Published online Aug 28, 2020. doi: 10.3748/wjg.v26.i32.4833
Peer-review started: April 2, 2020
First decision: April 29, 2020
Revised: May 4, 2020
Accepted: August 12, 2020
Article in press: August 12, 2020
Published online: August 28, 2020
Extrahepatic metastasis (EHM) of hepatocellular carcinoma (HCC) is associated with poor outcomes. However, the clinical features and risk factors of EHM of HCC after radiofrequency ablation (RFA) remain unclear.
To elucidate the characteristics and risk factors of EHM after RFA for HCC.
From January 2008 to December 2017, we retrospectively enrolled 661 patients who underwent RFA as first-line treatment for HCC at 2 tertiary hospitals. The inclusion criteria were age ≥ 18 years, a diagnosis of HCC, and treatment-naivety. Abdominal computed tomography (CT) or magnetic resonance imaging (MRI) and alpha-fetoprotein measurements were routinely performed at 1 mo after RFA and followed-up at intervals of 3-6 mo. Univariate analyses were performed using the chi-squared test or Student’s t-test, and univariate and multivariate analyses were performed via logistic regression, as appropriate.
EHM was diagnosed in 44 patients (6.7%) during a median follow-up period of 1204 days. The 10-year cumulative rate of HCC recurrence and EHM was 92.7% and 33.7%, respectively. Initial recurrence was most often intrahepatic, and the rate of extrahepatic recurrence at initial recurrence was only 1.2%. The median time to the diagnosis of EHM was 2.68 years, and 68.2% of patients developed EHM within 2 years of the first recurrence, regardless of recurrence-free survival and 75.0% of patients developed EHM within 5 years after first recurrence. EHM was mostly diagnosed via abdominal CT/MRI in 33 (75.0%) and 38 of 44 patients (86.4%) with EHM had either positive abdominal CT scan results or serum AFP level elevation. In multivariate analysis, recurrence-free survival < 2 years, ablation zone/tumor size < 2, and alpha-fetoprotein level > 400 IU/mL were associated with a high EHM risk.
EHM occurs following multiple intrahepatic recurrences after RFA and combined contrast-enhanced abdominal CT and serum AFP were useful for surveillance. Patients especially with high-risk factors require close follow-up for EHM.
Core tip: Extrahepatic metastasis (EHM) after radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC) takes place in substantial clinical situations and EHM of HCC is related with dismal prognosis. Our study provides characteristics and risk factors for EHM after RFA of HCC. EHM after RFA at the time of the first recurrence is rare; however, cumulative EHM frequently occurs following multiple intrahepatic recurrences. Most of the patients (86.4%) had either positive contrast-enhanced computed tomography scan results or serum alpha-fetoprotein level elevation at EHM. EHM turned out to be related with recurrence-free survival < 2 years, ablation zone/tumor size < 2, and alpha-fetoprotein level > 400 IU/mL.
- Citation: Yoon JH, Goo YJ, Lim CJ, Choi SK, Cho SB, Shin SS, Jun CH. Features of extrahepatic metastasis after radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol 2020; 26(32): 4833-4845
- URL: https://www.wjgnet.com/1007-9327/full/v26/i32/4833.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i32.4833
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related death[1]. Unlike other cancers that cause distant metastasis during progression, HCC has unique characteristics of loco-regional progression with an increase in size, intrahepatic metastasis, and vascular invasion. Therefore, current guidelines in Korea suggest follow-up surveillance after curative HCC treatment using abdominal imaging focusing on the liver and tumor marker such as serum alpha-fetoprotein (AFP) level[2]. Although enrolled subjects of previous studies represented all stages of HCC, the incidence of extrahepatic metastasis (EHM) of HCC ranged from 14%-37% and the presence of EHM was associated with poor clinical outcomes[3]. The reported median survival duration of patients with EHM is less than 6 (range, 4.9-5.9) mo, and the 1-year survival rate was reported as 24.9%[3,4]. Natsuizaka et al[3] suggested that the lung was the most common site of metastasis, and screening tests for lung metastases, as well as abdominal imaging, should be performed periodically for HCC patients. Lam et al[5] reported that when EHM is identified early and surgical resection is possible, the prognosis may be improved. Therefore, early diagnosis of EHM of HCC may help improve the prognosis.
Radiofrequency ablation (RFA) for the treatment of small HCC lesions (tumor diameter < 3 cm) has shown favorable clinical outcomes with less invasiveness and a low complication rate. RFA is recommended as one of the first-line treatment modalities for the management of HCC along with liver transplantation and surgery[6,7]. However, the recurrence-free survival (RFS) varies substantially (3.2%-38.2%) between studies, and cumulative extrahepatic recurrence rates were reported as 19.1% and 38.2% at 5 and 10 years, respectively[8,9]. Hence, careful monitoring for intra- and extrahepatic HCC recurrence after RFA is essential, but there is a lack of data regarding the effect of post-treatment surveillance on the prognosis of recurred HCC[10]. Moreover, few studies have been conducted on individualized post-treatment surveillance for EHM after RFA according to risk factors[11].
Therefore, we aimed to assess the characteristics and risk factors of EHM among patients who had undergone RFA as initial treatment for HCC and to investigate the appropriate surveillance tool for EHM detection.
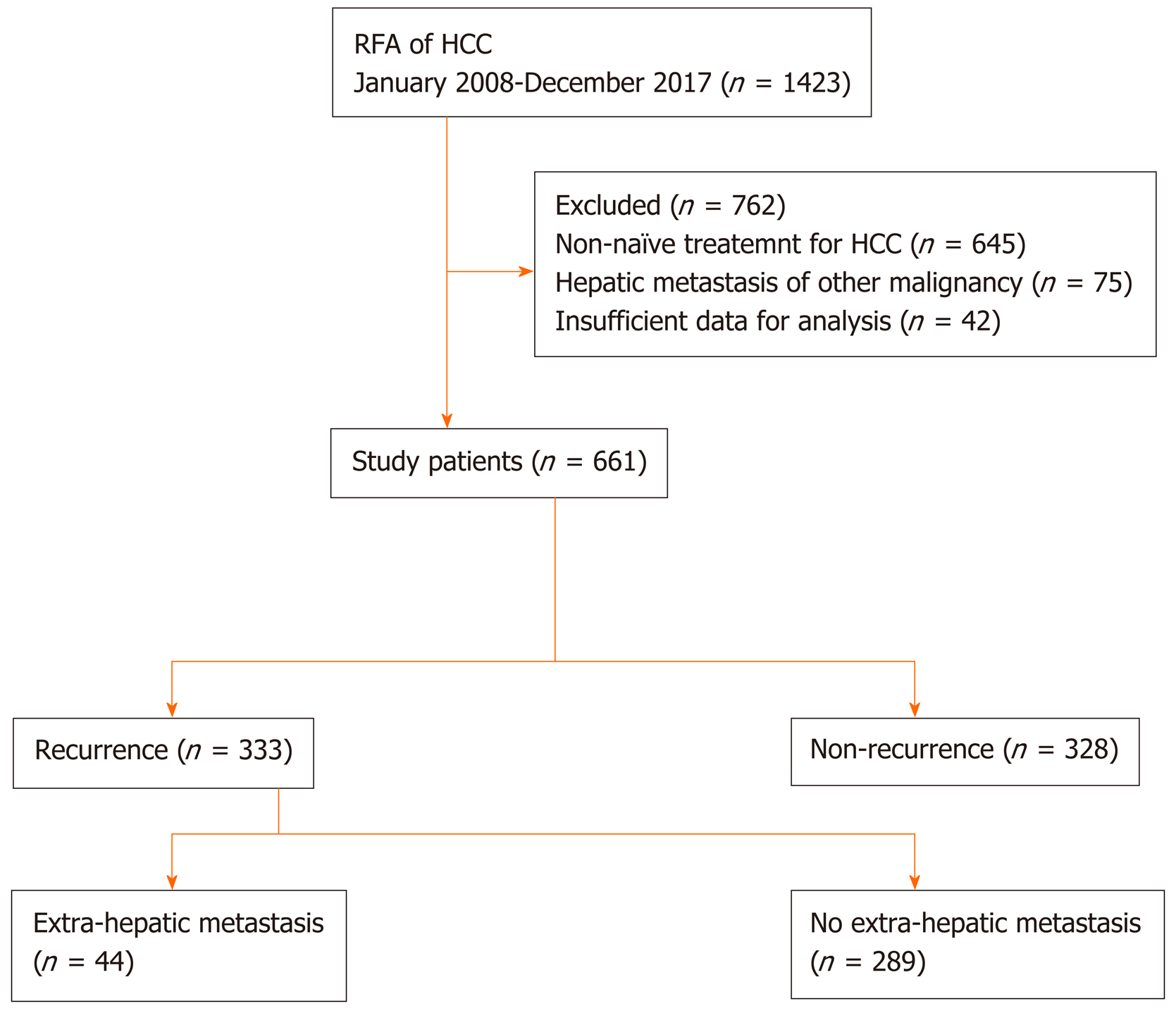
From January 2008 to December 2017, 1421 patients who underwent RFA for hepatic tumors at 2 tertiary hospitals were assessed. The inclusion criteria for this study were age ≥ 18 years, a diagnosis of HCC, and treatment-naivety. After excluding patients with hepatic tumors other than HCC or a history of HCC treatment, 661 patients were finally enrolled (Figure 1). Baseline clinical and tumor characteristics, complications of RFA, the status of recurrence, RFS, and rescue treatment methods for HCC recurrence were assessed retrospectively. The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-2019-203). The research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
The indications of RFA for HCC in our institutes were as follows: (1) Ineligible for surgical resection/liver transplantation or patient refusal for surgery; (2) Single nodular HCC < 5 cm in maximum diameter or multinodular HCC (3 in number, each < 3 cm in maximum diameter); (3) No EHM or vascular invasion; and (4) Prothrombin time ratio > 50% (international normalized ratio < 1.7).
HCC was diagnosed according to the guidelines proposed by the Korean Liver Cancer Study Group and the National Cancer Center[12]. HCC was staged at diagnosis according to the modified Union for International Cancer Control (mUICC) staging system[13] and the Barcelona Clinic Liver Cancer (BCLC) classification system[14]. The initial tumor size was based on planning ultrasonography performed before RFA, and ablation size was measured via computed tomography (CT) scan performed immediately after RFA. All measurements were reviewed by board-certified radiologists who were experts in abdominal imaging.
Abdominal CT or magnetic resonance imaging (MRI) and AFP measurements were routinely performed at 1 month after RFA and followed-up at intervals of 3-6 mo. Positron emission tomography (PET)-CT, bone scanning, spinal MRI, and chest CT were also performed in cases of clinically suspected metastasis or as intermittent routine follow-up based on the decision of the treating physician.
The time of EHM diagnosis was defined as the date on which EHM was detected via imaging study for the first time. Most cases of EHM were diagnosed during routine follow-up studies while few patients were diagnosed during the evaluation of new symptoms or significant AFP/serial AFP elevation without definite intrahepatic lesions. An increase in AFP > 15 ng/mL compared to the previous value within less than 6 months was considered significant; this was regarded as a feasible cutoff point for the prediction of long-term outcome among patients with HCC[15]. An AFP level that showed an increasing tendency more than twice was considered as serial elevation of AFP.
The data are expressed as means ± standard deviations or medians with ranges. Univariate analyses were performed using the chi-squared test or Student’s t-test, and univariate and multivariate analyses were performed via logistic regression, as appropriate. Variables with P values ≤ 0.05 in the univariate analysis were included in the multivariate logistic regression analysis. All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, United States). All analyses items with P < 0.05 were considered statistically significant.
The statistical methods of this study were reviewed by Ja Young Baek from Biomedical Research Institute, Hwasun Chonnam National University Hospital.
We identified 661 patients who underwent RFA as initial treatment for newly diagnosed HCC, and EHM occurred among 44 patients during the study period. We compared the baseline characteristics between the groups with and without EHM among the enrolled patients (Table 1). The mean age was 66.9 years, and 75.2% of the patients were male. The serum albumin level was lower among patients with EHM (4.3 mg/dL vs 3.9 mg/dL, P = 0.015). A higher proportion of patients in the EHM group had initial BCLC stage A and mUICC stage II disease compared to patients in the group without EHM who had a higher prevalence of BCLC stage 0 and mUICC stage I disease. There were no differences in tumor size and numbers between the 2 groups; however, the ratio of the ablation zone to tumor size was smaller in the group with EHM (2.07 vs 1.57, P = 0.001). There were no significant differences in the initial HCC recurrence site and mUICC tumor stage. The median follow-up duration of the enrolled patients was 1204 d.
| Total (n = 661) | Patients without extrahepatic metastasis (n = 617) | Patients with extrahepatic metastasis (n = 44) | P value | |
| Age (yr) | 66.9 ± 10.20 | 66.8 ± 10.2 | 68.4 ± 9.98 | 0.301 |
| Male, n (%) | 497 (75.2) | 469 (76.0) | 28 (63.6) | 0.066 |
| Etiology of liver cirrhosis, n (%) | 0.084 | |||
| Alcohol | 137 (20.7) | 131 (21.2) | 6 (13.6) | |
| HBV | 351 (53.1) | 322 (52.2) | 29 (65.9) | |
| HCV | 111 (16.8) | 108 (17.5) | 3 (6.8) | |
| Combined | 40 (6.1) | 38 (6.2) | 2 (4.5) | |
| Others | 22 (3.3) | 18 (2.9) | 4 (9.1) | |
| Platelet (× 103/μL) | 127.8 ± 55.1 | 127 ± 54.7 | 141.2 ± 63.1 | 0.304 |
| AST (IU/mL) | 49.0 ± 45.6 | 48.6 ± 43.2 | 54.7 ± 72.1 | 0.40 |
| ALT (IU/mL) | 37.2 ± 46.8 | 36.7 ± 44.9 | 43.0 ± 69.2 | 0.392 |
| ALP (U/L) | 97.4 ± 38.6 | 96.7 ± 38.7 | 107.4 ± 35.2 | 0.076 |
| Albumin (mg/dL) | 4.3 ± 2.9 | 4.3 ± 3.0 | 3.9 ± 0.6 | 0.015 |
| Total bilirubin (mg/dL) | 0.93 ± 0.83 | 0.92 ± 0.84 | 1.04 ± 0.67 | 0.378 |
| Serum AFP (IU/mL) | 209.7 ± 1558.3 | 185.5 ± 1503.8 | 548.3 ± 2179.0 | 0.283 |
| PIVKA-II (mAU/mL) | 331.9 ± 2901.4 | 336.3 ± 2973.6 | 246.1 ± 542.6 | 0.907 |
| BCLC stage, n (%) | 0.034 | |||
| 0 | 257 (39.0) | 248 (40.2) | 9 (20.5) | |
| A | 378 (57.1) | 345 (55.8) | 33 (75.0) | |
| B | 26 (3.9) | 24 (3.9) | 2 (4.5) | |
| mUICC stage, n (%) | 0.096 | |||
| I | 298 (45.0) | 28 (46.0) | 13 (29.5) | |
| II | 316 (47.7) | 288 (46.6) | 28 (63.6) | |
| III | 48 (7.3) | 45 (7.3) | 3 (6.8) | |
| Tumor size (cm) | 2.42 ± 1.02 | 2.41 ± 1.02 | 2.62 ± 1.05 | 0.18 |
| Ablation size/tumor size ratio | 2.04 ± 0.97 | 2.07 ± 0.97 | 1.57 ± 0.74 | 0.001 |
| Tumor number | 1.19 ± 0.45 | 1.19 ± 0.46 | 1.16 ± 0.37 | 0.356 |
| Encapsulated tumor, n (%) | 157 (23.7) | 146 (24.2) | 11 (25.6) | 0.840 |
| Subcapsular tumor, n (%) | 263 (39.7) | 240 (39.4) | 23 (53.5) | 0.069 |
| Follow-up duration, d (median, range) | 1204 (183-5016) | 1175 (183-5016) | 1379 (187-4541) | 0.270 |
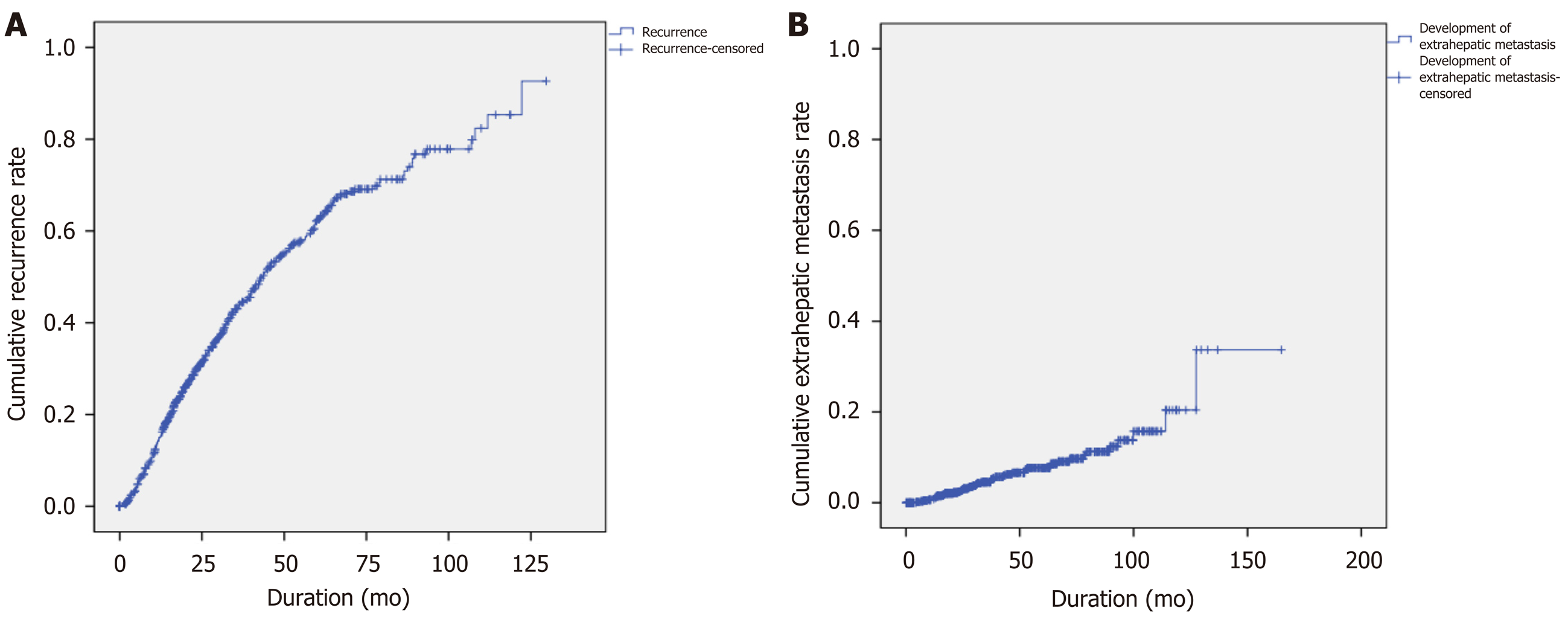
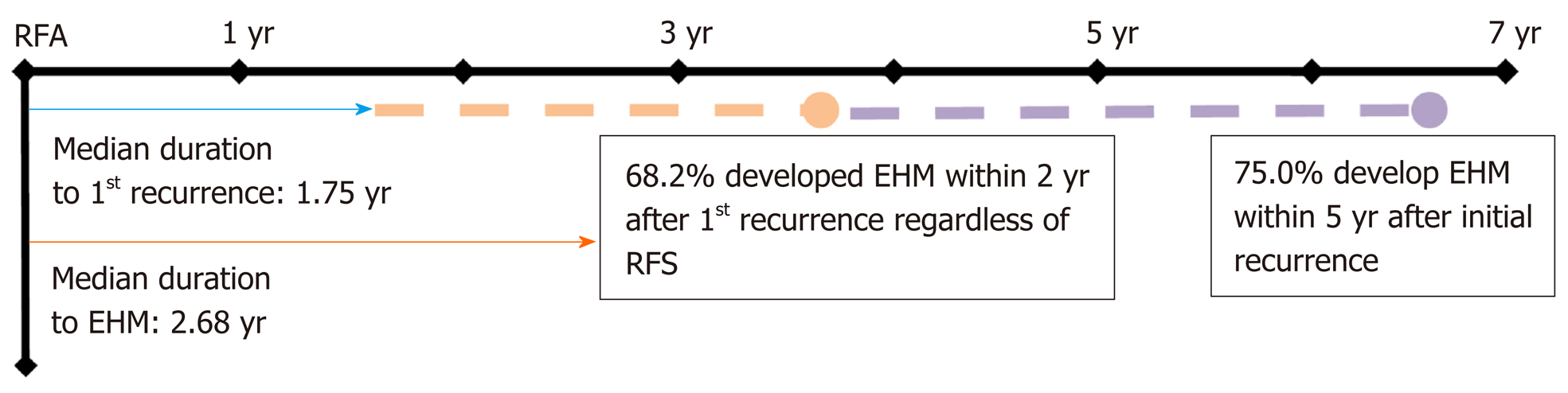
Among 661 enrolled patients, 289 (43.7%) developed recurrent lesions without EHM, and 44 (6.7%) were diagnosed with EHM during the follow-up period. The median AFP level was higher at diagnosis of EHM than before the diagnosis of EHM in 79.0% of patients. The 10-year cumulative rates of HCC recurrence and EHM were 92.7% and 33.7%, respectively (Figure 2). Among the 661 patients, the median time to the detection of first HCC recurrence after RFA was 1.75 years, and the median duration to the development of EHM was 2.68 years. In addition, 68.2% of patients developed EHM within 2 years after the first recurrence regardless of RFS, and 75.0% of patients developed EHM within 5 years of the first recurrence (Figure 3). The most common site of initial recurrence was intrahepatic; initial extrahepatic recurrence occurred in only 1.2% (8/661) of patients. Most cases of EHM occurred after multiple intrahepatic recurrences, and the HCC stages at first recurrence are shown in Table 2. In addition, the peritoneum was the most common site of first EHM among 8 patients with EHM (5/8, 62.5%) followed by the lymph nodes (3/8, 37.5%). There was no case of pulmonary metastasis as the first recurrence in this study. Transcatheter arterial chemoembolization and RFA were mostly used as rescue treatment modalities (45.9% and 36.9%, respectively) for recurrent HCC (Table 2).
| Patients without extrahepatic metastasis | Patients with extrahepatic metastasis | P value | |
| AFP level | |||
| Initial (median, range) | 8.13 (0.70-30000.0) | 21.750 (0.836-13148.0) | 0.291 |
| 1st recurrence (median, range) | 7.08 (0.93-50000.0) | 28.15 (0.73-70000.0) | 0.274 |
| CTP score, n (%) | 0.044 | ||
| A | 277 (95.8) | 39 (88.6) | |
| B | 12 (4.2) | 5 (11.4) | |
| Recurrence free survival, d (median, range) | 821 (49-3944) | 389 (79-2041) | < 0.001 |
| First recurred site, n (%) | < 0.001 | ||
| RFA site | 53 (18.3) | 7 (15.3) | |
| Same hepatic lobe | 145 (50.2) | 20 (45.5) | |
| Different hepatic lobe | 60 (20.8) | 8 (18.2) | |
| Both hepatic lobe | 31 (10.7) | 1 (3.1) | |
| Extrahepatic area | 0 (0.0) | 8 (18.2) | |
| Peritoneum | 5 | ||
| Lymph nodes | 3 | ||
| mUICC stage at 1st recurrence, n (%) | < 0.001 | ||
| I | 140 (48.4) | 17 (38.6) | |
| II | 101 (34.9) | 13 (29.5) | |
| III | 38 (13.1) | 4 (9.1) | |
| IVa | 5 (1.7) | 2 (4.5) | |
| IVb | 0 (0.0) | 8 (18.2) | |
| Rescue Treatment modalities, n (%) | 0.003 | ||
| TACE | 138 (47.7) | 15 (34.1) | |
| RFA | 110 (38.2) | 13 (29.5) | |
| Sorafenib | 1 (0.3) | - | |
| Surgery | 8 (2.8) | 3 (6.8) | |
| Radiotherapy | 1 (0.3) | 3 (6.8) | |
| Liver transplantation | 1 (0.3) | - | |
| TACE | 11 (3.8) | 3 (6.8) | |
| RFA PEIT | 1 (0.3) | - | |
| None | 9 (3.1) | 5 (11.4) | |
| Follow-up loss | 9 (3.1) | 2 (4.5) |
Forty-four patients with EHM were assessed for clinical features related to metastasis (Table 3). The location of metastasis was distributed evenly in the lymph nodes, bone, lung, and peritoneum. In 68.2% of patients, abdominal CT was used for the diagnosis of EHM (12 patients with lymph node, 11 patients with peritoneum, 6 patients with lung, and 5 patients with bone metastasis including 3 patients with multiple EHM), and 3 (6.8%) patients were diagnosed via chest X-rays during routine follow-up examinations. Three patients (6.8%) were diagnosed via abdominal MRI: 1 patient during HCC surveillance and 2 patients while evaluating elevated tumor marker levels without definite HCC recurrence on abdominal CT. Spine MRI was used for the diagnosis of spinal metastasis among 3 patients who presented with new-onset back pain. Five patients (11.4%) underwent PET-CT for re-staging of HCC without signs of intrahepatic recurrence, and EHM was detected in the lung, lymph nodes, and bone. In detail, 9 patients (20.5%) had multiple sites of EHM at first diagnosis. Among 13 patients with pulmonary metastasis, 7 (53.8%) were diagnosed via abdominal CT, 3 (23.1%) via chest X-ray, and 3 (23.1%) via chest CT.
| Clinical features | Value |
| Location of metastasis1 | |
| Lymph nodes | 16 (36.3) |
| Bone | 12 (27.3) |
| Lung | 13 (29.5) |
| Solitary/multiple | 4 (30.7)/9 (69.2) |
| Unilateral/Bilateral | 4 (30.7)/9 (69.2) |
| Lower lobe/non-lower lobe/all lobes | 2 (15.4)/9 (69.2)/2 (15.4) |
| Peritoneum | 12 (27.3) |
| Diagnostic modality | |
| Abdomen enhanced CT | 30 (68.2) |
| Abdomen enhanced MRI | 3 (6.8) |
| Spine MRI | 3 (6.8) |
| PET-CT | 5 (11.4) |
| Chest X-ray | 3 (6.8) |
| Chest enhanced CT | 3 (6.8%) |
| Tumor marker increment (AFP, PIVKA) | |
| Both | 14 (36.8) |
| Either | 17 (44.7) |
| AFP/PIVKA | 15 (39.5)/2 (5.3) |
| None | 7 (18.4) |
| N/A | 6 |
| Patients diagnosed of EHM with either of abdomen enhanced CT or serum AFP elevation (> 15 IU/mL) | 38 (86.4) |
| Time to extrahepatic metastasis (years, median, range) | 2.68 (0.38-10.6) |
| Intra-hepatic HCC status at diagnosis of EHM (mUICC T stage) | |
| T0/T1/T2 | 19 (43.2)/5 (11.4)/7 (15.9) |
| T3/T4a | 2 (4.5)/11 (25.0) |
Thirty-one patients (31/38, 81.5%) had increased tumor marker levels at the time of diagnosis of EHM. A diagnosis of EHM was made in most patients (86.4%) based on either contrast-enhanced CT findings or elevated serum AFP levels. Intrahepatic tumor status categorized according to the mUICC T stage at the time of EHM diagnosis was assessed; 43.2% of patients had no findings of intrahepatic HCC remnant tumor (T0), and 25.0% of patients had stage T4a disease.
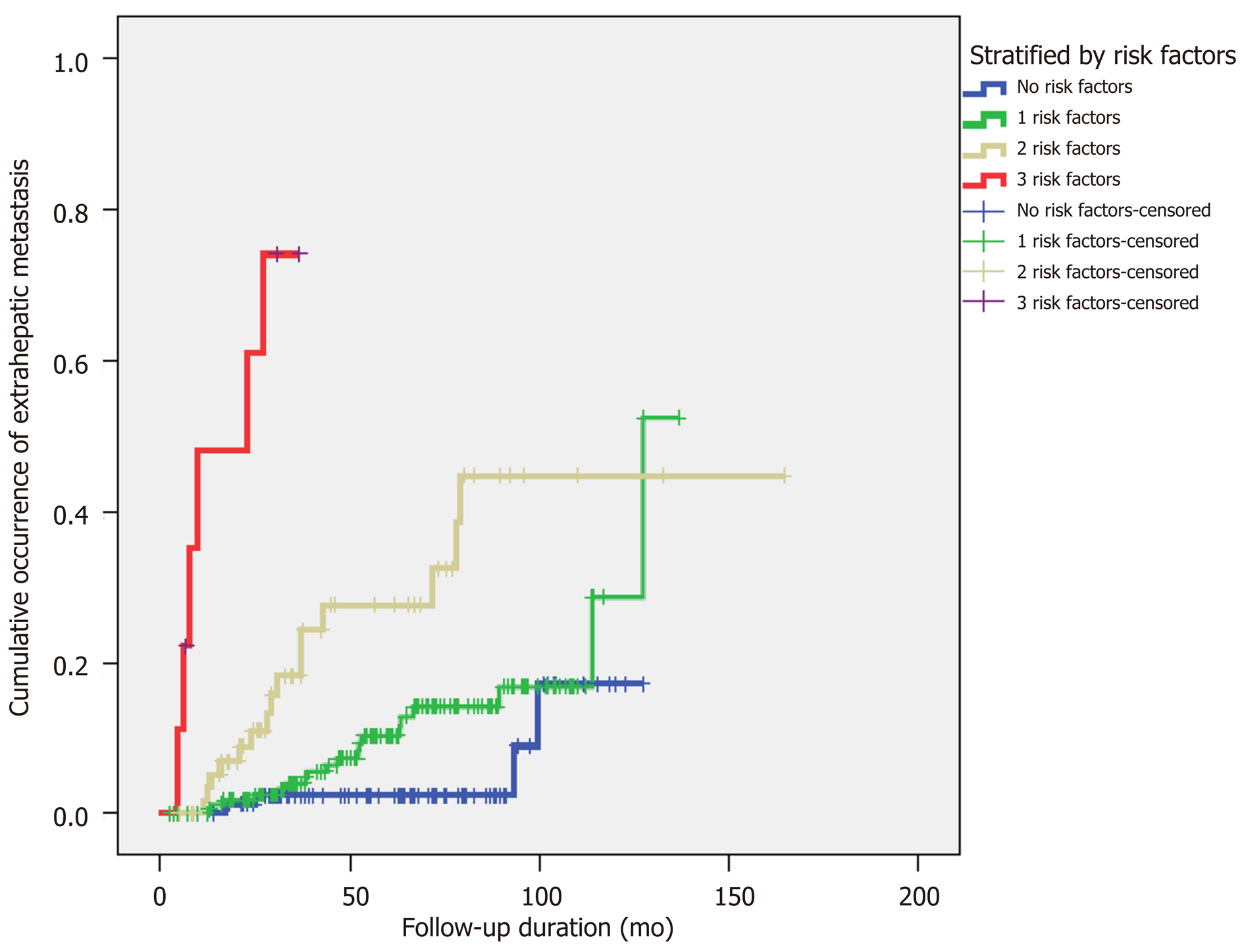
Multiple factors that might be relevant to EHM were assessed (Table 4). In the univariate analysis, more advanced stage, the presence of intrahepatic recurrence, serum alkaline phosphatase level > 97 U/L, first RFS within less than 2 years, ablation zone/tumor size > 2, and AFP level > 400 IU/mL at first HCC recurrence were associated with a high risk of EHM. Among these factors, a first HCC RFS < 2 years [odds ratio (OR), 2.44; P = 0.019], ablation zone/tumor size < 2 (OR, 3.33; P = 0.01), and AFP > 400 IU/mL at first recurrence (OR, 3.35; P = 0.01) were identified as significant factors in the multivariate analysis. We stratified the patients who had recurrence after RFA by numbers of risk factors related to EHM occurrence, and in proportion to the numbers of risk factors, the cumulative occurrence of EHM showed an increase (Figure 4).
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| BCLC stage (0 vs A, B) | 2.93 (1.38–6.20) | 0.003 | ||
| mUICC stage (I vs II, III) | 2.04 (1.04–3.96) | 0.034 | ||
| Post-RFA complication (fever, abscess) | 2.78 (1.31–5.92) | 0.050 | ||
| Presence of intra-hepatic recurrence | 48.8 (6.68–356.63) | 0.000 | ||
| ALP > 97 U/L | 2.29 (1.23-4.26) | 0.008 | ||
| 1st recurrence free survival < 2 yr | 2.88 (1.54-5.38) | 0.001 | 2.44 (1.16-5.14) | 0.019 |
| Ratio of ablation zone and tumor size < 2 | 3.84 (1.76-8.39) | 0.001 | 3.33 (1.34-8.27) | 0.010 |
| Presence of tumoral thrombosis | 2.57 (1.13-5.84) | 0.024 | ||
| AFP > 400 IU/mL at 1st recurrence | 4.52 (2.03-10.07) | 0.000 | 3.35 (1.33-8.43) | 0.010 |
| mUICC stage > 2 at 1st recurrence | 2.49 (1.22-5.06) | 0.012 | ||
Although RFA is one of the most effective curative treatment modalities for HCC along with surgical resection and liver transplantation, our study and previous studies showed that there is a substantial possibility of EHM even if intrahepatic lesions are stable[8,16,17]. Most other studies showed that the most common initial site of recurrence is intrahepatic and that most cases of EHM occur after multiple intrahepatic recurrences with several treatments[8]. This study focused on the characteristics and risk factors of extrahepatic recurrence after RFA and whether regular surveillance for EHM is needed. The results are of particular interest because data on the pattern of EHM in HCC after RFA and the role of regular surveillance for EHM are limited, and there have been no reports regarding surveillance methods for EHM in HCC.
In the present study, 289 patients experienced tumor recurrence without EHM, and 44 experienced EHM during a median follow-up period of 1204 d. The 1-, 3-, 5-, 8-, and 10-year cumulative rates of HCC recurrence and EHM development were concordant with the results of previous studies[8,9], but only 1.2% of the enrolled patients presented with EHM as the first recurrence of HCC. The median time to the detection of EHM was 2.68 years, and 68.2% of patients developed EHM within 2 years after the first recurrence regardless of RFS. Furthermore, 75% of patients developed EHM within 5 years of the first recurrence (Figure 3).
Other studies that reported the locations of EHM showed predominance in the lung (39%-54%), lymph nodes (34%-40%), and bone (25%-39%)[3,11,18]. In the present study, the location of EHM was relatively evenly distributed among the intra-abdominal lymph nodes (36.3%), bone (25.0%), lung (29.5%), and peritoneum (27.3%). The proportions of extrahepatic metastatic sites in our study may have differed from those identified in previous studies as our population comprised post-RFA patients who had early-stage disease, while the other studies included patients with diverse stages of HCC ranging from early to terminal. The median duration of peritoneal seeding was 883 d (range: 138–3878); however, in 3 patients, the time from RFA to EHM was less than 1 year, suggesting the possibility that peritoneal dissemination occurred via RFA in some patients. There was no case of pulmonary metastasis as the first recurrence in this study. Most pulmonary metastases occurred after intrahepatic recurrences.
The spread pattern of lung metastasis at the initial diagnosis of recurrent HCC is important because of the possible value of metastectomy, which was reported to yield favorable outcomes in some studies[19,20]. We found that at the time of HCC recurrence 69.2% of patients had multiple lung metastatic lesions, and 69.2% had bilateral lung metastasis for which metastectomy was not indicated. Thus, the option of metastectomy after RFA may be of limited value in the group we studied.
Regarding the detection method, EHM was diagnosed via abdominal imaging (CT/MRI) in most patients (75.0%). The backbone of surveillance after RFA for HCC is abdominal imaging, and only 14/661 patients (2.1%) required other diagnostic modalities such as PET-CT, spine MRI, chest CT, or chest X-ray. Considering the small proportion of diagnostic modalities other than abdominal imaging and the low incidence (1.2%) of EHM as the first recurrence of HCC, the need for regular surveillance tools other than abdominal imaging may not be very high. In particular, 38/44 patients (86.4%) with EHM had either positive abdominal CT scan results or serum AFP elevation, which we currently use as surveillance tools for HCC after RFA in clinical practice. Considering the high cost of spine MRI, PET-CT, and chest CT in general, the cost-effectiveness of routine surveillance using these modalities may be high however, an individualized approach in accordance with each country's reimbursement policy is needed.
A correlation between serum PIVKA-II levels and EHM was reported previously[21,22]. In our study, 31/38 patients (81.5%) had increasing tumor marker levels (serum AFP or protein induced by vitamin K absence-II [PIVKA-II]), and serum AFP levels were elevated in 29/38 (76.3%) which showed correlation with the development of EHM, as reported in other studies[23]. Although the PIVKA-II level was only assessed in 24 patients, 16 (66.7%) showed elevated serum levels at EHM development. Regarding the pattern of HCC recurrence, the most common initial site of recurrence was intrahepatic. Although the rate of extrahepatic initial recurrences in our population was only 1.2%, 43.2% of the patients had no sign of intrahepatic HCC at the time of diagnosis of EHM. This implies that even if loco-regionally managed HCC lesions are stable, close surveillance for possible EHM is warranted. In particular, when tumor marker levels increase without definite aggravation of previous HCC lesions, additional examination for EHM development should be considered. Refaat et al[17] reported that among 65 patients who underwent loco-regional therapy for HCC and had elevated serum AFP levels, 10 (15.4%) had EHM without intrahepatic tumor recurrence. In addition, Chen et al[16] reported that among 26 patients who had elevated serum AFP levels without findings of recurrence on conventional imaging studies, 8 (30.8%) experienced EHM.
Recurrence of HCC after curative treatment is reported to occur mostly within 2 years[24]; therefore, guidelines suggest surveillance for HCC recurrence within a short interval of 2-6 mo until the second year after treatment[25-27]. In the present study, the 1-, 3-, 5-, 8-, and 10-year cumulative rates of HCC recurrence were 15.1%, 43.8%, 62.5%, 77.9%, and 92.7% respectively, and those of EHM development were 1.0%, 2.9%, 8.1%, 15.7%, and 33.7%, respectively. Our results also showed a 1.75-year median time to first recurrence after RFA, a median time to the development of EHM of 2.68 years regardless of RFS, and 75.0% of patients experiencing EHM within 5 years after RFA. Thus, we suggest that it is prudent to pay attention to possible EHM occurrence for at least 5 years after RFA, and patients who experience tumor recurrence may require close observation for the development of EHM, particularly within 2 years after the first recurrence (Figure 3).
In the multivariate analysis of potential risk factors for EHM, first RFS < 2 years, ablation zone/tumor size < 2, and serum AFP level > 400 IU/mL at first recurrence were factors relevant to EHM development. Some studies have suggested that early recurrence is associated with vascular invasion, initial tumor staging, and poor prognosis, and our findings are consistent with these reports[28,29]. Other studies have assessed the risk of local tumor progression in relation to insufficiently ablated margins after RFA in liver malignancies, a minimal margin of < 2-5 mm being reported as an independent factor for local tumor progression[30-32]. Our study showed that ablation zone/tumor size was associated with the risk of EHM development. Most patients (97.9%) had minimal margins of > 5 mm, and the margin length between the tumor and ablation zones showed no relevance in the occurrence of EHM. Some studies have reported that higher AFP levels are associated with increased recurrence following liver transplantation for HCC, as well as worse disease-free survival and overall survival[33,34]. By stratifying patients according to the number of risk factors associated with EHM, the cumulative occurrence of EHM showed an increasing trend related to the number (≥ 2) of risk factors (Figure 4). Therefore, we suggest close surveillance for EHM after RFA, especially in these high-risk patients.
Our study had some limitations. First, this was a retrospective study based on medical records. Thus, there was no uniform post-treatment or surveillance schedule, and the surveillance modality used for each patient was at the physician’s discretion. Second, until the development of EHM, patients underwent different treatment modalities for local HCC recurrence depending on the tumor and patient’s status. There may be diverse statuses regarding tumor stage, liver reserve function, and patients’ physical performance status. However, we tried to overcome these limitations by using a considerable number of patients with a long-term follow-up duration in multiple tertiary centers.
In conclusion, EHM as the first recurrence after RFA was rare, but cumulative EHM occurred frequently following multiple intrahepatic recurrences. Thus, optimal surveillance for EHM after RFA for HCC is essential according to stratified risk factors (RFS < 2 years, ablation zone/tumor size < 2, and AFP level > 400 IU/mL) related to EHM, and combined contrast-enhanced abdominal CT and serum AFP level have been found useful for this purpose.
Extrahepatic metastasis (EHM) of hepatocellular carcinoma (HCC) is related to dismal prognosis.
The characteristics and risk factors of EHM of HCC after radiofrequency ablation are not elucidated.
To investigate the clinical features and risk factors of EHM after radiofrequency ablation for HCC.
Patients who underwent radiofrequency ablation for HCC were identified from the two tertiary hospitals in South Korea from 2008 to 2017. Univariate analyses were performed using the chi-squared test or Student’s t-test, and univariate and multivariate analyses were performed via logistic regression, as appropriate.
During a median follow-up period of 1,204 days, EHM was diagnosed in 44 patients (6.7%). The 10-year cumulative rate of HCC recurrence and EHM was 92.7% and 33.7%, respectively. The median time to the diagnosis of EHM was 2.68 years, and 68.2% of patients developed EHM within 2 years of the first recurrence, regardless of recurrence-free survival. EHM was mostly diagnosed via abdominal CT/MRI in 33 (75.0%) and 38 of 44 patients (86.4%) with EHM had either positive abdominal CT scan results or serum alpha-fetoprotein (AFP) level elevation. In multivariate analysis, recurrence-free survival < 2 years, ablation zone/tumor size < 2, and alpha-fetoprotein level > 400 IU/mL were associated with a high EHM risk.
EHM occurs following multiple intrahepatic recurrences after radiofrequency ablation and combined contrast-enhanced abdominal CT and serum AFP were useful for surveillance.
Patients especially with high-risk factors such as recurrence-free survival < 2 years, ablation zone/tumor size < 2, and alpha-fetoprotein level > 400 IU/mL, require close follow-up for EHM.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: Korean Association for the Study of the Liver.
P-Reviewer: Aurello P S-Editor: Gong ZM L-Editor: A P-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50885] [Article Influence: 8480.8] [Reference Citation Analysis (44)] |
| 2. | Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2019;20:1042-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 3. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 369] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 4. | Lee JI, Kim JK, Kim DY, Ahn SH, Park JY, Kim SU, Kim BK, Han KH, Lee KS. Prognosis of hepatocellular carcinoma patients with extrahepatic metastasis and the controllability of intrahepatic lesions. Clin Exp Metastasis. 2014;31:475-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4906] [Article Influence: 817.7] [Reference Citation Analysis (0)] |
| 7. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2677] [Article Influence: 446.2] [Reference Citation Analysis (1)] |
| 8. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Gwak GY, Yoo BC. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 10. | Hyder O, Dodson RM, Weiss M, Cosgrove DP, Herman JM, Geschwind JH, Kamel IR, Pawlik TM. Trends and patterns of utilization in post-treatment surveillance imaging among patients treated for hepatocellular carcinoma. J Gastrointest Surg. 2013;17:1774-1783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475-4483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 12. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ; Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1298] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 13. | Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 823] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 15. | Hsu CY, Liu PH, Lee YH, Hsia CY, Huang YH, Lin HC, Chiou YY, Lee FY, Huo TI. Using serum α-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One. 2015;10:e0118825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Chen YK, Hsieh DS, Liao CS, Bai CH, Su CT, Shen YY, Hsieh JF, Liao AC, Kao CH. Utility of FDG-PET for investigating unexplained serum AFP elevation in patients with suspected hepatocellular carcinoma recurrence. Anticancer Res. 2005;25:4719-4725. [PubMed] [Cited in This Article: ] |
| 17. | Refaat R, Basha MAA, Hassan MS, Hussein RS, El Sammak AA, El Sammak DAEA, Radwan MHS, Awad NM, Saad El-Din SA, Elkholy E, Ibrahim DRD, Saleh SA, Montasser IF, Said H. Efficacy of contrast-enhanced FDG PET/CT in patients awaiting liver transplantation with rising alpha-fetoprotein after bridge therapy of hepatocellular carcinoma. Eur Radiol. 2018;28:5356-5367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 272] [Cited by in F6Publishing: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Kuo TM, Chang KM, Cheng TI, Kao KJ. Clinical Factors Predicting Better Survival Outcome for Pulmonary Metastasectomy of Hepatocellular Carcinoma. Liver Cancer. 2017;6:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Mizuguchi S, Nishiyama N, Izumi N, Tsukioka T, Komatsu H, Iwata T, Tanaka S, Takemura S, Kubo S. Clinical Significance of Multiple Pulmonary Metastasectomy for Hepatocellular Carcinoma. World J Surg. 2016;40:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Bae HM, Lee JH, Yoon JH, Kim YJ, Heo DS, Lee HS. Protein induced by vitamin K absence or antagonist-II production is a strong predictive marker for extrahepatic metastases in early hepatocellular carcinoma: a prospective evaluation. BMC Cancer. 2011;11:435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Orita K, Sakamoto A, Okamoto T, Matsuda S. Solitary Muscle Metastasis of Hepatocellular Carcinoma to the Biceps Femoris Muscle with Only Elevated Serum PIVKA-II: A Case Report. Am J Case Rep. 2019;20:306-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Yokoo T, Patel AD, Lev-Cohain N, Singal AG, Yopp AC, Pedrosa I. Extrahepatic metastasis risk of hepatocellular carcinoma based on α-fetoprotein and tumor staging parameters at cross-sectional imaging. Cancer Manag Res. 2017;9:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16:792-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | He XX, Li Y, Ren HP, Tian DA, Lin JS. [2010 guideline for the management of hepatocellular carcinoma recommended by the American Association for the Study of Liver Diseases]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:249-250. [PubMed] [Cited in This Article: ] |
| 26. | European Association for the Study of the Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4345] [Article Influence: 362.1] [Reference Citation Analysis (2)] |
| 27. | Liu D, Chan AC, Fong DY, Lo CM, Khong PL. Evidence-Based Surveillance Imaging Schedule After Liver Transplantation for Hepatocellular Carcinoma Recurrence. Transplantation. 2017;101:107-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21:1207-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 72] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996;111:720-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 205] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Jiang C, Liu B, Chen S, Peng Z, Xie X, Kuang M. Safety margin after radiofrequency ablation of hepatocellular carcinoma: precise assessment with a three-dimensional reconstruction technique using CT imaging. Int J Hyperthermia. 2018;34:1135-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 32. | Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, Brown KT, Covey AM, Brody LA, Alago W, Thornton RH, Kemeny NE, Solomon SB. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 33. | Mahmud N, John B, Taddei TH, Goldberg DS. Pre-transplant alpha-fetoprotein is associated with post-transplant hepatocellular carcinoma recurrence mortality. Clin Transplant. 2019;33:e13634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |