Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4669
Peer-review started: April 19, 2020
First decision: June 8, 2020
Revised: June 21, 2020
Accepted: July 23, 2020
Article in press: July 23, 2020
Published online: August 21, 2020
Processing time: 123 Days and 14 Hours
Single incision plus one port left-side approach (SILS+1/L) totally laparoscopic distal gastrectomy (TLDG) is an emerging technique for the treatment of gastric cancer. Reduced port laparoscopic gastrectomy has a number of potential advantages for patients compared with conventional laparoscopic gastrectomy: relieving postoperative pain, shortening hospital stay and offering a better cosmetic outcome. Nevertheless, there are no previous reports on the use of SILS+1/L TLDG with uncut Roux-en-Y (uncut R-Y) reconstruction.
To investigate the initial feasibility of SILS+1/L TLDG with uncut Roux-en-Y digestive tract reconstruction (uncut R-Y reconstruction) to treat distal gastric cancer.
A total of 21 patients who underwent SILS+1/L TLDG with uncut R-Y reconstruction for gastric cancer were enrolled. All patients were treated at The Second Hospital of Shandong University. Reconstructions were performed intracorporeally with 60 mm endoscopic linear stapler and 45 mm no-knife stapler. The clinicopathological characteristics, surgical details, postoperative short-term outcomes, postoperative follow-up upper gastrointestinal radiography findings and endoscopy results were analyzed retrospectively.
All SILS+1/L operations were performed by SILS+1/L TLDG successfully. The patient population included 13 men and 8 women with a mean age of 48.2 years (ranged from 40 years to 70 years) and median body mass index of 22.8 kg/m2. There were no conversions to open laparotomy, and no other port was placed. The mean operation time was 146 min (ranged 130-180 min), and the estimated mean blood loss was 54 mL (ranged 20-110 mL). The mean duration to flatus and discharge was 2.3 (ranged 1-3.5) and 7.3 (ranged 6-9) d, respectively. The mean number of retrieved lymph nodes was 42 (ranged 30-47). Two patients experienced mild postoperative complications, including surgical site infection (wound at the navel incision) and mild postoperative pancreatic fistula (grade A). Follow-up upper gastrointestinal radiography and endoscopy were carried out at 3 mo postoperatively. No patients experienced moderate or severe food stasis, alkaline gastritis or bile reflux during the follow-up period. No recanalization of the biliopancreatic limb was found.
SILS+1/L TLDG with uncut R-Y reconstruction could be safely performed as a reduced port surgery.
Core tip: Single-incision plus one port left-side approach totally laparoscopic distal gastrectomy has become increasingly popular to treat gastric cancer because of fewer ports and shorter length of incisions. However, there are no previous reports on the use of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction. We reported our initial experience and demonstrated that single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction is safe and feasible.
- Citation: Zhou W, Dong CZ, Zang YF, Xue Y, Zhou XG, Wang Y, Ding YL. Initial experience of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction. World J Gastroenterol 2020; 26(31): 4669-4679
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4669.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4669
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related deaths among all malignant tumors in the world[1,2]. Although significant progress has been made in the diagnosis and comprehensive treatment of gastric cancer, the prognosis for patients with gastric cancer is still poor. Radical gastrectomy remains the first-choice treatment for patients with resectable gastric cancer. Laparoscopic techniques have recently been one of the main development directions for the surgical treatment of gastric cancer[3].
Laparoscopic distal gastrectomy has been widely accepted as a more effective approach than conventional open distal gastrectomy for early-stage gastric cancer according to several clinical studies[4-8]. The CLASS-01 randomized clinical trial by Yu et al[9] revealed that laparoscopic distal gastrectomy did not result in inferior 3-year disease-free survival compared with open distal gastrectomy among patients with a preoperative clinical stage indicating locally advanced gastric cancer[9]. As a type of reduced port surgery for patients with gastric cancer, single-incision laparoscopic surgery plus one port (SILS+1), in which fewer ports and shorter length of incisions are needed, has become increasingly popular in the past few years[10-14].
Digestive tract reconstruction after distal gastrectomy is still controversial because of the long-term postoperative complications that affect quality of life, such as Roux stasis syndrome and reflux gastritis. In 2005, Uyama et al[15] first outlined a laparoscopic-assisted uncut Roux-en-Y digestive tract (uncut R-Y reconstruction) procedure following distal gastrectomy for the digestive tract. There is an increasing trend towards laparoscopic uncut R-Y reconstruction for distal gastrectomy due to its more satisfactory postoperative outcomes (lower incidence of bile reflux and Roux stasis syndrome) than conventional R-Y reconstruction[16-21].
It might be safe and feasible to treat gastric cancer, colon cancer and rectal cancer with SILS+1 by experienced surgeons[22-26]. SILS+1 provides minimal invasion without compromising the principles of tumor treatment. However, an analysis of an uncut R-Y reconstruction method after SILS+1 has not been carried out. To the best of our knowledge, there are no previous reports on the use of SILS+1 left-side approach (SILS+1/L) totally laparoscopic distal gastrectomy (TLDG) with uncut R-Y reconstruction.
The aim of this report is to present our initial experience in performing SILS+1/L TLDG with uncut R-Y reconstruction as well as the short-term postoperative outcomes and endoscopic findings.
Twenty-one patients who had undergone SILS+1/L TLDG with uncut R-Y anastomosis reconstruction to treat distal gastric cancer were enrolled in this study. The inclusion criteria were a clinical diagnosis of gastric cancer and a depth of invasion of gastric cancer ≤ T3. The exclusion criteria included preoperative radiotherapy and chemotherapy, tumor perforation, fused lymph nodes leading to difficult dissection and severe medical illness. All operations were carried out in The Second Hospital of Shandong University between March 2019 and February 2020. All operations were performed by the same team of doctors. The perioperative details, surgical details and pathological data of all the selected patients were collected retrospectively from cancer registry data and the electronic medical records. The pathological stage of each specimen was classified according to the American Joint Cancer Committee 8th Edition tumor, node, metastasis classification[27].
Food stasis, gastritis and bile reflux of the remnant stomach were evaluated by upper gastrointestinal radiography and endoscopy at 3 mo postoperatively. These findings were reviewed by two expert radiologist or two endoscopists. If the two reviewers did not reach an agreement, consultations and discussions on individual cases were held until the two reviewers reached a consensus.
This study was approved by the Ethics Committee of the Second Hospital of Shandong University. Written informed consent was obtained from each patient prior to enrollment in the present study.
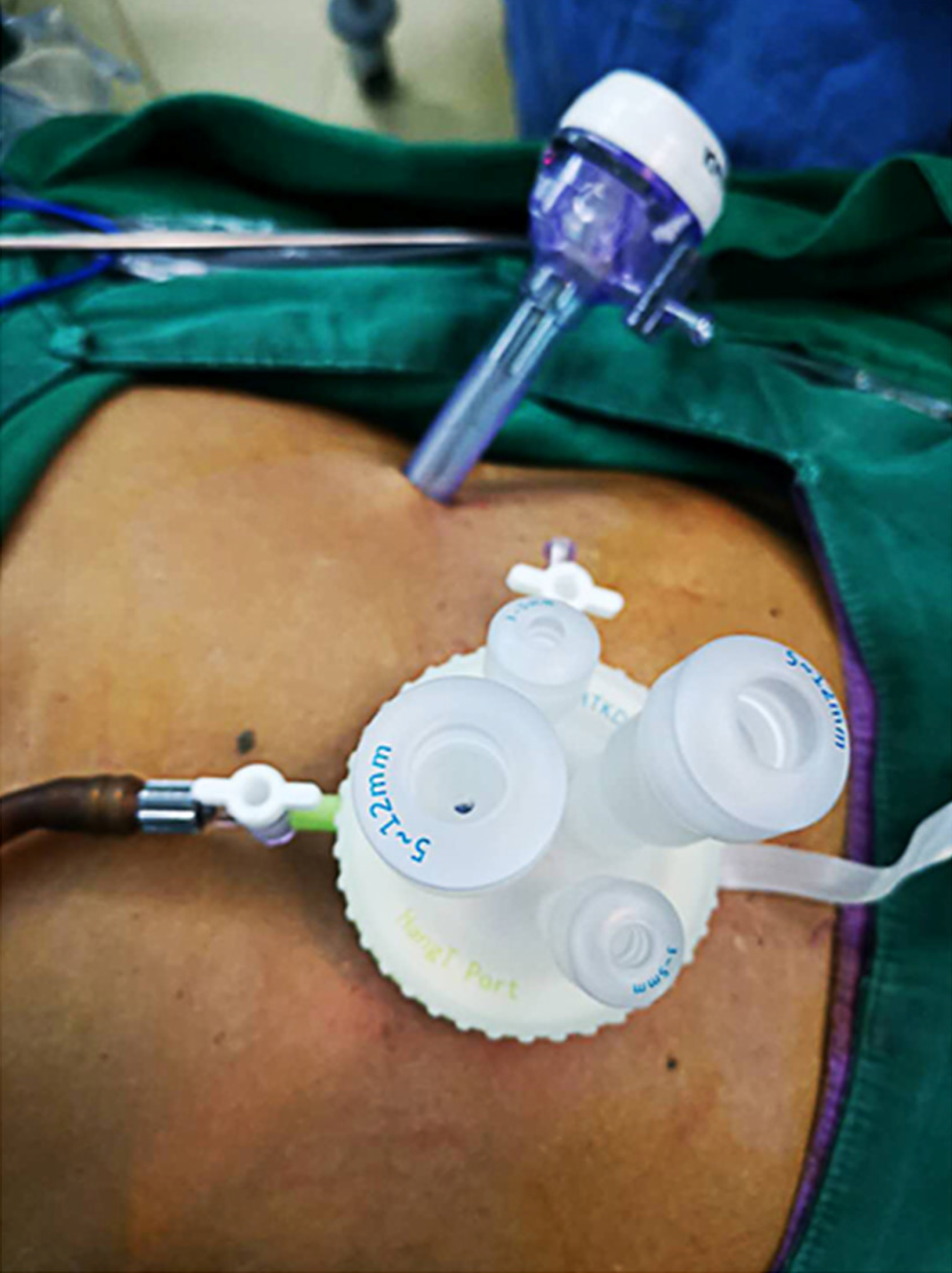
The patient was placed in a reverse Trendelenburg position under general anesthesia. A 3.0-3.5 cm incision was made around the navel to place the SILS portTM (Hangtian Kadi Technology, Beijing, China) with four in-built trocars. Another 12-mm trocar was placed in the left upper quadrant as operative port for the passage of the surgical instruments such as the ultrasonic shear, the endoscopic linear stapler and the postoperative drainage tube (Figure 1). A 10 mm scope was inserted through the 10 mm in-built trocar at the extreme caudal position of the SILS port for the duration of the operation. Other surgical instruments were inserted through the other three in-built trocars of the SILS port as necessary.
The operator and assistant stood on the left and right side of the patient respectively, and the scope operator was located between the patient’s legs, which could be spread out. The monitor screen was placed above the head of the patient. The surgeon used the left flank 12 mm port and either the extreme head-side 12 mm port or the left-side 5 mm trocar in the SILS portTM. The assistant used the right-side 5 mm trocar in the SILS portTM to provide support.
The falciform ligament was suspended and fixed at the anterior abdominal wall, drawing the round ligament away with polypropylene monofilament. All of the twenty-one patients underwent laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy, which was performed by the same operation team. After routine resection of the D2 group lymph nodes, the duodenum was cut 2 cm away from the pylorus, and the stomach was cut 5 cm from the upper edge of the tumor. Specimens were extracted from the umbilical port following a 3.0-3.5 cm vertical extension of the initial incision.
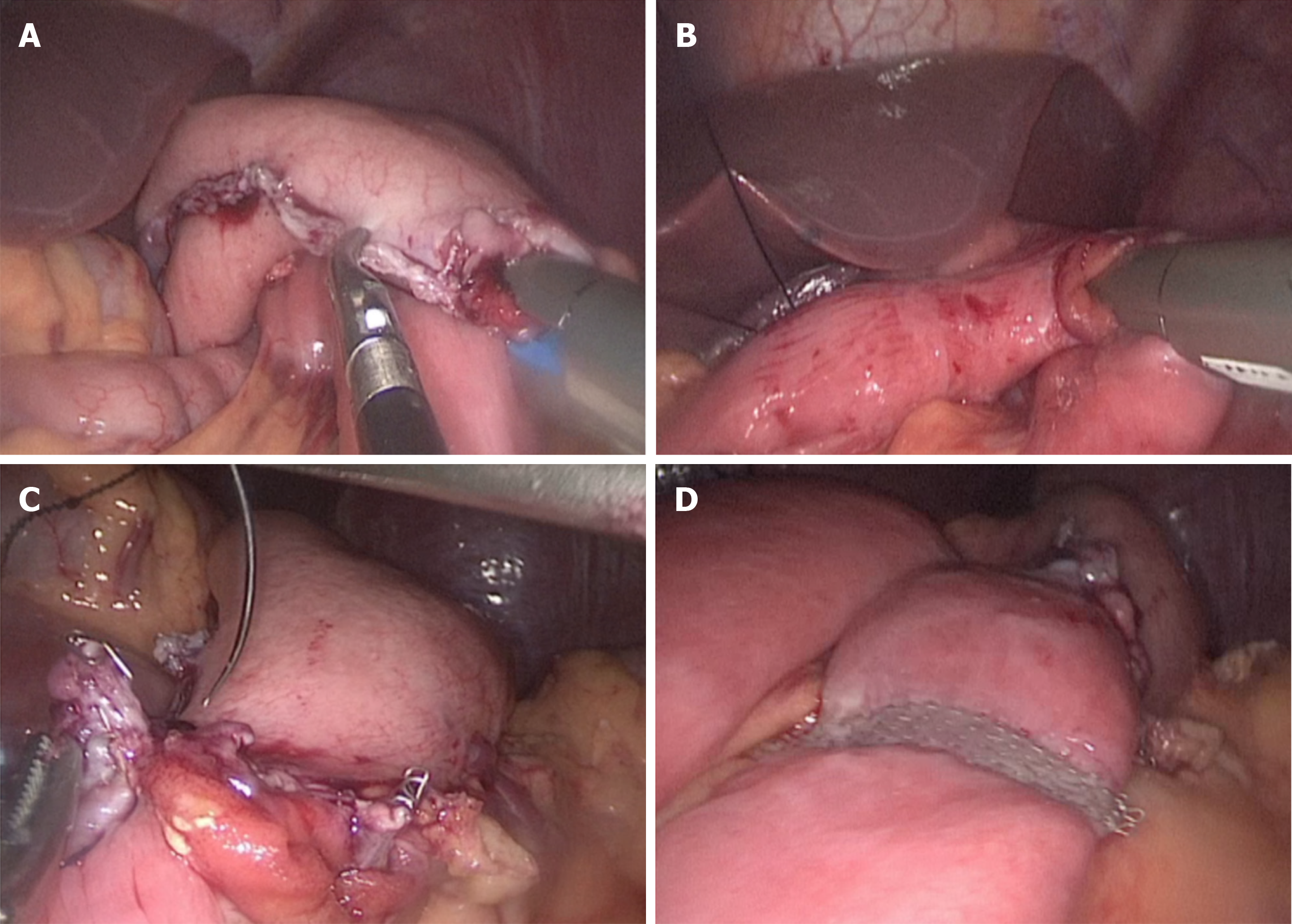
The uncut R-Y reconstruction was performed intracorporeally. The transverse colon was retracted cephalad to expose the ligament of Treitz. First, the gastrojejunal (GJ) anastomosis site was marked on the jejunum 20 cm distal to the ligament of Treitz and on the posterior wall of the greater curvature of the residual stomach. The GJ anastomosis was accomplished through the 12 mm port in the left upper quadrant, using 60 mm endoscopic linear stapler (Ethicon, United States), Then the jejunojejunal anastomosis site was marked 5 cm distal to the ligament of Treitz and 40 cm distal to the GJ anastomosis site. The jejunojejunal anastomosis with a length of 4 cm was made through the 12 mm port in the left upper quadrant using a 60 mm endoscopic linear stapler. Both the common openings of the GJ anastomosis and the jejunojejunal anastomosis were closed using 3-0 V-LOCTM thread (Covidien, Mansfield, MA, United States). Finally, the biliopancreatic limb was closed 3 cm proximal to the GJ anastomosis using a 45 mm non-knife stapler (Ethicon, United States) through the port in the left upper quadrant. The completed uncut R-Y reconstruction is shown in Figure 2A-D. The appearance of the abdomen after surgery is shown in Figures 3 and 4.
All patients received patient-controlled analgesia postoperatively. Nonsteroidal anti-inflammatory drugs were used when the patient needed additional pain control. Water was provided after the first exhaust, followed by liquid diet and then soft diet. The patient was discharged when the diet was acceptable, and there was no adverse reaction.
Data exploration was used as appropriate. All statistical analyses were performed using SPSS for Windows (ver.22.0; SPSS, Inc., Chicago, IL, United States).
The patient population included 13 men and 8 women with a mean age of 48.2 years (ranged from 40 years to 70 years) and median BMI of 22.8 kg/m2. All patients had no previous history of abdominal surgery or preoperative chemoradiotherapy. None of the patients needed conversion to laparotomy or additional port insertion. The mean length of the proximal and distal margins was 4.2 and 6.4 cm, respectively. The number of retrieved lymph nodes ranged from 30-47 with a mean of 42. Six patients were in stage I and 15 patients were in stage II.
All patients underwent D2 radical gastrectomy with uncut R-Y reconstruction. No intraoperative complications occurred in any cases. The mean operating time was 146 min (ranged 130-180 min), and the estimated mean blood loss was 54 mL (ranged 20-110 mL). The mean duration to flatus and discharge was 2.3 d (ranged 1-3.5 d) and 7.3 d (ranged 6-9 d), respectively. One patient experienced mild postoperative pancreatic fistula ileus (grade A) and was managed without medication or surgical intervention. One patient experienced surgical site infection (wound at the navel incision) and was treated conservatively. The other complications such as postoperative pneumonia, postoperative small bowel ileus, bleeding, duodenal residual leakage, anastomotic complications and postoperative mortality did not occur in our study.
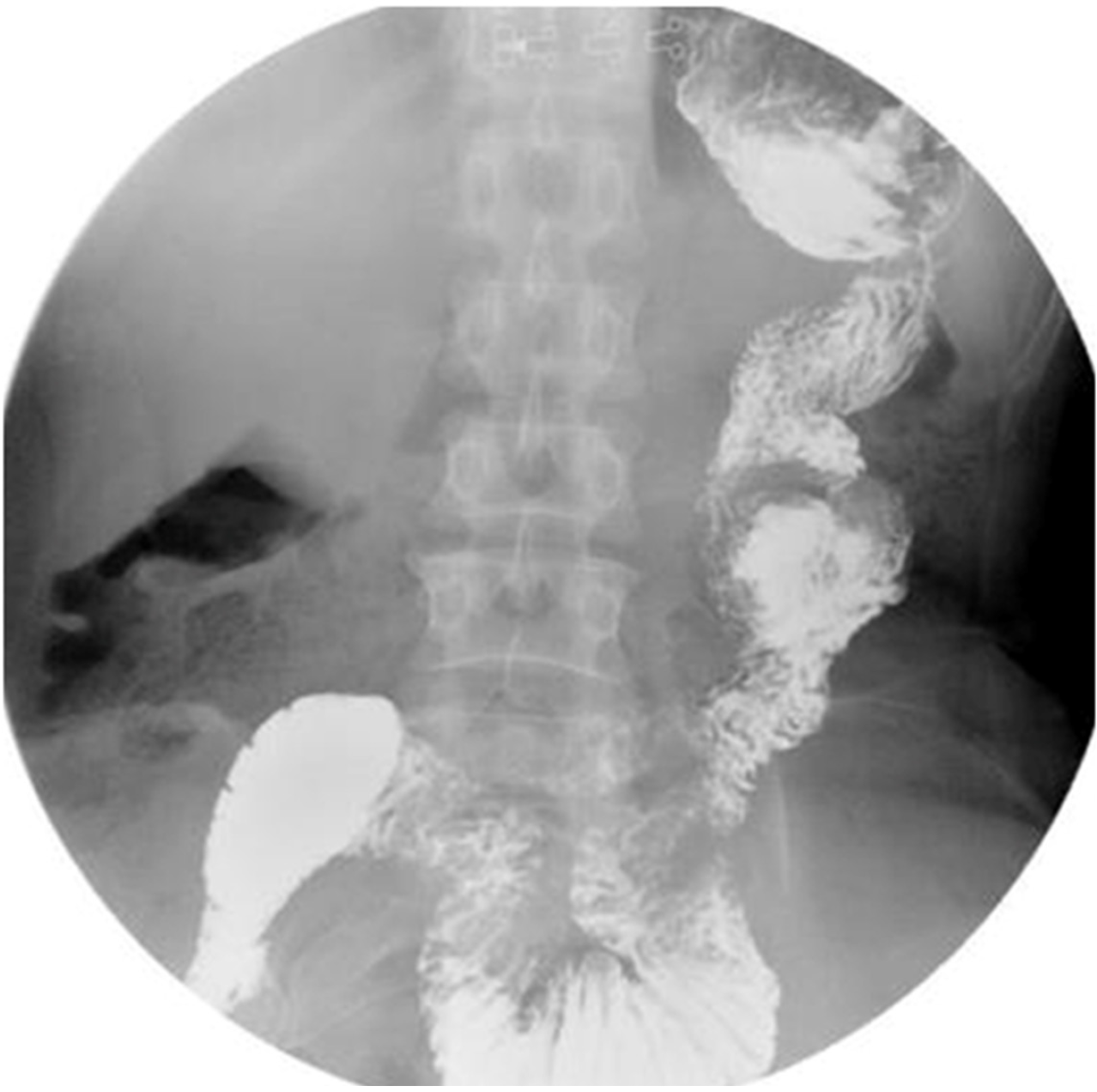
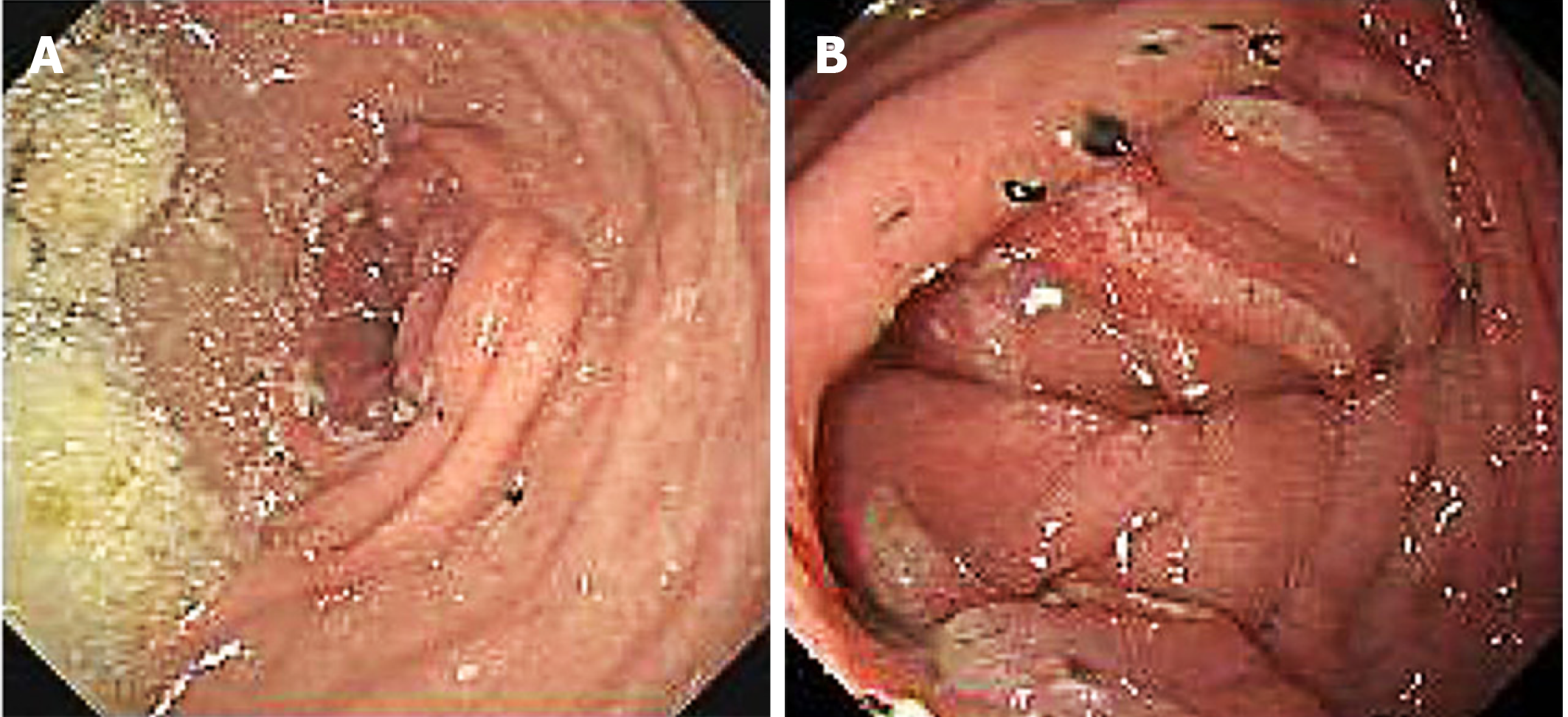
All patients underwent upper gastrointestinal imaging examination using oral meglumine diatrizoate and gastroscopy examination 3 mo after surgery. At the 3-mo postoperative follow-up, no jejunal recanalization of the uncut staple line was observed by upper gastrointestinal imaging examination (Figure 5). Endoscopic examination showed that minimal food stasis was present in two patients (Figure 6A). No patient had moderate or severe food stasis. One patient had alkaline gastritis around the GJ anastomosis (Figure 6B). There was no moderate or severe alkaline gastritis, and no patients had bile reflux.
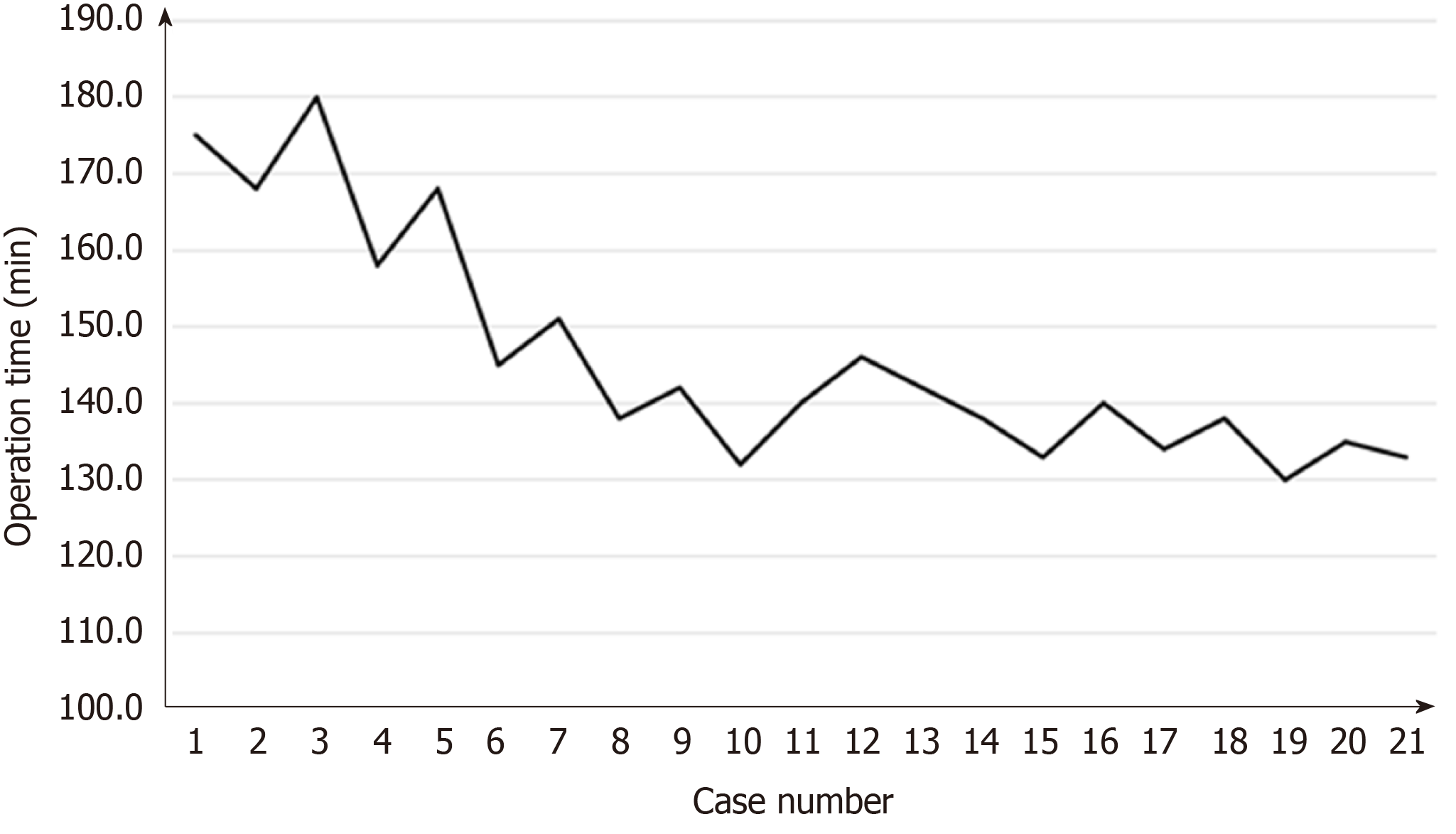
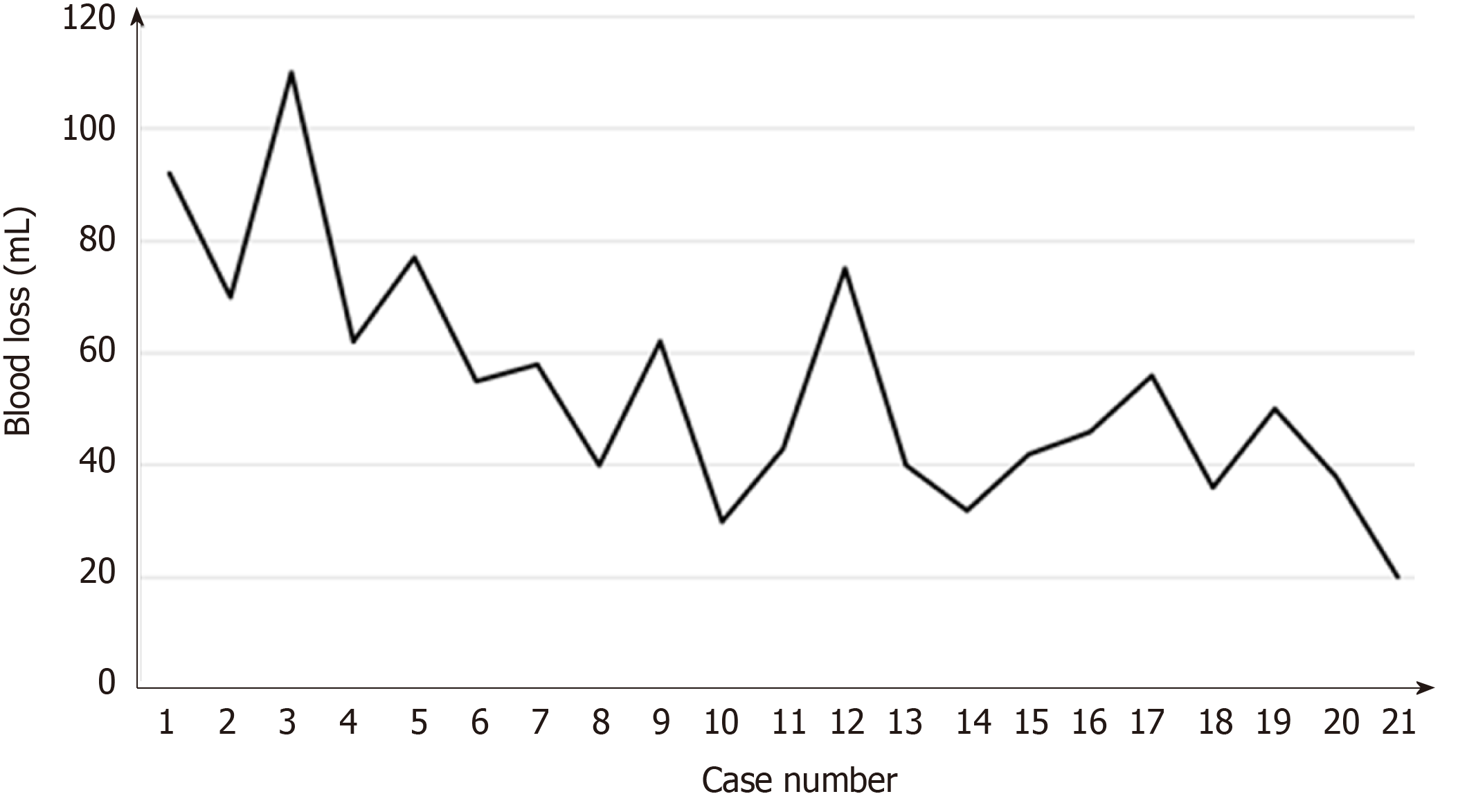
Surgical learning curve in SILS+1/L TLDG was reviewed. The data of operation time and blood loss were collected and plotted (Figures 7 and 8). With the case number increasing, operation time and blood loss gradually decreased, and the relatively stable plateau emerged. Experienced laparoscopic gastrectomy surgeons also need a long learning curve.
The digestive tract reconstruction procedure after distal gastrectomy is one of the most important factors that affects the quality of postoperative life. Although the reconstruction technique was a very hot spot issue in recent years, the optimal approach has not yet been proven by any trial or research yet. In most cases, B (Billroth)-I, B-II or R-Y are used[28,29]. The unique advantages and disadvantages of each method are determined by the location of the tumor, the progression of the cancer, the condition of the patient and the preference of the operator[30].
B-I gastroduodenal anastomosis is the most commonly used method for gastrointestinal reconstruction after distal gastrectomy[29]. However, this method requires enough remnant stomach to relieve anastomotic tension and has several disadvantages, including an increased incidence of food stasis, bile reflux, alkaline gastritis, dumping syndrome and reflux esophagitis[31-33]. Furthermore, the principle of tumor treatment should be fully considered. Once local recurrence occurs, a second opportunity for an operation may be lost.
B-II gastrojejunostomy is more suitable for extensive resection of advanced gastric antrum cancer and lymph node dissection. B-II type gastric jejunostomy is more likely to cause duodenal juice and bile reflux and residual gastritis. Roux-en-Y stomach jejunostomy can prevent these complications, may effectively control the blood sugar level of patients with type 2 diabetes and play a role in the treatment of diabetes. However, the additional anastomosis, the complexity of the procedure and the longer anastomosis time remain disadvantages of this technique. In addition, Roux stasis can occur in up to 30% of patients who undergo the R-Y procedure[34].
The uncut Roux-en-Y reconstruction preserves the advantages of Roux-en-Y gastrojejunostomy and builds a bridge that maintains neuromuscular continuity between the proximal jejunum and the Roux limb instead of cutting it off. Roux stasis is known to be an electrical and motor coordination problem that occurs after jejunal transection[35]. Uncut Roux-en-Y reconstruction may theoretically reduce the incidence of Roux retention syndrome[36].
Single-port and reduced-port laparoscopic radical gastrectomy as an innovative surgery for gastric cancer and has been gradually accepted and increasing attention has been paid to single-port and reduced-port laparoscopic radical gastrectomy due to its better cosmetic effect, less pain, lower incidence rate of surgical site infection and more advantages in enhanced recovery after surgery[37].
Pure SILS has some technical limitations such as collision between instruments and inadequate countertraction. As a type of reduced port surgery, SILS+1 is more convenient than pure SILS. In the setting of SILS+1/L, the umbilical multichannel port is used to extend the lens, through which surgeons can manipulate forceps into the abdominal cavity for the operation with their left hand, and their right hand can manipulate energy instrument through the auxiliary port in the left upper quadrant. In Japan, surgeons are accustomed to adopting single-incision laparoscopic surgery plus one port in the right upper quadrant[38]. Therefore, the auxiliary operation port is set at 2 cm below the costal margin on the right side of the clavicle midline. The dis-advantage of SILS+1 in the right upper quadrant is that the lymph node dissection in the subpyloric area is too close and the operation is inconvenient; the omentum is difficult to disperse without the help of an assistant. When standing on the right side, the energy instrument and lens in the surgeon’s right hand are passed through the multichannel puncture device, which is relatively close and prone to rear-end collisions. Conversely, the port in the upper left abdomen is the main operation hole in the case of SILS+1/L. The energy instrument and other surgical devices can enter the abdominal cavity through this hole and maintain a certain distance from the lens. Thus, this approach makes some surgical procedures, such as lymph node dissection and digestive tract reconstruction, easier without the need to replace instruments with the left and right hands.
In this study, no severe postoperative complications were found according to the Clavien-Dindo classification of surgical complications. Minimal to mild and limited gastritis were seen in 4.8% of the patients, and no patients had bile reflux. Food stasis was minimal or mild (9.5% of patients), and there was no moderate or severe food stasis. Moreover, no patients complained of nausea, vomiting or postprandial pain during the follow-up period. Our results are similar to the study by Yang et al[39]. This suggests that an uncut R-Y reconstruction after laparoscopic distal gastrectomy maintains the advantages of conventional R-Y in reducing the incidence of gastritis and bile acid reflux except for preventing roux stasis. In addition, we did not find any recanalization of the jejunum-jejunum anastomosis during the postoperative follow-up.
Standardized lymph node dissection and a sufficient number of lymph nodes for pathological examinations are the prerequisite and basis for radical surgery for gastric cancer. The number of lymph nodes recommended for pathological tumor, node, metastasis staging of gastric cancer in the 8th edition of American Joint Cancer Committee is ≥ 16[27]. In this study, the average number of retrieved lymph nodes was 42. This number meets the needs of judging tumor, node, metastasis stage in terms of oncology.
In conclusion, SILS+1/L with the uncut R-Y digestive reconstruction procedure in TLDG is secure and feasible. The uncut technique can be done by experienced surgeons in large medical center for patients with gastric cancer. Additional long-term follow-up is needed to evaluate the tumor effect compared to conventional laparoscopic radical gastrectomy.
Single-incision laparoscopic surgery plus one port (SILS+1), in which fewer ports and shorter length of incisions are needed, has become increasingly popular in the past few years. However, the safety of SILS+1 left-side approach (SILS+1/L) totally laparoscopic distal gastrectomy (TLDG) with uncut Roux-en-Y reconstruction is not clear.
An analysis of an uncut R-Y reconstruction method after SILS+1 has not been carried out. To the best of our knowledge, there are no previous reports on the use of SILS+1/L TLDG with uncut R-Y reconstruction.
This study aimed to evaluate the safety and feasibility of SILS+1/L with the uncut R-Y digestive reconstruction procedure in TLDG. This report is to present our initial experience in performing SILS+1/L TLDG with uncut R-Y reconstruction as well as the short-term postoperative outcomes and endoscopic findings.
The statistics of 21 patients who had undergone SILS+1/L TLDG with uncut R-Y anastomosis reconstruction to treat distal gastric cancer were collected. Data exploration was used as appropriate. All statistical analyses were performed using SPSS for Windows.
The mean operating time was 146 min (ranged 130-180 min), and the estimated mean blood loss was 54 mL (ranged 20-110 mL). The mean length of the proximal and distal margins was 4.2 and 6.4 cm, respectively. The number of retrieved lymph nodes ranged from 30-47 with a mean of 42. One patient experienced mild postoperative pancreatic fistula ileus, and one patient experienced surgical site infection. Other complications did not occur in our study.
We found that SILS+1/L TLDG with uncut Roux-en-Y reconstruction is safe and effective and should be popularized.
From this study, we can found that SILS+1/L TLDG with uncut R-Y reconstruction can be used not only for colon surgery but also for gastric surgery. In the future, the direction of the research is that an effective perioperative management program specific for gastric cancer is developed. The best method is to conduct a large-scale clinical trial to verify it.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Motoyama K S-Editor: Liu JH L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Zheng XH, Zhang W, Yang L, Du CX, Li N, Xing GS, Tian YT, Xie YB. Role of D2 gastrectomy in gastric cancer with clinical para-aortic lymph node metastasis. World J Gastroenterol. 2019;25:2338-2353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55722] [Article Influence: 7960.3] [Reference Citation Analysis (132)] |
| 3. | Liao T, Deng L, Yao X, Ouyang M. Comparison of the safety and efficacy between linear stapler and circular stapler in totally laparoscopic total gastrectomy: protocol for a systematic review and meta-analysis. BMJ Open. 2019;9:e028216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 288] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306-S311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 459] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 9. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 522] [Article Influence: 87.0] [Reference Citation Analysis (1)] |
| 10. | Kim SM, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, Bae JM, Kim S, Lee JH. Comparison of Reduced Port Totally Laparoscopic Distal Gastrectomy (Duet TLDG) and Conventional Laparoscopic-Assisted Distal Gastrectomy. Ann Surg Oncol. 2015;22:2567-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Omori T, Fujiwara Y, Moon J, Sugimura K, Miyata H, Masuzawa T, Kishi K, Miyoshi N, Tomokuni A, Akita H, Takahashi H, Kobayashi S, Yasui M, Ohue M, Yano M, Sakon M. Comparison of Single-Incision and Conventional Multi-Port Laparoscopic Distal Gastrectomy with D2 Lymph Node Dissection for Gastric Cancer: A Propensity Score-Matched Analysis. Ann Surg Oncol. 2016;23:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kim SM, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, Bae JM, Kim S, Lee JH. Comparison of single-port and reduced-port totally laparoscopic distal gastrectomy for patients with early gastric cancer. Surg Endosc. 2016;30:3950-3957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kunisaki C, Makino H, Yamaguchi N, Izumisawa Y, Miyamato H, Sato K, Hayashi T, Sugano N, Suzuki Y, Ota M, Tsuburaya A, Kimura J, Takagawa R, Kosaka T, Ono HA, Akiyama H, Endo I. Surgical advantages of reduced-port laparoscopic gastrectomy in gastric cancer. Surg Endosc. 2016;30:5520-5528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Liu R, Wang Y, Zhang Z, Li T, Liu H, Zhao L, Deng H, Li G. Assessment of treatment options for rectosigmoid cancer: single-incision plus one port laparoscopic surgery, single-incision laparoscopic surgery, and conventional laparoscopic surgery. Surg Endosc. 2017;31:2437-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Uyama I, Sakurai Y, Komori Y, Nakamura Y, Syoji M, Tonomura S, Yoshida I, Masui T, Inaba K, Ochiai M. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer. Gastric Cancer. 2005;8:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 16. | Shim JH, Oh SI, Yoo HM, Jeon HM, Park CH, Song KY. Roux-en-Y gastrojejunostomy after totally laparoscopic distal gastrectomy: comparison with Billorth II reconstruction. Surg Laparosc Endosc Percutan Tech. 2014;24:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Park JY, Kim YJ. Uncut Roux-en-Y Reconstruction after Laparoscopic Distal Gastrectomy Can Be a Favorable Method in Terms of Gastritis, Bile Reflux, and Gastric Residue. J Gastric Cancer. 2014;14:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Ma JJ, Zang L, Yang A, Hu WG, Feng B, Dong F, Wang ML, Lu AG, Li JW, Zheng MH. A modified uncut Roux-en-Y anastomosis in totally laparoscopic distal gastrectomy: preliminary results and initial experience. Surg Endosc. 2017;31:4749-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Wang Q, Ni Q, Yang K, Ji S, Fan Y, Wang C, Zhang W, Yan S, Ma Q, Wei Q, Zhang D, Yu J, Ji G. Laparoscopic uncut Roux-en-Y for radical distal gastrectomy: the study protocol for a multirandomized controlled trial. Cancer Manag Res. 2019;11:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | In Choi C, Baek DH, Lee SH, Hwang SH, Kim DH, Kim KH, Jeon TY, Kim DH. Comparison Between Billroth-II with Braun and Roux-en-Y Reconstruction After Laparoscopic Distal Gastrectomy. J Gastrointest Surg. 2016;20:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Huang Y, Wang S, Shi Y, Tang D, Wang W, Chong Y, Zhou H, Xiong Q, Wang J, Wang D. Uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 22. | Kim HO, Choi DJ, Lee D, Lee SR, Jung KU, Kim H, Chun HK. Hybrid Single-Incision Laparoscopic Colon Cancer Surgery Using One Additional 5 mm Trocar. J Laparoendosc Adv Surg Tech A. 2018;28:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Li J, Wang Y, Liu D, Zhou H, Mou T, Li G, Deng H. Multidimensional analyses of the learning curve for single-incision plus one port laparoscopic surgery for sigmoid colon and upper rectal cancer. J Surg Oncol. 2018;117:1386-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Hirano Y, Hattori M, Douden K, Shimada M, Hashizume Y. Short-Term Clinical and Oncological Outcomes after Single-Incision Plus One Port Laparoscopic Anterior Resection for Rectal Cancer. Dig Surg. 2018;35:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Du G, Jiang E, Qiu Y, Wang W, Wang S, Li Y, Peng K, Li X, Yang H, Xiao W. [Feasibility and preliminary technical experience of single incision plus one port laparoscopic total gastrectomy combined with π-shaped esophagojejunal anastomosis in surgical treatment of gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Deng H, Mou T, Li J, Liu H, Zhou H, Li G. Short-term outcomes of single-incision plus one-port laparoscopic versus conventional laparoscopic surgery for rectosigmoid cancer: a randomized controlled trial. Surg Endosc. 2019;33:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Amin MB, Edge SB, Greene FL. AJCC Cancer Staging Manual. 8th ed. New York: Springer 2016; 203-220. |
| 28. | Seo HS, Jung YJ, Kim JH, Park CH, Lee HH. Three-Port Right-Side Approach-Duet Totally Laparoscopic Distal Gastrectomy for Uncut Roux-en-Y Reconstruction. J Laparoendosc Adv Surg Tech A. 2018;28:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Chen XJ, Chen YZ, Chen DW, Chen YL, Xiang J, Lin YJ, Chen S, Peng JS. The Development and Future of Digestive Tract Reconstruction after Distal Gastrectomy: A Systemic Review and Meta-Analysis. J Cancer. 2019;10:789-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Lee J, Kim D, Kim W. Comparison of laparoscopy-assisted and totally laparoscopic Billroth-II distal gastrectomy for gastric cancer. J Korean Surg Soc. 2012;82:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M, Doki Y; Osaka University Clinical Research Group for Gastroenterological Study. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Nunobe S, Okaro A, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Billroth 1 versus Roux-en-Y reconstructions: a quality-of-life survey at 5 years. Int J Clin Oncol. 2007;12:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Gustavsson S, Ilstrup DM, Morrison P, Kelly KA. Roux-Y stasis syndrome after gastrectomy. Am J Surg. 1988;155:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Tu BN, Kelly KA. Elimination of the Roux stasis syndrome using a new type of "uncut Roux" limb. Am J Surg. 1995;170:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Yan S, Ma XF, Zhao K. An analysis of technical difficulties of single-port and reduced-port laparoscopic radical gastrectomy for gastric cancer. Chin J Dig Surg. 2019;18:222-228. [DOI] [Full Text] |
| 38. | Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg. 2015;8:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. 2017;23:6350-6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |