Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4442
Peer-review started: April 9, 2020
First decision: May 26, 2020
Revised: July 8, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 14, 2020
Processing time: 127 Days and 8.6 Hours
Transarterial chemoembolization (TACE) is the first-line treatment for patients with unresectable liver cancer; however, TACE is associated with postembolization pain.
To analyze the risk factors for acute abdominal pain after TACE and establish a predictive model for postembolization pain.
From January 2018 to September 2018, all patients with liver cancer who underwent TACE at our hospital were included. General characteristics; clinical, imaging, and procedural data; and postembolization pain were analyzed. Postembolization pain was defined as acute moderate-to-severe abdominal pain within 24 h after TACE. Logistic regression and a classification and regression tree were used to develop a predictive model. Receiver operating characteristic curve analysis was used to examine the efficacy of the predictive model.
We analyzed 522 patients who underwent a total of 582 TACE procedures. Ninety-seven (16.70%) episodes of severe pain occurred. A predictive model built based on the dataset from classification and regression tree analysis identified known invasion of blood vessels as the strongest predictor of subsequent performance, followed by history of TACE, method of TACE, and history of abdominal pain after TACE. The area under the receiver operating characteristic curve was 0.736 [95% confidence interval (CI): 0.682-0.789], the sensitivity was 73.2%, the specificity was 65.6%, and the negative predictive value was 92.4%. Logistic regression produced similar results by identifying age [odds ratio (OR) = 0.971; 95%CI: 0.951-0.992; P = 0.007), history of TACE (OR = 0.378; 95%CI: 0.189-0.757; P = 0.007), history of abdominal pain after TACE (OR = 6.288; 95%CI: 2.963-13.342; P < 0.001), tumor size (OR = 1.978; 95%CI: 1.175-3.330; P = 0.01), multiple tumors (OR = 2.164; 95%CI: 1.243-3.769; P = 0.006), invasion of blood vessels (OR = 1.756; 95%CI: 1.045-2.950; P = 0.034), and TACE with drug-eluting beads (DEB-TACE) (OR = 2.05; 95%CI: 1.260-3.334; P = 0.004) as independent predictive factors for postembolization pain.
Blood vessel invasion, TACE history, TACE with drug-eluting beads, and history of abdominal pain after TACE are predictors of acute moderate-to-severe pain. The predictive model may help medical staff to manage pain.
Core tip: Transarterial chemoembolization (TACE) is associated with postembolization pain. We analyzed the risk factors for acute abdominal pain after TACE and established a predictive model for it. The predictive model built based on the dataset from a classification and regression tree identified known invasion of blood vessels as the strongest predictor of subsequent performance, followed by history of TACE, method of TACE, and history of abdominal pain after TACE. Our predictive model is simple to use and provides a more rational reference to improve the quality of pain management after TACE.
- Citation: Bian LF, Zhao XH, Gao BL, Zhang S, Ge GM, Zhan DD, Ye TT, Zheng Y. Predictive model for acute abdominal pain after transarterial chemoembolization for liver cancer. World J Gastroenterol 2020; 26(30): 4442-4452
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4442
Primary liver cancer (PLC) is the seventh most common carcinoma worldwide and the third most common cause of cancer-related mortality[1]. More than half of new cases of liver cancer occur in China. Transarterial chemo-embolization (TACE) is the most widely used treatment for unresectable PLC. TACE plays an important role in the treatment of tumors, improving quality of life and prolonging patient survival[2]. According to statistics, more than 600000 people undergo TACE in China each year[3].
TACE is a procedure that consists of local delivery of a high dose of chemotherapeutic agents to the tumor, which can be associated with particulate and/or oily embolization of feeding arteries, which results in exposure of the tumor to a higher concentration of chemotherapeutic agent and subsequent tumor infarction and necrosis due to vascular occlusion[4,5]. Postembolization syndrome, which is characterized by abdominal pain, nausea, vomiting, and fever, is the most frequently reported adverse event after TACE[6]. Approximately 60%-80% of patients complained of different levels of pain after TACE. Among those patients, more than 25% experienced moderate-to-severe pain[7,8]. While TACE is generally understood to require hospital admission and at least a one-night in-patient stay[9], postembolization pain is primarily associated with an extended hospital stay[10]. Painkillers, such as opioids, are effective and safe[11]. Clearly identifying factors associated with postembolization pain could help predict its occurrence and improve analgesic treatment.
At present, few studies have examined the related risk factors or predictive models for postembolization pain after TACE; thus, no conclusions about the risk factors for postembolization pain have been reached. The present study aimed to evaluate the risk factors for postembolization pain and to establish a predictive model for postembolization pain in patients undergoing TACE.
This single-center retrospective cohort study was approved as an expedited chart review study and obtained ethical approval from the institutional review board at our hospital.
Patients with PLC who underwent TACE at our hospital between January 2018 and September 2018 were analyzed retrospectively. Some patients underwent the procedure more than once during this period. A diagnosis of liver cancer was confirmed either histologically or based on consistent findings obtained from at least two imaging techniques, including ultrasonography, computed tomography, magnetic resonance imaging, and selective hepatic arterial angiography[12]. The exclusion criteria were: (1) PLC in patients aged < 18 years; (2) Emergency embolization for rupture of liver cancer; (3) Severe complications such as bleeding after TACE; (4) Use of additional analgesics to relieve increased pain during TACE; (5) Cognitive impairment; (6) Use of psychiatric medications; and (7) Drug or alcohol abuse.
All procedures were performed at a single tertiary center by board-certified interventional radiologists. All patients were administered with 10 mL of 2% lidocaine to achieve local anesthesia, and 5 mg of dezocine during surgery. An arterial catheter was inserted into the femoral artery using the Seldinger technique and subsequently placed in the hepatic artery. Tumor-feeding vessels were super-selected whenever possible. Chemotherapy drugs used were pirarubicin hydrochloride (10 mg/bottle; Shenzhen Main Luck Pharmaceuticals Inc., Shenzhen, China) at a dose of 30 mg mixed with Lipiodol (Laboratoire Guerbet, Aulnay-sous-Bois, France) for TACE, or at 60 mg for drug-eluting beads (DEB-TACE) treatment. Oxaliplatin (150 mg; Jiangsu Hengrui Medicine Co., Ltd.) was used for arterial perfusion chemotherapy. Lipiodol and/or polyvinyl alcohol particles (350-510 μm; Ailikang Medicine Co., Ltd, Hangzhou, China) and Embosphere (Biosphere Medical, Rockland, MA, United States) were used as embolization materials. All patients received supportive treatment after TACE, including non-steroidal anti-inflammatory drugs or dezocine, liver protection, antacid agents, and antiemetics. If moderate-to-severe abdominal pain was observed, the patient received tramadol (100 mg) or other opioids by intravenous administration.
The aim of our study was to analyze the risk factors that helped to predict moderate-to-severe postembolization pain. The numerical rating scale pain scores at rest were assessed in all patients within 24 h of embolization. The numerical rating scale pain score was used as the standard subjective evaluation using a score of 0-10, where 0 = painless; < 3 = mild pain; 4-6 = moderate pain; and 7-10 = severe pain. Additional painkillers were administered when the pain score was ≥ 4. The pain scores were recorded 0, 2, 4, 6, 12, and 24 h after TACE. The highest score through the overall time was defined as a pain score in each patient.
Independent variables included demographics and clinical, imaging, and procedural data. Seventeen registered variables were included for each TACE procedure, including: Age and gender of the patient; tumor location (distance to liver capsule); tumor size and number; pathological properties of the tumor; invasion of blood vessels; disease type; history and number of TACE procedures; history of postembolization pain; drug delivery method (traditional TACE vs DEB-TACE); dosage of lipiodol; and complementary embolization (blank microsphere and/or polyvinyl alcohol particles; and postoperative prophylactic analgesics).
Quantitative data are described as the mean ± standard deviation or as medians (min, max). Qualitative data are described by the number of cases (proportion, %). Patient characteristics were compared using the χ2 test or Fisher’s exact test for categorical data, and the Wilcoxon rank-sum test or t-test were used to analyze continuous data. Two statistical methods were used to develop the predictive model: A primary analysis using classification and regression tree (CART), and a conjoint predictive model using logistic regression. In this research, cross-validation was used to select the regression model in which the mean cross-validated error was within one standard error of the minimum. The area under the receiver operating characteristic (ROC) curve, specificity, sensitivity, and negative predictive value of the predictive model were calculated by ROC curve analysis to evaluate its performance. Two-sided P values of < 0.05 indicated statistical significance. Analyses were conducted using statistical software (IBM SPSS Statistics 22.0).
A total of 522 patients who underwent a total of 582 TACE procedures were enrolled in the study. Patient demographics, baseline clinical and laboratory data, and procedural details are listed in Table 1 and 2. As shown in Table 1, the age range of patients was 23-87 years (average, 60.1 ± 11.4 years). The median number of TACE procedures in the 582 patients was two. As shown in Table 2, the data set comprised 81 females (13.9%) and 501 males (86.1%). Ninety-seven (16.7%) patients had acute moderate-to-severe abdominal pain after TACE. A total of 57.2% (333/582) of patients had a history of TACE and 12.5% (73/582) had a history of abdominal pain after TACE. Blood vessel invasion occurred in 176 (30.2%) patients. Approximately 57.7% (336/582) of patients used traditional TACE and 42.3% (246) used DEB-TACE.
| Variable | n | mean ± SD | Median (IQR) | Min | Max |
| Age (yr) | 582 | 60.1 ± 11.4 | 60 (53, 67) | 23 | 87 |
| Number of TACE | 582 | 2.5 ± 2.2 | 2 (1, 3) | 1 | 15 |
| Dose of Lipiodol (mL) | 582 | 4.7 ± 6.8 | 3 (0, 6) | 0 | 30 |
| Variable | n | % |
| Sex | ||
| Female | 81 | 13.9 |
| Male | 501 | 86.1 |
| Disease type | ||
| No surgery | 371 | 63.7 |
| Recurrence after surgery | 211 | 36.3 |
| TACE history | 333 | 57.2 |
| History of abdominal pain after TACE | 73 | 12.5 |
| Pathological properties | ||
| HCC | 280 | 48.1 |
| ICC | 13 | 2.2 |
| Unknown | 265 | 45.5 |
| Other | 24 | 4.1 |
| Tumor size | ||
| ≤ 5 cm | 334 | 57.4 |
| > 5 cm | 248 | 42.6 |
| Number of tumors | ||
| ≤ 2 (non-multiple) | 197 | 33.8 |
| > 2 (multiple) | 385 | 66.2 |
| Invasion of blood vessels | 176 | 30.2 |
| Blank microsphere | 138 | 23.7 |
| PVA particles | 63 | 10.8 |
| Method of TACE | ||
| Traditional TACE | 336 | 57.7 |
| DEB-TACE | 246 | 42.3 |
| Prophylactic analgesics | ||
| Tenay | 74 | 12.7 |
| Kaffin | 112 | 19.2 |
| Dezocine | 252 | 43.3 |
| Pentam | 29 | 5 |
| None | 58 | 10 |
| Two painkillers | 57 | 9.8 |
| Pain after TACE | ||
| No pain/mild pain | 485 | 83.3 |
| Moderate pain/severe pain | 97 | 16.7 |
The results of the univariate analysis are shown in Table 3. Younger patients (P = 0.002) and those patients who had not undergone hepatectomy (P = 0.010) were more likely to have acute moderate-to-severe abdominal pain after TACE compared with older patients and those who had tumor recurrence after hepatectomy. History of TACE (P < 0.001), history of abdominal pain after TACE (P < 0.001), tumor size (P < 0.001), tumor number (P = 0.010), invasion of blood vessels (P < 0.001), use of the DEB-TACE method (P < 0.001), and the number of TACE procedures (P < 0.001) were significantly associated with moderate-to-severe abdominal pain. The pathological properties of the tumor was not associated with moderate-to-severe abdominal pain.
| Variable | No pain/mild pain (n = 485) | Moderate pain/severe pain (n = 97) | P value |
| Age (yr), mean ± SD | 60.79 ± 11.39 | 56.87 ± 10.99 | 0.002 |
| Sex (%) | 0.870 | ||
| Female | 68 (14) | 13 (13.4) | |
| Male | 417 (86) | 84 (86.6) | |
| Disease type (%) | 0.010 | ||
| No surgery | 298 (61.4) | 73 (75.3) | |
| Recurrence after surgery | 187 (38.6) | 24 (24.7) | |
| TACE history (%) | 293 (60.4) | 40 (41.2) | < 0.001 |
| History of abdominal pain after TACE (%) | 51 (10.5) | 22 (22.7) | < 0.001 |
| Pathological properties (%) | 0.110 | ||
| HCC | 231 (47.6) | 49 (50.5) | |
| ICC | 8 (1.6) | 5 (5.2) | |
| Unknown | 224 (46.2) | 41 (42.3) | |
| Other | 22 (4.5) | 2 (2.1) | |
| Tumor location (distance to liver capsule) (%) | 0.085 | ||
| > 1 cm | 245 (81.40) | 50 (90.91) | |
| ≤ 1 cm | 56 (18.60) | 5 (9.09) | |
| Tumor size (%) | < 0.001 | ||
| ≤ 5 cm | 294 (60.6) | 40 (41.2) | |
| > 5 cm | 191 (39.4) | 57 (58.8) | |
| Number of tumors (%) | 0.010 | ||
| ≤ 2 (non-multiple) | 175 (36.1) | 22 (22.7) | |
| > 2 (multiple) | 310 (63.9) | 75 (77.3) | |
| Invasion of blood vessels (%) | 131 (27) | 45 (46.4) | < 0.001 |
| Blank microsphere (%) | 112 (23.1) | 26 (26.8) | 0.430 |
| Polyvinyl alcohol particles (%) | 54 (11.1) | 9 (9.3) | 0.590 |
| Method of TACE (%) | < 0.001 | ||
| Traditional TACE | 296 (61) | 40 (41.2) | |
| DEB-TACE | 189 (39) | 57 (58.8) | |
| Prophylactic analgesics (%) | 0.780 | ||
| Parecoxib Na | 58 (12) | 16 (16.5) | |
| Flurbiprofen | 97 (20) | 15 (15.5) | |
| Dezocine | 209 (43.1) | 43 (44.3) | |
| Pentazocine | 24 (4.9) | 5 (5.2) | |
| None | 48 (9.9) | 10 (10.3) | |
| Two painkillers | 49 (10.1) | 8 (8.2) | |
| Number of TACE, median (IQR) | 2 (1, 3) | 1 (1, 2) | < 0.001 |
| Dosage of lipiodol, median (IQR) | 3 (0, 6) | 0 (0, 8) | 0.179 |
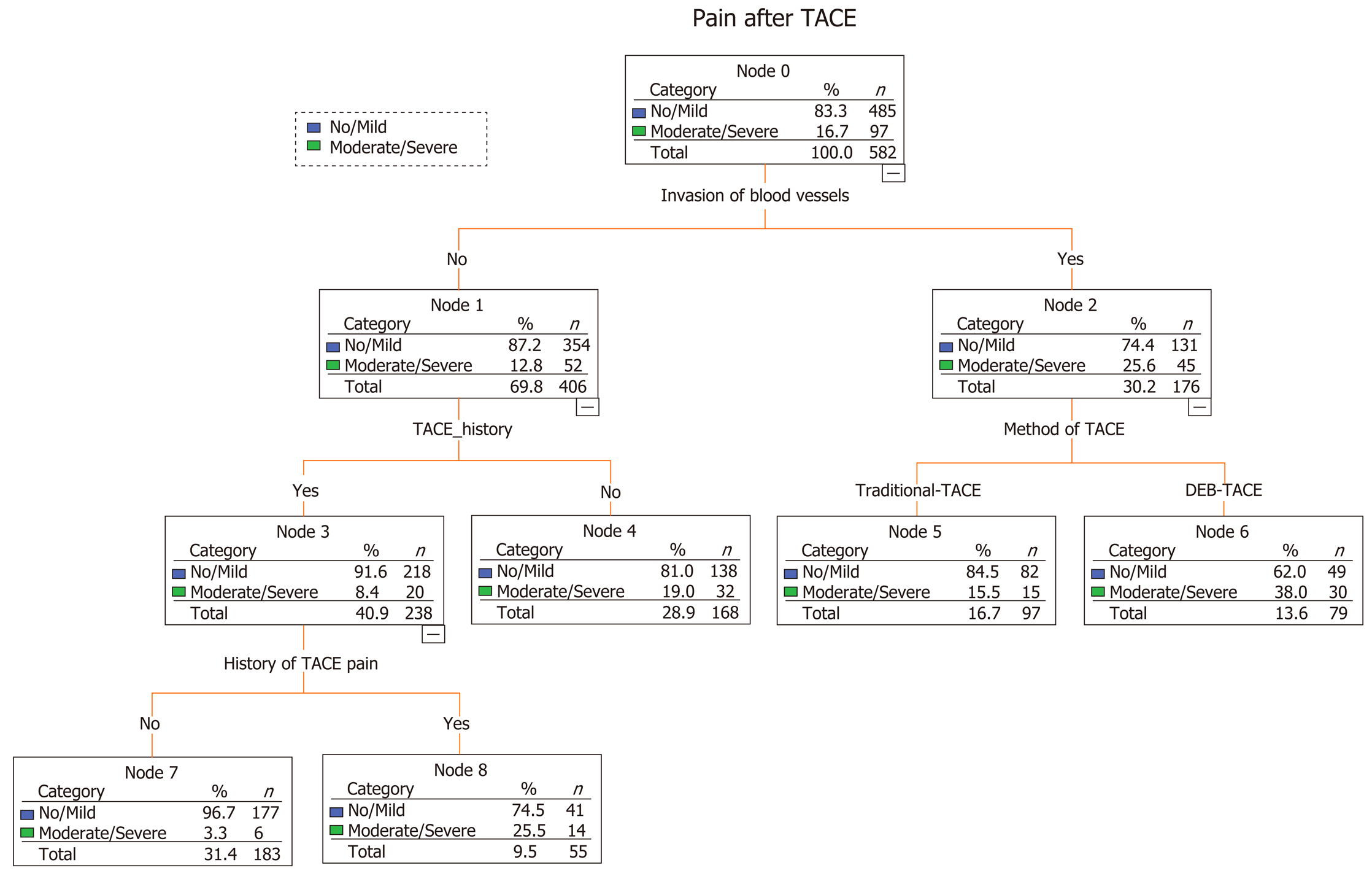
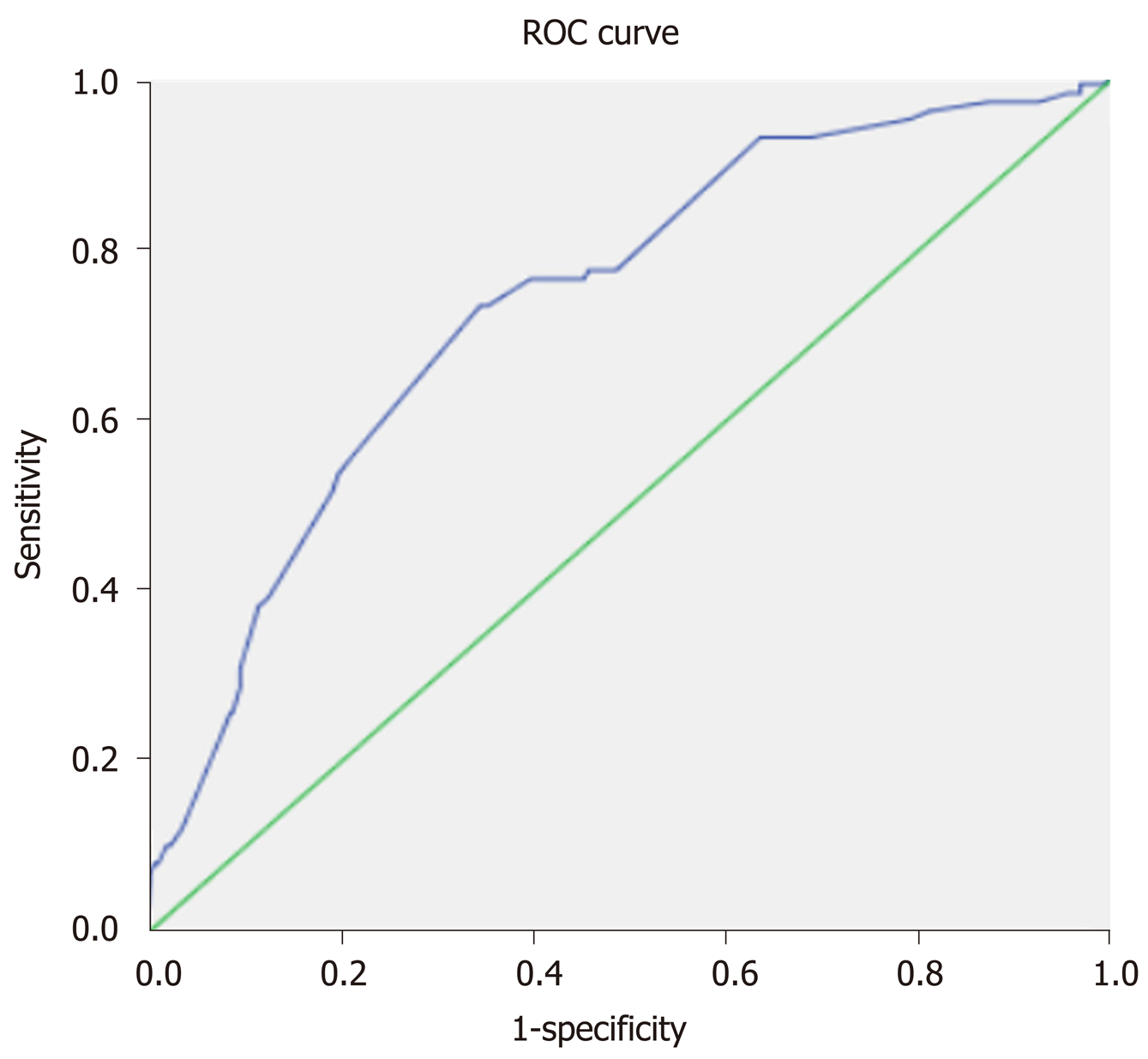
A predictive model built based on the dataset from the classification and regression trees identified known blood vessel invasion as the strongest predictor of subsequent performance, followed by history of TACE, method of TACE, and history of abdominal pain after TACE (Figure 1). We used ROC curve analysis to examine the efficacy of the predictive model. We set an optimal predictive probability threshold of 0.18, and demonstrated a sensitivity of 73.2% (71/97; 95% confidence interval [CI]: 64.4%-82.0%), specificity of 65.6% (318/485; 95%CI: 61.3%-69.8%), negative predictive value of 92.4% (318/344; 95%CI: 89.6%-95.2%), and area under the curve of 0.736 (95%CI: 0.682-0.789) (Figure 2). Logistic regression produced similar results by identifying age (odds ratio [OR] = 0.971; 95%CI: 0.951-0.992; P = 0.007), history of TACE (OR = 0.378; 95%CI: 0.189-0.757; P = 0.007), history of abdominal pain after TACE (OR = 6.288; 95%CI: 2.963-13.342; P < 0.001), tumor size (OR = 1.978; 95%CI: 1.175-3.330; P = 0.01), multiple tumors (OR = 2.164; 95%CI: 1.243-3.769; P = 0.006), blood vessel invasion (OR = 1.756; 95%CI: 1.045-2.950; P = 0.034), and the DEB-TACE method (OR = 2.05; 95%CI: 1.260-3.334; P = 0.004) as independent predictive factors for postembolization pain.
Although painkillers were used prophylactically during and after TACE in our study, the incidence of moderate-to-severe abdominal pain in the first 24 h after TACE procedures remained as high as 16.7%. This conclusively demonstrated that use of a single non-steroidal anti-inflammatory drug or dezocine is often not sufficient for effective pain control. Multimodal analgesia was associated with superior pain relief and decreased opioid consumption when compared with use of a single pain medication[13]. Guo et al[8] demonstrated that patients who used preemptive parecoxib and a sufentanil-based multimodal analgesia regimen had better pain relief, evidenced by a lower incidence of severe pain (11.9%)[8]. Similar to a previous study[14], we observed that effective pain management could reduce the length of hospital stay. The prediction model can be used to predict the risk of postembolization pain after TACE, thus providing medical staff with a reference for pain management.
The cause of postembolization pain is not fully understood; however, it is believed to be caused by local tissue hypoxia, tumor necrosis, swelling of the capsule, ectopic embolization, or consequent cytokine release and the inflammatory response[15,16]. Identification of preoperative predictors of postembolization pain is challenging. In our pain predictive model using CART methods, blood vessel invasion was the strongest predictor of postembolization pain, followed by history of TACE, the DEB-TACE method, and history of abdominal pain after TACE. Besides these four risk factors, age, tumor size (> 5 cm), and presence of multiple tumors were identified as predictors of postembolization pain by logistic regression.
Primary liver cancer has a great propensity to invade the portal venous system, which leads to portal vein tumor thrombosis. Portal vein tumor thrombosis is found in the trunk or branches of the portal vein, and TACE is considered if the portal vein trunk is not completely blocked or portal collateral circulation is already present in the hepatic hilar region[17]. No sources of data on blood vessel invasion as a risk factor for pain were found when conducting a literature review; thus, our study is the first in this respect, identifying blood vessel invasion as a predictor of pain. Tumor invasion of the portal vein is more common in the late stages of cancer[17], and is often accompanied by tumors that are large in size and/or numerous, which may be attributable to tumor necrosis and a more marked inflammatory response, which is caused by embolization of a larger site[18-20].
The conclusions that can be drawn from the two statistical approaches are generally consistent. The tree graph output from CART is intuitive and easy to explain in terms of the interaction between variables and the influence of different factors on outcome variables. The four predictors of the model can be easily extracted as predictive risk factors prior to TACE. It is beneficial to provide a comprehensive analgesic plan for patients who are at a high risk of postembolization pain. The risk factors for postembolization pain identified in this study are similar to those identified by Khalaf et al[21].
Although DEB-TACE is considered less toxic and better standardized compared with traditional lipiodol-TACE, tolerance caused by DEB-TACE is controversial[22]. Traditional TACE is performed using lipiodol loaded with chemotherapy drugs to embolize blood vessels and kill tumor cells. DEB-TACE depends on drug-loaded microspheres to precisely control the release of drugs to maximize tumor necrosis and minimize adverse effects[23]. A randomized study performed by Lammer et al[23] reported that tolerance was better with DEB-TACE compared with traditional TACE[23]. The Precision Italia Study Group compared two types of TACE in 177 patients. The results showed that the probability of abdominal pain with DEB-TACE is lower than that of traditional TACE[24]. In contrast to this result, our analysis showed that patients who underwent DEB-TACE experienced increased postembolization pain and required more painkillers within 24 h of the procedure. This is in accordance with the data from two other studies, which showed that severe pain occurred significantly more frequently in patients who underwent DEB-TACE than in the traditional TACE group[21,25]. Some studies showed that the total dose of chemotherapeutic agents administered for TACE is related to the pain score[19,21,26]. In this study, the chemotherapy drug used was pirarubicin hydrochloride administered at a dose of 30 mg for traditional TACE and 60 mg for DEB-TACE, similar to the report of Benzakoun et al[19], and that may be one of the reasons why postembolization pain was worse for DEB-TACE.
Our findings suggest that first-time TACE patients were more likely to experience pain than those with previous experience of TACE and this is consistent with a recent study[27]. There are two possible reasons for this; first, the pain threshold is increased according to the time that TACE treatment is carried out and second, the tolerance to TACE is increased by repeated treatments. Patients with pain after TACE are more likely to develop pain in the future, which may be related to the individual’s pain threshold and the presence of liver disease.
The predictive model that used CART was examined by ROC curve analysis. The area under the ROC curve was used to predict postembolization pain (0.736; 95%CI: 0.682-0.789). The model had a good sensitivity and specificity, and a high negative predictive value of 92.4%.
Our study has several notable limitations common to retrospective, single-center studies. First, prior epidemiologic findings indicate that chronic liver disease, performance status, and psychological factors may contribute to postembolization pain after TACE. However, we did not control for or investigate these factors as part of our analysis. Future investigations with larger sample sizes should aim to develop more robust prediction models that include other potential contributing factors to further elucidate the risk factors for this disorder. Second, our patient population was obtained from a regional tertiary care center, which may not be representative of the general population. Finally, our model was not validated using an external population. Therefore, future studies with a larger sample size, a multicenter design, and using an external cohort are needed to confirm our findings.
Despite these limitations, our predictive model is simple to use and provides a more rational reference to improve the quality of pain management after TACE. It is suggested that more comprehensive analgesic interventions should be provided for patients who are at a high risk of pain, such as multimodal analgesic therapy.
Transarterial chemoembolization (TACE) is the first-line treatment for patients with unresectable liver cancer. However, approximately 60%-80% of patients complain of different levels of postembolization pain after TACE.
Clearly identifying factors associated with postembolization pain could help predict its occurrence. Prediction model could be used to predict the risk of abdominal pain after TACE, thus providing medical staff with a reference for pain management.
To analyze the risk factors for acute abdominal pain after TACE and establish a predictive model for postembolization pain.
From January 2018 to September 2018, all patients with liver cancer who underwent TACE at our hospital were included. General characteristics; clinical, imaging, and procedural data; and postembolization pain were analyzed. Postembolization pain was defined as acute moderate-to-severe abdominal pain within 24 h after TACE. Logistic regression and a classification and regression tree were used to develop a predictive model. Receiver operating characteristic curve analysis was used to examine the efficacy of the predictive model.
We analyzed 522 patients who underwent a total of 582 TACE procedures. Ninety-seven (16.70%) episodes of severe pain occurred. A predictive model built based on the dataset from classification and regression tree analysis identified known invasion of blood vessels as the strongest predictor of subsequent performance, followed by history of TACE, method of TACE, and history of abdominal pain after TACE. The area under the receiver operating characteristic curve was 0.736, the sensitivity was 73.2%, the specificity was 65.6%, and the negative predictive value was 92.4%.
Blood vessel invasion, TACE history, TACE with drug-eluting beads, and history of abdominal pain after TACE are predictors of acute moderate-to-severe pain. Our predictive model is simple to use and provides a more rational reference to improve the quality of pain management.
Future studies with a larger sample size, a multicenter design, and using an external cohort are needed to confirm our findings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hata M S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O, Artaman A, Asgedom SW, Assadi R, Atey TM, Avila-Burgos L, Awasthi A, Ba Saleem HO, Barac A, Bennett JR, Bensenor IM, Bhakta N, Brenner H, Cahuana-Hurtado L, Castañeda-Orjuela CA, Catalá-López F, Choi JJ, Christopher DJ, Chung SC, Curado MP, Dandona L, Dandona R, das Neves J, Dey S, Dharmaratne SD, Doku DT, Driscoll TR, Dubey M, Ebrahimi H, Edessa D, El-Khatib Z, Endries AY, Fischer F, Force LM, Foreman KJ, Gebrehiwot SW, Gopalani SV, Grosso G, Gupta R, Gyawali B, Hamadeh RR, Hamidi S, Harvey J, Hassen HY, Hay RJ, Hay SI, Heibati B, Hiluf MK, Horita N, Hosgood HD, Ilesanmi OS, Innos K, Islami F, Jakovljevic MB, Johnson SC, Jonas JB, Kasaeian A, Kassa TD, Khader YS, Khan EA, Khan G, Khang YH, Khosravi MH, Khubchandani J, Kopec JA, Kumar GA, Kutz M, Lad DP, Lafranconi A, Lan Q, Legesse Y, Leigh J, Linn S, Lunevicius R, Majeed A, Malekzadeh R, Malta DC, Mantovani LG, McMahon BJ, Meier T, Melaku YA, Melku M, Memiah P, Mendoza W, Meretoja TJ, Mezgebe HB, Miller TR, Mohammed S, Mokdad AH, Moosazadeh M, Moraga P, Mousavi SM, Nangia V, Nguyen CT, Nong VM, Ogbo FA, Olagunju AT, Pa M, Park EK, Patel T, Pereira DM, Pishgar F, Postma MJ, Pourmalek F, Qorbani M, Rafay A, Rawaf S, Rawaf DL, Roshandel G, Safiri S, Salimzadeh H, Sanabria JR, Santric Milicevic MM, Sartorius B, Satpathy M, Sepanlou SG, Shackelford KA, Shaikh MA, Sharif-Alhoseini M, She J, Shin MJ, Shiue I, Shrime MG, Sinke AH, Sisay M, Sligar A, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tessema GA, Topor-Madry R, Tran TT, Tran BX, Ukwaja KN, Vlassov VV, Vollset SE, Weiderpass E, Williams HC, Yimer NB, Yonemoto N, Younis MZ, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1105] [Cited by in RCA: 1167] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 2. | Miyayama S, Matsui O. Superselective Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma: Rationale, Technique, and Outcome. J Vasc Interv Radiol. 2016;27:1269-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (34)] |
| 3. | Teng Gaojun. Standardization of interventional therapy for liver cancer [EB/OL]. Available from: http://www.sohu.com/a/240698530_377345, 2018-7-12. |
| 4. | Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E. Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol. 2012;35:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Boulin M, Adam H, Guiu B, Aho LS, Cercueil JP, Di Martino C, Fagnoni P, Minello A, Jouve JL, Hillon P, Bedenne L, Lepage C. Predictive factors of transarterial chemoembolisation toxicity in unresectable hepatocellular carcinoma. Dig Liver Dis. 2014;46:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Blackburn H, West S. Management of Postembolization Syndrome Following Hepatic Transarterial Chemoembolization for Primary or Metastatic Liver Cancer. Cancer Nurs. 2016;39:E1-E18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 7. | Kogut MJ, Chewning RH, Harris WP, Hippe DS, Padia SA. Postembolization syndrome after hepatic transarterial chemoembolization: effect of prophylactic steroids on postprocedure medication requirements. J Vasc Interv Radiol. 2013;24:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Guo JG, Zhao LP, Rao YF, Gao YP, Guo XJ, Zhou TY, Feng ZY, Sun JH, Lu XY. Novel multimodal analgesia regimen improves post-TACE pain in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2018;17:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Prajapati HJ, Rafi S, El-Rayes BF, Kauh JS, Kooby DA, Kim HS. Safety and feasibility of same-day discharge of patients with unresectable hepatocellular carcinoma treated with doxorubicin drug-eluting bead transcatheter chemoembolization. J Vasc Interv Radiol. 2012;23:1286-93.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Lv N, Kong Y, Mu L, Pan T, Xie Q, Zhao M. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol. 2016;26:3492-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, Griffith S, Manworren R, McCarberg B, Montgomery R, Murphy J, Perkal MF, Suresh S, Sluka K, Strassels S, Thirlby R, Viscusi E, Walco GA, Warner L, Weisman SJ, Wu CL. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1787] [Article Influence: 198.6] [Reference Citation Analysis (0)] |
| 14. | Zhou B, Wang J, Yan Z, Shi P, Kan Z. Liver cancer: effects, safety, and cost-effectiveness of controlled-release oxycodone for pain control after TACE. Radiology. 2012;262:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011;22:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Dhand S, Gupta R. Hepatic transcatheter arterial chemoembolization complicated by postembolization syndrome. Semin Intervent Radiol. 2011;28:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Li T, Liu C, He JT, Sui KD, Zhang ZB, Hong D, Su HY, Shao HB. Portal stent with endovascular brachytherapy improves the efficacy of TACE for hepatocellular carcinoma with main portal vein tumor thrombus. Hepatobiliary Pancreat Dis Int. 2020;19:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Arslan M, Degirmencioglu S. Risk Factors for Postembolization Syndrome After Transcatheter Arterial Chemoembolization. Curr Med Imaging Rev. 2019;15:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Benzakoun J, Ronot M, Lagadec M, Allaham W, Garcia Alba C, Sibert A, Vilgrain V. Risks factors for severe pain after selective liver transarterial chemoembolization. Liver Int. 2017;37:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Lima M, Dutra S, Gomes FV, Bilhim T, Coimbra É. Risk Factors for the Development of Postembolization Syndrome after Transarterial Chemoembolization for Hepatocellular Carcinoma Treatment. Acta Med Port. 2018;31:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Khalaf MH, Sundaram V, AbdelRazek Mohammed MA, Shah R, Khosla A, Jackson K, Desai M, Kothary N. A Predictive Model for Postembolization Syndrome after Transarterial Hepatic Chemoembolization of Hepatocellular Carcinoma. Radiology. 2019;290:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J Gastroenterol. 2018;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 23. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 24. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F, PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (1)] |
| 25. | Baur J, Ritter CO, Germer CT, Klein I, Kickuth R, Steger U. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med. 2016;8:69-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Lu W, Li YH, Yu ZJ, He XF, Chen Y, Zhao JB, Zhu ZY. A comparative study of damage to liver function after TACE with use of low-dose versus conventional-dose of anticancer drugs in hepatocellular carcinoma. Hepatogastroenterology. 2007;54:1499-1502. [PubMed] |
| 27. | Zhang X, Zhou J, Zhu DD, Huang J, Sun JH, Li TF, Shi CS, Sun ZC, Hou QM, Peng ZY, Yu WQ, Ji JS, Gu WJ, Zhou GH, Xie XX, Guo XH, Cao GH, Yu ZH, Xu HH, Fang J, Ying SH, Hu WH, Ji WB, Han J, Wu X, Zheng JP, Luo J, Chen YT, Hu TY, Li L, Hu HJ, Du HJ, Shao GL. CalliSpheres® drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol. 2019;21:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |