Published online Sep 14, 2020. doi: 10.3748/wjg.v26.i34.5074
Peer-review started: May 28, 2020
First decision: June 4, 2020
Revised: June 14, 2020
Accepted: August 22, 2020
Article in press: August 22, 2020
Published online: September 14, 2020
Processing time: 104 Days and 19.5 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. At the molecular level, GISTs can be categorized into two groups based on the causative oncogenic mutations. Approximately 85% of GISTs are caused by gain-of-function mutations in the tyrosine kinase receptor KIT or platelet-derived growth factor receptor alpha (PDGFRA). The remaining GISTs, referred to as wild-type (WT) GISTs, are often deficient in succinate dehydrogenase complex (SDH), a key metabolic enzyme complex in the tricarboxylic acid (TCA) cycle and electron transport chain. SDH deficiency leads to the accumulation of succinate, a metabolite produced by the TCA cycle. Succinate inhibits α-ketoglutarate-dependent dioxygenase family enzymes, which comprise approximately 60 members and regulate key aspects of tumorigenesis such as DNA and histone demethylation, hypoxia responses, and m6A mRNA modification. For this reason, succinate and metabolites with similar structures, such as D-2-hydroxyglutarate and fumarate, are considered oncometabolites. In this article, we review recent advances in the understanding of how metabolic enzyme mutations and oncometabolites drive human cancer with an emphasis on SDH mutations and succinate in WT GISTs.
Core Tip: The connection between altered cellular metabolism and tumorigenesis has gained increased attention in recent years. Deficiency in succinate dehydrogenase (SDH), a key metabolic enzyme, drives tumorigenesis of a subset of gastrointestinal stromal tumors (GISTs) by accumulating the oncometabolite succinate. Oncometabolites such as succinate, D-2-hydroxyglutarate, and fumarate competitively inhibit the α-ketoglutarate-dependent dioxygenase family enzymes, which regulate epigenetic status, hypoxia responses, RNA metabolism, and DNA damage repair. In this article, we review the recent advances in understanding how metabolic enzyme mutations and oncometabolites drive human cancer with an emphasis on the SDH deficiency and succinate in GISTs.
- Citation: Zhao Y, Feng F, Guo QH, Wang YP, Zhao R. Role of succinate dehydrogenase deficiency and oncometabolites in gastrointestinal stromal tumors. World J Gastroenterol 2020; 26(34): 5074-5089
- URL: https://www.wjgnet.com/1007-9327/full/v26/i34/5074.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i34.5074
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract. At the molecular level, GISTs can be categorized into two groups based on the causative oncogenic mutations. The major group, which accounts for approximately 85% of total GISTs and mainly occurs in adults, is caused by gain-of-function mutations in the receptor tyrosine kinase (RTK) KIT or platelet-derived growth factor receptor alpha (PDGFRA)[1,2]. The remaining 15% of GISTs, known as wild-type (WT) GISTs, do not contain mutations in RTKs[1-3]. Consequently, tyrosine kinase inhibitors such as imatinib, which have been effective in treating GISTs with RTK mutations[4,5], show little benefit in patients with WT GISTs[6-8]. Surgery remains the primary and recommended treatment of nonmetastatic WT GISTs[9,10].
In 2011, Janeway et al[11] reported that deficiency of the succinate dehydrogenase complex (SDH), either caused by loss-of-function mutations or reduced expression of genes encoding SDH, was associated with most WT GISTs. This discovery was soon confirmed by multiple independent studies[12-19]. Similar to those of other gastric tumors, the clinical manifestations of SDH-deficient GISTs include bleeding and discomfort in the GI tract[20]. In a molecular genetic study that analyzed more than 1100 patient samples, the patients with SDH-deficient GISTs were most frequently children and young adults (approximately 85%-90% of patients) and were more frequently in female patients with a predilection ratio over 2:1[14]. Furthermore, SDH-deficient GISTs occur primarily in the stomach, particularly in the distal stomach and antrum[14].
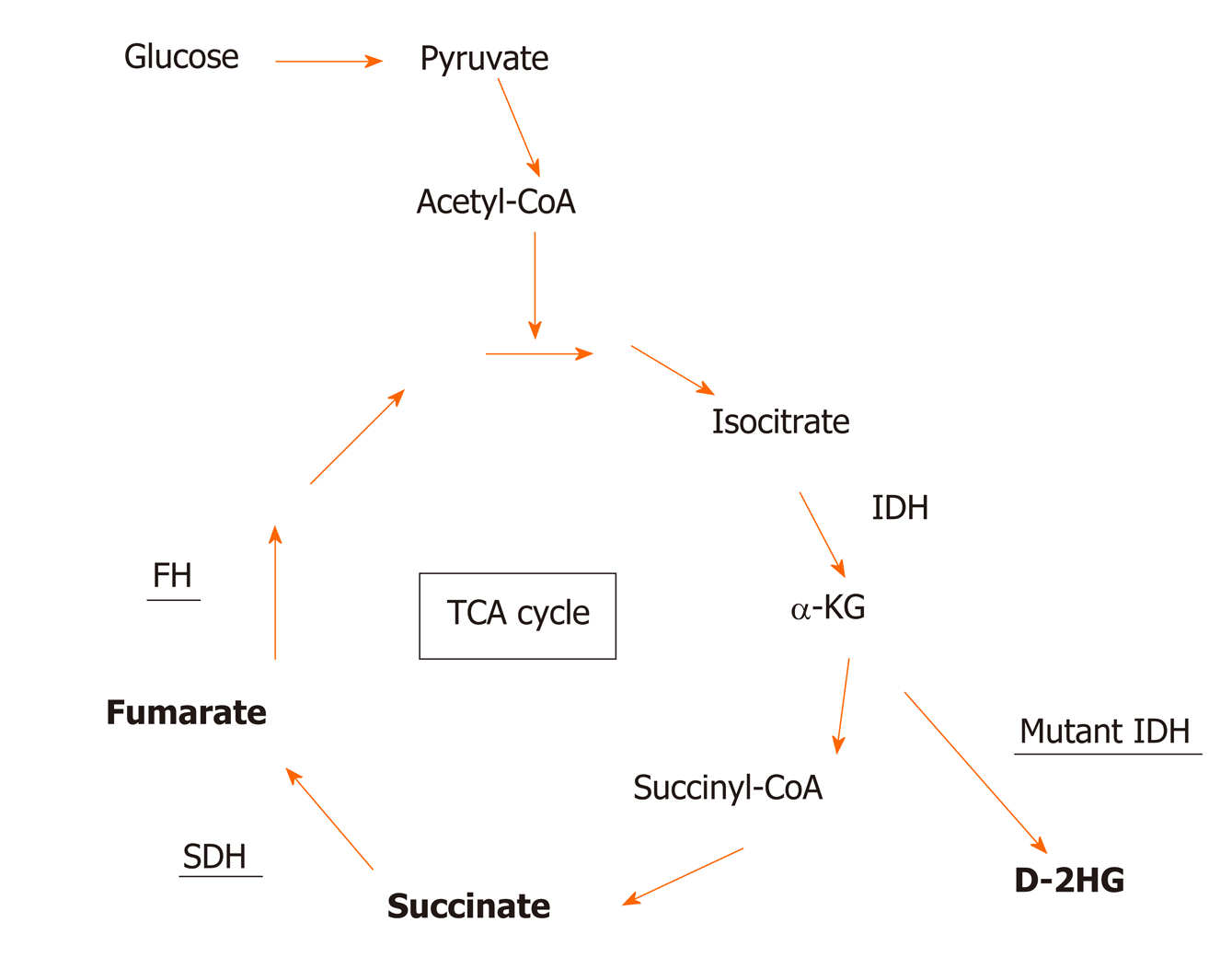
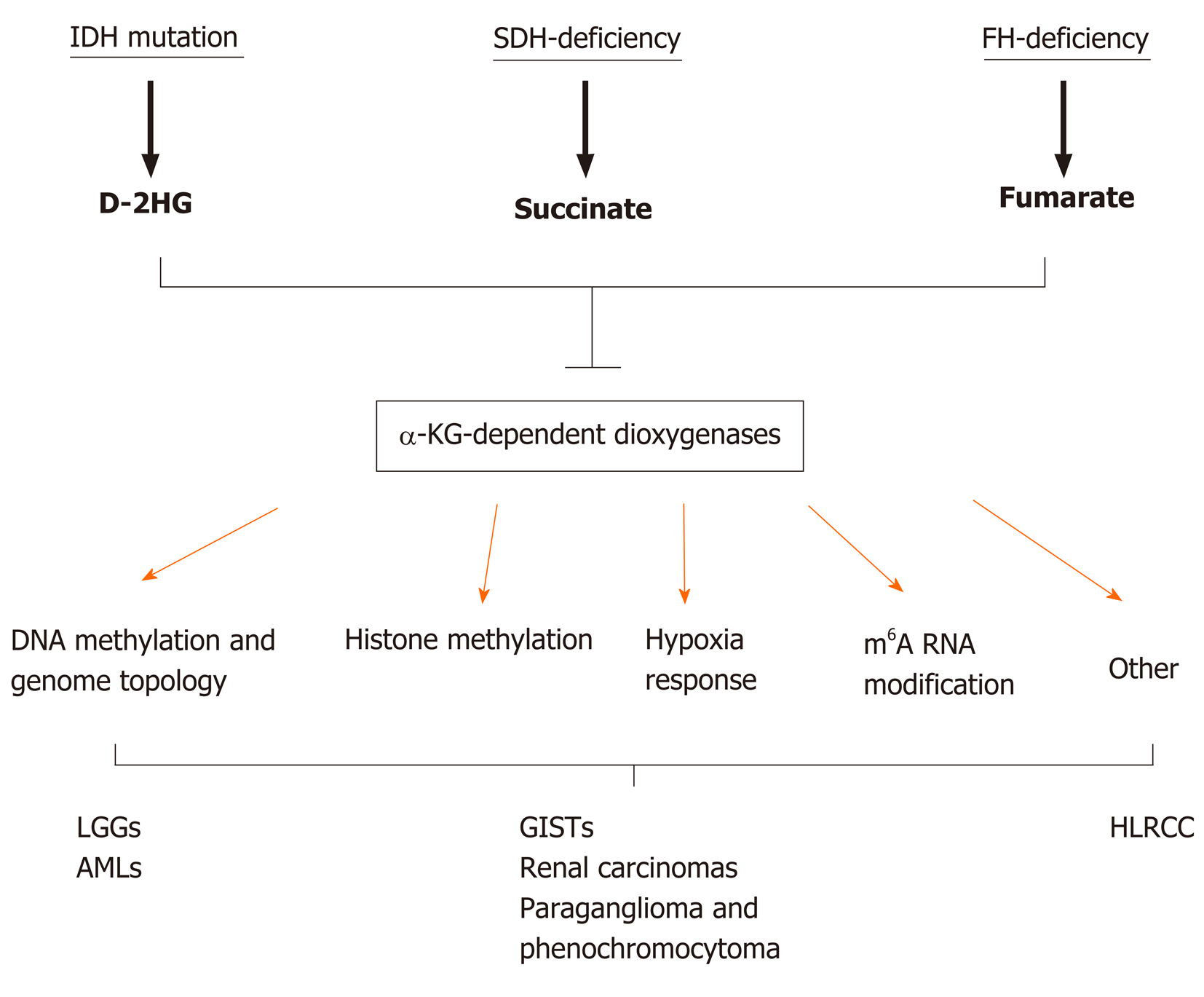
SDH, also known as succinate-coenzyme Q reductase or mitochondrial complex II, is a key metabolic enzyme complex in the tricarboxylic acid (TCA) cycle and electron transport chain. SDH oxidizes succinate to fumarate, which is a key step of the TCA cycle that regulates carbon metabolism and energy production in the cells (Figure 1). SDH deficiency changes cellular metabolism, such as the demand for extracellular pyruvate and the biosynthesis of aspartate[21,22]. Metabolic reprogramming in cancer cells has long been noticed but is primarily considered the consequence of tumorigenesis[23-27]. Emerging evidence, however, has demonstrated that metabolic changes caused by mutations in metabolic enzymes, such as SDH mutations in GISTs, serve as the driver of human cancer. Additional oncogenic mutations in metabolic enzymes include isocitrate dehydrogenase (IDH1 or IDH2) mutations in low-grade gliomas (LGGs) and acute myeloid leukemia (AML) and fumarate hydratase (FH) mutations in hereditary leiomyomatosis and renal cell cancer[28-35]. How these metabolic enzyme mutations drive tumorigenesis is only partially understood. It has been proposed that these mutations can distort mitochondria-mediated apoptosis and activate the hypoxia response pathway. In recent years, emerging evidence indicates that these mutations accumulate metabolites affecting the α-ketoglutarate (α-KG)-dependent dioxygenase family enzymes, which regulate key aspects of tumorigenesis, such as epigenetic modification, hypoxia responses, and RNA methylation (Figure 2). In this article, we review recent advances in understanding how metabolic enzyme mutations and oncometabolites drive human cancer with an emphasis on SDH deficiency and succinate in WT GISTs.
SDH is a key component of both the TCA cycle and the electron transport chain (ETC). Localized in the inner membrane of mitochondria, the SDH holoenzyme consists of four subunits, SDHA, SDHB, SDHC, and SDHD, and two assembly factors, SDHF1 and SDHF2[36]. Among the four subunits, SDHA catalyzes succinate to fumarate in the TCA cycle. SDHB is involved in the oxidation of ubiquinone to ubiquinol in the ETC, while SDHC and SDHD are mainly responsible for anchoring the SDH protein complex to mitochondria. Loss-of-function mutation in any of the four subunits destabilizes the SDH protein complex and eliminates the entire SDH enzymatic activity. Mutations in all SDH subunits have been identified in GISTs as well as several other human cancers such as rental carcinoma, leukemia, and familial paraganglioma and pheochromocytoma[37-42]. Among the SDH subunits, mutations in SDHA are most frequent, accounting for approximately 30% of total SDH-deficient GISTs[12-19,43]. Notably, approximately 50% of SDH-deficient GISTs are not caused by genetic mutations in any of the SDH subunits. Instead, SDH deficiency in these GISTs results from a lack of expression of the SDH enzyme complex, presumably by mutations elsewhere that affect the expression or turnover of the SDH subunits[15,20].
The loss of SDH enzymatic activity by a loss-of-function mutation or a lack of gene expression leads to the accumulation of succinate[44,45], a metabolite produced from the TCA cycle (Figure 1). Under normal conditions, SDH rapidly converts succinate into fumarate by passing two protons to ubiquinone to initiate the ETC, which is the major process to generate the energy-carrying molecule adenosine triphosphate (ATP). This process is disrupted in SDH-deficient cells. The blockage of succinate conversion to fumarate leads to consequences beyond simply affecting the efficiency of the TCA cycle and the ETC. To adapt to the disruption of the TCA cycle, cells must rewire cellular metabolism by initiating compensation pathways. For example, SDH-deficient cells increase activities in glycolysis, lactate production, and pentose phosphate pathways[46]. More importantly, succinate also functions as a competitive inhibitor of α-KG, which is not only a metabolite in the TCA cycle for energy metabolism but also a co-factor required by the α-KG-dependent dioxygenases. α-KG-dependent dioxygenases catalyze hydroxylation reactions on biomolecule substrates, including DNA, RNA, protein, and lipids[47,48]. Members of the α-KG-dependent dioxygenase family include DNA hydroxylases, histone demethylases, RNA demethylases, and prolyl hydroxylases, which regulate cellular processes such as the demethylation of DNA, histone and nonhistone proteins, and RNA molecules and the responses to hypoxic conditions (Figure 2)[49,50]. Dysregulation of these processes has been considered the driving force of human cancers[51,52]. Because of this tumor-promoting role, succinate together with D-2-hydroxyglutarate (D-2HG) and fumarate, which are produced by IDH and FH mutations, respectively, are dubbed oncometabolites[53].
Mutations in key metabolic enzymes invariably alter the composition and concentration of metabolites in cells. Generally, there are two nonexclusive ways that metabolites can epigenetically reprogram the affected cells. First, changes in the abundance of metabolites such as acetyl-CoA and S-adenosyl methionine (SAM), which are substrates for key biochemical reactions such as acetylation and methylation, can affect the epigenetic status of the entire genome. Second, the accumulation of oncometabolites can affect the activities of α-KG-dependent dioxygenases, which are involved in the regulation of specific epigenetic modifications and related biological pathways.
Acetylation and methylation of histone proteins and methylation of genomic DNA are the major modifications that shape the epigenetic landscape of cells. Acetylation involves covalently linking an acetyl group to the Nε amino group of lysine residues of histone and nonhistone proteins. By adding an acetyl group, acetylation neutralizes the positively charged lysine residue, which often causes changes in the enzymatic activity, localization, stability, and interaction profiles of the modified proteins[54-56]. More than 8000 unique acetylation sites have been identified in histone and nonhistone proteins in mammalian cells[57-59]. Histone acetylation, catalyzed by histone acetyltransferases, is associated with genomic regions with active transcription[54,60,61]. As acetyl-CoA is the sole acetyl group donor for protein acetylation, its abundance is one of the key determinants of acetylation reactions. There are three major pathways to produce acetyl-CoA: Pyruvate oxidation, β-oxidation of fatty acids, and ATP-citrate lyase-mediated production from citrate[62]. As a result, cells with active acetyl-CoA production are often associated with increased levels of histone acetylation and gene transcription[63].
Methylation can occur on both protein and genomic DNA, and both forms of modification utilize SAM as the primary donor of the methyl group[64]. Histone methylation involves the covalent addition of one to three methyl groups onto lysine or arginine residues of N-terminal tails by protein methyltransferases, while DNA methylation involves covalent modification of cytosine (C) residues by DNA methyltransferases (DNMTs). In contrast to histone acetylation, which is always associated with active transcription, histone methylation could impact transcription positively or negatively, depending on the specific lysine residues that are modified. For example, histone H3K9 methylation is associated with heterochromatin structures with little transcriptional activity, while histone H3K4 methylation often indicates actively transcribed promoters or enhancers[65]. DNA methylation, particularly within the promoter regions, is associated with transcriptional silencing. Biosynthesis of SAM involves the conjugation of an ATP molecule to homocysteine by methionine adenosyltransferases. The cellular abundance of SAM, presumably via modulating DNA and histone methylation modifications, has been suggested to regulate the proliferation and viability of liver cancer cells[66-68].
Metabolites can regulate epigenetic modifying enzymes in two mechanisms. First, epigenetic modifying enzymes can be directly methylated or acetylated, which leads to changes in protein conformation, localization, and enzymatic activities. For example, p300, a histone acetyltransferase implicated in several human cancers, including gastric carcinomas[69-72], activates its catalytic activity by autoacetylation[73].
In the second mechanism, metabolites serve as cofactors or antagonists of epigenetic modifying enzymes. The best-known example is α-KG and the related oncometabolites (i.e., succinate, D-2HG, and fumarate) in the regulation of the α-KG-dependent dioxygenase family[74]. Mutations in metabolic enzymes such as SDH, IDH, and FH lead to excessive accumulation of succinate, D-2HG, and fumarate, respectively, all of which share structural similarity with α-KG and competitively inhibit α-KG-dependent dioxygenases (Figure 2)[75-79]. Such inhibition affects epigenetic processes, including DNA and histone demethylation, responses to hypoxia, and other biological processes, such as RNA demethylation and DNA damage repair.
Oncometabolites that regulate DNA methylation and genome topology: D-2HG produced by IDH mutations in LGGs and AMLs is the best-studied oncometabolite. Long before the discovery of IDH mutations as the genetic drivers of LGGs, the genome of a subset of brain tumors was noted to be hypermethylated, referred to as the glioma CpG island methylator phenotype[80]. Subsequent genetic studies associated this group of gliomas with brain tumors harboring neomorphic mutations in the IDH1 or IDH2 genes[33,81-83]. Under normal conditions, IDH1 and IDH2 convert isocitrate into α-KG (Figure 1). In LGG cells, neomorphic IDH mutations acquire the novel function to further oxidize α-KG into D-2HG, which competitively inhibits all α-KG dependent dioxygenases including the TET-family DNA methylcytosine hydroxylases (i.e., TET1, TET2, and TET3)[75,84]. The methylation status of the genome is determined by the dynamic balance between DNA methylation by DNMTs and demethylation by TETs. The TET enzymes convert 5-methylcytosine to 5-hydroxymethylcytosine[85], which is the first step in the active DNA demethylation pathway[86]. Gain-of-function mutations in DNMTs and loss-of-function mutations in the TET family enzymes lead to genome hypermethylation and have been identified in malignancies such as leukemia, lymphoma, melanoma, and colorectal cancer[87]. Therefore, the hypermethylation phenotype likely contributes to the tumorigenicity of LGGs. SDH-deficient GISTs and FH-deficient tumors similarly exhibit a genome-wide hypermethylated phenotype because of inhibition of the TET family enzymes[11,78,88,89]. Methylation on the promoter of the O6-methylguanine DNA methyltransferase (MGMT) gene has been a biomarker for the efficacy of alkylating agents in LGGs, colorectal carcinomas, and several other cancers[90-92]. Recently, methylation of the MGMT promoter has been detected in approximately two-thirds of SDH-deficient GISTs, which is significantly more frequent than the SDH-proficient counterparts[93]. However, whether the MGMT promoter methylation status can similarly predict the response of SDH-deficient GISTs to alkylating agents remains to be investigated.
DNA methylation usually decreases transcription activity by reducing the binding of methylation-sensitive transcription factors and/or by recruiting additional chromatin modification enzymes to create a nonpermissive chromatin environment[94]. The altered transcriptional profile may change cellular physiology that favors tumorigenesis. For example, SDH-deficient tumors with DNA hypermethylation are more invasive than SDH-WT tumors, which is at least partially due to transcriptional dysregulation of genes associated with epithelial-to-mesenchymal transition[88,95].
Very recently, DNA methylation has been demonstrated to regulate transcription by altering genomic topology. This primarily works through CTCF, a DNA-binding insulator protein that only binds to unmethylated DNA recognition motifs[96,97]. CTCF forms protein complexes with cohesins that define chromatin boundaries and establish topologically associated domains[98-100]. One of the key functions of insulators is to prevent promiscuous interactions between enhancer elements and promoters of oncogenes[101]. In IDH-mutant LGG cells, CTCF fails to bind a fraction of putative binding sites, which leads to altered chromatin topology and aberrant expression of associated genes[102]. Gain-of-function mutations in PDGFRA are among the most common driver mutations of glioblastomas (GBMs) but are rare in LGGs[103-105]. CTCF failing to bind to recognition motifs adjacent to the PDGFRA promoter in IDH-mutant LGG cells allows interaction between the PDGFRA promoter and a nearby enhancer element, which leads to increased expression of PDGFRA in LGG tumor cells[102]. Interestingly, treating LGG cells with 5-azacytidine, an FDA-approved DNA-demethylating agent[106], not only partially restores CTCF binding but also reduces PDGFRA expression, suggesting that modulating DNA methylation status and PDGFRA expression could be a potential strategy to treat IDH-mutant LGGs[102].
Similarly, DNA hypermethylation in SDH-deficient GIST cells leads to a loss of over a thousand potential CTCF binding sites[107]. Importantly, the expression of genes adjacent to the affected CTCF binding sites changes significantly, indicating that SDH-deficient cells similarly acquire promiscuous enhancer-promoter interactions and an altered genome topology. Expression of KIT, one the most frequently mutated RTKs in most GISTs but not WT-GISTs[1], is significantly upregulated by the altered CTCF binding profile[107]. This discovery raised the interesting possibility that activation of KIT and downstream signaling pathways are an underlying cause of both RTK-mutant and WT GISTs.
In addition to affecting KIT, disruption of CTCF binding increased the expression of FGF3 and FGF4, both of which are known to drive human cancer[42]. FGF3 and FGF4 are ligands activating the tyrosine kinase receptor FGFR, which plays a known role in the pathogenesis of GISTs[108,109]. Expression analysis revealed that KIT and FGFR are the most abundantly expressed RTKs in SDH-deficient GISTs. CTCF binding to the recognition motifs adjacent to FGF3 and FGF4 genes is disrupted in SDH-deficient GISTs, which leads to an increase in FGF3 and FGF4 expression by several magnitudes[110]. The upregulation of FGF3 and FGF4 likely plays a redundant role with the gain-of-function mutation of KIT because both activate the MAPK pathway[108,109]. KIT inhibition by imatinib showed no efficacy in treating WT GISTs[6-8]. Interestingly, combinatorial treatment with the FDA-approved KIT inhibitor sunitinib and the FGFR inhibitor BGJ-398 effectively reduced SDH-deficient GISTs in a patient-derived xenograft (PDX) mouse model[107]. More intriguingly, while treatment with sunitinib alone had only a marginal effect, which is consistent with the previous observation that WT-GISTs are refractory to KIT inhibitors[7], treatment with BGJ-398 could completely suppress tumor growth in this model[107].
Oncometabolites regulate histone methylation: Jumonji domain-containing lysine demethylases (KDMs) belong to the α-KG-dependent dioxygenase family and can be inhibited by succinate, fumarate, and D-2HG[77-79]. Loss-of-function or reduced expression of Jumonji domain-containing KDMs has been correlated with several human cancers, such as renal cell carcinoma, GBM, and multiple myeloma[111-113]. These KDMs remove the methyl group on lysine in histone tails, which can either activate or repress transcription depending on the specifically modified lysine residues. For example, these oncometabolites inhibit the activities of both KDM4A and KDM7A, which remove methylation on histone H3K36 and H3K9, respectively[77,78]. Methylation of histone H3K36 often occurs in the gene body region and is known to antagonize silencing and safeguard transcription fidelity[114], while methylation of H3K9 has been associated with transcriptional silencing and heterochromatin formation[94]. Therefore, the impact of oncometabolite accumulation on histone tail modification is complex. Nonetheless, dysregulation of histone methylation has been associated with poor prognosis of human malignancies such as prostate cancer, lung cancer, breast cancer, colon adenocarcinoma, and gastric adenocarcinoma[65,115-117]. Experimental inactivation of SDH and FH causes increased H3K9 methylation in paraganglioma, phe-ochromocytoma and smooth muscle tumors[118]. In LGGs, IDH mutations suppress the histone H3K9 demethylase KDM4C[119]. LGG cells exhibit increased H3K9 methylation and downregulation of the glial differentiation transcriptional program, suggesting that IDH mutation-induced D-2HG accumulation leads to a differentiation blockage of LGG cells[119,120]. However, the specific target genes affected by oncometabolites that are critical in tumorigenesis remain largely unknown. Recently, inhibition of H3K9 demethylases in IDH mutation-bearing LGGs and AMLs was found to reduce the expression of the ataxia telangiectasia mutated (ATM) gene[121], which encodes a key regulator involved in the DNA damage response (DDR) pathway. Consequently, AML cells with IDH mutations are defective in DDR responses and accumulate unrepaired DNA damage[121]. SDH-deficient hereditary paraganglioma and pheochromocytoma are also known to have reduced capacities in the DDR pathway[122]. However, whether SDH deficiency similarly leads to reduced expression of ATM and whether an impaired DDR contributes to the tumorigenesis of GISTs are still unknown, and these questions represent directions for future investigation.
Activation of the hypoxia response pathway by overexpression of hypoxia-inducible factor 1α (HIF1α) plays an important role in the progression of SDH-deficient tumors[11,45,123,124]. Under normoxic conditions, HIF1α turns over rapidly by proteasomal degradation that is dependent on the ubiquitin E3 ligase VHL[125]. The VHL recognition of HIF1α requires hydroxylation on proline residues on HIF1α, which is mediated by prolyl hydroxylases (PHDs)[125]. Under hypoxic conditions, HIF1α degradation is blocked because the enzymatic activities of PHDs are suppressed by low levels of oxygen. HIF1α then translocates into the nucleus, forms the HIF1 heterodimer complex with HIF1β, and activates the hypoxia response pathway[125]. Transcriptional targets of HIF1 control several aspects of tumor biology, including angiogenesis, proliferation and survival, glucose metabolism, invasion, and metastasis[124-130]. Because PHDs belong to the α-KG-dependent dioxygenase family, oncometabolites can effectively activate the hypoxia response pathway by competitively inhibiting PHDs under normoxic conditions. Forced expression of LGG-inducing IDH mutations elevated levels of HIF1α and its transcriptional targets in cells, which is consistent with the observation that the HIF1α pathway is more active in IDH-mutant glioma patient samples[131]. Similarly, HIF1α and its transcriptional targets, such as VEGF and IGF, are more abundantly expressed in SDH-deficient GISTs than in RTK-mutant GISTs[45,132,133]. These observations suggest that the hypoxia response pathway is more active in SDH-deficient GISTs than in GISTs with normal SDH activity, which is likely caused by succinate-mediated PHD inhibition.
RNA methylation, particularly the N6-methyladenine (m6A) modification in mRNA, has emerged as an important regulatory mechanism of RNA metabolism in recent years[134]. Genome-wide profiling studies have demonstrated that mRNA produced by approximately 15000 human genes has m6A modifications, which affect the splicing, stability, and translation efficiency of the modified mRNA molecules[135,136]. The m6A modification is deposited on mRNA molecules by the RNA methyltransferase writer enzyme complex METTL3-METTL14, removed by the eraser enzymes FTO and ALKBH5, and decoded by reader proteins such as YTHDF1 and YTHDF2[134]. Dysregulation of m6A modification has been suggested to drive human cancers[137]. Among the genes involved in m6A modification are the eraser enzymes FTO and ALKBH5, which belong to the α-KG-dependent dioxygenase family[138]. It has been demonstrated that the enzymatic activity of FTO is inhibited in IDH mutation-bearing AML cells, which correlates with a significant increase in m6A levels[139]. The inhibition of FTO in AML cells seems to have a positive effect on all-trans retinoic acid (ATRA)-induced leukemic cell differentiation[139]. Given the similar inhibitory roles of IDH mutations and SDH deficiency, it is highly expected that SDH-deficient GISTs will have similarly elevated m6A levels. However, how such changes may contribute to tumorigenesis and the treatment of GISTs is awaiting future investigation.
The alkB homolog (ALKBH) family enzymes are α-KG-dependent dioxygenases involved in repairing DNA damage caused by alkylating agents[140,141]. In IDH-mutant LGG cells, the activities of ALKBH enzymes are suppressed, which makes these cells sensitive to alkylation damage[142]. Consistently, combinatorial treatment of the DNA alkylating agents procarbazine and lomustine with the microtubule polymerization inhibitor vincristine is beneficial in some glioma patients[143,144]. In contrast, experimental restoration of the enzymatic activities by forced expression of ALKBH family members in IDH-mutant cells blunts the responses to alkylating agents[142]. However, the activities of ALKBH enzymes in SDH-deficient GISTs and the benefit of their inhibition in treating these tumors have not yet been investigated.
Oncometabolites may also function in mechanisms independent of α-KG-dependent dioxygenases. Because of the structural similarity, D-2HG produced by IDH mutations competitively inhibits SDH[145]. Inhibition or genetic inactivation of SDH results in the accumulation of succinate, which increases levels of succinyl-CoA, a metabolite immediately upstream of succinate and fumarate in the TCA cycle (Figure 1)[145]. Succinyl-CoA is the substrate for lysine succinylation, a common posttranslational modification that affects cellular stress responses[146]. In tumor cells with IDH or SDH mutations, proteins are preferentially hypersuccinylated in the mitochondria, which induces mitochondrial depolarization and cancerous metabolism[145]. Hyper-succinylation confers tumor cells with apoptosis resistance via deposition of the prosurvival factor BCL-2 onto the mitochondrial membrane[145]. Notably, inhibition of hypersuccinylation by overexpression of SIRT5, the enzyme that reverses succinylation, slows IDH mutation-bearing tumor growth in a mouse xenograft
GISTs with RTK mutations are resistant to standard chemotherapy but show responsiveness to the first-line KIT inhibitor imatinib[150]. Because of the lack of RTK mutations, WT GISTs, including all SDH-deficient GISTs, are resistant to the first-generation RTK inhibitor imatinib[6-8]. However, second-generation RTK inhibitors, such as sunitinib, have shown only moderate effectiveness in SDH-deficient GISTs[151]. Patients with SDH-deficient GISTs are recommended to undergo complete tumor resection[9,10]. As suggested by studies in preclinical animal models[107], SDH-deficient GISTs may be more responsive to combinatorial treatment with the KIT inhibitor sunitinib and an FGFR inhibitor than to monotherapy. This serves as a great example of how research on the basic biological mechanism may shed light on the potential treatment of human diseases.
Cancer is a complex disease that involves genetic mutations in multiple genes. The connection between altered cellular metabolism and tumorigenesis has gained increased attention in recent years. The discovery that mutations in metabolic enzymes such as IDH, SDH, or FH mutations drive tumorigenesis indicates that metabolic reprogramming likely plays an underappreciated role in human cancer. On-cometabolites such as D-2HG, succinate, and fumarate competitively inhibit α-KG-dependent dioxygenases, which has a profound impact on epigenetic status, hypoxia response, RNA metabolism, and DNA repair capacity (Figure 2). We are only beginning to understand how these metabolic enzyme mutations and oncometabolites promote cancerous changes at the molecular and cellular levels. Further elucidation of the underlying mechanisms will lead to discoveries of novel approaches to treat SDH-deficient GISTs and other tumors with similar mutations.
We sincerely thank Drs. Rui Ji, Zhao-Feng Chen, Xi Gou, and Jun Wang for their insightful discussion.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sandhu DS S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 2. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1723] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 3. | Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 4. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3111] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 5. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1919] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 6. | Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, Benjamin RS, Bramwell VH, Demetri GD, Bertagnolli MM, Fletcher JA. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360-5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 460] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Janeway KA, Albritton KH, Van Den Abbeele AD, D'Amato GZ, Pedrazzoli P, Siena S, Picus J, Butrynski JE, Schlemmer M, Heinrich MC, Demetri GD. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, McKinley A, Ou WB, Fletcher JA, Fletcher CD, Huang X, Cohen DP, Baum CM, Demetri GD. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352-5359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 579] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 9. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42-4. [PubMed] |
| 10. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 672] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 11. | Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, Lai AH, Kelly L, Hornick JL; NIH Pediatric and Wild-Type GIST Clinic, O'Sullivan M, de Krijger RR, Dinjens WN, Demetri GD, Antonescu CR, Fletcher JA, Helman L, Stratakis CA. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 463] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 12. | Italiano A, Chen CL, Sung YS, Singer S, DeMatteo RP, LaQuaglia MP, Besmer P, Socci N, Antonescu CR. SDHA loss of function mutations in a subset of young adult wild-type gastrointestinal stromal tumors. BMC Cancer. 2012;12:408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Belinsky MG, Rink L, von Mehren M. Succinate dehydrogenase deficiency in pediatric and adult gastrointestinal stromal tumors. Front Oncol. 2013;3:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35:1712-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Miettinen M, Killian JK, Wang ZF, Lasota J, Lau C, Jones L, Walker R, Pineda M, Zhu YJ, Kim SY, Helman L, Meltzer P. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol. 2013;37:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Dwight T, Benn DE, Clarkson A, Vilain R, Lipton L, Robinson BG, Clifton-Bligh RJ, Gill AJ. Loss of SDHA expression identifies SDHA mutations in succinate dehydrogenase-deficient gastrointestinal stromal tumors. Am J Surg Pathol. 2013;37:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Oudijk L, Gaal J, Korpershoek E, van Nederveen FH, Kelly L, Schiavon G, Verweij J, Mathijssen RH, den Bakker MA, Oldenburg RA, van Loon RL, O'Sullivan MJ, de Krijger RR, Dinjens WN. SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors. Mod Pathol. 2013;26:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Wagner AJ, Remillard SP, Zhang YX, Doyle LA, George S, Hornick JL. Loss of expression of SDHA predicts SDHA mutations in gastrointestinal stromal tumors. Mod Pathol. 2013;26:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Pantaleo MA, Astolfi A, Urbini M, Nannini M, Paterini P, Indio V, Saponara M, Formica S, Ceccarelli C, Casadio R, Rossi G, Bertolini F, Santini D, Pirini MG, Fiorentino M, Basso U, Biasco G; GIST Study Group. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur J Hum Genet. 2014;22:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Janeway KA, Liegl B, Harlow A, Le C, Perez-Atayde A, Kozakewich H, Corless CL, Heinrich MC, Fletcher JA. Pediatric KIT wild-type and platelet-derived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67:9084-9088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Cardaci S, Zheng L, MacKay G, van den Broek NJ, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V, Kamphorst JJ, Vazquez A, Fleming S, Schiavi F, Kalna G, Blyth K, Strathdee D, Gottlieb E. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol. 2015;17:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Lussey-Lepoutre C, Hollinshead KE, Ludwig C, Menara M, Morin A, Castro-Vega LJ, Parker SJ, Janin M, Martinelli C, Ottolenghi C, Metallo C, Gimenez-Roqueplo AP, Favier J, Tennant DA. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat Commun. 2015;6:8784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | WARBURG O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9936] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 24. | Gaude E, Frezza C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat Commun. 2016;7:13041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 25. | Reznik E, Miller ML, ÅženbabaoÄŸlu Y, Riaz N, Sarungbam J, Tickoo SK, Al-Ahmadie HA, Lee W, Seshan VE, Hakimi AA, Sander C. Mitochondrial DNA copy number variation across human cancers. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 377] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 26. | Reznik E, Wang Q, La K, Schultz N, Sander C. Mitochondrial respiratory gene expression is suppressed in many cancers. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Frezza C. Metabolism and cancer: the future is now. Br J Cancer. 2020;122:133-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA; Multiple Leiomyoma Consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1121] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 29. | Castro-Vega LJ, Buffet A, De Cubas AA, Cascón A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre O, Currás-Freixes M, Franco-Vidal V, Guillaud-Bataille M, Simian C, Morin A, Letón R, Gómez-Graña A, Pollard PJ, Rustin P, Robledo M, Favier J, Gimenez-Roqueplo AP. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23:2440-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 30. | Clark GR, Sciacovelli M, Gaude E, Walsh DM, Kirby G, Simpson MA, Trembath RC, Berg JN, Woodward ER, Kinning E, Morrison PJ, Frezza C, Maher ER. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014;99:E2046-E2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, Wilkinson MR, Vadodaria B, Chen X, McGee RB, Hines-Dowell S, Nuccio R, Quinn E, Shurtleff SA, Rusch M, Patel A, Becksfort JB, Wang S, Weaver MS, Ding L, Mardis ER, Wilson RK, Gajjar A, Ellison DW, Pappo AS, Pui CH, Nichols KE, Downing JR. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med. 2015;373:2336-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 914] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 32. | Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4786] [Cited by in RCA: 4469] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 33. | Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4838] [Cited by in RCA: 4498] [Article Influence: 281.1] [Reference Citation Analysis (0)] |
| 34. | Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1782] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 35. | Tommasini-Ghelfi S, Murnan K, Kouri FM, Mahajan AS, May JL, Stegh AH. Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease. Sci Adv. 2019;5:eaaw4543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 36. | Van Vranken JG, Na U, Winge DR, Rutter J. Protein-mediated assembly of succinate dehydrogenase and its cofactors. Crit Rev Biochem Mol Biol. 2015;50:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peçzkowska M, Morrison CD, Lehtonen R, Januszewicz A, Järvinen H, Juhola M, Mecklin JP, Pukkala E, Herva R, Kiuru M, Nupponen NN, Aaltonen LA, Neumann HP, Eng C. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 38. | Baysal BE. A recurrent stop-codon mutation in succinate dehydrogenase subunit B gene in normal peripheral blood and childhood T-cell acute leukemia. PLoS One. 2007;2:e436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1212] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 40. | Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 648] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 41. | Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG, Gygi SP, Winge DR, Kremer H, Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 42. | Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Sköldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 798] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 43. | Charville GW, Longacre TA. Surgical Pathology of Gastrointestinal Stromal Tumors: Practical Implications of Morphologic and Molecular Heterogeneity for Precision Medicine. Adv Anat Pathol. 2017;24:336-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, Sleddens HF, Derkx P, Rivière J, Dannenberg H, Petri BJ, Komminoth P, Pacak K, Hop WC, Pollard PJ, Mannelli M, Bayley JP, Perren A, Niemann S, Verhofstad AA, de Bruïne AP, Maher ER, Tissier F, Méatchi T, Badoual C, Bertherat J, Amar L, Alataki D, Van Marck E, Ferrau F, François J, de Herder WW, Peeters MP, van Linge A, Lenders JW, Gimenez-Roqueplo AP, de Krijger RR, Dinjens WN. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 391] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 45. | Pollard PJ, Brière JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, Hargreaves IP, Heales SJ, Chung YL, Griffiths JR, Dalgleish A, McGrath JA, Gleeson MJ, Hodgson SV, Poulsom R, Rustin P, Tomlinson IP. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 669] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 46. | Aspuria PP, Lunt SY, Väremo L, Vergnes L, Gozo M, Beach JA, Salumbides B, Reue K, Wiedemeyer WR, Nielsen J, Karlan BY, Orsulic S. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Guengerich FP. Introduction: Metals in Biology: α-Ketoglutarate/Iron-Dependent Dioxygenases. J Biol Chem. 2015;290:20700-20701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Hausinger RP. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39:21-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 765] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 49. | Markolovic S, Wilkins SE, Schofield CJ. Protein Hydroxylation Catalyzed by 2-Oxoglutarate-dependent Oxygenases. J Biol Chem. 2015;290:20712-20722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 50. | Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 51. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19516] [Article Influence: 780.6] [Reference Citation Analysis (0)] |
| 52. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47165] [Article Influence: 3368.9] [Reference Citation Analysis (5)] |
| 53. | Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652-3658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 54. | Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2495] [Cited by in RCA: 2601] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 55. | Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 56. | Narita T, Weinert BT, Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol. 2019;20:156-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 813] [Article Influence: 162.6] [Reference Citation Analysis (0)] |
| 57. | Chen Y, Zhao W, Yang JS, Cheng Z, Luo H, Lu Z, Tan M, Gu W, Zhao Y. Quantitative acetylome analysis reveals the roles of SIRT1 in regulating diverse substrates and cellular pathways. Mol Cell Proteomics. 2012;11:1048-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 58. | Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3070] [Cited by in RCA: 3260] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 59. | Schölz C, Weinert BT, Wagner SA, Beli P, Miyake Y, Qi J, Jensen LJ, Streicher W, McCarthy AR, Westwood NJ, Lain S, Cox J, Matthias P, Mann M, Bradner JE, Choudhary C. Acetylation site specificities of lysine deacetylase inhibitors in human cells. Nat Biotechnol. 2015;33:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 60. | Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1318] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 61. | Kurdistani SK, Grunstein M. Histone acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol. 2003;4:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 62. | Rathmell JC, Newgard CB. Biochemistry. A glucose-to-gene link. Science. 2009;324:1021-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1833] [Cited by in RCA: 1720] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 64. | Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer. 2013;13:497-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 431] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 65. | Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1642] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 66. | Frau M, Feo F, Pascale RM. Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J Hepatol. 2013;59:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 67. | Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92:1515-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 68. | García-Trevijano ER, Latasa MU, Carretero MV, Berasain C, Mato JM, Avila MA. S-adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565-1569. [PubMed] |
| 70. | Gayther SA, Batley SJ, Linger L, Bannister A, Thorpe K, Chin SF, Daigo Y, Russell P, Wilson A, Sowter HM, Delhanty JD, Ponder BA, Kouzarides T, Caldas C. Mutations truncating the EP300 acetylase in human cancers. Nat Genet. 2000;24:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 434] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 71. | Bandyopadhyay D, Okan NA, Bales E, Nascimento L, Cole PA, Medrano EE. Down-regulation of p300/CBP histone acetyltransferase activates a senescence checkpoint in human melanocytes. Cancer Res. 2002;62:6231-6239. [PubMed] |
| 72. | Borrow J, Stanton VP, Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dubé I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 593] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 73. | Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, An W, Ge Q, Roeder RG, Wong J, Levrero M, Sartorelli V, Cotter RJ, Cole PA. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 74. | Rose NR, McDonough MA, King ON, Kawamura A, Schofield CJ. Inhibition of 2-oxoglutarate dependent oxygenases. Chem Soc Rev. 2011;40:4364-4397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 75. | Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3236] [Cited by in RCA: 3021] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 76. | Sciacovelli M, Frezza C. Oncometabolites: Unconventional triggers of oncogenic signalling cascades. Free Radic Biol Med. 2016;100:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 77. | Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2370] [Cited by in RCA: 2257] [Article Influence: 161.2] [Reference Citation Analysis (0)] |
| 78. | Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan KL. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 862] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 79. | Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, Leung IK, Li XS, Woon EC, Yang M, McDonough MA, King ON, Clifton IJ, Klose RJ, Claridge TD, Ratcliffe PJ, Schofield CJ, Kawamura A. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 827] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 80. | Malta TM, de Souza CF, Sabedot TS, Silva TC, Mosella MS, Kalkanis SN, Snyder J, Castro AVB, Noushmehr H. Glioma CpG island methylator phenotype (G-CIMP): biological and clinical implications. Neuro Oncol. 2018;20:608-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 81. | Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wesseling P, Reifenberger G, von Deimling A. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 872] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 82. | Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1485] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 83. | Liu Z, Che P, Mercado JJ, Hackney JR, Friedman GK, Zhang C, You Z, Zhao X, Ding Q, Kim K, Li H, Liu X, Markert JM, Nabors B, Gillespie GY, Zhao R, Han X. Characterization of iPSCs derived from low grade gliomas revealed early regional chromosomal amplifications during gliomagenesis. J Neurooncol. 2019;141:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016;18:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 85. | Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4860] [Cited by in RCA: 4388] [Article Influence: 274.3] [Reference Citation Analysis (0)] |
| 86. | Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017;18:517-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 1081] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 87. | Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 788] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 88. | Letouzé E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, Buffet A, Marcaillou C, Bertherat J, Amar L, Rustin P, De Reyniès A, Gimenez-Roqueplo AP, Favier J. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 573] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 89. | Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI, Jahromi MS, Xekouki P, Szarek E, Walker RL, Lasota J, Raffeld M, Klotzle B, Wang Z, Jones L, Zhu Y, Wang Y, Waterfall JJ, O'Sullivan MJ, Bibikova M, Pacak K, Stratakis C, Janeway KA, Schiffman JD, Fan JB, Helman L, Meltzer PS. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 90. | Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1643] [Cited by in RCA: 1655] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 91. | Inno A, Fanetti G, Di Bartolomeo M, Gori S, Maggi C, Cirillo M, Iacovelli R, Nichetti F, Martinetti A, de Braud F, Bossi I, Pietrantonio F. Role of MGMT as biomarker in colorectal cancer. World J Clin Cases. 2014;2:835-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 92. | Esteller M, Gaidano G, Goodman SN, Zagonel V, Capello D, Botto B, Rossi D, Gloghini A, Vitolo U, Carbone A, Baylin SB, Herman JG. Hypermethylation of the DNA repair gene O(6)-methylguanine DNA methyltransferase and survival of patients with diffuse large B-cell lymphoma. J Natl Cancer Inst. 2002;94:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 93. | Ricci R, Martini M, Ravegnini G, Cenci T, Milione M, Lanza P, Pierconti F, Santini D, Angelini S, Biondi A, Rosa F, Alfieri S, Clemente G, Persiani R, Cassano A, Pantaleo MA, Larocca LM. Preferential MGMT methylation could predispose a subset of KIT/PDGFRA-WT GISTs, including SDH-deficient ones, to respond to alkylating agents. Clin Epigenetics. 2019;11:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Michalak EM, Burr ML, Bannister AJ, Dawson MA. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat Rev Mol Cell Biol. 2019;20:573-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 95. | Loriot C, Domingues M, Berger A, Menara M, Ruel M, Morin A, Castro-Vega LJ, Letouzé É, Martinelli C, Bemelmans AP, Larue L, Gimenez-Roqueplo AP, Favier J. Deciphering the molecular basis of invasiveness in Sdhb-deficient cells. Oncotarget. 2015;6:32955-32965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 96. | Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1284] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 97. | Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1125] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 98. | Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell. 2013;152:1270-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 544] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 99. | Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4690] [Cited by in RCA: 4836] [Article Influence: 372.0] [Reference Citation Analysis (0)] |
| 100. | Dekker J, Mirny L. The 3D Genome as Moderator of Chromosomal Communication. Cell. 2016;164:1110-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 658] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 101. | Hnisz D, Weintraub AS, Day DS, Valton AL, Bak RO, Li CH, Goldmann J, Lajoie BR, Fan ZP, Sigova AA, Reddy J, Borges-Rivera D, Lee TI, Jaenisch R, Porteus MH, Dekker J, Young RA. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science. 2016;351:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 102. | Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suvà ML, Bernstein BE. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 974] [Cited by in RCA: 940] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 103. | Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, Iavarone A, Aldape K, Brennan CW, Jabado N, Pfister SM. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 104. | Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O'Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3089] [Cited by in RCA: 3745] [Article Influence: 312.1] [Reference Citation Analysis (0)] |
| 105. | Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5060] [Cited by in RCA: 5599] [Article Influence: 373.3] [Reference Citation Analysis (0)] |
| 106. | Howell PM, Liu Z, Khong HT. Demethylating Agents in the Treatment of Cancer. Pharmaceuticals (Basel). 2010;3:2022-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Flavahan WA, Drier Y, Johnstone SE, Hemming ML, Tarjan DR, Hegazi E, Shareef SJ, Javed NM, Raut CP, Eschle BK, Gokhale PC, Hornick JL, Sicinska ET, Demetri GD, Bernstein BE. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature. 2019;575:229-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 108. | Javidi-Sharifi N, Traer E, Martinez J, Gupta A, Taguchi T, Dunlap J, Heinrich MC, Corless CL, Rubin BP, Druker BJ, Tyner JW. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Cancer Res. 2015;75:880-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 109. | Li F, Huynh H, Li X, Ruddy DA, Wang Y, Ong R, Chow P, Qiu S, Tam A, Rakiec DP, Schlegel R, Monahan JE, Huang A. FGFR-Mediated Reactivation of MAPK Signaling Attenuates Antitumor Effects of Imatinib in Gastrointestinal Stromal Tumors. Cancer Discov. 2015;5:438-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 110. | Arao T, Ueshima K, Matsumoto K, Nagai T, Kimura H, Hagiwara S, Sakurai T, Haji S, Kanazawa A, Hidaka H, Iso Y, Kubota K, Shimada M, Utsunomiya T, Hirooka M, Hiasa Y, Toyoki Y, Hakamada K, Yasui K, Kumada T, Toyoda H, Sato S, Hisai H, Kuzuya T, Tsuchiya K, Izumi N, Arii S, Nishio K, Kudo M. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 111. | Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, Teague J, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Forbes S, Jia M, Jones D, Knott H, Kok CY, Lau KW, Leroy C, Lin ML, McBride DJ, Maddison M, Maguire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, O'Meara S, Pleasance E, Rajasingham A, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turrell K, Dykema KJ, Khoo SK, Petillo D, Wondergem B, Anema J, Kahnoski RJ, Teh BT, Stratton MR, Futreal PA. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 950] [Cited by in RCA: 939] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 112. | van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, Edkins S, Hardy C, O'Meara S, Teague J, Butler A, Hinton J, Latimer C, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Cole J, Forbes S, Jia M, Jones D, Kok CY, Leroy C, Lin ML, McBride DJ, Maddison M, Maquire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, Pleasance E, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turner R, Turrell K, Varian J, West S, Widaa S, Wray P, Collins VP, Ichimura K, Law S, Wong J, Yuen ST, Leung SY, Tonon G, DePinho RA, Tai YT, Anderson KC, Kahnoski RJ, Massie A, Khoo SK, Teh BT, Stratton MR, Futreal PA. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 622] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 113. | Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 114. | Huang C, Zhu B. Roles of H3K36-specific histone methyltransferases in transcription: antagonizing silencing and safeguarding transcription fidelity. Biophys Rep. 2018;4:170-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 115. | Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6156] [Cited by in RCA: 6154] [Article Influence: 246.2] [Reference Citation Analysis (0)] |
| 116. | Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1368] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 117. | Borun TW, Pearson D, Paik WK. Studies of histone methylation during the HeLa S-3 cell cycle. J Biol Chem. 1972;247:4288-4298. [PubMed] |
| 118. | Hoekstra AS, de Graaff MA, Briaire-de Bruijn IH, Ras C, Seifar RM, van Minderhout I, Cornelisse CJ, Hogendoorn PC, Breuning MH, Suijker J, Korpershoek E, Kunst HP, Frizzell N, Devilee P, Bayley JP, Bovée JV. Inactivation of SDH and FH cause loss of 5hmC and increased H3K9me3 in paraganglioma/pheochromocytoma and smooth muscle tumors. Oncotarget. 2015;6:38777-38788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, Wellen KE, O'Rourke DM, Berger SL, Chan TA, Levine RL, Mellinghoff IK, Thompson CB. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1564] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 120. | Venneti S, Felicella MM, Coyne T, Phillips JJ, Gorovets D, Huse JT, Kofler J, Lu C, Tihan T, Sullivan LM, Santi M, Judkins AR, Perry A, Thompson CB. Histone 3 lysine 9 trimethylation is differentially associated with isocitrate dehydrogenase mutations in oligodendrogliomas and high-grade astrocytomas. J Neuropathol Exp Neurol. 2013;72:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Inoue S, Li WY, Tseng A, Beerman I, Elia AJ, Bendall SC, Lemonnier F, Kron KJ, Cescon DW, Hao Z, Lind EF, Takayama N, Planello AC, Shen SY, Shih AH, Larsen DM, Li Q, Snow BE, Wakeham A, Haight J, Gorrini C, Bassi C, Thu KL, Murakami K, Elford AR, Ueda T, Straley K, Yen KE, Melino G, Cimmino L, Aifantis I, Levine RL, De Carvalho DD, Lupien M, Rossi DJ, Nolan GP, Cairns RA, Mak TW. Mutant IDH1 Downregulates ATM and Alters DNA Repair and Sensitivity to DNA Damage Independent of TET2. Cancer Cell. 2016;30:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 122. | Sulkowski PL, Sundaram RK, Oeck S, Corso CD, Liu Y, Noorbakhsh S, Niger M, Boeke M, Ueno D, Kalathil AN, Bao X, Li J, Shuch B, Bindra RS, Glazer PM. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet. 2018;50:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 123. | Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nosé V, Li C, Stiles CD. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 124. | King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675-4682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 519] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 125. | Esteban MA, Maxwell PH. HIF, a missing link between metabolism and cancer. Nat Med. 2005;11:1047-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1363] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 127. | Hong SS, Lee H, Kim KW. HIF-1alpha: a valid therapeutic target for tumor therapy. Cancer Res Treat. 2004;36:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 128. | Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 710] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 129. | Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, Farese RV, Freeman RS, Carter BD, Kaelin WG, Schlisio S. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 406] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 130. | Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med. 2019;51:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 131. | Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, Ding J, Lei Q, Guan KL, Xiong Y. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 945] [Cited by in RCA: 932] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 132. | Burnichon N, Brière JJ, Libé R, Vescovo L, Rivière J, Tissier F, Jouanno E, Jeunemaitre X, Bénit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 506] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 133. | Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X; COMETE Network. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615-5621. [PubMed] |
| 134. | Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1625] [Article Influence: 270.8] [Reference Citation Analysis (0)] |
| 135. | Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2600] [Cited by in RCA: 3644] [Article Influence: 280.3] [Reference Citation Analysis (0)] |
| 136. | Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2265] [Cited by in RCA: 3192] [Article Influence: 245.5] [Reference Citation Analysis (0)] |
| 137. | Deng X, Su R, Weng H, Huang H, Li Z, Chen J. RNA N6-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 138. | Zhang X, Wei LH, Wang Y, Xiao Y, Liu J, Zhang W, Yan N, Amu G, Tang X, Zhang L, Jia G. Structural insights into FTO's catalytic mechanism for the demethylation of multiple RNA substrates. Proc Natl Acad Sci U S A. 2019;116:2919-2924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 139. | Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, Qin X, Tang L, Wang Y, Hong GM, Huang H, Wang X, Chen P, Gurbuxani S, Arnovitz S, Li Y, Li S, Strong J, Neilly MB, Larson RA, Jiang X, Zhang P, Jin J, He C, Chen J. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 1162] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 140. | Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. The AlkB Family of Fe(II)/α-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond. J Biol Chem. 2015;290:20734-20742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 310] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 141. | Bian K, Lenz SAP, Tang Q, Chen F, Qi R, Jost M, Drennan CL, Essigmann JM, Wetmore SD, Li D. DNA repair enzymes ALKBH2, ALKBH3, and AlkB oxidize 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine in vitro. Nucleic Acids Res. 2019;47:5522-5529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 142. | Wang P, Wu J, Ma S, Zhang L, Yao J, Hoadley KA, Wilkerson MD, Perou CM, Guan KL, Ye D, Xiong Y. Oncometabolite D-2-Hydroxyglutarate Inhibits ALKBH DNA Repair Enzymes and Sensitizes IDH Mutant Cells to Alkylating Agents. Cell Rep. 2015;13:2353-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 143. | Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 799] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 144. | Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, Buckner JC, Fink KL, Souhami L, Laperriere NJ, Huse JT, Mehta MP, Curran WJ. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 145. | Li F, He X, Ye D, Lin Y, Yu H, Yao C, Huang L, Zhang J, Wang F, Xu S, Wu X, Liu L, Yang C, Shi J, He X, Liu J, Qu Y, Guo F, Zhao J, Xu W, Zhao S. NADP(+)-IDH Mutations Promote Hypersuccinylation that Impairs Mitochondria Respiration and Induces Apoptosis Resistance. Mol Cell. 2015;60:661-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 146. | Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1104] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 147. | Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 814] [Cited by in RCA: 1029] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 148. | Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinié V, Richard S, Tan PH, Teh BT, Furge KA. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell. 2011;20:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 149. | Smestad J, Erber L, Chen Y, Maher LJ. Chromatin Succinylation Correlates with Active Gene Expression and Is Perturbed by Defective TCA Cycle Metabolism. iScience. 2018;2:63-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 150. | Gold JS, van der Zwan SM, Gönen M, Maki RG, Singer S, Brennan MF, Antonescu CR, De Matteo RP. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol. 2007;14:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 151. | Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, Maki RG, DeMatteo RP, Besmer P, Antonescu CR. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |