Supplementary Figure 1.
We performed a review of the literature reporting the largest series of GIT complications published to date, highlighting the features that may help clinicians in the detection and management of these feared events (Table 1). Published studies are mainly retrospective and extremely heterogeneous as regards information on tumour size and location, comorbidities and treatment approach, which are often missing, or otherwise include lesions other than HCC.
| Ref. | Study design | Number of patients | Overall complication rate(%) | GIT injury rate (%) | Type of GIT injury | Thermoablative treatment | Timing of GIT injury | Management of GIT injury | Outcome | Associated conditions |
| Livraghi et al[29], 2003 | Multicentre, retrospective, questionnaire-based1 | 2320 | 7.1 (2.42) | 0.72 | 5 colonic perforations; 1 jejunal perforation; 1 gastric perforation | Percutaneous RFA | 2 d-4 d | Surgery (7/7) | 2 deaths after colonic perforation | Gut wall distance < 1 cm (7/7); adherence due to previous abdominal surgery or inflammatory chronic cholecystitis (6/7); large superficial HCC in left lobe + aggressive treatment (1/7) |
| De Baere et al[31], 2003 | Multicentre, prospective1 | 312 | 12 (5.72) | 0.32 | 1 colonic perforation | RFA3 | 4 d | Surgery | Death | NA |
| Curley et al[57], 2004 | Multicentre, prospective1 | 608 | 9.5 | 0.162 | 1 stomach wall necrosis | Open RFA | Immediate | Surgery | Recovery | Left lobe |
| Jansen et al[58], 2005 | Multicentre, prospective1 | 122 | 9.8 (6.32) | 2.5 (02) | 2 transient paralitic ileus | RFA3 | NA | Spontaneous resolution (2/2) | Recovery | NA |
| Casaril et al[32], 2007 | Single-centre, retrospective1 | 83 | 25 (7.22) | 0.72 | 1 colonic perforation | Percutaneous RFA | 36 d | NA | Death | Superficial HCC in Sg4; Child-Pugh B |
| Kasugai et al[22], 2007 | Multicentre, retrospective, questionnaire-based | 2614 | 7.92 | 0.22 | 1 duodenum injury; 1 stomach injury; 1 colonic perforation | RFA3 | NA | External drainage (1/3) | Recovery | NA |
| Chen et al[34], 2008 | Single-centre, retrospective1 | 104 | 5.22 | 0.62 | 1 colonic peroration with fistula and abscess | Percutaneous RFA | 3 wk | External drainage | Recovery | Superficial HCC in Sg4; previous surgery for Denver shunt |
| Liang et al[30], 2009 | Single-centre, retrospective1 | 1136 | 2.62 | 0.22 | 2 colonic perforations | Percutaneous MWA | 3 d-5 d | Surgery (2/2) | Recovery | HCC located < 1 cm from colonic wall + prior right partial hepatectomy (2/2) |
| Livraghi et al[33], 2012 | Multicentre, retrospective, questionnaire-based1 | 736 | 10.2 (2.92) | 0.22 | 1 ileal perforation; 1 colonic perforation | Percutaneous MWA | NA | Surgery (2/2) | Recovery | Superficial HCC in Sg4 + abdominal adhesions (2/2) |
| Koda et al[53], 2012 | Multicentre, retrospective, questionnaire-based | 13283 | 3.52 | 0.05 | 1 colonic perforation; 3 stomach injuries; 2 duodenum injuries | RFA3 | NA | Surgery (3/6) | 1 Death after colonic perforation | NA |
| Ding et al[19], 2013 | Single-centre, retrospective1 | 879 | 8.8-9.4 (3.1-3.52) | 0.32 | 1 bowel perforation | Percutaneous RFA | Immediate | External drainage | Recovery | Previous Whipple procedure |
| Park et al[54], 2017 | Single-centre, retrospective | 1211 | 6.8 (22) | 0.22 | 1 colonic perforation | Percutaneous RFA | NA | NA | Recovery | NA |
| Jeong et al[28], 2017 | Single-centre, retrospective1 | 3933 | NA | 1.32 | 28 stomach injuries; 16 colonic injuries; 6 small bowel injuries; 1 small bowel perforation; 1 colonic perforation | Percutaneous RFA | 2 d-13 d (perforations) | Surgery (2/2 perforations) | Recovery | Subcapsular HCC (47/52); previous percutaneous treatments (7/52) or abdominal surgery (19/52) |
| Maeda et al[59], 2020 | Multicentre, retrospective, questionnaire based | 9411 | 3.52 | 0.042 | 2 colonic perforations | NA | NA | NA | 1 Death | NA |
The reported prevalence of GIT injuries ranges from 0.04% to 2.5%, particularly when thermal ablative therapies were administered percutaneously.
The great variability in the GIT injury rate depends on whether minor injuries were included in the studies. Most literature concerns only major complications, strictly defined as those that increase the level of care, leading to significant morbidity and disability[27]. This probably plays down the effect that thermal ablation may have on GIT. Two previous reviews on the complications of thermoablative treatments found an extremely low prevalence of GIT injuries (0.11%-0.5%)[23,24]. However, a subsequent study on radiological predictors of major GIT injury reported a rate of 1.32%[28] and in a review that included also minor injuries, this rate rose to 3.2%[25].
Outcome is often unfavourable, as confirmed by previous studies. After sepsis and liver failure, GIT injuries were more frequently associated with death than other more commonly-observed complications[24,29,30]. GIT complications accounted for two in six fatal adverse events in one Italian multicentre study[29], one in five in the series from De Baère et al[31], and the only lethal event observed by Casaril et al[32].
Associated conditions that may represent precipitating factors for GIT injury are HCC nodules or gut wall close to liver capsule (< 1 cm), previous abdominal surgery or percutaneous treatments.
It is well known that structures located within 0.5 cm-1 cm from the tumour margin are at-risk of heat-induced damage, since ablative treatment usually includes an area of healthy peritumoral tissue, in order to eradicate any microscopic satellites and prevent local recurrence[12]. However, this is not the only factor involved, as stressed by Liang et al[30] in their retrospective analysis, which did not identify any significant difference in tumour location between patients with vs without major complications[30]. The association of an unfavourable location and a predisposing history is clearly shown by the data reported in the literature. In two studies analysing complications after both RFA and MWA, Livraghi et al[29,33] reported nine major GIT complications. All occurred after the treatment of superficial lesions, mostly in patients who had had previous abdominal surgery or had inflammatory abdominal processes (89%, 8/9 patients). Other studies confirmed that GIT injuries exclusively followed the treatment of subcapsular tumours in a specific subset of patients, whose history included Whipple resection, right partial hepatectomy or peritoneovenous shunt[19,30,34]. In line with these data, a more recent Korean study found that of 52 patients who experienced GIT complications 47 (90.4%) had a tumour located in a subcapsular portion of the liver and in half of these patients previous abdominal surgery or repeated percutaneous treatments were reported[28].
In the majority of the studies examined, the colon appears the most commonly involved organ, with a delay in presentation of several days. Its vulnerability is associated with its thin wall and relatively stable position compared to the stomach’s thick wall and to the small intestine’s active peristalsis[20,35]. In case of aggressive treatments with repeated or prolonged sessions, particularly in high-risk settings as previously discussed, caution is warranted as other organs than the colon may be involved[36-38].
GIT injuries can be difficult to recognise because their clinical presentation is subtler than classic digestive perforation and they may be misdiagnosed as post-ablation syndrome. Unresolved or delayed-onset fever, pain or increase in white blood cell count several days after the procedure, should raise suspicions and careful patient assessment is warranted. The presence of an abscess, although more commonly related with other aetiologies, could be the result of a contaminating digestive tract perforation, and consequently close monitoring of GIT is also recommended in these cases[39].
Some authors advocate using of imaging techniques soon after RFA. Gastrointestinal wall thickening, fat stranding and free fluid can be found around the injured area as a result of minimal insult[39,40]. Similarly, the presence of free air does not always indicate a major perforation, as it is found in more than half of all patients with minor complications and usually subsides within one month[41]. On the other hand, immediate post-treatment computed tomography, may show concentric bowel wall thickening with mucosal disruption, which significantly correlates with the risk of major GIT injury requiring surgery[28]. However, immediate imaging is not routinely performed in all centres, as it is not always possible to make a distinction between transient hyperaemia and the residual unablated tumour, which hampers the evaluation of treatment efficacy[41]. Nevertheless, in selected cases, when dealing with tumours with a high-risk location in high-risk patients, close imaging parallel to clinical follow-up could be advised.
Few studies focused on the detailed course of these complications and their management, although the impact on patient morbidity and quality of life is non-negligible. Whenever possible, patients were treated minimally-invasively with fasting and percutaneous drain placement, but most required surgical intervention to repair the injured GIT[39]. To the best of our knowledge, the case reported here is the first case of gastric perforation after thermal ablation therapies to be managed endoscopically with an OTSC.
OTSCs differ from traditional through-the-scope clips in several characteristics, namely higher compression force, larger diameter and grasping accessories that allow the closure of wall defects of up to 30 mm, including the muscle layer[42-44]. They have been successfully used and validated in literature in different settings, i.e., haemostasis in acute GI bleeding, closure of GI perforations, leaks and fistulas or as anchor to prevent stent migration[45]. While clinical success is nearly 100% for haemostasis, it decreases to 40%-75% for GI defect closure, with best outcomes in acute perforations, which have fresh edges with less fibrosis[44,46,47]. Nevertheless, their use has been increasingly reported in postoperative leakages or fistulas as well, owing to the attractive possibility of avoiding complex surgery[48-50]. Clinicians must take into account that a multidisciplinary approach should always be taken, since surgical or radiological placement of a drain is often advisable to prevent abscess formation after defect closure.
An interesting issue is whether preventive measures may reduce the incidence of GIT injuries caused by thermal ablation therapies. These lesions are mainly associated with the percutaneous route and surgical management showed the presence of fibrotic adhesions that affixed the GI wall to the liver. Thus, a laparoscopic or intraoperative thermal ablation approach may allow the mechanical separation of the GIT from the surface of the liver and protect it from subsequent thermal damage[32,51]. Nevertheless, the rate of other complications of these invasive routes is higher than for the percutaneous route[21].
One well-established, easy and safe procedure is the use of artificial ascites. This technique allows the displacement of the liver, with its considered high-risk ablation area, from the adjacent organs[52]. Authors who routinely perform this procedure reported a lower incidence of GIT complications despite dealing with high-risk tumours[53,54]. Nevertheless, the presence of perihepatic adhesions due to previous abdominal surgery, other locoregional treatments such as transarterial chemoembolization or intra-abdominal inflammation, represent a limit for technical success. Moreover, this technique may be of limited efficacy in tumours located in left liver, since the nearby stomach is not easy to displace[55]. Two other tips to help minimise adjacent organ injury are the interposition of thermocouples that ensure controlled temperature increase[30] and the use of straight needle electrodes, which are more appropriate for monitoring the distance from GIT than expandable devices[56].
This is the first reported case of RFA-related GIT injury endoscopically treated with OTSC. OTSCs have become part of the endoscopist’s armamentarium and are now widely used to treat GI defects. This application may help reduce the need for complex surgery for this rare yet severe complication.
Our review raises awareness on an overlooked but severe complication of thermal ablative treatments. Nowadays, following the expansion of ablation criteria for HCC, these therapeutic modalities are gaining wider application. Therefore, clinicians must consider possible complications and accurately weigh up the risks and benefits, choosing the best treatment option not only according to fixed algorithms for HCC, but also tailored to the specific patient.
Since GIT injuries are rare but have an unfavourable prognosis and outcome, careful patient evaluation may help detect the tumour-related (location < 1 cm from the GI tract) or patient-related (previous surgery or locoregional treatments) risk factors that may trigger this event. These features do not represent an absolute contraindication to thermal ablation therapies, since these treatments remain safe and have an acceptable complication rate. However, according to local expertise, a non-percutaneous (laparoscopic or intraoperative) route should be chosen or preventive measures such as artificial ascites should be used. Otherwise, other ablative methods for HCC should be preferred.
In the case of GIT injury occurrence, the initial subtle clinical presentation warrants a low threshold for GI imaging. Finally, prompt identification of the injury is mandatory to avoid diagnostic delay and provide timely management.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun JH, Tolga E S-Editor: Yan JP L-Editor: A P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55742] [Article Influence: 7963.1] [Reference Citation Analysis (132)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3591] [Article Influence: 276.2] [Reference Citation Analysis (4)] |
| 3. | Gadsden MM, Kaplan DE. Multidisciplinary Approach to HCC Management: How Can This Be Done? Dig Dis Sci. 2019;64:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 5. | Fan SY, Eiser C, Ho MC. Health-related quality of life in patients with hepatocellular carcinoma: a systematic review. Clin Gastroenterol Hepatol. 2010;8:559-64.e1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Das A, Gabr A, O'Brian DP, Riaz A, Desai K, Thornburg B, Kallini JR, Mouli S, Lewandowski RJ, Salem R. Contemporary Systematic Review of Health-Related Quality of Life Outcomes in Locoregional Therapies for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2019;30:1924-1933.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (4)] |
| 8. | Habib A, Desai K, Hickey R, Thornburg B, Lewandowski R, Salem R. Locoregional therapy of hepatocellular carcinoma. Clin Liver Dis. 2015;19:401-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20:4141-4150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Faria SC, Szklaruk J, Kaseb AO, Hassabo HM, Elsayes KM. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: concepts, perspectives, and radiologic implications. Abdom Imaging. 2014;39:1070-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6025] [Article Influence: 860.7] [Reference Citation Analysis (3)] |
| 12. | Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 14. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 15. | Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther. 2016;7:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (2)] |
| 17. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, Solé M, Rodés J, Bruix J; Barcelona Clínic Liver Cancer (BCLC) Group. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 18. | Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005;92:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, Du Z. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7:2578-2589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 21. | Kong WT, Zhang WW, Qiu YD, Zhou T, Qiu JL, Zhang W, Ding YT. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651-2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Kasugai H, Osaki Y, Oka H, Kudo M, Seki T; Osaka Liver Cancer Study Group. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology. 2007;72 Suppl 1:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 24. | Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Baker EH, Thompson K, McKillop IH, Cochran A, Kirks R, Vrochides D, Martinie JB, Swan RZ, Iannitti DA. Operative microwave ablation for hepatocellular carcinoma: a single center retrospective review of 219 patients. J Gastrointest Oncol. 2017;8:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Maida M, Cabibbo G, Brancatelli G, Genco C, Alessi N, Genova C, Romano P, Raineri M, Giarratano A, Midiri M, Cammà C. Assessment of treatment response in hepatocellular carcinoma: a review of the literature. Future Oncol. 2013;9:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan J, Phillips DA, Rhim H, Silverman SG, Solbiati L, Vogl TJ, Wood BJ, Vedantham S, Sacks D; Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377-S390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Jeong YS, Kim SH, Lee JM, Lee JY, Kim JH, Lee DH, Kang HJ, Yoon CJ, Han JK. Gastrointestinal tract complications after hepatic radiofrequency ablation: CT prediction for major complications. Abdom Radiol (NY). 2018;43:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 932] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 30. | Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 31. | de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Casaril A, Abu Hilal M, Harb A, Campagnaro T, Mansueto G, Nicoli N. The safety of radiofrequency thermal ablation in the treatment of liver malignancies. Eur J Surg Oncol. 2008;34:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol. 2008;23:e445-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Meloni MF, Goldberg SN, Moser V, Piazza G, Livraghi T. Colonic perforation and abscess following radiofrequency ablation treatment of hepatoma. Eur J Ultrasound. 2002;15:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Falco A, Orlando D, Sciarra R, Sergiacomo L. A case of biliary gastric fistula following percutaneous radiofrequency thermal ablation of hepatocellular carcinoma. World J Gastroenterol. 2007;13:804-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Frich L, Edwin B, Brabrand K, Rosseland AR, Mala T, Mathisen O, Gladhaug I. Gastric perforation after percutaneous radiofrequency ablation of a colorectal liver metastasis in a patient with adhesions in the peritoneal cavity. AJR Am J Roentgenol. 2005;184:S120-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Yamane T, Imai K, Umezaki N, Yamao T, Kaida T, Nakagawa S, Yamashita YI, Chikamoto A, Ishiko T, Baba H. Perforation of the esophagus due to thermal injury after laparoscopic radiofrequency ablation for hepatocellular carcinoma: a case for caution. Surg Case Rep. 2018;4:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Kwon HJ, Kim PN, Byun JH, Kim KW, Won HJ, Shin YM, Lee MG. Various complications of percutaneous radiofrequency ablation for hepatic tumors: radiologic findings and technical tips. Acta Radiol. 2014;55:1082-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, Teratani T, Shiina S, Ohtomo K. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25 Suppl 1:S57-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Kim YS, Rhim H, Lim HK. Imaging after radiofrequency ablation of hepatic tumors. Semin Ultrasound CT MR. 2009;30:49-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc. 2012;76:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 43. | Manta R, Galloro G, Mangiavillano B, Conigliaro R, Pasquale L, Arezzo A, Masci E, Bassotti G, Frazzoni M. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endosc. 2013;27:3162-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Lee HL, Cho JY, Cho JH, Park JJ, Kim CG, Kim SH, Han JH. Efficacy of the Over-the-Scope Clip System for Treatment of Gastrointestinal Fistulas, Leaks, and Perforations: A Korean Multi-Center Study. Clin Endosc. 2018;51:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Donatelli G, Cereatti F, Dhumane P, Vergeau BM, Tuszynski T, Marie C, Dumont JL, Meduri B. Closure of gastrointestinal defects with Ovesco clip: long-term results and clinical implications. Therap Adv Gastroenterol. 2016;9:713-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Goenka MK, Rai VK, Goenka U, Tiwary IK. Endoscopic Management of Gastrointestinal Leaks and Bleeding with the Over-the-Scope Clip: A Prospective Study. Clin Endosc. 2017;50:58-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Verlaan T, Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc. 2015;82:618-28.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Kim JS, Kim BW, Kim JI, Kim JH, Kim SW, Ji JS, Lee BI, Choi H. Endoscopic clip closure versus surgery for the treatment of iatrogenic colon perforations developed during diagnostic colonoscopy: a review of 115,285 patients. Surg Endosc. 2013;27:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Haito-Chavez Y, Law JK, Kratt T, Arezzo A, Verra M, Morino M, Sharaiha RZ, Poley JW, Kahaleh M, Thompson CC, Ryan MB, Choksi N, Elmunzer BJ, Gosain S, Goldberg EM, Modayil RJ, Stavropoulos SN, Schembre DB, DiMaio CJ, Chandrasekhara V, Hasan MK, Varadarajulu S, Hawes R, Gomez V, Woodward TA, Rubel-Cohen S, Fluxa F, Vleggaar FP, Akshintala VS, Raju GS, Khashab MA. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc. 2014;80:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 50. | Morrell DJ, Winder JS, Johri A, Docimo S, Juza RM, Witte SR, Alli VV, Pauli EM. Over-the-scope clip management of non-acute, full-thickness gastrointestinal defects. Surg Endosc. 2020;34:2690-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Swan RZ, Sindram D, Martinie JB, Iannitti DA. Operative microwave ablation for hepatocellular carcinoma: complications, recurrence, and long-term outcomes. J Gastrointest Surg. 2013;17:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, Shibata H, Shimoe T, Matsuda T, Toshikuni N, Fujioka S, Ohmoto K, Nakamura S, Kariyama K, Aikata H, Kobayashi Y, Tsutsui A. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Park JG, Park SY, Tak WY, Kweon YO, Jang SY, Lee YR, Hur K, Lee HJ, Lee HW. Early complications after percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1,843 ablations in 1,211 patients in a single centre: experience over 10 years. Clin Radiol. 2017;72:692.e9-692.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006;93:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS, Kim YH, Park CM, Kim PN, Byun JY. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123-34; discussion 134-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 57. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, Loyer E, Vallone P, Fiore F, Scordino F, De Rosa V, Orlando R, Pignata S, Daniele B, Izzo F. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 58. | Jansen MC, van Duijnhoven FH, van Hillegersberg R, Rijken A, van Coevorden F, van der Sijp J, Prevoo W, van Gulik TM. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg. 2005;92:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Maeda M, Saeki I, Sakaida I, Aikata H, Araki Y, Ogawa C, Kariyama K, Nouso K, Kitamoto M, Kobashi H, Sato S, Shibata H, Joko K, Takaki S, Takabatake H, Tsutsui A, Takaguchi K, Tomonari T, Nakamura S, Nagahara T, Hiraoka A, Matono T, Koda M, Mandai M, Mannami T, Mitsuda A, Moriya T, Yabushita K, Tani J, Yagi T, Yamasaki T. Complications after Radiofrequency Ablation for Hepatocellular Carcinoma: A Multicenter Study Involving 9,411 Japanese Patients. Liver Cancer. 2020;9:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |