Published online Aug 7, 2020. doi: 10.3748/wjg.v26.i29.4316
Peer-review started: March 4, 2020
First decision: April 12, 2020
Revised: June 2, 2020
Accepted: July 1, 2020
Article in press: July 1, 2020
Published online: August 7, 2020
Processing time: 155 Days and 21.2 Hours
Spontaneous bacterial peritonitis (SBP) is a detrimental infection of the ascitic fluid in liver cirrhosis patients, with high mortality and morbidity. Early diagnosis and timely antibiotic administration have successfully decreased the mortality rate to 20%-25%. However, many patients cannot be diagnosed in the early stages due to the absence of classical SBP symptoms. Early diagnosis of asymptomatic SBP remains a great challenge in the clinic.
To establish a multivariate predictive model for early diagnosis of asymptomatic SBP using positive microbial cultures from liver cirrhosis patients with ascites.
A total of 98 asymptomatic SBP patients and 98 ascites liver cirrhosis patients with negative microbial cultures were included in the case and control groups, respectively. Multiple linear stepwise regression analysis was performed to identify potential indicators for asymptomatic SBP diagnosis. The diagnostic performance of the model was estimated using the receiver operating characteristic curve.
Patients in the case group were more likely to have advanced disease stages, cirrhosis related-complications, worsened hematology and ascites, and higher mortality. Based on multivariate analysis, the predictive model was as follows: y (P) = 0.018 + 0.312 × MELD (model of end-stage liver disease) + 0.263 × PMN (ascites polymorphonuclear) + 0.184 × N (blood neutrophil percentage) + 0.233 × HCC (hepatocellular carcinoma) + 0.189 × renal dysfunction. The area under the curve value of the established model was 0.872, revealing its high diagnostic potential. The diagnostic sensitivity was 73.5% (72/98), the specificity was 86.7% (85/98), and the diagnostic efficacy was 80.1%.
Our predictive model is based on the MELD score, polymorphonuclear cells, blood N, hepatocellular carcinoma, and renal dysfunction. This model may improve the early diagnosis of asymptomatic SBP.
Core tip: This retrospective study established a multivariate diagnostic model for asymptomatic spontaneous bacterial peritonitis in liver cirrhosis patients with ascites. The multivariate predictive model constructed by multiple linear stepwise regression analysis was as follows: y (P) = 0.018 + 0.312 × MELD (model of end-stage liver disease) + 0.263 × PMN (ascites polymorphonuclear) + 0.184 × N (blood neutrophil percentage) + 0.233 × HCC (hepatocellular carcinoma) + 0.189 × renal dysfunction. The diagnostic efficacy of the model was 80.1%, sensitivity was 73.5% and specificity was 86.7%. This model may improve the early diagnosis of asymptomatic spontaneous bacterial peritonitis.
- Citation: Tu B, Zhang YN, Bi JF, Xu Z, Zhao P, Shi L, Zhang X, Yang G, Qin EQ. Multivariate predictive model for asymptomatic spontaneous bacterial peritonitis in patients with liver cirrhosis. World J Gastroenterol 2020; 26(29): 4316-4326
- URL: https://www.wjgnet.com/1007-9327/full/v26/i29/4316.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i29.4316
Spontaneous bacterial peritonitis (SBP) is a detrimental infection of the ascitic fluid in liver cirrhosis patients, with a prevalence of 10%-30% among hospitalized patients[1,2]. SBP worsens the outcomes of chronic liver diseases and increases the risk of complications, including renal and hepatic failure and portal hypertension[3,4]. Patients with SBP usually present with fever, shivering, and abdominal pain, but up to 30% of patients can also be asymptomatic[5]. SBP diagnosis follows the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases guidelines, which indicate that ascites polymorphonuclear (PMN) leukocyte counts combined with no intra-abdominal source of infection are suggestive of SBP[6,7]. However, some SBP cases caused by gram-positive cocci often have PMN counts less than 250/mm3[8].
SBP leads to 30%-90% mortality within the first year. However, early diagnosis and timely administration of antibiotics have successfully decreased the mortality rate to 20%-25% in the past three decades[9,10]. In patients with PMN > 250/mm3, antibiotics are promptly administered, while in patients with PMN < 250/mm3, antibiotics are usually administered following typical SBP clinical manifestations[11]. However, many patients cannot be diagnosed in the early stages due to the absence of classical SBP symptoms[12]. Some SBP cases are diagnosed only based on clinical symptoms, leading to possible antibiotic abuse. Therefore, there is an urgent need to establish a more specific and accurate model for early SBP diagnosis, especially for asymptomatic SBP patients. Increasing the diagnosis sensitivity can promote early discovery of SBP, and enhancing the specificity of the current diagnostic tools could inhibit antibiotics abuse.
Accumulating evidence has demonstrated that SBP is regulated by a variety of risk factors, including decreased activity of the reticuloendothelial system, advanced liver dysfunction, medications, and genetic factors[1]. To date, effective multivariate prediction models for asymptomatic SBP are not available. Therefore, the present retrospective cohort study aimed to establish an effective predictive model for early screening of asymptomatic SBP in liver cirrhosis patients with ascites. Early diagnosis of asymptomatic SBP will improve antibiotic management strategies and reduce SBP associated mortality.
A total of 371 cirrhotic patients with ascites who had no SBP symptoms were recruited from the 302 People’s Liberation Army Hospital in the Beijing area from January 2015 to December 2018. Liver cirrhosis diagnoses were confirmed via clinical, laboratory, histological, and imaging findings. A diagnostic paracentesis was performed to confirm ascites according to standard methodology. This study was approved by the ethics committee of the Beijing 302 People’s Liberation Army Hospital. All patients provided informed written consent.
Liver cirrhosis patients with ascites who were enrolled in this study met the following inclusion criteria: (1) Adult population; (2) Ascites PMN count < 250/mm3; (3) Absence of typical SBP symptoms such as fever, abdominal pain, diarrhea, tenderness, or rebound pain; (4) No active infection signs, such as infections of the respiratory tract, digestive tract, urinary tract and central nervous system, etc.; (5) No antibiotics administered in the two weeks preceding the study; and (6) Available microbiological results of ascites specimens. The exclusion criteria included incomplete medical record and medication history.
Patients were divided into the case and control groups according to microbiological results. Under aseptic conditions, the ascites specimens were inoculated in aerobic and anaerobic bottles (BACTEC 9120, BD, United States) at the bedside. The bottles were then incubated at 35°C for 5 d. The culture results were confirmed positive if the same type of pathogen was isolated from both the aerobic and anaerobic cultures. The patients with negative microbiological examination results were placed in the control group. Patients with positive cultures were designated as the case group. Moreover, all the individuals in the case group could present signs of disease deterioration, such as ascites PMN > 250/mm3, fever, and other physical signs of infection, or liver and kidney function deterioration, and antibiotic treatments were necessary for these patients. Patients in the control group did not receive antibiotic treatments.
Demographic features and clinical information including age, gender, complications, and etiology of cirrhosis were collected from the initial medical records. Disease severity was estimated using the Child-Pugh stage and model of end-stage liver disease (MELD) score, as described previously[13,14]. Hematological factors included white blood cell (WBC) count, platelet count (PLT), and neutrophil percentage (N). Indicators for ascites included ascites leukocyte and polymorphonuclear percentage, as well as the PMN. Finally, patient condition at discharge was recorded.
The differences between the case and control groups were calculated using the Student’s t test (normal distribution) or the rank sum test (abnormal distribution). Data (continuous variables) are presented as mean ± SD. Categorical variables are presented as percentages and analyzed using the chi-square test. The predictive model was constructed using the multiple linear stepwise regression method of the logistic regression model. The diagnostic yield of the model was estimated using a receiver operating characteristic (ROC) curve. All analyses were two sided. In the multiple linear analysis, a P value < 0.01 was considered statistically significant, while P < 0.05 was considered significant in other analyses. All statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, United States).
A total of 371 liver cirrhosis patients were initially enrolled in this study. However, during the course of our study, 28 patients dropped out. Thus, 343 patients were included (259 males and 84 females) with an average age of 54.60 ± 12.79 years (Table 1). Among them, 220 (64.14%) patients were diagnosed with cirrhosis due to viral hepatitis, 65 (18.95%) cases were confirmed as having alcoholic cirrhosis, and 27 (7.87%) cirrhosis cases were caused by autoimmune diseases. According to Child-Pugh stages, the majority of patients were at stages B (175, 51.02%) and C (149, 43.44%), and the remaining patients (19, 5.54%) were diagnosed with stage A. The mean MELD score was 12.04 ± 8.87. Liver failure was observed in 51 (14.87%) patients. Additionally, 64 (18.66%) patients were diagnosed with hepatocellular carcinoma (HCC), while hepatic encephalopathy (HE) was detected in 29 (8.45%) patients. A total of 68 (19.82%) patients presented with cirrhosis combined with diabetes, 68 (19.82%) cases presented with renal dysfunction, and 22 (6.41%) cases had upper gastrointestinal bleeding (UGB) (Table 1).
| Parameters | Patients (n = 343, %) |
| Age (yr) | 54.60 ± 12.79 |
| Gender | |
| Male | 259 (75.51) |
| Female | 84 (24.49) |
| Clinical characteristics | |
| Etiology of cirrhosis | |
| Viral hepatitis | 220 (64.14) |
| Alcohol | 65 (18.95) |
| Autoimmune | 27 (7.87) |
| Others | 31 (9.04) |
| Child-Pugh | |
| A | 19 (5.54) |
| B | 175 (51.02) |
| C | 149 (43.44) |
| MELD score | 12.04 ± 8.87 |
| Complications | |
| Liver failure | 51 (14.87) |
| HCC | 64 (18.66) |
| HE | 29 (8.45) |
| Diabetes | 68 (19.82) |
| Renal dysfunction | 68 (19.82) |
| UGB | 22 (6.41) |
| Hematological factors | |
| WBC (× 109/L) | 5.19 ± 3.62 |
| N (100%) | 0.64 ± 0.12 |
| PLT (× 109/L) | 89.93 ± 60.52 |
| WBC/PLT | 0.082 ± 0.013 |
| Ascites examinations | |
| Leukocyte (mm3) | 258.92 ± 247.46 |
| Polymorphonuclear (100%) | 0.10 ± 0.098 |
| PMN (mm3) | 28.22 ± 39.46 |
| Microbiological examinations | |
| Positive | 111 (32.36) |
| Gram-negative | 21 (18.92) |
| Gram-positive | 80 (72.07) |
| Mixture | 10 (9.01) |
| Negative | 232 (67.64) |
| Clinical outcomes | |
| Non-survivors | 37 (10.79) |
| Improved | 297 (86.59) |
| Invalid | 9 (2.62) |
The mean WBC count was 5.19 ± 3.62 × 109/L, the average N was 0.64 ± 0.12 (× 100%), PLT was 89.93 ± 60.52 × 109/L, and the average ratio of WBC/PLT was 0.082 ± 0.013. In the ascites specimens, the leukocyte count was 258.92 ± 247.46 /mm3, polymorphonuclear cell percentage was 0.100 ± 0.098 (× 100%), and PMN was 28.22 ± 39.46 /mm3 (Table 1).
Microbiological investigations demonstrated that 111 (32.26%) patients were positive for pathogens, while no pathogens were isolated from the other 232 (67.64%) patients. Among the patients with positive cultures, gram-positive bacteria were isolated from 80 (72.07%) patients, and gram-negative bacteria were observed in 21 (18.92%) patients. Additionally, 10 (9.01%) infectious ascites cultures were the result of mixed pathogens. Among patients with positive microbial cultures, 13 patients improved without antibiotic administration. At the time of discharge, 297 (86.58%) patients had improved clinical outcomes, 37 (10.79%) patients died, and 9 (2.62%) showed deteriorating symptoms (these 9 patients were discharged upon their request) (Table 1).
Among the study subjects, 98 patients were positive for bacterial pathogens and were administered antibiotics. These patients were designated as the case group. In addition, 98 patients were randomly recruited from the study population into the control group according to the proportion of 1:1 using SPSS 18.0 software. The case and control groups were matched for age and gender (P > 0.05 for both).
Next, we compared the baseline characteristics between both groups (Table 2). Compared to the control group, the MELD scores, WBC, N, WBC/PLT, ascites polymorphonuclear and PMN were significantly higher in the case group (P < 0.05). Patients in the case group were more likely to develop advanced Child-Pugh stages (P = 0.004), liver failure (P < 0.001), HCC (P < 0.001), HE (P < 0.001), renal dysfunction (P < 0.001), and UGB (P < 0.001). Moreover, the death rate was significantly higher in the case group (33.67% vs 3.06%, P < 0.001) (Table 2).
| Characteristics | Asymptomatic SBP (n = 98, %) | Control group (n = 98, %) | P value |
| Age (yr) | 54.01 ± 13.60 | 53.56 ± 13.47 | 0.817 |
| Gender | 0.178 | ||
| Male | 79 (80.61) | 71 (72.45) | |
| Female | 19 (19.39) | 27 (27.55) | |
| Clinical characteristics | |||
| Etiology of cirrhosis | 0.280 | ||
| Viral hepatitis | 70 (71.43) | 61 (62.24) | |
| Alcohol | 16 (16.33) | 19 (19.39) | |
| Autoimmune | 5 (5.10) | 12 (12.24) | |
| Others | 7 (7.14) | 6 (6.12) | |
| Child-Pugh | 0.004 | ||
| A | 2 (2.04) | 2 (2.04) | |
| B | 35 (35.71) | 58 (59.18) | |
| C | 61 (62.24) | 38(38.78) | |
| MELD score | 18.20 ± 11.04 | 9.92 ± 5.88 | < 0.001 |
| Complications | |||
| Liver failure | 28 (28.57) | 7 (7.14) | < 0.001 |
| HCC | 37 (37.75) | 10 (10.20) | < 0.001 |
| HE | 21 (21.43) | 3 (3.06) | < 0.001 |
| Diabetes | 22 (22.45) | 13 (13.26) | 0.093 |
| Renal dysfunction | 39 (39.80) | 10 (10.20) | < 0.001 |
| UGB | 19 (19.39) | 0 (0.00) | < 0.001 |
| Hematological factors | |||
| WBC (× 109/L) | 7.25 ± 4.66 | 3.97 ± 1.99 | < 0.001 |
| N (100%) | 0.71 ± 0.13 | 0.60 ± 0.13 | 0.007 |
| PLT (×109/L) | 92.08 ± 71.37 | 82.44 ± 43.27 | 0.244 |
| WBC/PLT | 0.14 ± 0.22 | 0.057 ± 0.04 | 0.001 |
| Ascites examinations | |||
| Leukocyte (/mm3) | 314.41 ± 351.67 | 254.08 ± 231.51 | 0.151 |
| Polymorphonuclear (100%) | 0.15 ± 0.12 | 0.086 ± 0.10 | < 0.001 |
| PMN (/mm3) | 45.83 ± 52.25 | 21.44 ± 28.71 | < 0.001 |
| Clinical outcomes | |||
| Non-survivors | 33 (33.67) | 3 (3.06) | < 0.001 |
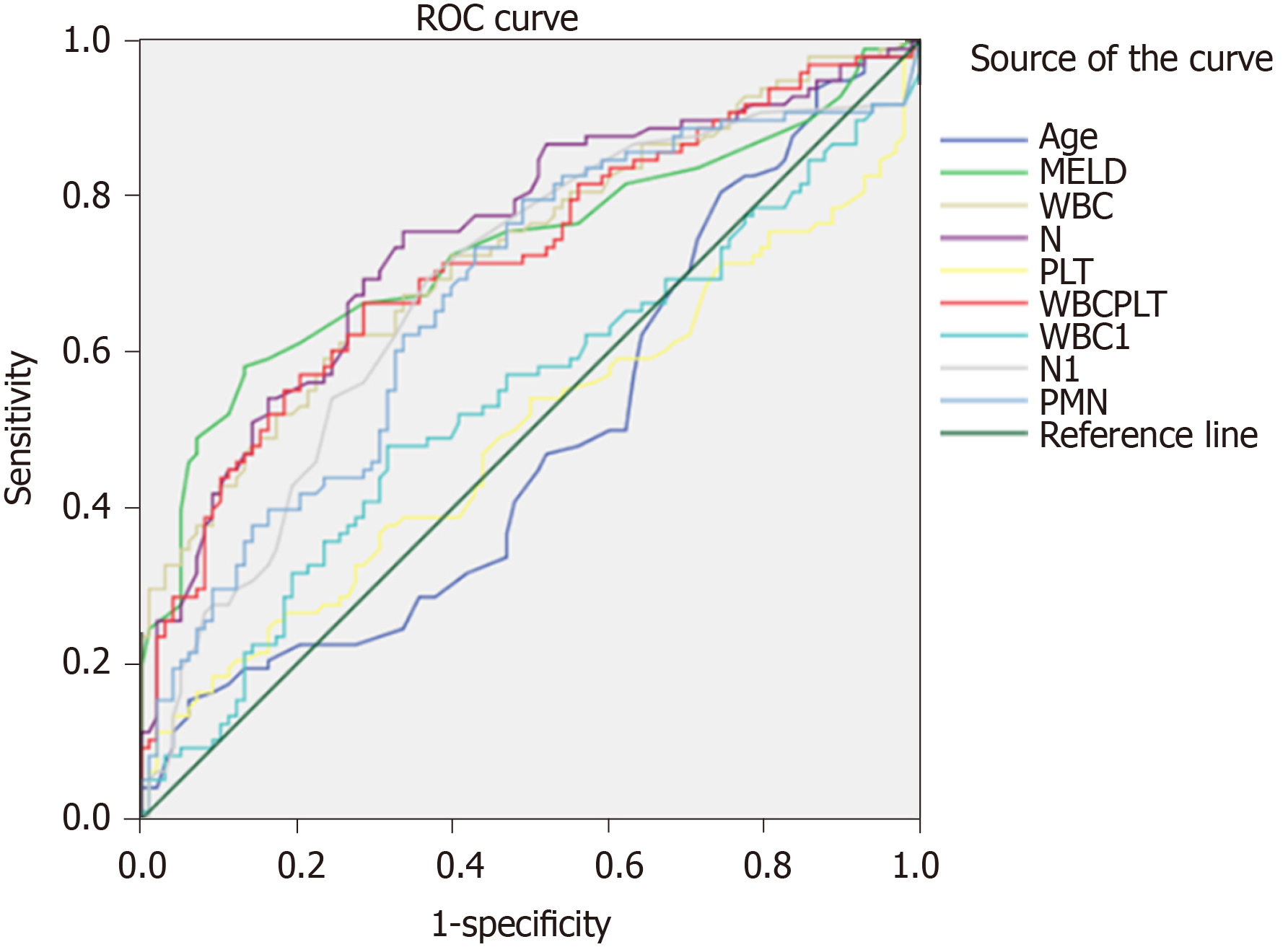
ROC curves were plotted for the selected 196 patients according to the continuous variables, including age, MELD scores, WBC, N, PLT, WBC/PLT, ascites leukocyte, polymorphonuclear, and PMN (Figure 1 and Table 3). The area under curve (AUC) values for age and PLT were 0.465 and 0.490, respectively, and their corresponding cut-off values were 71.50 years and 187.50 × 109/L, respectively. The AUC values of MELD scores, WBC, N, WBC/PLT, ascites leukocyte, polymorphonuclear, and PMN were more than 0.5, with the corresponding cut-off values at 15.50, 4.73 × 109/L, 66.15%, 0.0619, 230/mm3, 7.50%, and 10.95/mm3, respectively. Subsequently, based on the ROC cut-off values, these factors were entered into the multiple linear stepwise regression analysis as categorized variables.
| Variables | AUC | Cut-off value |
| Age (yr) | 0.489 | 71.50 |
| MELD score | 0.733 | 15.50 |
| WBC (× 109/L) | 0.729 | 4.73 |
| N (100%) | 0.747 | 0.6615 |
| PLT (× 109/L) | 0.494 | 187.50 |
| WBC/PLT | 0.720 | 0.0619 |
| Leukocyte (mm3) | 0.538 | 230.00 |
| Polymorphonuclear (100%) | 0.683 | 0.075 |
| PMN (mm3) | 0.671 | 10.95 |
Based on the multivariate analysis results (Table 4), blood neutrophil percentage, HCC, MELD, PMN, and renal dysfunction were included in the predictive model. The equation was as follows: y (P) = 0.018 + 0.312 × MELD (0: < 15.50; 1: > 15.50) + 0.263 × PMN (0: < 10.95; 1: > 10.95) + 0.184 × N (0: < 0.6615; 1: > 0.6615) + 0.233 × HCC (0: no; 1: yes) + 0.189 × renal dysfunction (0: no; 1: yes).
| Parameters | Unstandardized Coefficients | Standardized Coefficients | t | P value | |
| B | Std. Error | Beta | |||
| Constant | 0.018 | 0.055 | 0.331 | 0.741 | |
| MELD | 0.312 | 0.064 | 0.299 | 4.901 | 0.000 |
| PMN | 0.263 | 0.059 | 0.252 | 4.474 | 0.000 |
| N | 0.184 | 0.062 | 0.183 | 2.963 | 0.003 |
| HCC | 0.233 | 0.067 | 0.199 | 3.480 | 0.001 |
| Renal dysfunction | 0.189 | 0.071 | 0.164 | 2.671 | 0.008 |
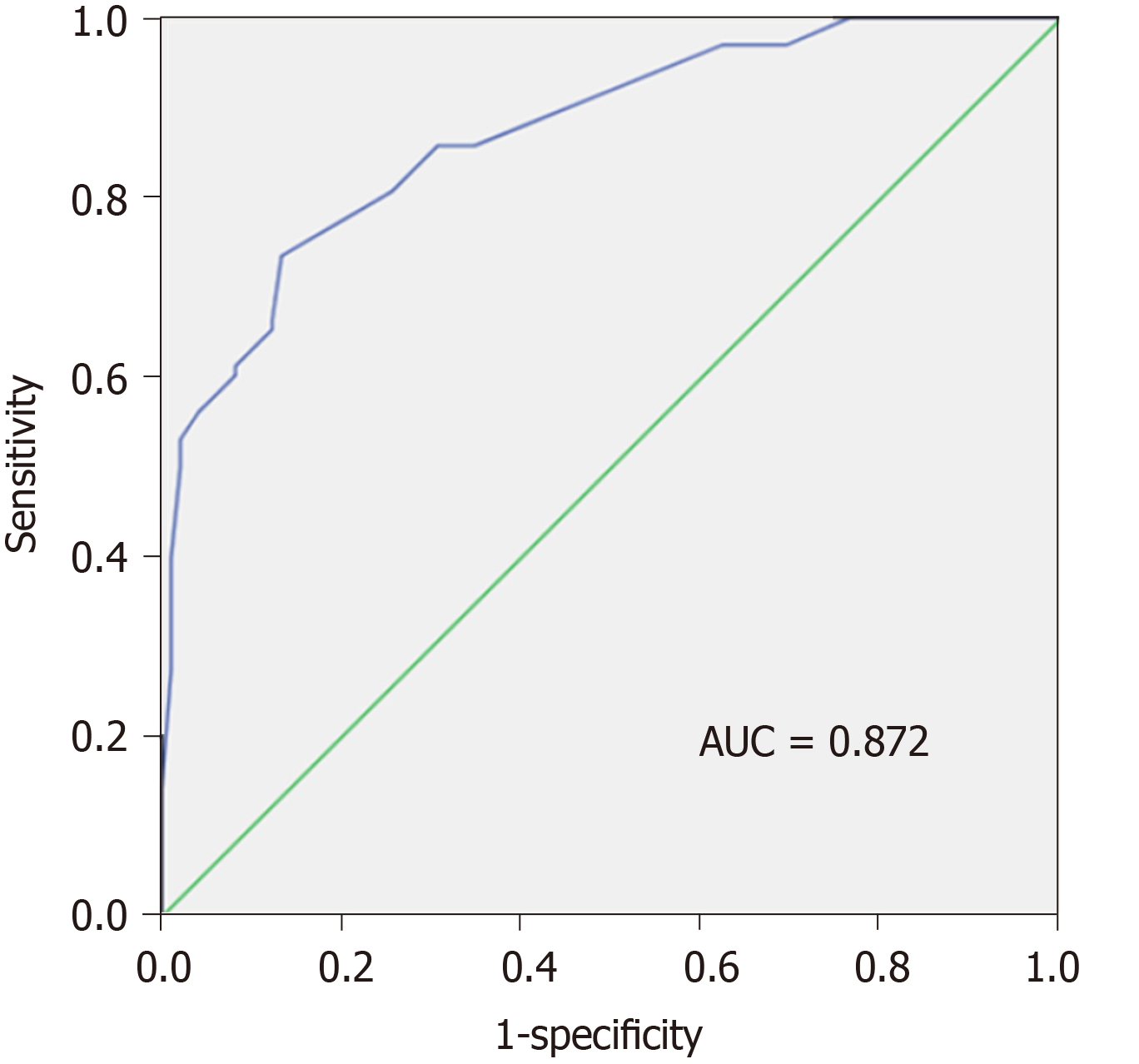
In our study, we also calculated y (P) value of each patient based on the original data using the constructed equation. The ROC curve was plotted based on the calculated P values (Figure 2). The AUC value was 0.872, revealing the high diagnostic value of the model with diagnostic sensitivity and specificity at 73.5%, and 86.7%, respectively.
In addition, P = 0.5 was identified as the optimum cut-off value for asymptomatic SBP diagnosis. The patients with P values > 0.5 were confirmed as having asymptomatic SBP. Accordingly, there were 72 patients in the asymptomatic SBP group with P > 0.5, while 26 patients in the control group did (P < 0.001). Our analysis showed that the diagnostic sensitivity was 73.5% (72/98), the specificity was 86.7% (85/98), and the diagnostic efficacy was 80.1% (Table 5). The Youden's index was 0.602, more than 0.5, suggesting a high application value of the model.
| Predictive results | Observed results | Percentage correct | |
| Asymptomatic SBP | Control group | ||
| Asymptomatic SBP | 72 | 26 | 73.5% |
| Control group | 13 | 85 | 86.7% |
SBP is associated with increased mortality in liver cirrhosis patients[15]. Early diagnosis followed by appropriate antibiotic administration can significantly improve the survival of SBP patients. However, based on the current diagnostic strategies, many asymptomatic SBP cases can be misdiagnosed leading to a delay in treatment or antibiotic abuse[16]. Therefore, in the present study, we established a multivariate predictive model by comparing 98 asymptomatic SBP patients with positive microbial culture (case group) and 98 cirrhotic ascites liver patients with negative microbial culture (control group). We compared the clinical characteristics and hematological factors, as well as ascites examination results between both groups. Our results demonstrated that patients in the case group were more likely to develop advanced Child-Pugh stages and high MELD scores. In agreement with previous reports, our results indicate that liver cirrhosis patients with advanced conditions are more likely to develop SBP. SBP was independently associated with liver disease severity according to Child-Pugh stage and high MELD score[17]. Additionally, the frequencies of liver failure, HCC, HE, renal dysfunction, and UGB were significantly higher in the case group, suggesting a close association of these factors with increased risk of SBP. SBP might not only contribute to the progression of liver disease, but also increase the risk of other complications, such as HE, septic shock, and hepatorenal syndrome. In addition, the WBC levels and the WBC/PLT ratios were significantly increased in asymptomatic SBP cases compared to the controls. The mortality of asymptomatic SBP cases was also higher than that of controls. Our findings are consistent with previous reports suggesting that SBP might aggravate dysregulation of the immune system, thus contributing to disease-related complications and increasing the risk of mortality among cirrhosis patients[18-20].
In this study, multiple linear stepwise regression analysis was performed to identify the potential indicators for early diagnosis of asymptomatic SBP in liver cirrhosis patients. Our results showed that blood neutrophil percentage, HCC, MELD, PMN, and renal dysfunction could be included in the predictive model. Liver cirrhosis patients are more likely to be infected by pathogens due to their compromised immune response. Abnormal stimulation of neutrophils induced by impaired phagocytic and oxidative burst function may be responsible for dysregulation of the immune system in liver cirrhosis patients[21,22]. Therefore, blood neutrophils and PMN in ascitic fluid might be effective factors for early SBP screening in liver cirrhosis patients. MELD is an indicator of disease severity, and its positive association with SBP initiation has been previously reported. Na et al[23] demonstrated that patients with SBP had higher MELD scores than those without SBP. Additionally, the MELD score can function as an independent factor for occurrence and clinical outcomes of SBP[24,25]. However, some studies may hold different opinions. In the study by Haddad et al[26], MELD was confirmed to have no association with SBP. Although MELD had no direct association with SBP, MELD might be employed as a predictive biomarker for SBP. Moreover, liver disease-related complications may contribute to the development of SBP. Tsung et al[27] reported that HCC and renal dysfunction could increase the death rate in liver cirrhosis patients with SBP[27]. Taken together, the predictive model constructed in our study is feasible, and ROC analysis confirmed that the predictive model had high application potential for early SBP diagnosis. According to Project Leonardo, a health follow-up file may be established for patients with liver cirrhosis, especially those who have ascites. The special care manager will follow up the patients on a regular basis to gain the trust and cooperation of the patients, in order to discover changes in the condition with time. Based on our diagnostic model, time treatments could be supplied for patients to improve the long-term prognosis[28].
There were several limitations in our study. First, the sample size was relatively small, which may have influenced the accuracy of the established model. SBP patients might not have been admitted to hospital without obvious clinical symptoms; moreover, the positive rate of ascites culture was relatively low. This resulted in a relatively small sample size. Second, the diagnostic performance of the predictive model was only verified in our patient cohort. Only 98 cases met the case conditions; thus, these patients could not be divided into a modeling group and a validation group. Therefore, the validation group was not adopted to verify our model. The cross-validation with a larger sample size will be required to confirm the efficacy and further improve our predictive model. Third, the present study was a retrospective investigation, and some indicators that may have been significant for early diagnosis of SBP could not be obtained. Thus, some potential biomarkers were not taken into consideration in our study, such as inflammatory factors. Therefore, well-designed prospective studies are required to improve and verify our study.
In conclusion, a multivariate predictive model was established for the early diagnosis of asymptomatic SBP patients with positive microbiological results to determine which patients should be treated with antibiotics. Our predictive model was based on MELD, PMN, blood N, HCC, and renal dysfunction, which may enhance antibiotic treatment in asymptomatic SBP patients.
Spontaneous bacterial peritonitis (SBP) is a detrimental infection of the ascitic fluid in liver cirrhosis patients, with high mortality and morbidity. Early diagnosis and timely antibiotic administration have successfully decreased the mortality rate to 20%-25%. Early diagnosis of asymptomatic SBP remains a great challenge in the clinic.
Currently, SBP cases are diagnosed based only on clinical symptoms, leading to possible antibiotic abuse. SBP is regulated by a variety of risk factors, including decreased activity of the reticuloendothelial system, advanced liver dysfunction, medications, and genetic factors. A multivariate predictive model may be effective for early screening of asymptomatic SBP.
The present retrospective cohort study aimed to establish an effective predictive model for early screening of asymptomatic SBP in liver cirrhosis patients with ascites. Early diagnosis of asymptomatic SBP will improve antibiotic management strategies and reduce SBP-associated mortality.
Liver cirrhosis patients with ascites who had no typical SBP symptoms were included in the current study, and divided into the case (positive cultures) and control (negative cultures) groups according to microbiological results. The demographic features, clinical information, disease activity, hematological and ascites factors were compared between the case and control groups to identify potential indicators of asymptomatic SBP. The multiple linear stepwise regression method of the logistic regression model was adopted to construct the multivariate predictive model. The diagnostic performance of the model was estimated by the receiver operating characteristic curve.
Patients in the case group were more likely to have advanced disease stages, cirrhosis related-complications, worsened hematology and ascites, and higher mortality. Based on multivariate analysis, the predictive model was as follows: y (P) = 0.018 + 0.312 × MELD (model of end-stage liver disease) + 0.263 × PMN (ascites polymorphonuclear) + 0.184 × N (blood neutrophil percentage) + 0.233 × HCC (hepatocellular carcinoma) + 0.189 × renal dysfunction. The area under curve value of the established model was 0.872, revealing its high diagnostic potential. The diagnostic sensitivity was 73.5% (72/98), the specificity was 86.7% (85/98), and the diagnostic efficacy was 80.1%.
The multivariate predictive model based on model of end-stage liver disease, polymorphonuclear, blood neutrophil percentage, hepatocellular carcinoma, and renal dysfunction exerts high diagnostic efficacy which may improve the early diagnosis of asymptomatic SBP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Vidal S S-Editor: Zhang L L-Editor: Webster JR E-Editor: Ma YJ
| 1. | Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol. 2016;8:307-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (2)] |
| 2. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 848] [Article Influence: 77.1] [Reference Citation Analysis (1)] |
| 3. | Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | How J, Azar MM, Meyer JP. Are Nectarines to Blame? A Case Report and Literature Review of Spontaneous Bacterial Peritonitis Due to Listeria monocytogenes. Conn Med. 2015;79:31-36. [PubMed] |
| 5. | Koulaouzidis A, Bhat S, Saeed AA. Spontaneous bacterial peritonitis. World J Gastroenterol. 2009;15:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 6. | de Mattos AA, Costabeber AM, Lionço LC, Tovo CV. Multi-resistant bacteria in spontaneous bacterial peritonitis: a new step in management? World J Gastroenterol. 2014;20:14079-14086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 562] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 8. | Mohan P, Venkataraman J. Prevalence and risk factors for unsuspected spontaneous ascitic fluid infection in cirrhotics undergoing therapeutic paracentesis in an outpatient clinic. Indian J Gastroenterol. 2011;30:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Bernardi M. Spontaneous bacterial peritonitis: from pathophysiology to prevention. Intern Emerg Med. 2010;5 Suppl 1:S37-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 11. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1132] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 12. | Sundaram V, Manne V, Al-Osaimi AM. Ascites and spontaneous bacterial peritonitis: recommendations from two United States centers. Saudi J Gastroenterol. 2014;20:279-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 519] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 14. | Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Runyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 16. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5736] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 17. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 18. | Botwin GJ, Morgan TR. Bacterial infections in cirrhosis. Hepatol Int. 2014;8 Suppl 2:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Fiore M, Maraolo AE, Leone S, Gentile I, Cuomo A, Schiavone V, Bimonte S, Pace MC, Cascella M. Spontaneous peritonitis in critically ill cirrhotic patients: a diagnostic algorithm for clinicians and future perspectives. Ther Clin Risk Manag. 2017;13:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Miozzo SAS, John JA, Appel-da-Silva MC, Dossin IA, Tovo CV, Mattos AA. Influence of proton pump inhibitors in the development of spontaneous bacterial peritonitis. World J Hepatol. 2017;9:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Strauss E. The impact of bacterial infections on survival of patients with decompensated cirrhosis. Ann Hepatol. 2013;13:7-19. [PubMed] |
| 22. | Nieto JC, Sánchez E, Romero C, Román E, Poca M, Guarner C, Juárez C, Soriano G, Vidal S. Impaired innate immune response of leukocytes from ascitic fluid of patients with spontaneous bacterial peritonitis. J Leukoc Biol. 2015;98:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Na SH, Kim EJ, Nam EY, Song KH, Choe PG, Park WB, Bang JH, Kim ES, Park SW, Kim HB, Oh MD, Kim NJ. Comparison of clinical characteristics and outcomes of spontaneous bacterial peritonitis and culture negative neutrocytic ascites. Scand J Gastroenterol. 2017;52:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 25. | Engelmann C, Becker C, Boldt A, Herta T, Boehlig A, Splith K, Schmelzle M, Mueller N, Krohn S, Tautenhahn HM, Bartels M, Sack U, Berg T. Ascites' neutrophil function is significantly impaired in patients with decompensated cirrhosis but can be restored by autologous plasma incubation. Sci Rep. 2016;6:37926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Haddad L, Conte TM, Ducatti L, Nacif L, D'Albuquerque LA, Andraus W. MELD Score Is Not Related to Spontaneous Bacterial Peritonitis. Gastroenterol Res Pract. 2015;2015:270456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Tsung PC, Ryu SH, Cha IH, Cho HW, Kim JN, Kim YS, Moon JS. Predictive factors that influence the survival rates in liver cirrhosis patients with spontaneous bacterial peritonitis. Clin Mol Hepatol. 2013;19:131-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Ciccone MM, Aquilino A, Cortese F, Scicchitano P, Sassara M, Mola E, Rollo R, Caldarola P, Giorgino F, Pomo V, Bux F. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc Health Risk Manag. 2010;6:297-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |