Published online Jan 21, 2020. doi: 10.3748/wjg.v26.i3.291
Peer-review started: October 28, 2019
First decision: December 12, 2019
Revised: December 20, 2019
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: January 21, 2020
Processing time: 79 Days and 20.6 Hours
Enterotoxigenic Bacteroides fragilis (ETBF) causes colitis and diarrhea, and is considered a candidate pathogen in inflammatory bowel diseases as well as colorectal cancers. These diseases are dependent on ETBF-secreted toxin (BFT). Dendritic cells (DCs) play an important role in directing the nature of adaptive immune responses to bacterial infection and heme oxygenase-1 (HO-1) is involved in the regulation of DC function.
To investigate the role of BFT in HO-1 expression in DCs.
Murine DCs were generated from specific pathogen-free C57BL/6 and Nrf2−/− knockout mice. DCs were exposed to BFT, after which HO-1 expression and the related signaling factor activation were measured by quantitative RT-PCR, EMSA, fluorescent microscopy, immunoblot, and ELISA.
HO-1 expression was upregulated in DCs stimulated with BFT. Although BFT activated transcription factors such as NF-κB, AP-1, and Nrf2, activation of NF-κB and AP-1 was not involved in the induction of HO-1 expression in BFT-exposed DCs. Instead, upregulation of HO-1 expression was dependent on Nrf2 activation in DCs. Moreover, HO-1 expression via Nrf2 in DCs was regulated by mitogen-activated protein kinases such as ERK and p38. Furthermore, BFT enhanced the production of reactive oxygen species (ROS) and inhibition of ROS production resulted in a significant decrease of phospho-ERK, phospho-p38, Nrf2, and HO-1 expression.
These results suggest that signaling pathways involving ROS-mediated ERK and p38 mitogen-activated protein kinases-Nrf2 activation in DCs are required for HO-1 induction during exposure to ETBF-produced BFT.
Core tip: Enterotoxigenic Bacteroides fragilis is associated with non-invasive diarrheal diseases, inflammatory bowel diseases, and colorectal cancers. Bacteroides fragilis enterotoxin (BFT) is responsible for these diseases. The present study demonstrated that signaling pathways involving reactive oxygen species-mediated ERK, p38 mitogen-activated protein kinases and Nrf2 activation in dendritic cells are required for heme oxygenase-1 (HO-1) induction during exposure to BFT. This signaling pathway is different from our previous report that BFT upregulates HO-1 in intestinal epithelial cells via a p38 mitogen-activated protein kinases- and NF-κB-dependent pathway. Therefore, this is the first report concerning the effects of BFT on the HO-1 induction pathway in dendritic cells.
- Citation: Ko SH, Jeon JI, Woo HA, Kim JM. Bacteroides fragilis enterotoxin upregulates heme oxygenase-1 in dendritic cells via reactive oxygen species-, mitogen-activated protein kinase-, and Nrf2-dependent pathway. World J Gastroenterol 2020; 26(3): 291-306
- URL: https://www.wjgnet.com/1007-9327/full/v26/i3/291.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i3.291
Enterotoxigenic Bacteroides fragilis (ETBF) not only causes colitis and diarrhea but is also implicated in inflammatory bowel diseases and colorectal cancer[1-3]. ETBF secrete a single unique virulence factor called ETBF enterotoxin (B. fragilis toxin; BFT) that causes those diseases[1-3]. The secreted BFT first contacts the intestinal epithelial cells. Since BFT is a metalloprotease, it can destroy the tight junctions in the intestinal epithelium by cleaving E-cadherin, resulting in loss of tight junctions[3-5]. Therefore, after passing through the destroyed area of the intestinal epithelial barrier, BFT may be in direct contact with immune cells distributed in the lamina propria of the gut.
Among immune cells present in the lamina propria, dendritic cells (DCs) play an important role in mucosal immune responses to bacterial pathogens. In addition to antigen uptake through the above methods, luminal bacterial antigens such as virulence factors can enter into the mucosal tissue when lamina propria DCs extend their dendrites into the lumen[6,7]. Therefore, secreted BFT may contact DCs distributed within the intestinal mucosa. Although BFT has been reported not to directly induce maturation in bone marrow (BM)-derived DCs[8], it is presumed that cellular responses may occur after DCs contact BFT. Nevertheless, little is known about BFT-induced DC responses.
Heme oxygenase-1 (HO-1) is an inducible enzyme that catalyzes the degradation of free heme into carbon monoxide, biliverdin, and free iron[9-11]. HO-1 plays a major protective role in various disease models through anti-inflammatory actions[12-14]. In addition, HO-1 has been associated with DC function regulation. For example, the upregulation of HO-1 endues DCs with more potent and durable immunoregulatory properties[15]. In addition, the upregulation of HO-1 in murine Kupffer cells inhibits DC migration in vitro[16]. HO-1-expressing DCs also promote the differentiation of Foxp3+ regulatory T cells and then induce less severe airway inflammation in murine models[17]. We previously demonstrated that BFT upregulates HO-1 expression in intestinal epithelial cells, which may play a role in protection from apoptotic cell death[12]. However, there are no reports regarding BFT-induced HO-1 expression in DCs.
Several studies have demonstrated that HO-1 expression is regulated via signaling pathways from transcription factors, including nuclear factor-kappaB (NF-κB), activator protein-1 (AP-1), and NF-E2-related factor 2 (Nrf2)[9,11,12]. Although BFT can activate such transcription factor signaling in intestinal epithelial cells[12], there is no evidence that signals associated with BFT-induced transcription factors are related to HO-1 induction in DCs. In the studies reported here, we investigated HO-1 induction in response to stimulation of DCs with BFT and found that signaling pathways involving reactive oxygen species (ROS)-mediated mitogen-activated protein kinases (MAPKs)-Nrf2 in DCs are required for HO-1 induction following exposure to BFT.
GIBCO BRL (Gaithersburg, MD, USA) supplied Ca2+ and Mg2+-free Hank's balanced salt solution (HBSS), antibiotics, L-glutamine, and Trizol. LPS-free fetal bovine serum (FBS) was purchased from American Type Culture Collection (ATCC, Manassas, VA, United States). RPMI 1640 media, DMEM media, N-Acetyl-L-cysteine (NAC), sodium pyruvate, non-essential amino acids, collagenase XΙa, dispase, bovine serum albumin (BSA), and soybean trypsin inhibitor were obtained from Sigma Chemical Co. (St. Louis, MO, United States). Rabbit monoclonal antibodies (mAbs) against phospho-IκBα and phospho-IKKα/β, and rabbit polyclonal Abs against phospho-c-jun, phospho-p65, phospho-ERK1/2, phospho-Elk1, pan-extracellular signal-regulated kinase 1/2 (ERK1/2), phospho-p38, pan-p38, phospho-JNK, pan-JNK, and were acquired from Cell Signaling Technology, Inc. (Beverly, MA, United States). Rabbit polyclonal Ab against phospho-Nrf2 was gained from Bioss Antibodies Inc. (Woburn, MA, United States). Santa Cruz Biotechnology (Santa Cruz, CA, United States) supported rabbit polyclonal Abs against Nrf2 and HO-1, and mouse mAbs against actin and lamin B, and goat anti-mouse and anti-rabbit secondary Abs conjugated to horseradish peroxidase. Thermo Fisher Scientific (Waltham, MA, United States) and AbCAM (Cambridge, MA, United States) supplied Alexa Fluor 488 and DyLight 549 secondary Abs, respectively. Chemical inhibitors such as PD98059, SP600125, SB203580, and Bay 11-7085 were acquired from Calbiochem (La Jolla, CA, United States). Tocris Bioscience (Bristol, United Kingdom) supported SR11302. Murine recombinant IL-4 and GM-CSF were gained from PeproTech (Rocky Hill, NJ, United States).
BFT was purified from culture supernatants of a toxigenic strain of ETBF (ATCC 43858) as described previously[18-20]. An immature murine DC cell line DC2.4 cells was cultured in RPMI 1640 supplemented with 10% FBS, 1% antibiotics, L-glutamine (2 mmol/L), sodium pyruvate (1 mmol/L) and non-essential amino acids (2 mmol/L). These cells were grown at 37°C with 5% CO2 as previously described[9,21].
All animal experiments were approved from the Institutional Animal Care and Use Committee of Hanyang University and Ewha Womans University. Specific pathogen-free C57BL/6 and breeding pairs of Nrf2−/− knockout mice were obtained from Orient Experimental Animals (Seoungnam, South Korea) and RIKEN BioResource Center (Tsukuba, Japan)[22], respectively. Generation of primary murine bone marrow (BM)-derived DCs was performed as previously described[9]. Briefly, femurs, and tibias of male mice (8-12 wk of age, body mass of 20-25 g) were harvested and cells were then cultured in RPMI 1640 media supplemented with 10% FBS, 1% antibiotics, L-glutamine (2 mmol/L), 2-mercaptoethanol (55 μmol/L), murine recombinant GM-CSF (10 ng/mL), and murine recombinant IL-4 (10 ng/mL). After six days in culture, DCs were harvested and stimulated with BFT[9]. The purity of CD11c+ cells was greater than 95% as determined by flow cytometric analysis.
Primary murine colonic epithelial cells were also isolated as described previously[12]. Briefly, intestines were cut into 1-mm fragments and treated with HBSS containing an enzyme solution [dispase (0.02 mg/mL), collagenase XΙa (60 units/mL), soybean trypsin inhibitor (0.2 mg/mL), and BSA (2%)]. Cells were suspended in DMEM containing FBS (10%) with antibiotics, plated on mouse fibronectin-coated dishes, and incubated in 5% CO2 at 37 °C. Cells were then cultivated in medium containing equal volumes of DMEM and Ham's 12 medium supplemented with FBS (10%) and antibiotics[12]. At least 90% of primary colonic epithelial cells were viable for 2 wk in culture as determined by trypan blue exclusion. This procedure was supported by Dr. Sang Hoon Lee of the University of California, Los Angeles, CA[12,23].
Total RNA was extracted from BFT-treated cells using Trizol. Reverse transcription and PCR amplification were performed as described previously[12]. The primers and expected PCR product sizes were as follows: mouse HO-1, 5'-AAG AGG CTA AGA CCG CCT TC-3' (sense), 5'-GTC GTG GTC AGT CAA CAT GG-3' (antisense), 591 bp [NM_010442.2 Mus musculus heme oxygenase (decycling) 1 (Hmox1), mRNA][24]; mouse β-actin, 5'-GTG GGC CGC TCT AGG CAC CAA-3' (sense) and 5'-CTC TTT GAT GTC ACG CAC GAT TTC-3' (antisense), 540 bp [NM_007393.4 Mus musculus actin, beta (Actb), mRNA][25]. For quantifying mRNA molecules, reverse transcription and PCR amplification were performed as described previously[12]. The sizes of PCR products generated from standard RNAs for mouse HO-1 and β-actin are 478 bp and 746 bp, respectively[12].
Cells were harvested and nuclear extracts were prepared as described previously[9,26]. The concentration of protein in extracts was determined using a Bradford assay (Bio-Rad, Hercules, CA, United States). Electrophoretic mobility shift assays were performed according to the manufacturer’s instructions (Promega, Madison, WI, United States)[9]. In brief, 5 μg of nuclear extract was incubated for 30 min at room temperature with γ32P-labeled oligonucleotide probes (5'-AGT TGA GGG GAC TTT CCC AGG C-3' for the NF-κB binding site; 5'-CGC TTG ATG ACT CAG CCG GAA-3' for the AP-1 binding site; 5'- TGG GGA ACC TGT GCT GAG TCA CTG GAG-3' for the Nrf2 binding site). Oligonucleotide probes for the NF-κB- and AP-1-binding assays were purchased from Promega. Santa Cruz Biotechnology supplied Nrf2 oligonucleotides. Nrf2 supershift and competition assay were performed as previously described[9,12].
were used to For suppression of NF-κB, AP-1, Nrf2, or MAPK signals, lentiviral systems containing mammalian expression vectors were used as previously described[9,12]. Transfection assays using lentiviral systems were performed according to the manufacturer’s instructions, as described previously[9,12]. Small interfering RNA (siRNA) against the NF-κB p65 subunit and c-jun, and negative (non-silencing) siRNA control (NS-RNA) were obtained (Qiagen, Valencia, CA, United States) as described previously[9,12]. Briefly, cells were cultured in 6-well plates to 50%-80% confluence and then transfected with siRNA using Fugene 6 (Roche, Mannheim, Germany) as a transfection reagent, as described previously[9,12]. Transfected cells were incubated for 48 h before the assay.
For immunoblot, cells were washed with ice-cold PBS and lysed in 0.5 mL/well lysis buffer (150 mmol/L NaCl, 20 mmol/L Tris pH 7.5, 0.1% Triton X-100, 1 mmol/L PMSF, and 10 μg/mL aprotinin). Fifteen to 50 µg of protein per lane was size-fractionated on a polyacrylamide minigel (Mini-PROTEIN II, Bio-Rad) and electrophoretically transferred to a nitrocellulose membrane (0.1-μm pore size). Immunoreactive proteins to which primary Abs bound were visualized using goat anti-rabbit or anti-mouse secondary Abs conjugated to horseradish peroxidase, followed by enhanced chemiluminescence (ECL system; Amersham Life Science, Buckinghamshire, United Kingdom) and exposure to X-ray film[9,12].
The level of HO-1 proteins was measured using a commercially available kit (R&D Systems, Inc., Minneapolis, MN, United States). An ELISA kit of the TransAM Nrf2 (Active Motif, Carlsbad, CA, United States) was also used[9,25]. For detecting expression of phospho-Elk1, a p44/42 MAP kinase assay kit (Cell Signaling Technology) was used[12]. Briefly, immobilized phospho-p44/42 MAPK (Thr202/Tyr204) mAb was used to immunoprecipitate active p44/42 MAPK from cell extracts, after which an in vitro kinase assay was performed using Elk-1 protein as a substrate. Elk-1 phosphorylation was then detected by Western blotting using phospho-Elk-1 (Ser383) Ab. Each assay was performed according to each manufacturer’s instructions.
The level of intracellular ROS was determined using a commercially available kit (Thermo Fisher Scientific). Briefly, BFT-exposed cells were washed with PBS and were then incubated with 5 μmol/L CellROX® Reagent (Thermo Fisher Scientific) at 37 °C for 30 min. Fluorescence was measured using a Varioskan Flash spectral scanning multimode reader (Thermo Fisher Scientific). The assay was performed according to the manufacturer’s protocol.
Immunofluorescence assay was performed, as described previously[9,12,19]. Briefly, cells were cultured (5 × 104 cells in 0.2 mL of RPMI 1640/well) in microslides coated with poly-D-lysine (Santa Cruz). After stimulation with BFT for the indicated period, cells were treated with goat anti-HO-1 and rabbit anti-phospho-Nrf2 Abs as primary Abs for 2 h. Cells were then treated with Alexa fluor 488-conjugated secondary Ab (green color) against goat IgG and DyLight 549-conjugated secondary Ab (red color) against rabbit IgG for 1 h. Images were captured using a fluorescence microscope (DMI4000B, Leica Microsystems GmbH, Wetzlar, Germany)[9,12,19].
Data from quantitative RT-PCR assays are presented as mean ± SD and data from ELISA and ROS assay are presented as mean ± SE. Mann-Whitney t-test was used for statistical analysis. P values < 0.05 were considered statistically significant.
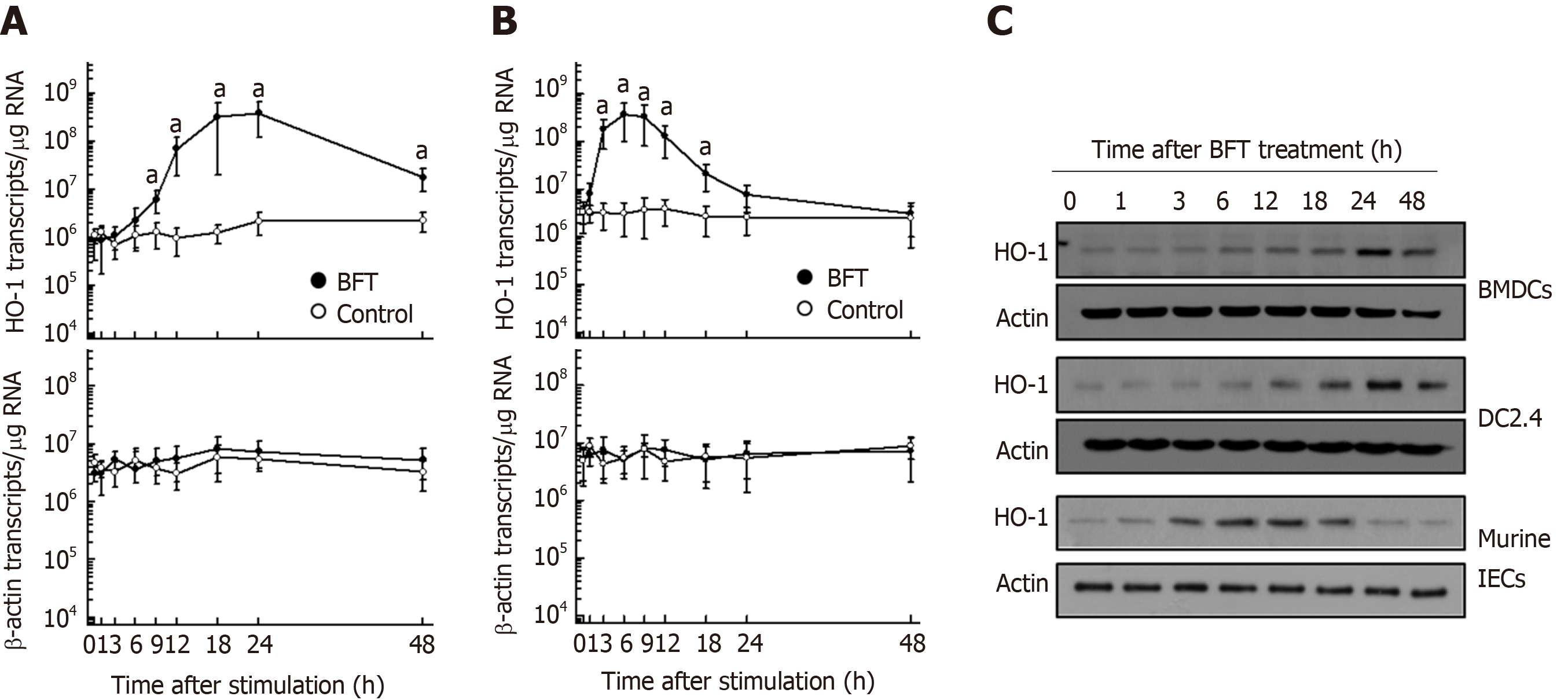
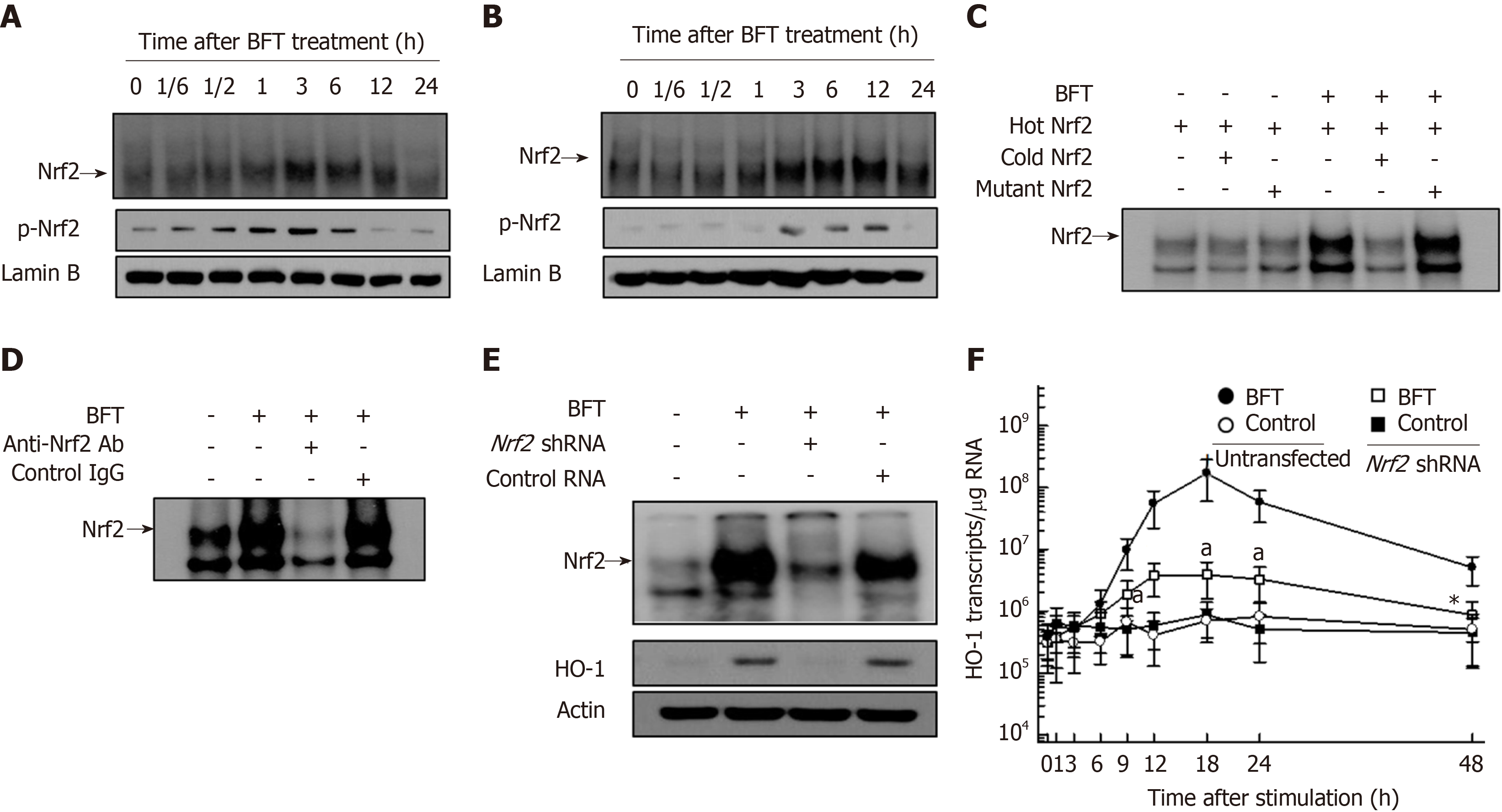
BFT enhanced HO-1 mRNA expression in murine BMDCs (Figure 1A). Significant increases in HO-1 mRNA expression in DC were first noted 9 h after stimulation with BFT, the expression continued to increase for 24 h before decreasing thereafter. Since BFT was known to upregulate HO-1 expression in intestinal epithelial cells[12], we observed differenced in the kinetics of HO-1 mRNA expression between DCs and intestinal epithelial cells. As shown in Figure 1B, compared with DCs, primary murine intestinal epithelial cells showed early expression of HO-1 mRNA, with peak expression approximately 6 h post-stimulation that decreased to baseline level at 24 h post-stimulation. These results suggest that the kinetics of HO-1 mRNA expression in DCs differs from that of intestinal epithelial cells. To determine whether increased levels of mRNA transcripts were parallel with protein expression, we performed immunoblot analyses. As shown in Figure 1C, BFT increased the expression of HO-1 proteins in BMDCs (top panel). Similar results were obtained in the murine DC cell line DC2.4 when stimulated with BFT (Figure 1C, middle panel). In contrast to DCs, primary murine intestinal epithelial cells first noted upregulated expression HO-1 proteins 3 h after stimulation with BFT (Figure 1C, bottom panel). In the present study, we used 100 ng/mL of BFT after previously establishing that the EC50 of BFT was 99.8 ng/mL for expression of HO-1[12].
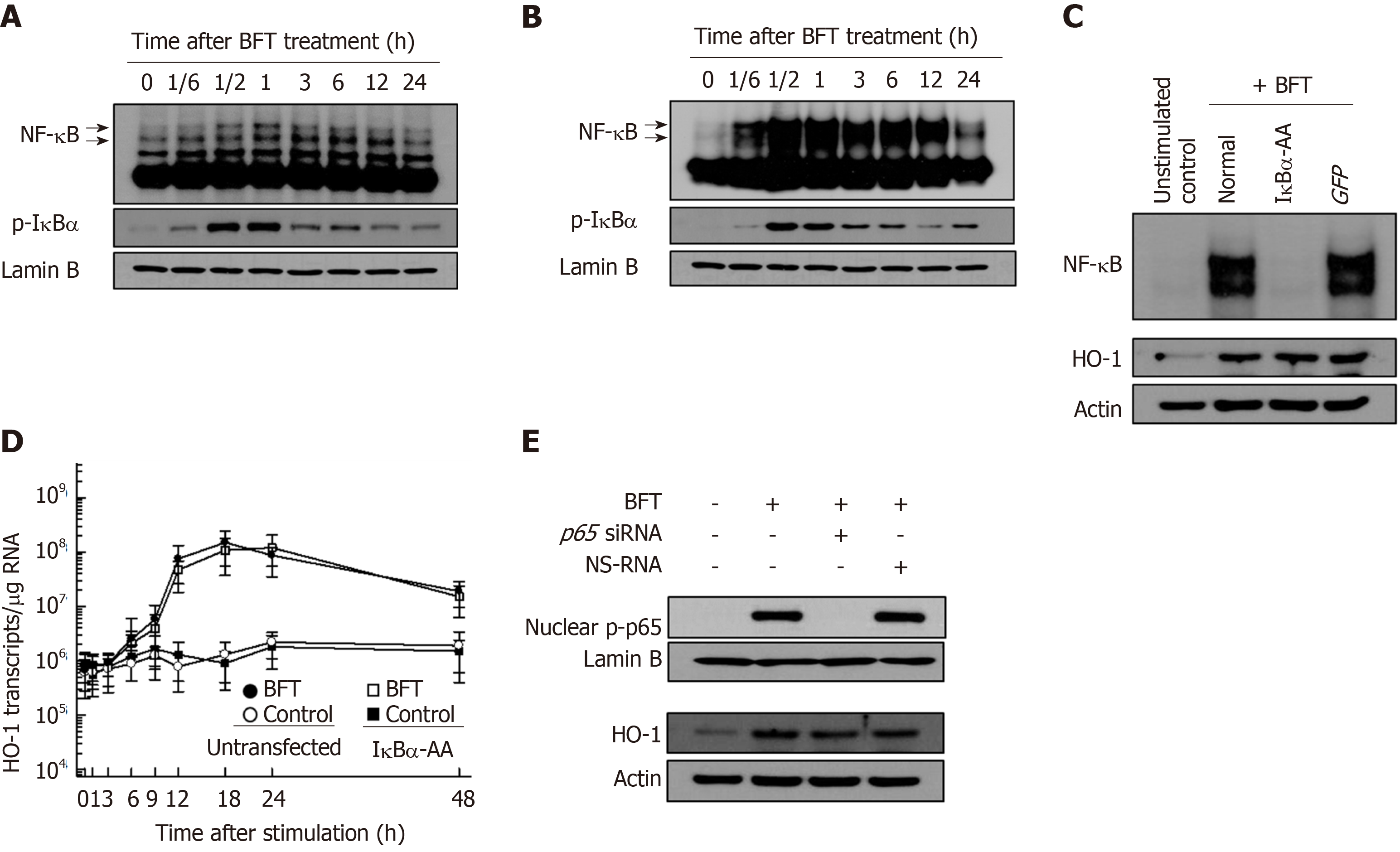
Since there is no evidence for BFT-induced activation of NF-κB and AP-1 signaling in DCs, we first examined whether BFT stimulation could activate NF-κB and AP-1 signaling in DCs. As shown in Figure 2A, treatment of BM-derived DCs with BFT increased NF-κB DNA binding, as assessed by EMSA. In addition, enhanced expression of phosphorylated IκBα was observed in BFT-treated DCs. Similar results were also observed in DC 2.4 cells exposed to BFT (Figure 2B). Based on these results, we determined whether BFT-induced activation of NF-κB signal was related to HO-1 expression in DCs. For these experiments, we used DC2.4 cells transfected with lentivirus-IκBα-AA. As shown in Figure 2C, transfection with lentivirus-IκBα-AA suppressed NF-κB activity to the control level under BFT-treated condition (Figure 2C, top panel). In this experimental system, HO-1 expression induced by stimulation with BFT did not differ between untransfected and transfected cells (Figure 2C, bottom panel). Consistent with these results, transfection with lentivirus-IκBα-AA did not significantly alter HO-1 mRNA expression in DC2.4 cells under BFT exposure (Figure 2D). To confirm these results, another experiment using p65 siRNA was performed to inhibit NF-κB activity. As shown in Figure 2E, BFT-induced nuclear phospho-p65 protein expression was reduced in p65 siRNA-transfected cells compared with untransfected cells (Figure 2E, top panel). In contrast, no difference of BFT-induced HO-1 expression was observed between cells with p65 siRNA transfection and cells without transfection (Figure 2E, bottom panel).
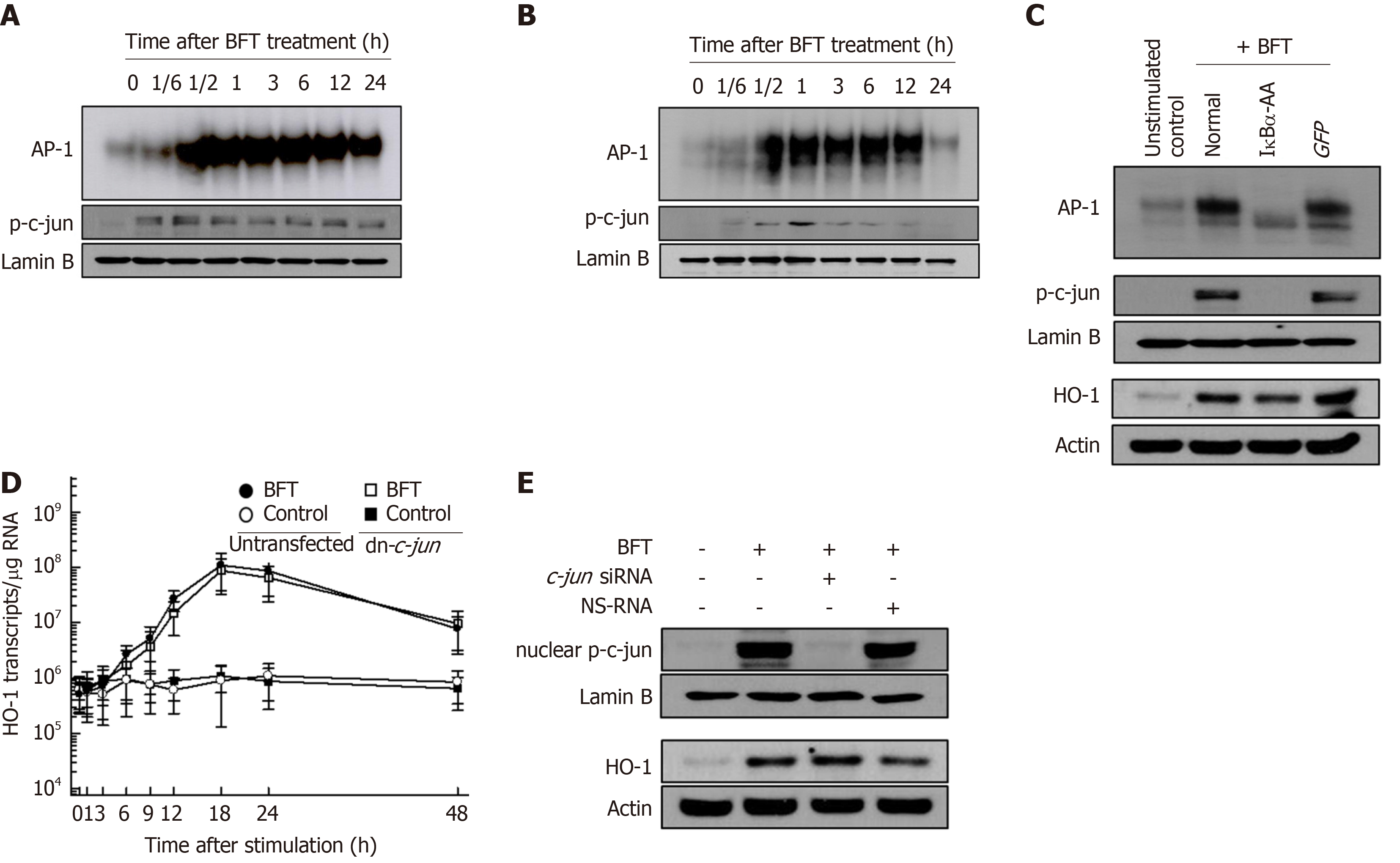
BFT can activate AP-1 signaling in DCs. As shown in Figure 3, stimulation of DCs with BFT increased AP-1-DNA binding activity in murine BMDCs (Figure 3A) and DC2.4 cells (Figure 3B), as shown by EMSA. Concurrently, increased expression of phosphorylated c-jun was observed in BFT-treated murine BMDCs and DC2.4 cells. The effects of transfection with lentivirus-dn-c-jun on HO-1 expression in BFT-stimulated DC2.4 cells were assessed next. As shown in Figure 3C, transfection with lentivirus-dn-c-jun suppressed AP-1 activity to control levels in DC2.4 cells stimulated with BFT (top panel). Consistent with this, expression of nuclear phospho-c-jun was reduced in lentivirus-dn-c-jun-transfected cells compared with untransfected cells (Figure 3C, middle panel), while transfection with lentivirus-dn-c-jun did not change the BFT-induced HO-1 expression (Figure 3C, bottom panel). In addition, lentivirus-dn-c-jun-transfected cells did not significantly alter HO-1 mRNA expression compared with untransfected cells under BTT-exposed condition (Figure 3D). In another experiment, we used c-jun siRNA to suppress AP-1 activity. As shown in Figure 3E, c-jun siRNA almost completely suppressed the expression of nuclear phospho-c-jun in DC2.4 cells (top panel). However, no change was observed in BFT-induced HO-1 protein expression between AP-1-suppressed and unsuppressed cells (Figure 3E, bottom panel).
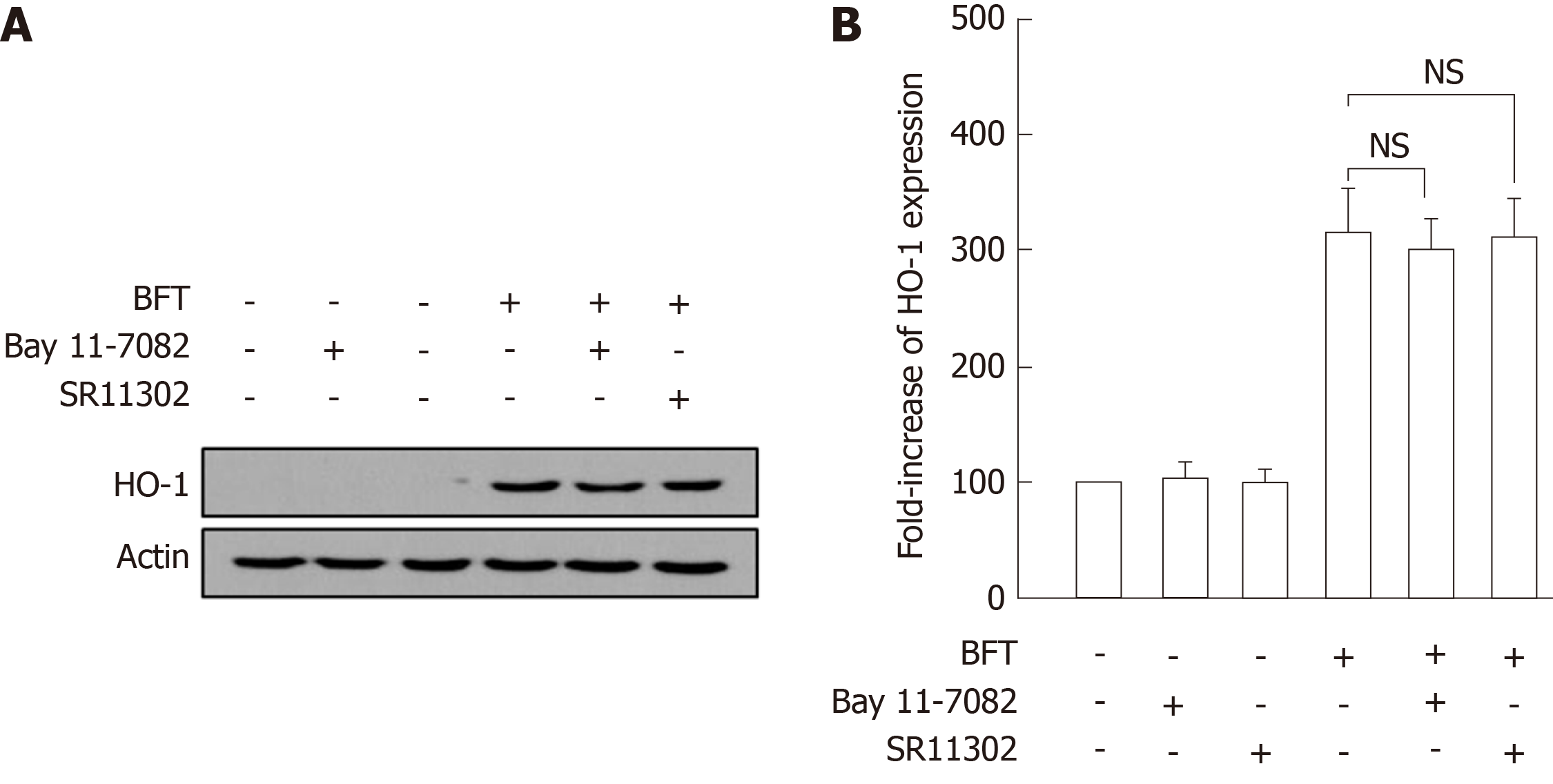
To confirm these results, BM-derived DCs were preincubated with NF-κB inhibitor Bay 11-7082 or AP-1 inhibitor SR11302 for 30 min, and BFT was then added to the culture system. As shown in Figure 4A, there was no significant difference between the group treated with Bay 11-7082 + BFT and the group treated with BFT alone. A similar result was observed between the group treated with SR11302 + BFT and the group treated with BFT alone. Consistent with these findings, no significant changes in BFT-induced HO-1 expression in BM-derived DCs were observed between the three groups consisting of combined treatment with Bay 11-7082 and BFT, combined treatment with SR11302 and BFT, and BFT treatment alone (Figure 3B).
Since there was no evidence for BFT-induced activation of Nrf2 signaling in DCs, we first examined whether BFT stimulation could activate Nrf2 signals in DCs. As shown in Figure 5A, BFT enhanced DNA-binding activity of Nrf2 in murine BM-derived DCs. Similar results were found in BFT-exposed DC2.4 cells (Figure 5B). To confirm the specificity of Nrf2-DNA binding, two assays, including a competition assay using cold Nrf2 and an inhibition assay using anti-Nrf2 Ab, were performed. In the first experiment, the addition of excess Nrf2 oligomer (cold Nrf2) markedly decreased Nrf2-DNA binding in comparison to no addition of Nrf2 oligomer (hot Nrf2) under BFT-exposed condition (Figure 5C). In the second experiment, treatment with anti-Nrf2 Ab definitely inhibited Nrf2-DNA binding in nuclear extracts from BM-derived DCs, as assessed by supershift assay (Figure 5D).
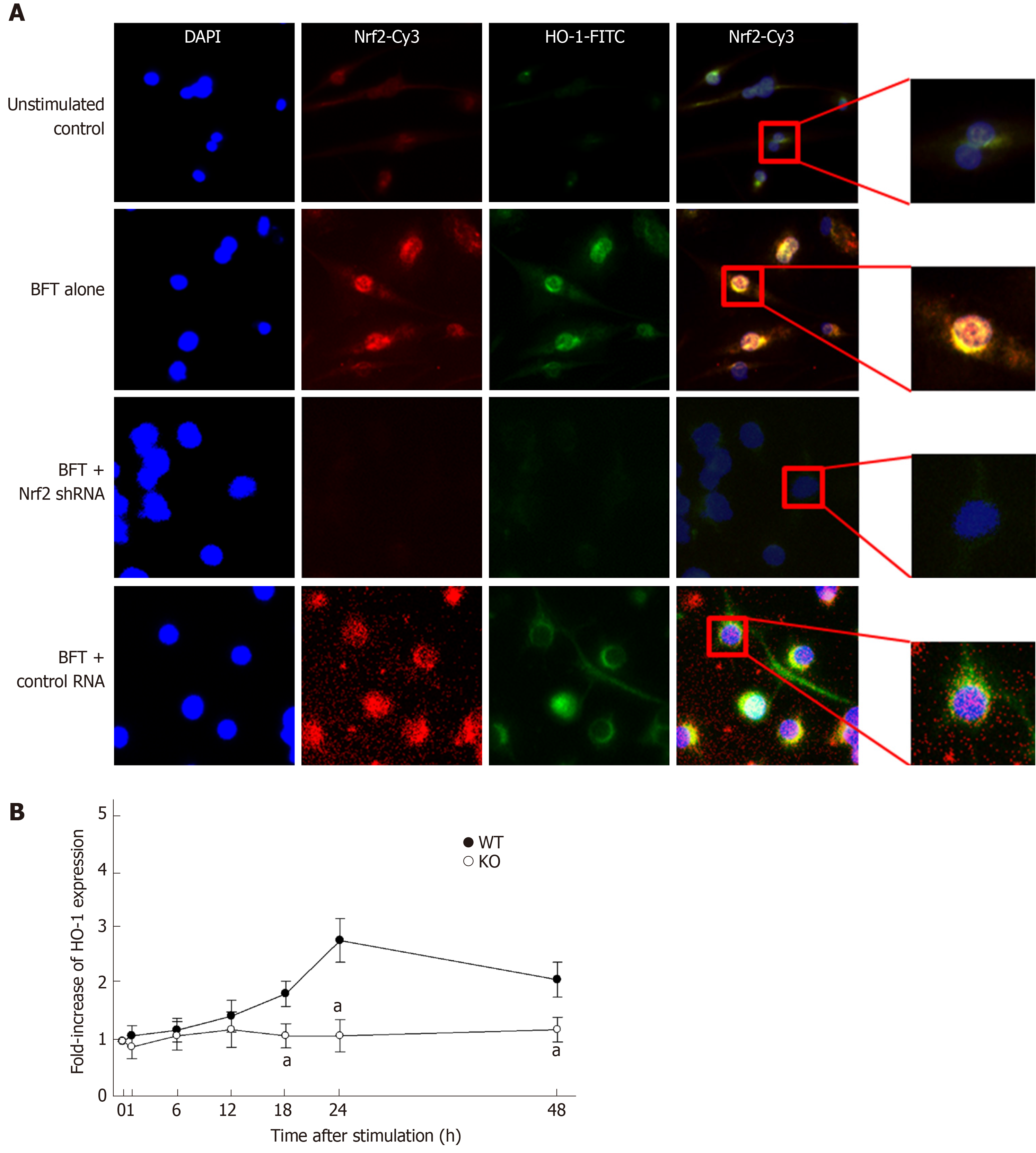
Based on these results, we next evaluated whether BFT-induced HO-1 expression was related to Nrf2 activation. For these experiments, DC2.4 cells transfected with lentivirus containing Nrf2 shRNA were used. Transfection with Nrf2 shRNA decreased BFT-induced activity of Nrf2-DNA binding in DC2.4 cells (Figure 5E, top panel). In this experimental system, enhanced expression of HO-1 protein in BFT-treated DC2.4 cells were reduced with transfection of Nrf2 shRNA compared with no transfection (Figure 5E, bottom panel). Consistent with this, transfection with Nrf2 shRNA significantly inhibited HO-1 mRNA expression in BFT-stimulated DC2.4 cells compared with no transfection (Figure 5F). An additional experiment using immunofluorescent microscopy showed that phospho-Nrf2 and HO-1 signals increased in BFT-exposed DC2.4 cells, while Nrf2 shRNA reduced the extent of phospho-Nrf2 and HO-1 signals (Figure 6A). To confirm these results, BM-derived DCs obtained from Nrf2−/− knockout mice were used. As shown in Figure 6B, there was a significant difference in HO-1 expression between DCs from wild-type mice and those derived from Nrf2−/− knockout mice.
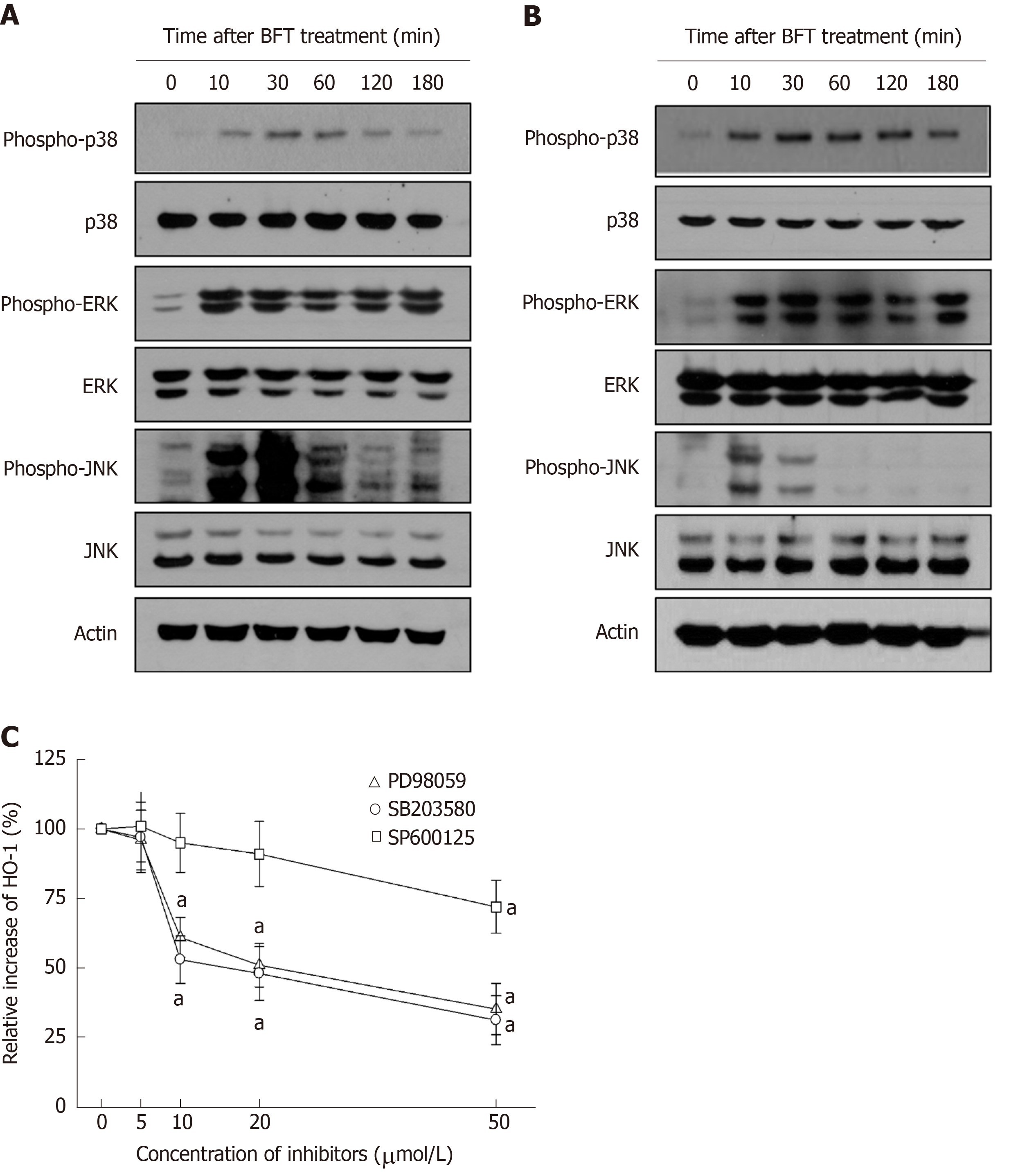
We further evaluated whether MAPK signaling could be associated with Nrf2 and HO-1 expression in DCs. As shown in Figure 7A, BFT increased phosphorylated signals of MAPKs, including phospho-ERK1/2, phospho-p38, and phospho-JNK, in BM-derived DCs. Similar results were observed in DC2.4 cells (Figure 7B). We next examined whether inhibition of MAPK activity could influence expression of HO-1 in BFT-exposed DCs. As shown in Figure 7C, pretreatment of BM-derived DCs with PD98059 (≥ 10 μmol/L), SB203580 (≥ 10 μmol/L), or SP600125 (≥ 50 μmol/L) significantly inhibited BFT-induced expression of HO-1.
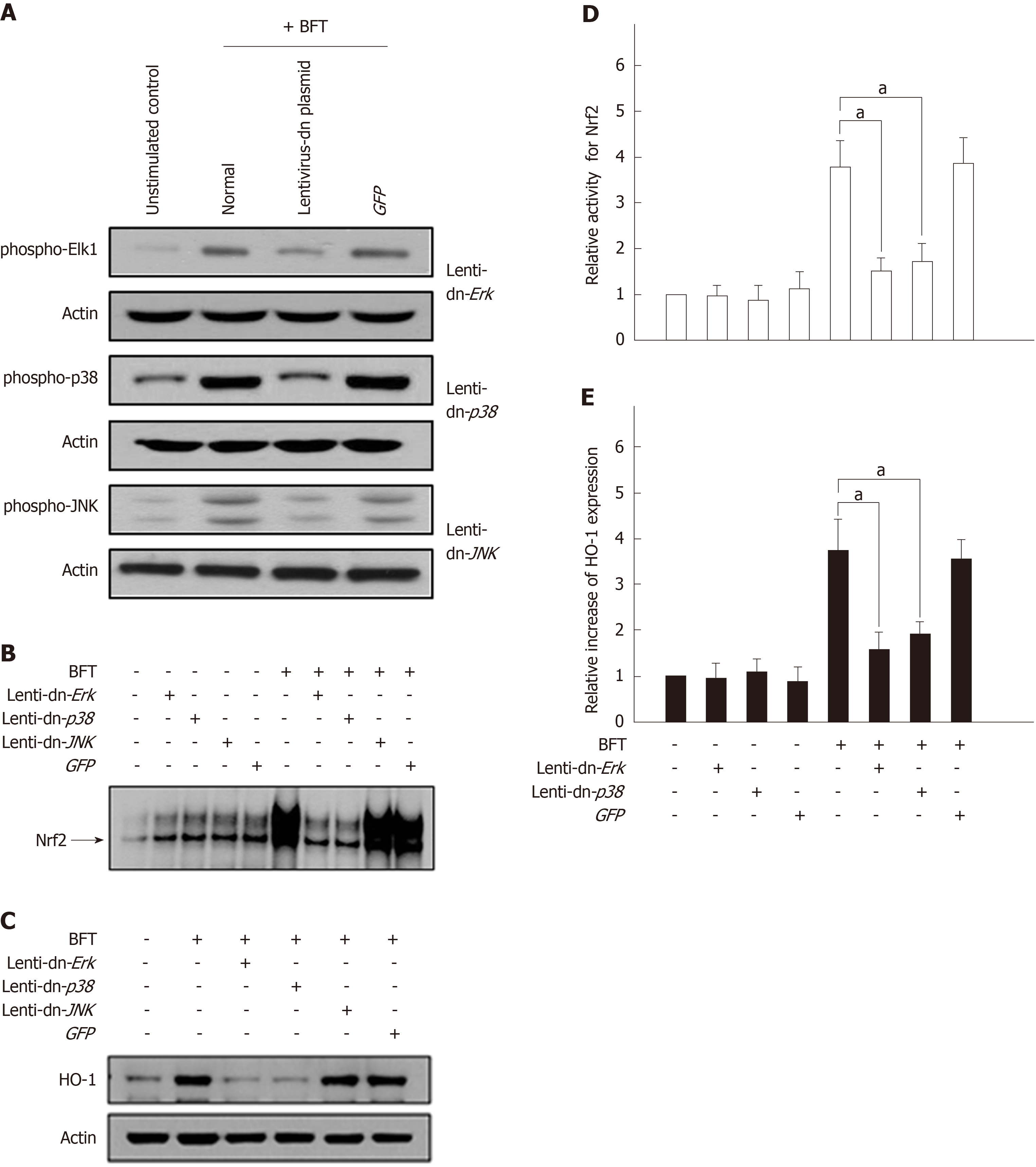
To confirm these results, lentiviral systems containing dominant-negative plasmids were used. Phosphorylation of each MAPK protein was markedly suppressed in DC2.4 cells transfected with lentiviruses containing each dominant-negative plasmid (Figure 8A). Transfection with lentivirus-dn-Erk2 and lentivirus-dn-p38 significantly decreased BFT-induced Nrf2 activation, as assessed by EMSA (Figure 8B). In these experimental systems, cells transfected with lentivirus-dn-Erk2 and lenti-dn-p38 reduced the expression of HO-1 compared with untransfected cells under BFT-treated conditions (Figure 8C). Moreover, lentivirus-dn-Erk2 and lentivirus-dn-p38 significantly inhibited the activities of both phosphorylated Nrf2 and HO-1 in BFT-stimulated cells (Figure 8D and 8E). These results suggest that exposure of DCs to BFT activates a signaling cascade involving ERK and p38 MAPKs, leading to Nrf2 activation and finally to HO-1 induction.
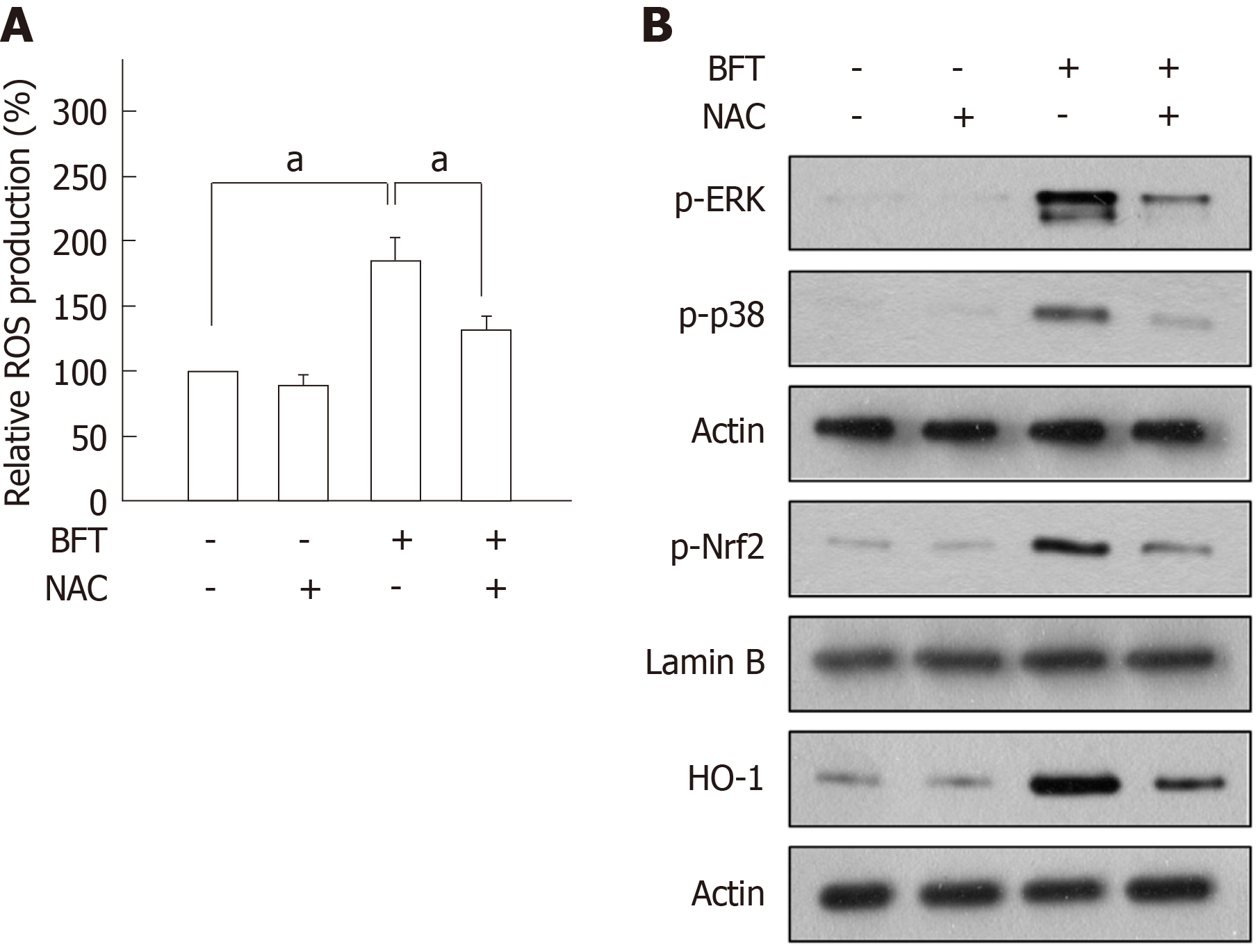
Although BFT is reported to induce the generation of ROS in T84 intestinal epithelial cells[27], there are no reports regarding BFT-induced ROS generation in DCs. In the present study, we demonstrated that BFT markedly increased intracellular ROS generation in DC2.4 cells, while antioxidant NAC significantly inhibited it (Figure 9A). In this experimental system, treatment with NAC attenuated the increased levels of phospho-ERK and phospho-p38 in DC2.4 cells exposed to BFT (Figure 9B). Upregulated expression of both phospho-Nrf2 and HO-1 were also reduced in the presence of NAC under BFT-stimulated conditions, indicating that ROS seems to regulate the overall signaling process of inducing HO-1 expression in BFT-exposed DCs.
ETBF is a noninvasive bacterium associated with pathogenic effects in the intestine[2]. Since invasion of ETBF into the surface epithelium from the intestine has not been reported and no bacteremia has been observed in infected rabbit models[2,28], the produced BFT seems to be primarily present in the lumen. Organized and diffuse lymphoid tissues are found in the intestinal mucosa layer and DCs are abundant in the lamina propria. DCs can extend dendritic processes between intestinal epithelial cells into the lumen to sample antigens. In addition, BFT can destroy the tight junctions in the intestinal epithelium by cleaving E-cadherin[3-5] and the secreted BFT may come into direct contact with DCs. Therefore, BFT may be exposed to DCs at the site of ETBF infection. In experiments reported here, we demonstrated that the exposure of DCs to BFT could upregulate expression of HO-1 at the mRNA and protein levels.
Transcription factors such as NF-κB, AP-1, and Nrf2 regulate a variety of inflammatory responses and their promoter regions of HO-1 contain binding sites for these transcription factors[9,12,29]. We previously demonstrated that stimulation of intestinal epithelial cells with BFT activate NF-κB and AP-1 signaling[12]. However, there have been no reports on whether BFT may activate these transcription factors in DCs. The present study showed that signals of NF-κB, AP-1, and Nrf2 were activated by exposure of DCs to BFT. However, whether HO-1 induction of DCs may be regulated by NF-κB, AP-1 or Nrf2 remains controversial. In the present study, suppression of NF-κB activity either by transfection with lentivirus-IκBα-AA and p65 siRNA, or pretreatment with chemical inhibitor Bay 11-7082 did not significantly reduce BFT-induced HO-1 expression in DCs. In addition, the suppression of AP-1 signals did not result in a significant change in HO-1 expression. In contrast, the suppression of BFT-induced activation of Nrf2 led to the downregulation of HO-1 in DCs assessed by transfection with Nrf2 shRNA. These results were confirmed by experiments using DCs isolated from Nrf2−/− knockout mice. Our results differ from previous findings that LPS-induced HO-1 expression is mediated by an NF-κB-dependent pathway in DCs[30]. In addition, the present results are different from our previous study, in which H. pylori outer membrane vesicles induced HO-1 expression through both NF-κB- and Nrf2-dependent pathway in DCs[9]. Therefore, this Nrf2-dependent and both NF-κB- and AP-1-independent expression of HO-1 may be a distinctive signature of DCs exposure to BFT.
Kinetics of both NF-κB and AP-1 signaling in DCs resemble those in intestinal epithelial cells stimulated with BFT when comparing the present and previous results[12]. However, the kinetics of Nrf2 signals induced by BFT are different in DCs and intestinal epithelial cells. The present study showed that Nrf2 signals in DCs continued to increase over the ensuing 24 h following stimulation. In contrast, our previous study showed that the activation of Nrf2 in intestinal epithelial cells treated with BFT peaked between 1 h and 6 h after stimulation and then decreased[12]. Compared with previous results, the activation of Nrf2 in DCs was delayed compared to the response of intestinal epithelial cells to BFT stimulation. The delayed activation of Nrf2 seems to be associated with the delayed upregulation of HO-1 expression in BFT-exposed DCs.
MAPK signaling is known to be an important event underlying HO-1 expression[11,31,32]. In the present study, suppression of ERK or p38 MAPK signals in BFT-treated DCs resulted in significant inhibitions of both Nrf2 activation and HO-1 expression. Considering that suppression of p38 MAPK results in significant attenuation of BFT-induced NF-κB and HO-1 activities in intestinal epithelial cells[12], two differential pathways may be involved in BFT-induced HO-1 expression. That is, the exposure of DCs to BFT can activate a signaling cascade involving ERK and p38 MAPKs, leading to Nrf2 activation and HO-1 induction. In contrast, BFT-exposed intestinal epithelial cells can activate a signaling cascade involving p38 MAPKs, leading to NF-κB activation and finally to HO-1 induction.
Since ROS production is known to be related to the activation of MAPK signaling[33] and BFT increase ROS production in intestinal epithelial cells[27], we investigated the role of ROS in the regulation of MAPK and Nrf2 signaling. In the present study, exposure of DCs to BFT enhanced ROS production and treatment of BFT-exposed DCs with the antioxidant NAC significantly inhibited the activation of ERK and p38 MAPK signals. In addition, both Nrf2 activation and HO-1 expression were attenuated in the presence of NAC under BFT-stimulated conditions. Based on these results, increased intracellular ROS is proposed as the mechanism allowing BFT to contribute to ERK/p38 MAPK pathway-mediated Nrf2 activation and HO-1 expression in DCs.
BFT has been shown to not directly induce DC maturation[8]. Nevertheless, many papers have demonstrated that HO-1 expression is associated with inhibition of DC maturation[31,34,35]. Therefore, BFT-induced HO-1 upregulation seems to affect the maturation process of DCs and consequently inhibit maturation. Further studies are needed to clarify the role HO-1 plays in DC maturation in ETBF infection.
Upregulated HO-1 is likely to attenuate acute inflammation in ETBF infection. The rationale for this supposition is that HO-1 controls a variety of infections in mice, including Mycobacterium avium[36], Listeria monocytogenes[37], and Salmonella Typhimurium[38]. Further, HO-1 expression induces anti-inflammatory cytokine IL-10 production[34,39] and HO-1 mediates the anti-inflammatory effect of IL-10[40]. Based on these results, upregulated HO-1 may be an important mediator of the anti-inflammatory effects of DCs in acute ETBF infection. However, further studies are required to clarify the anti-inflammatory effects in BFT-stimulated DCs.
In summary, we demonstrated that exposure of DCs to BFT resulted in ROS-mediated activation of MAPK signaling, which then led to the induction of HO-1 molecules via Nrf2 signaling pathway in DCs.
Enterotoxigenic Bacteroides fragilis (ETBF) causes colitis and diarrhea, and is considered a candidate pathogen in inflammatory bowel diseases as well as colorectal cancers. These diseases are dependent on ETBF-secreted toxin (BFT). Dendritic cells (DCs) play an important role in directing the nature of adaptive immune responses to bacterial infection and heme oxygenase-1 (HO-1) is involved in the regulation of DC function.
Although BFT can activate such transcription factor signaling in intestinal epithelial cells, there is no evidence that signals associated with BFT-induced transcription factors are related to HO-1 induction in DCs. These led us to this study.
The present study aimed to investigate the role of BFT in HO-1 expression in DCs.
Murine DCs were generated from specific pathogen-free C57BL/6 and Nrf2−/− knockout mice. DCs were exposed to BFT, after which HO-1 expression and the related signaling factor activation were measured by quantitative RT-PCR, EMSA, fluorescent microscopy, immunoblot, and ELISA.
HO-1 expression was upregulated in DCs stimulated with BFT. Although BFT activated transcription factors such as NF-κB, AP-1, and Nrf2, activation of NF-κB and AP-1 was not involved in the induction of HO-1 expression in BFT-exposed DCs. Instead, upregulation of HO-1 expression was dependent on Nrf2 activation in DCs. Moreover, HO-1 expression via Nrf2 in DCs was regulated by mitogen-activated protein kinases (MAPKs) such as ERK and p38. Furthermore, BFT enhanced the production of reactive oxygen species (ROS) and inhibition of ROS production resulted in a significant decrease of phospho-ERK, phospho-p38, Nrf2, and HO-1 expression.
These results suggest that signaling pathways involving ROS-mediated ERK and p38 MAPK-Nrf2 activation in DCs are required for HO-1 induction during exposure to ETBF-produced BFT.
Upregulated HO-1 expression in DCs will help explain the pathogenesis of the ETBF infection. In addition, upregulated HO-1 may be an important mediator of the anti-inflammatory effects of DCs in acute ETBF infection. However, further studies are required to clarify the anti-inflammatory effects in BFT-stimulated DCs.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sorio C S-Editor: Gong ZM L-Editor: A E-Editor: Ma YJ
| 1. | Sears CL. The toxins of Bacteroides fragilis. Toxicon. 2001;39:1737-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349-369, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Sears CL, Geis AL, Housseau F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest. 2014;124:4166-4172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA. 1998;95:14979-14984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 289] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20:593-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1499] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 6. | MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1870] [Cited by in RCA: 1821] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 8. | Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, Sartor RB, Golenbock D, Hamad AR, Gan CM, Housseau F, Sears CL. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Ko SH, Rho DJ, Jeon JI, Kim YJ, Woo HA, Kim N, Kim JM. Crude Preparations of Helicobacter pylori Outer Membrane Vesicles Induce Upregulation of Heme Oxygenase-1 via Activating Akt-Nrf2 and mTOR-IκB Kinase-NF-κB Pathways in Dendritic Cells. Infect Immun. 2016;84:2162-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Liu XM, Peyton KJ, Durante W. Physiological cyclic strain promotes endothelial cell survival via the induction of heme oxygenase-1. Am J Physiol Heart Circ Physiol. 2013;304:H1634-H1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 12. | Ko SH, Rho da J, Jeon JI, Kim YJ, Woo HA, Lee YK, Kim JM. Bacteroides fragilis Enterotoxin Upregulates Heme Oxygenase-1 in Intestinal Epithelial Cells via a Mitogen-Activated Protein Kinase- and NF-κB-Dependent Pathway, Leading to Modulation of Apoptosis. Infect Immun. 2016;84:2541-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Katada K, Takagi T, Uchiyama K, Naito Y. Therapeutic roles of carbon monoxide in intestinal ischemia-reperfusion injury. J Gastroenterol Hepatol. 2015;30 Suppl 1:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Ryter SW, Choi AM. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res. 2016;167:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 15. | Zhao Y, Jia Y, Wang L, Chen S, Huang X, Xu B, Zhao G, Xiang Y, Yang J, Chen G. Upregulation of Heme Oxygenase-1 Endues Immature Dendritic Cells With More Potent and Durable Immunoregulatory Properties and Promotes Engraftment in a Stringent Mouse Cardiac Allotransplant Model. Front Immunol. 2018;9:1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ma YY, Yang MQ, He ZG, Fan MH, Huang M, Teng F, Wei Q, Li JY. Upregulation of heme oxygenase-1 in Kupffer cells blocks mast cell degranulation and inhibits dendritic cell migration in vitro. Mol Med Rep. 2017;15:3796-3802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wong TH, Chen HA, Gau RJ, Yen JH, Suen JL. Heme Oxygenase-1-Expressing Dendritic Cells Promote Foxp3+ Regulatory T Cell Differentiation and Induce Less Severe Airway Inflammation in Murine Models. PLoS One. 2016;11:e0168919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-kappa B plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001;123:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Ko SH, Jeon JI, Myung HS, Kim YJ, Kim JM. Bacteroides fragilis Enterotoxin Induces Formation of Autophagosomes in Endothelial Cells but Interferes with Fusion with Lysosomes for Complete Autophagic Flux through a Mitogen-Activated Protein Kinase-, AP-1-, and C/EBP Homologous Protein-Dependent Pathway. Infect Immun. 2017;85:pii: e00420-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Roh HC, Yoo do Y, Ko SH, Kim YJ, Kim JM. Bacteroides fragilis enterotoxin upregulates intercellular adhesion molecule-1 in endothelial cells via an aldose reductase-, MAPK-, and NF-κB-dependent pathway, leading to monocyte adhesion to endothelial cells. J Immunol. 2011;187:1931-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Lee JY, Kim H, Cha MY, Park HG, Kim YJ, Kim IY, Kim JM. Clostridium difficile toxin A promotes dendritic cell maturation and chemokine CXCL2 expression through p38, IKK, and the NF-kappaB signaling pathway. J Mol Med (Berl). 2009;87:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3209] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 23. | Choi YJ, Im E, Chung HK, Pothoulakis C, Rhee SH. TRIF mediates Toll-like receptor 5-induced signaling in intestinal epithelial cells. J Biol Chem. 2010;285:37570-37578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580-11584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 25. | Ko SH, Jeon JI, Kim H, Kim YJ, Youn J, Kim JM. Mitogen-activated protein kinase/IκB kinase/NF-κB-dependent and AP-1-independent CX3CL1 expression in intestinal epithelial cells stimulated with Clostridium difficile toxin A. J Mol Med (Berl). 2014;92:411-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Yoo do Y, Ko SH, Jung J, Kim YJ, Kim JS, Kim JM. Bacteroides fragilis enterotoxin upregulates lipocalin-2 expression in intestinal epithelial cells. Lab Invest. 2013;93:384-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci USA. 2011;108:15354-15359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 441] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 28. | Myers LL, Shoop DS, Collins JE, Bradbury WC. Diarrheal disease caused by enterotoxigenic Bacteroides fragilis in infant rabbits. J Clin Microbiol. 1989;27:2025-2030. [PubMed] |
| 29. | Aw Yeang HX, Hamdam JM, Al-Huseini LM, Sethu S, Djouhri L, Walsh J, Kitteringham N, Park BK, Goldring CE, Sathish JG. Loss of transcription factor nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to dysregulation of immune functions, redox homeostasis, and intracellular signaling in dendritic cells. J Biol Chem. 2012;287:10556-10564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Jung ID, Lee JS, Lee CM, Noh KT, Jeong YI, Park WS, Chun SH, Jeong SK, Park JW, Son KH, Heo DR, Lee MG, Shin YK, Kim HW, Yun CH, Park YM. Induction of indoleamine 2,3-dioxygenase expression via heme oxygenase-1-dependant pathway during murine dendritic cell maturation. Biochem Pharmacol. 2010;80:491-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Al-Huseini LM, Aw Yeang HX, Hamdam JM, Sethu S, Alhumeed N, Wong W, Sathish JG. Heme oxygenase-1 regulates dendritic cell function through modulation of p38 MAPK-CREB/ATF1 signaling. J Biol Chem. 2014;289:16442-16451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Roy Chowdhury S, Sengupta S, Biswas S, Sinha TK, Sen R, Basak RK, Adhikari B, Bhattacharyya A. Bacterial fucose-rich polysaccharide stabilizes MAPK-mediated Nrf2/Keap1 signaling by directly scavenging reactive oxygen species during hydrogen peroxide-induced apoptosis of human lung fibroblast cells. PLoS One. 2014;9:e113663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 353] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 33. | Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 35. | Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A. Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase-1. Front Immunol. 2019;10:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect Immun. 2013;81:2536-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Tachibana M, Hashino M, Nishida T, Shimizu T, Watarai M. Protective role of heme oxygenase-1 in Listeria monocytogenes-induced abortion. PLoS One. 2011;6:e25046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Onyiah JC, Sheikh SZ, Maharshak N, Steinbach EC, Russo SM, Kobayashi T, Mackey LC, Hansen JJ, Moeser AJ, Rawls JF, Borst LB, Otterbein LE, Plevy SE. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology. 2013;144:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Riquelme SA, Carreño LJ, Espinoza JA, Mackern-Oberti JP, Alvarez-Lobos MM, Riedel CA, Bueno SM, Kalergis AM. Modulation of antigen processing by haem-oxygenase 1. Implications on inflammation and tolerance. Immunology. 2016;149:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 856] [Article Influence: 37.2] [Reference Citation Analysis (0)] |