Published online Aug 7, 2020. doi: 10.3748/wjg.v26.i29.4198
Peer-review started: May 18, 2020
First decision: June 4, 2020
Revised: June 10, 2020
Accepted: July 23, 2020
Article in press: July 23, 2020
Published online: August 7, 2020
Processing time: 80 Days and 13.2 Hours
Gastrointestinal leaks and fistulae are serious, potentially life threatening conditions that may occur with a wide variety of clinical presentations. Leaks are mostly related to post-operative anastomotic defects and are responsible for an important share of surgical morbidity and mortality. Chronic leaks and long standing post-operative collections may evolve in a fistula between two epithelialized structures. Endoscopy has earned a pivotal role in the management of gastrointestinal defects both as first line and as rescue treatment. Endotherapy is a minimally invasive, effective approach with lower morbidity and mortality compared to revisional surgery. Clips and luminal stents are the pioneer of gastrointestinal (GI) defect endotherapy, whereas innovative endoscopic closure devices and techniques, such as endoscopic internal drainage, suturing system and vacuum therapy, has broadened the indications of endoscopy for the management of GI wall defect. Although several endoscopic options are currently used, a standardized evidence-based algorithm for management of GI defect is not available. Successful management of gastrointestinal leaks and fistulae requires a tailored and multidisciplinary approach based on clinical presentation, defect features (size, location and onset time), local expertise and the availability of devices. In this review, we analyze different endoscopic approaches, which we selected on the basis of the available literature and our own experience. Then, we evaluate the overall efficacy and procedural-specific strengths and weaknesses of each approach.
Core tip: Early diagnosis of gastrointestinal leaks and fistulae is associated with better outcomes. Endoscopic minimally invasive management is becoming the treatment of choice for gastrointestinal wall defects. It is more effective and safer than surgery. Several endoscopic devices and techniques are available, and they include endoclip, metal or plastic stent, tissue sealants, suturing systems and vacuum therapy. The choice of one procedure over another should depend on clinical presentation, defect features and local expertise. Early leaks have a higher rate of longstanding healing compared to late leaks and fistulae. A close collaboration between surgeons, interventional radiologists and therapeutic endoscopists is recommended to assure a favorable outcome.
- Citation: Cereatti F, Grassia R, Drago A, Conti CB, Donatelli G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J Gastroenterol 2020; 26(29): 4198-4217
- URL: https://www.wjgnet.com/1007-9327/full/v26/i29/4198.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i29.4198
Gastrointestinal (GI) leaks and fistulae constitute a disruption of the GI wall. GI leaks and fistulae refer to two well distinct entities.
Leak is defined as a pathological communication between intra and extra-luminal compartments as a result of a defect in the integrity of the GI wall, which often lead to egression of luminal contents. They are mostly related to anastomotic defect after surgical procedures[1] and are associated with a high risk of morbidity and mortality. They constitute the single adverse event (AE), which is responsible for the majority of surgical mortality occurring in up to 60% of cases if the treatment is delayed[2]. Prevalence of GI leaks has increased in recent years most probably due to an increased complexity of GI surgery. Post-operative leaks after oncological surgery has been reported in 8% to 26% of cases after distal esophagectomy and in 3% to 12 % after total gastrectomy[3,4]. Leaks represent a major concern even in bariatric surgery with a prevalence of 1%-2% after sleeve gastrectomy (SG) and from 2% to 8% after Roux-Y-Gastric bypass[5,6] (Figure 1). Whereas anastomotic leakage after colorectal surgery has been observed in approximately 11% of cases with a mortality around 12%. Proctocolectomy and total mesorectal excision, followed by ileoanal or coloanal anastomosis, may reach a rate of leaks as high as 20%[7].
Fistula is defined as an abnormal communication between two epithelialized surfaces. A fistula may involve many adjacent structures: Entero-enteric, entero-bronchial/tracheal, entero-vaginal, entero-vescical, entero-cutaneous (Figure 2). Prolonged anastomotic leaks, especially if coupled with extra-luminal fluid spillage and abscess, may evolve in a chronic fistula[8] (Table 1).
| Definition | |
| Leak | Pathological communication between intra and extra-luminal compartments |
| Fistula | Abnormal communication between two epithelialized surfaces |
The fundamental principles of GI leak and fistula management are identification of the site of defect, drainage of any leaked luminal contents and avoidance of further spillage either by diversion of luminal contents flow or by closure of the defect[9].
Mainstay of conservative management include bowel rest, adequate nutritional support and appropriate antibiotic therapy[10]. Historically, conservative management and revisional surgery with surgical drainage, defect repair or redo anastomosis, had been the mainstay treatment of GI leaks and fistulae. However, surgical interventions may be difficult and associated with a high risk of morbidity and mortality[11]. Therefore, the last decades have witnessed an increasing interest in endoscopic management. Recent advances in interventional endoscopy allowed a paradigm shift in the management of GI wall defect from surgery to minimally invasive endoscopic approaches. Endoscopy showed to be an effective and less invasive alternative to primary surgery. Several endoscopic options are available in order to re-establish GI continuity, avoid further luminal spillage thus preventing infections, drain/prevent collection and provide nutritional support. Available endoscopic treatments include: Through the scope (TTS) or over the scope (OTS) clip, stent deployment, endoscopic internal drainage (EID), suturing systems, vacuum assisted therapy (EVT) and sealants[12].
The aforementioned techniques may be applied alone or in combination, and as first line or as salvage treatment after failure of previous approaches. Unfortunately, a standardized approach that fits for all possible scenarios does not exist. Each treatment should be tailored according to several variables, such as the clinical presentation and patient’s general status, size of the defect, time of onset, defect location, endoscopic accessibility, ability to drain or avoid any associated collection and local expertise/accessories availability. In reason of technical complexity of most procedures and the relative learning curve, difficult cases should be managed in referral centers with adequate caseload, whenever possible.
Surgery as first line treatment should nowadays be reserved to patients with severe sepsis or multi organ failure. Revisional surgery plays a major role in case of generalized or extensive peritonitis because it allows to perform a complete peritoneal washout and drainage with prompt reduction of the bacterial load. Early diagnosis is of paramount importance because it is associated to better outcomes. Diagnosis should be reached based on a combination of clinical presentation, radiological findings and endoscopic evaluation. Pre-procedural assessment of defect site is mandatory in order to evaluate the feasibility of proposed endoscopic approach and features of the defect and surrounding tissue (e.g., healthy, inflamed, ischemic or chronic). Defect orifice and cavity features should be assessed not only by means of intra-procedural contrast study but even, whenever possible, by means of direct endoscopic cavity exploration.
This review aims to describe the main endoscopic available techniques to manage the GI defects and to describe the pros and cons of their application in case of fistulae and leaks.
Endoscopic clips are routinely used in clinical practice for a wide variety of GI conditions. Although endoclips have shown to be very effective in the management of acute intra-procedural GI perforation[13] their role in closure of chronic leak and fistula is controversial. Two main types of endoclips are available: Through-the scope clips and Over-the scope clips.
TTS clip is a widely available accessory, routinely used in endoscopy, in different designs and sizes, and it is inserted through the operative channel of the scope. There are two main types of TTS clips: Reusable and single use clip. The first type is the most commonly used and it has a reloading manually device to load the clip onto a small hook at the end of a metal cable running through a plastic sheath. Once put in the scope, the clip arms can be aligned to the tissue that the operator wishes to grasp, by rotating the handle and cannot be reopened. Conversely, the single use clip is a preloaded accessory. This type of TTS has a wider opening then the reusable ones and its arms can be closed and reopened several times, before the definitive release of the clip. These different models make TTS clips easy to use and adaptable to different scenarios. However, clip performance in closure of chronic defects is hampered by its limited pressure applied to tissues and its “natural” tendency to dislodge spontaneously. Therefore, if necrotic or inflamed tissue is present, TTS clip may easily result in a suboptimal closure. Nonetheless, in a case series of 20 patients with anastomotic leak after gastric surgery Lee et al[14] reported a 95% success rate after TTS clip deployment. A mean number of 3.4 ± 1.46 clips were used. Clip deployment was coupled with fibrin glue in 14 cases whereas in 2 patients detachable snare plus clip were used.
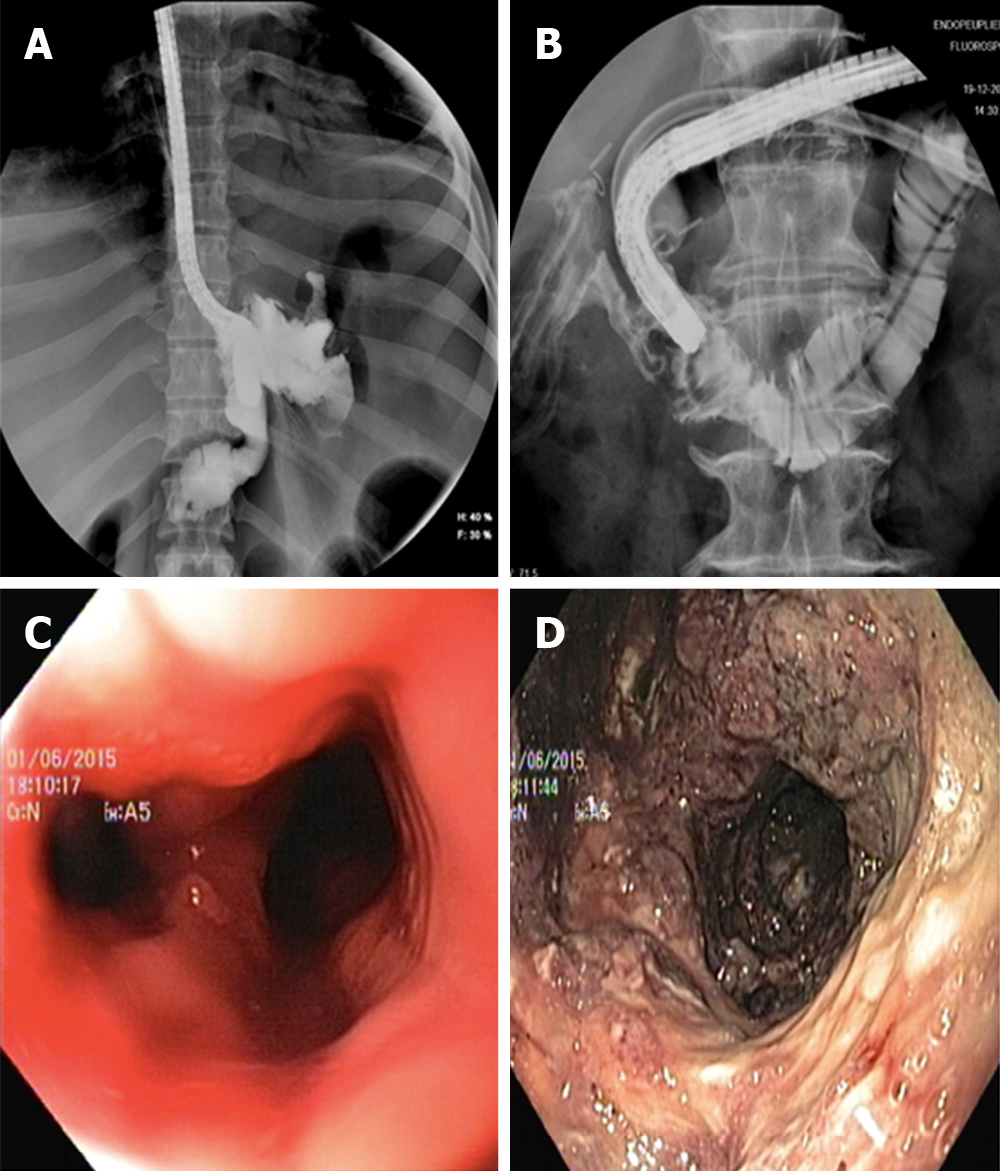
OTS clip is a biocompatible nitinol clip with a bear-trap shape design. It is mounted on a cap installed at the tip of the endoscope allowing full-thickness closure of GI defects up to 2 cm in size. The most common commercially available OTS clip are the over-the-scope clips (OTSC) system (OTSC, Ovesco Endoscopy AG, Tubingen, Germany) (Figure 3) and Padlock clip (Aponos Medical Corp, Kingstone, New Hampshire). OTS clips are available in different sizes and different teeth designs according to required indication.
These are the advantages of OTS over TTS clip: It consists in a clip with wider arms and it has higher mechanical tissue compression allowing long-lasting full-thickness closure[15] (Figure 4). These are the shortcomings of OTS: It requires a challenging removal procedure in case of treatment failure; it displays a high rate of fistula recurrence after initial clinical success[16] and it may cause interference with subsequent surgical procedure.
Some authors suggest to de-epithelialize the edges of the defect and surrounding mucosa with Argon Plasma Coagulation or with a cytology brush before OTS clip deployment, in order to guarantee a stronger and more durable tissue grasp. A long indwelling time of OTS reflects its correct deployment over a suitable tissue and translates into a higher long-term clinical success. Donatelli et al[17] reported OTS clip outcome in a retrospective study comprising 45 patients, who presented both iatrogenic acute perforation (15 pts) as well as post-surgical leak and fistula (30 pts). In the latter group OTS clips were used as a rescue therapy after previous endoscopic treatments. Clinical success rate in the chronic setting group was significantly lower (36.6%) compared to the success rate in the acute setting group (100%). The largest multicenter series of OTS clip for management of GI wall defects highlighted a similar trend. Considering 188 patients, the rate of successful closure of perforations (90%) and leaks (73.3%) were significantly higher than that of fistulae (42.9%) (P < 0.05). Long-term success was significantly higher when OTSCs were applied as primary therapy (primary 69.1% vs rescue 46.9%; P = 0.004)[18]. In a recent retrospective study, Morrel et al[19] reported overall success rate of 64.4% in OTS deployment. Long-term success was significantly higher for leaks than for fistulae (79.6% vs 55.0%, P = 0.007) and, more patients with fistulae ultimately underwent definitive operative management (16.9% vs 3.9%, P = 0.0253). A recent systematic review, which accounted for 1517 cases retrieved from 30 studies published between 2010 and 2018, summarized OTS clip results for various GI indications. Out of 1517 cases, 388 fistulae and 97 anastomotic leaks were treated with OTS clip. The review reported an overall success rate of 51.5% in case of fistulae and 66% for anastomotic leaks[20].
The use of temporary endoscopic stent has emerged has an effective and safe treatment option for the management of upper gastrointestinal leaks and fistula with acceptable morbidity and low mortality[21,22]. The rationale of stent deployment is to seal the defect and divert luminal content thus allowing mucosal wall healing. Further advantages consist in the possibility of early oral intake and reduced risk of stricture formation[23]. Complete drainage of any extra-luminal collection is mandatory before stent deployment, in order to allow successful closure and reduce septic com-plications[24]. Different types of stent may be used, namely: Self-expandable plastic stents (SEPS) and self-expandable metal stent (SEMS) both fully covered (FCSEMS) or partially covered (PCSEMS).
SEPS are endoscopic stent made of a polyester netting fully covered with silicone. They were initially developed for the management of esophageal stricture[25] and later deployed with satisfactory results for the management of esophageal leaks[26-28].
SEMS may be composed either of Elgiloy, an alloy of cobalt, nickel and chromium or of Nitinol, an alloy of nickel and titanium. SEMS presents a flexible delivery system and a higher radial force compared to SEPS[29]. FCSEMS has a membrane (pol-yurethane, polyethylene or silicone rubber) along its full length whereas PCSEMS has uncovered distal and proximal ends.
Comparison between SEPS and SEMS: Presumed benefits of SEPS over SEMS are easier removability, lower costs and reduced tendency to induce hyperplastic tissue formation.
In a systematic review comprising 267 patients treated with luminal stent (FCSEMS vs PCSEMS vs SEPS) for benign esophageal rupture or leak, van Boeckel et al[30] showed a similar efficacy between the different stents (SEPS 84%; FCSEMS 85%; PCSEMS 86%; P = 0.97). These data are in accordance with other studies showing a clinical success of SEPS ranging from 66% to 100%[31-33]. However, the disadvantages of SEPS over SEMS are its large diameter, the need to mount the stent on a delivery system that may hamper its deployment if strictures or angulation are present and a high rate of migration, reaching up to 40% of cases in long term follow up[34]. Although the existing literature shows a similar efficacy of SEPS and SEMS, in recent years the use of SEMS has substantially replaced the use of SEPS. A recent expert international survey[12] on endoscopic treatment of upper gastrointestinal (UGI) leaks, identified SEMS deployment as the most frequently used technique.
The clinical use of SEMS in upper GI tract: Clinical success ranges in literature from 48 to 100%[35-37]. van Halsema et al[34] reported an overall clinical success of 76.8% (480/625) and, according to etiology 81.4% (201/247) for post-surgical leaks and 64.7% for fistulae (11/17).
A short interval time between index surgery, leak diagnosis and SEMS deployment seems to be a fundamental factor for a successful treatment[38]. Considering UGI leak, Freeman et al[39] identified 4 factors associated with treatment failure: Leak of the proximal cervical esophagus, stent trasversing gastroesophageal junction, esophageal rupture longer than 6 cm and anastomotic leak associated with a more distal conduit leak. Optimal stent indwelling time is not well established. Although animal studies suggested that an indwelling time of 30 d is sufficient to guarantee healing[40] a pooled analysis of 20 retrospective studies from 2013 to 2015 showed a median indwell time of 5 to 7 wk for FCSEMS and an indwell time of 7 to 10 wk for PCSEMS[37]. Lately there is a tendency to reduce the stent dwell time to 4-5 wk[12] in order to guarantee a proper time for complete closure but at the same time reduce stent related AE. Unfortunately, SEMS treatment is burdened by an AE rate that ranges in literature from 20% to 72% (Figure 5) with a stent related mortality ranging from 0 to 28%[34,35,41-44], which is lower however, than those reported after surgical management, which ranges from 12% to 50%[34].
Stent migration is a major limitation, since it is responsible for up to one third of cases needing re-intervention, thus increasing costs. Stent migration may be responsible for further AEs such as perforation or obstruction[45] and it is related to altered anatomy and absence of stenosis coupled with physiologically large diameter of GI tract. FCSEMS are more susceptible to migration than PCSEMS. A systematic review from 2011[30] reported a migration rate of 26% for FCSEMS and 13% for PCSEMS (P ≤ 0.001). In one study endoscopic re-treatment was necessary for stent migration in 50% of cases[45]. These results suggest that in order to achieve clinical success of leaks and fistula, multiple stent deployment may be necessary.
Fixating the proximal flange of the stent to the esophageal wall by means of through-the scope (TTS) clips, OTSC or endosuturing devices has been proposed[46-50]. Fixation techniques are used in 80% of expert centers, particularly in case of previous stent migration, when incomplete sealing between stent and esophageal wall is present or when stents are placed across jejunal anastomoses[12]. In a multicenter retrospective study, Ngamruengphong et al[51] evaluated 74 patients underwent to stent deployment for benign UGI conditions (strictures, leaks, fistulae and perforations). All subjects were treated either with PCSEMS (28 pts) or with FCSEMS sutured to the esophageal wall with the Overstitch suturing device (Apollo Endosurgery, Austin, TX, United States). The study detected no statistically significant difference in stent migration rate between the 2 techniques (adjusted odds ratio 0.56; 95%CI 0.15-2.00; P = 0.37). However, the rate of other stent-related AEs was higher in the PCSEMS group (46% vs 21%; P = 0.37)[51].
Tissue hyperplasia within the mesh (ingrowth) or at stent edges (overgrowth) has been reported as high as 41% to 53% after PCSEMS deployment[52,53]. Granulation tissue may hamper stent removal or induce stricture formation. Different methods to remove partially embedded PCSEMS has been described. The most common one is the so called “stent-in-stent” technique in which a second stent is deployed inside the embedded one in order to induce pressure necrosis of hyperplastic tissue thus allowing stent removal. Swinnen et al[54] demonstrated a successful rate of 97.8% for stent removal after SEPS deployment for 6 to 10 wk. Use of Argon Plasma Coagulation in order to ablate the ingrowing tissue has been proposed as well[55]. Nonetheless, hemorrhage and esophageal rupture have been described after stent removal[23].
The literature describes the following stent related AEs as well: Stent rupture, food impaction, severe pain, mucosal erosion with fistulae formation or massive bleeding due to erosion into major vessels[56].
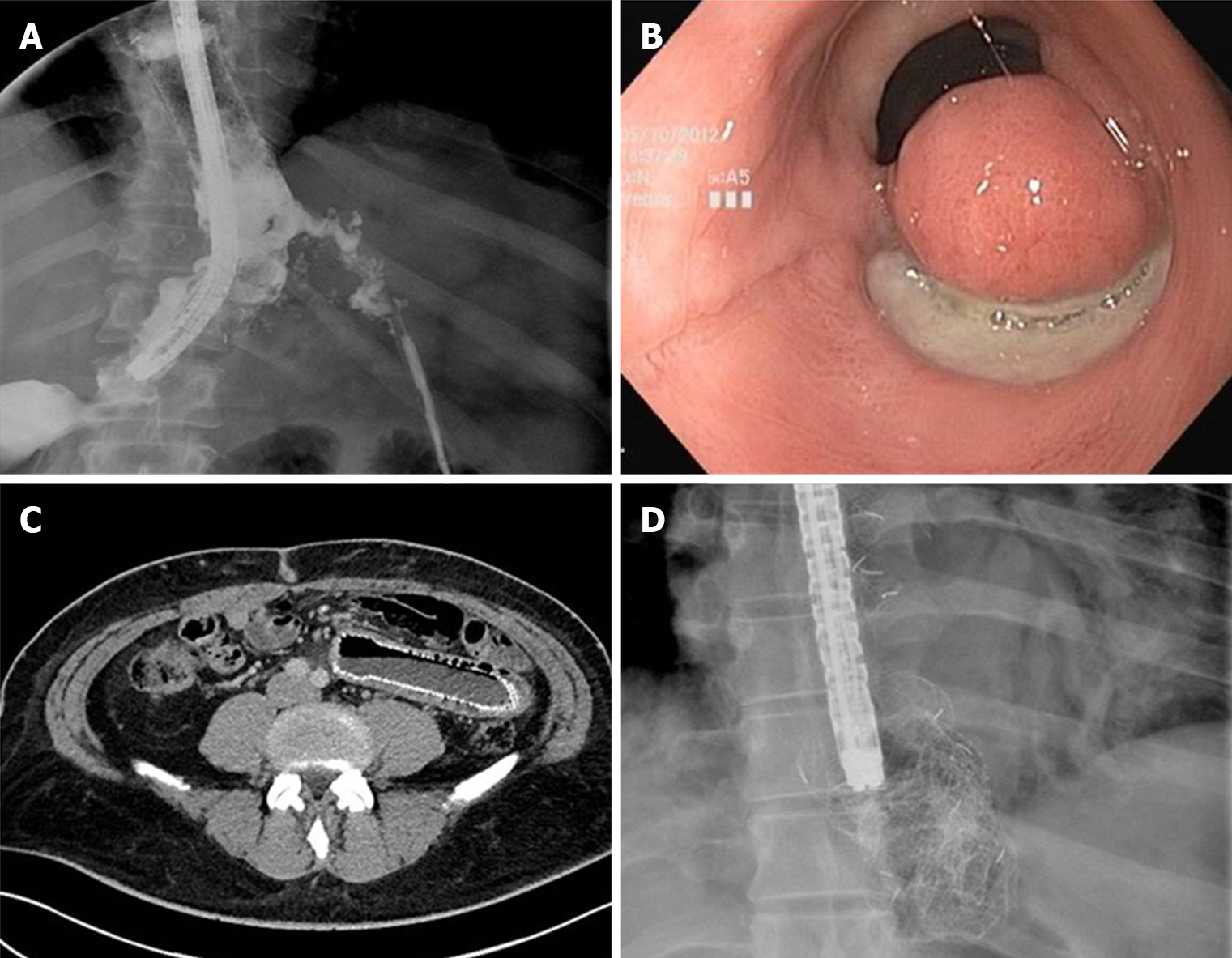
The clinical use of SEMS in bariatric surgery: Specifically designed SEMS have been recently developed for the management of leaks after bariatric surgery. The most common used are: Mega Stent (Taewoong medical, Seoul, South Korea) a fully covered ultra large and long (18-24 cm) stent with a design studied to reduce migration and to give additional flexibility to better adapt to post sleeve gastrectomy anatomy (Figure 6) and Niti-S-Beta stent (Taewoong medical, Seoul, South Korea) a fully covered stent with a proximal flange and a double-bump in the proximal third in order to reduce migration. Nonetheless, data from literature showed a similar success rate without statistically significant differences in migration rate[57,58]. Moreover, special attention should be taken when placing a stent across gastro-jejunal anastomosis after Roux-Y-Gastric bypass because its migration in the small bowel may hamper endoscopic removal causing obstruction or perforations. In similar scenario, if stent management is decided, proximal fixation is advised to reduce the risk of migration.
The clinical use of SEMS in lower GI tract: The role of SEMS has been investigated even in the management of colorectal leaks and fistulae. A meta-analysis considering 17 studies including 68 patients treated with SEMS showed a success rate in approximately 75% of cases[59]. A case series considering 22 patients treated for anastomotic leakage (at least 30% of circumference) reported a healing rate with diverting stoma reversal of 84%[60]. However, due to vigorous motility and luminal diameter, stent migration may occur in approximately 40% of cases, reaching up to 80% of cases if a concomitant stricture is not present[61]. The following general consideration must be kept in mind if SEMS treatment is decided: Mandatory use of FCSEMS, avoid use of stent closer than 1 cm from the anal verge due to patient discomfort, prior drainage of any nearby collection and avoid if sepsis is present[62].
In recent years, endoscopic management of leak and fistula after bariatric surgery started to shift from stent deployment to EID. Nonetheless, SEMS remains the most used technique although it is associated with significant rate of AE. Moreover, long term success after stent management may not be reached in more than 70% of cases, independently from the type of stent or combination of different endoscopic approaches[63,64].
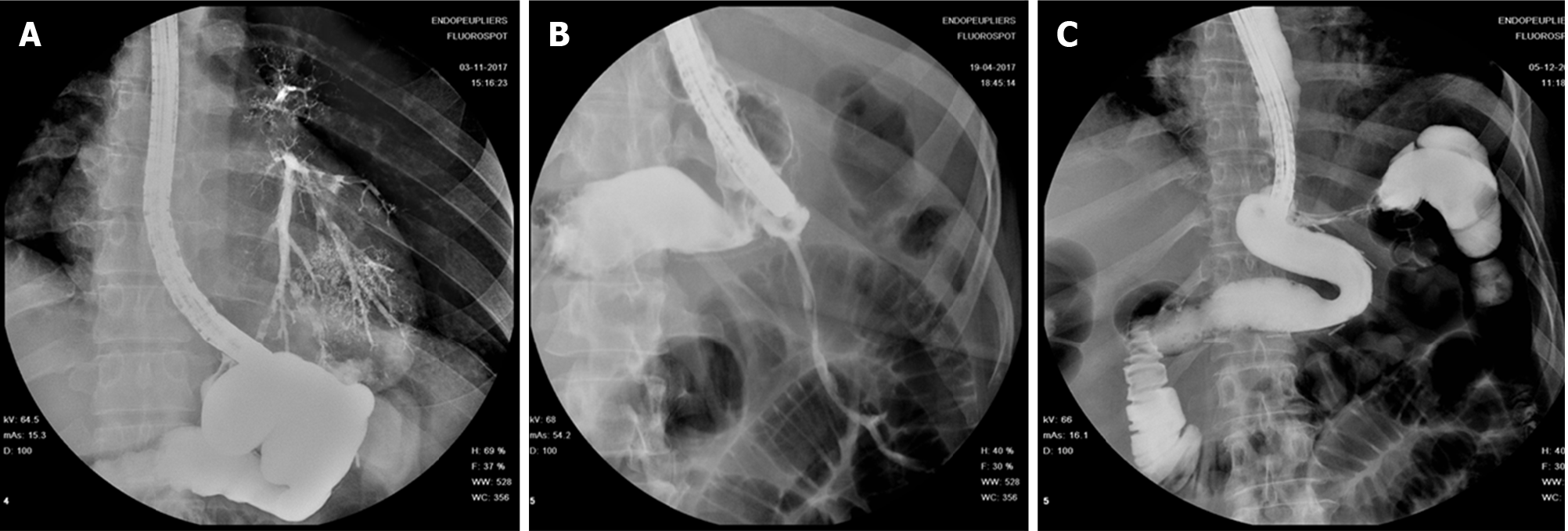
Pequignot et al[65] in 2012 described for the first time the use of double pigtail stent or naso-biliary drain across leak orifice in order to guide drainage toward GI lumen and promote healing while favoring leak orifice closure. In their case series, 25 patients presenting with gastric leak after SG were treated either with SEMS deployment or EID. EID was mainly used in case of late onset of gastric leak and after failure of the other techniques. In their study EID was more effective and safer than SEMS. The authors reported that pigtail stents were better tolerated, requiring less procedures per patient with a shorter healing time, lower morbidity and mortality.
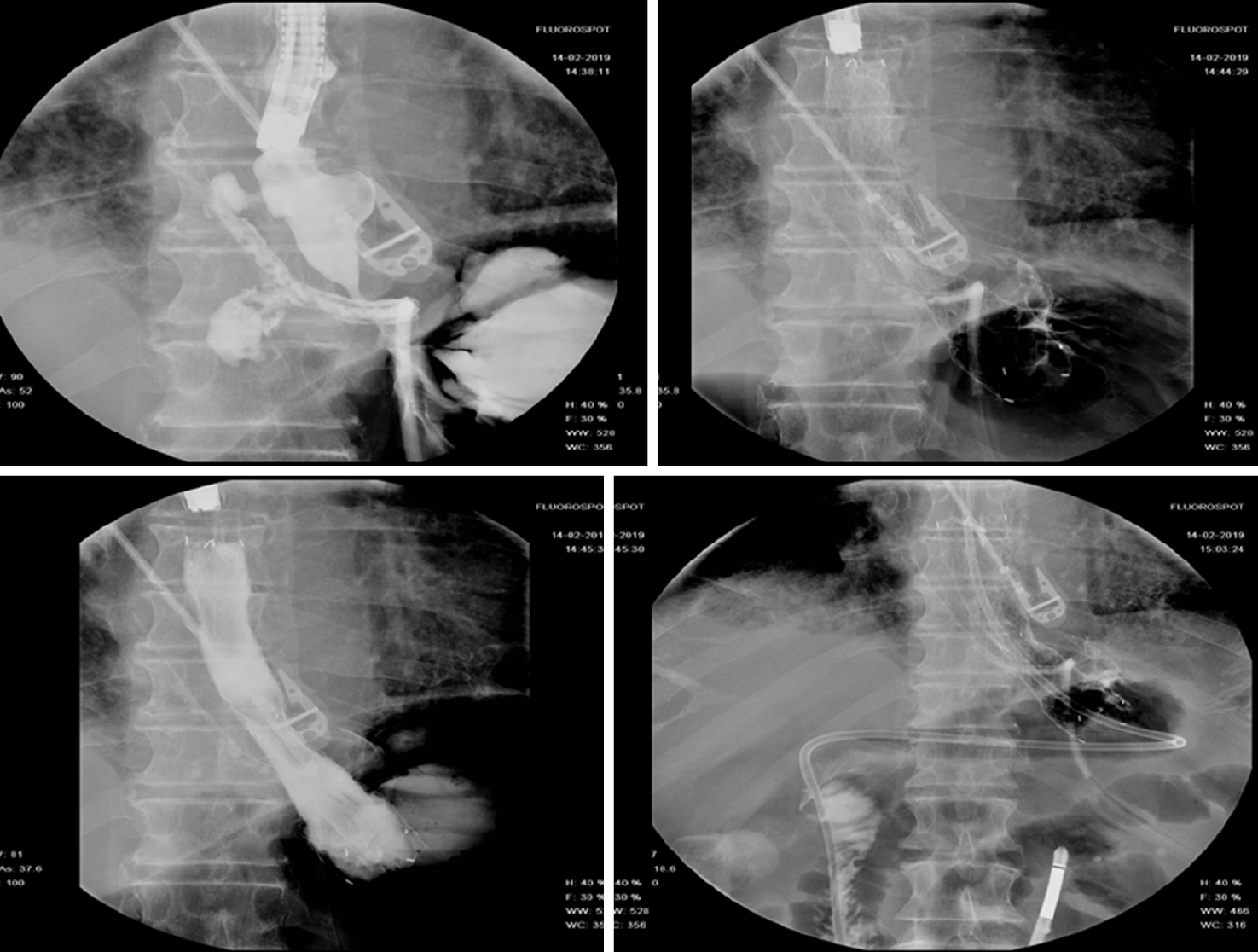
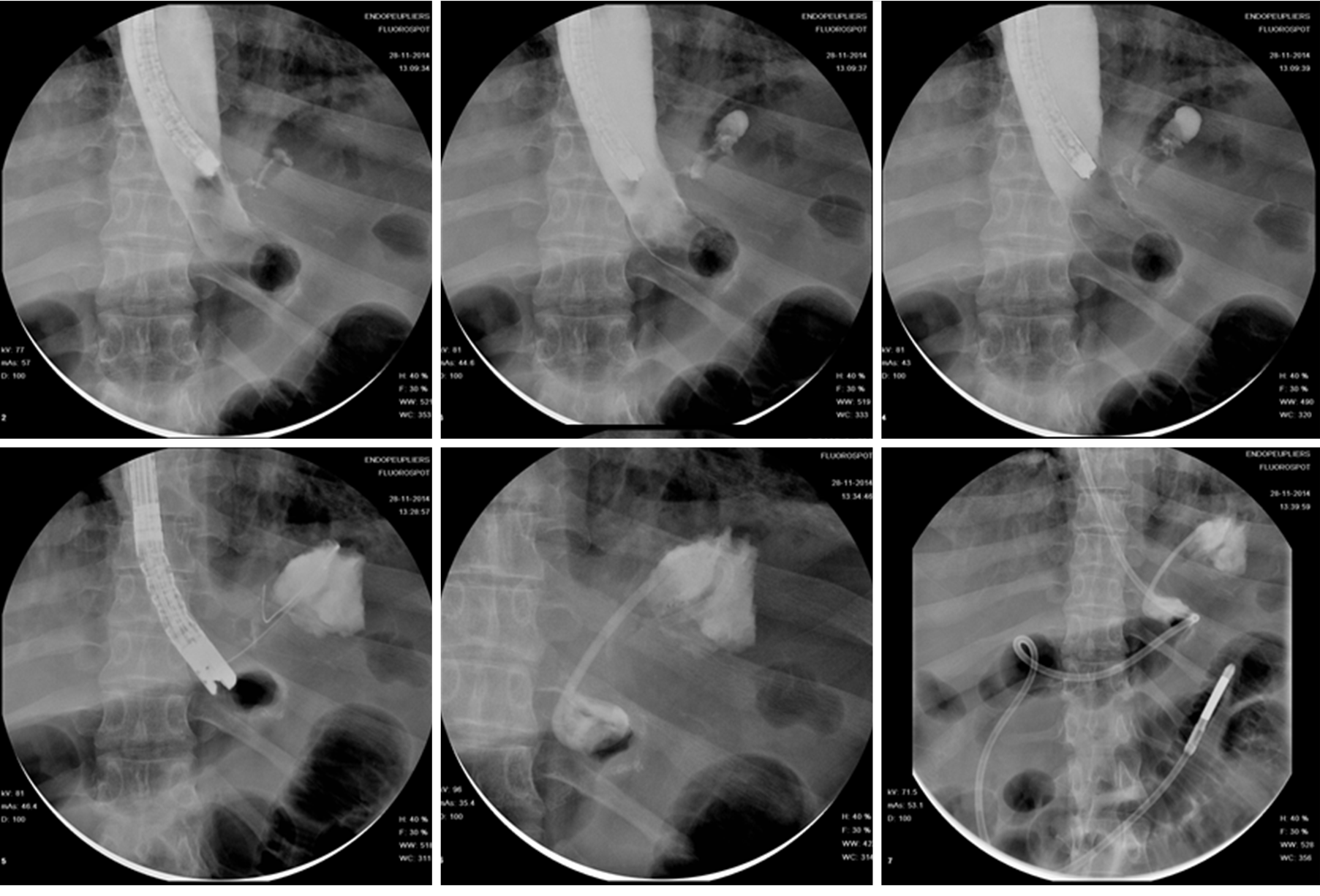
The rationale of EID with deployment of one or more pigtail plastic stents across leak orifice is to internally drain any fluid collection, obstruct the leak orifice thus allowing early oral intake and to induce mechanical re-epithelization of the fistula tract[66]. According to Donatelli et al[67] pigtail stents acting as a foreign body promotes re-epithelialization while guarantying internal drainage. Moreover, stents allow in most cases early removal of surgical drainage, thus reducing the risk of chronic fistula formation along drainage tract[67]. Before deciding the number, length and diameter of pigtail stent, it is of paramount importance to adequately assess orifice and cavity features not only by means of intra-procedural contrast study but even, whenever possible, by means of direct endoscopic cavity exploration. Donatelli et al[68], differently from other authors, advises enteral nutrition by means of feeding tube placed in the third part of the duodenum for the first 4 wk in order to allow hyper-alimentation. Systematic endoscopic review is advisable after 4 to 6 wk to avoid stent obstruction and to induce fistula traumatism (Figure 7). Lorenzo et al[69] in 2018 published a study comparing the outcomes of internal drainage versus closure (SEMS, glue or OTSC) for the management of fistula after SG in 100 patients. The efficacy of EID was significantly higher than that in the closure group (86% vs 64%; P = 0.55) and the mean (± SD) number of endoscopic sessions needed were 3.7 ± 3.4 per patient. The authors identified, in accordance with previous studies, the following risk factors associated to treatment failure: Delay of more than 21 d between diagnosis and treatment, large fistula, late patient referral, sepsis, presence of gastro-bronchial fistula, previous OTSC deployment. In the largest series of patients treated solely with EID consisting of 67 patients, clinical success was achieved in 78.2% of cases, after a mean time of 57.5 d (10-206) and an average of 3.14 sessions (2-16), whereas 9 patients were still under treatment at the end of the study after an average of 36 d of treatment. Clinical failure was observed in 5 patients (7.8%), all with a chronic fistula, whereas 6 patients presented a stricture after a mean period of 36 d from the end of the treatment. They were thus successfully treated with endoscopic dilation[67]. In a case series of 11 patients, Donatelli et al[70] proposed EID as first line treatment for fistula following GI surgery different from bariatric procedures. Leaks were as follow: 4 duodenal leaks (biliopancreatic cancer), 2 colonic leaks (colorectal surgery) and 5 esophagogastric-jejunal fistulas (foregut surgery). The overall clinical success was achieved in 9 patients (82%) after an average of 44 d (28-90) and a median of 2.3 endoscopic session (2-4).
In the past two decades several suturing systems have been developed for full-thickness closure of GI defect. However, most of them have shown major limitations preventing their widespread clinical use.
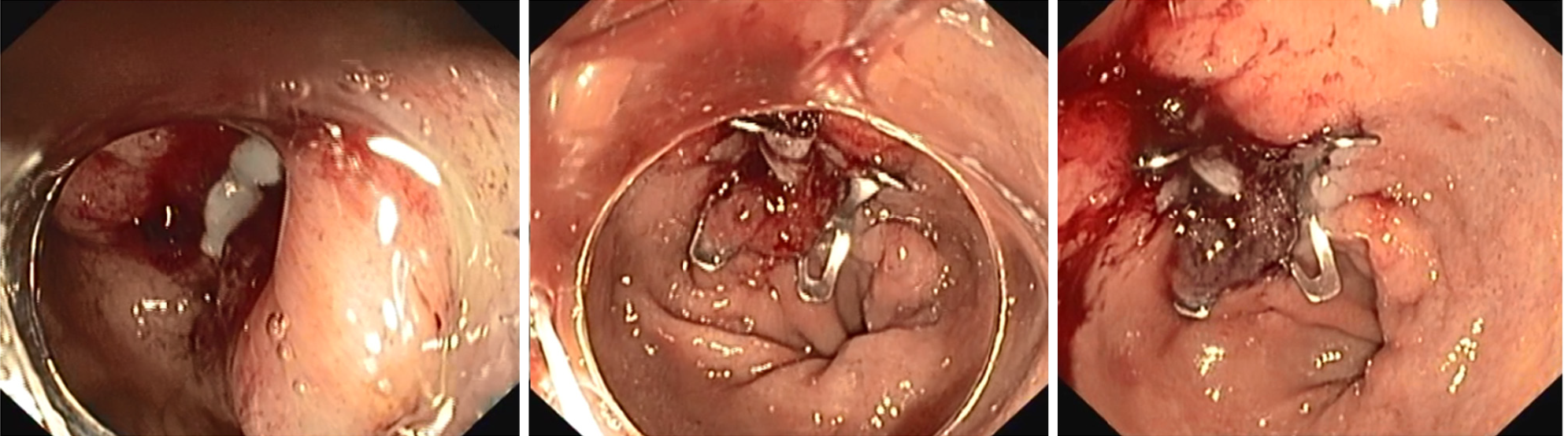
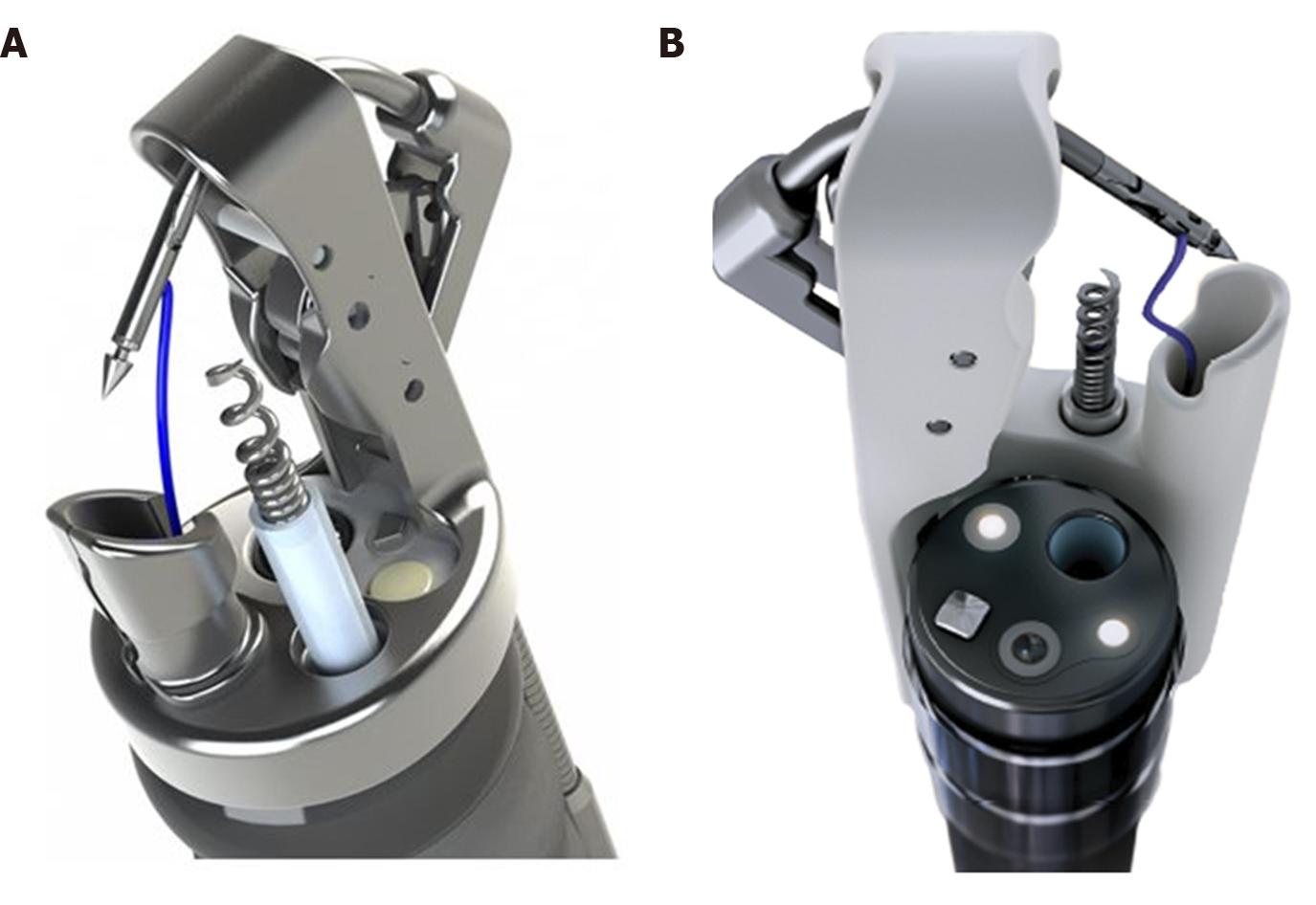
Currently OverStitch[71] (Apollo Endosurgery, TX, United States) has become the main endoscopic suturing platform enabling single operator surgical suturing with a flexible endoscope. The original Overstitch is a single use disposable platform that is mounted on a double therapeutic channel endoscope (Olympus only), allowing full-thickness uninterrupted or continuous suturing with both non-absorbable or re-absorbable stitches. The main components of the platform are: The needle driver handle, the cap mounted on top of the endoscope and an anchor exchange catheter. Grasping forceps or tissue retracting helix device may be used to aid tissue apposition. An important innovation was carried out with the recent introduction of Overstich SX device (Apollo Endosurgery, TX, United States) that can be mounted on single channel endoscope and it is compatible with over 20 single-channel endoscopes and 4 platforms (Figure 8). Nonetheless, Overstitch requires expertise and a specific training limiting its use to tertiary centers only. Sutures may be particularly demanding when endoluminal space is tight and suturing site is tangential; moreover, especially in case of large defect, similarly to surgical sutures, a robust and healthy tissue is necessary for successful primary closure[72]. The overstitch system has been successfully used for a growing variety of indications, including sleeve gastroplasty in obese patients, trans-oral outlet reduction after bariatric surgery, stent anchorage, and closure of mucosal defects after endoscopic resections[73-76]. However only a small amount of literature evaluated the role OverStitch for primary closure of GI leaks and fistula.
In a multicenter retrospective study Sharaiha et al[77] analyzed the results of endoscopic suturing in 122 patients. Among these, 40 fistulae (32.7%) and 15 leaks (12.3%) were treated. Although high technical success was reported, long term clinical success was obtained in respectively 80% and 27% of the cases. Mukewar et al[78], in the largest series of endoscopic suturing management for a wide variety of GI fistula (51.8% gastro-gastric fistulae), showed an immediate success rate of 100% and a sustained clinical success for nearly 40% of patients, with 13 patients requiring an additional endoscopic procedure. Despite multiple endoscopic attempts, the fistula of many patients (26 out of 56; 46%) failed to close or surgical treatment was required.
Before attempting endoscopic closure of an epithelialized fistula is of paramount importance to de-epithelialize it in order to guarantee a liable closure. Coagulation of the defect perimeter by means of deployment is the most common technique followed by mechanical abrasion of the fistula tract[79] by means of brush catheter. Modified endoscopic submucosal dissection technique to completely ablate the mucosa of the fistula or multiple endoscopic mucosal resections around the fistula opening has been described[80,81] as well. Granata et al[82], in a recent case series of 20 patients with post-operative leaks, described an interesting multimodality approach. The therapeutic approach was stratified in 3 groups based on structural condition of the wall defect layers (tissue status and suture feasibility). The study proposed the following strategies: Pure endoscopic direct suture (Group A: Healthy tissue and feasible suture), combined therapy with endoscopic direct suture + FC-SEMS placement + anchoring (Group B: Unhealthy tissue and feasible sutures) and FC-SEMS placement + anchoring (Group C: Unhealthy tissue and suture not feasible). The overall long-term clinical success was 80% (16/20 patients). Considering the results in each group success rate was 77% (7/9) in group A, 85% (6/7) in group B and 75% (3/4) in group C. AEs occurred in 4 cases consisting in short strictures of the distal esophagus.
In conclusion, literature shows that OverStitch is a minimally invasive endoscopic technique with interesting results in the management of leak and fistula, since it allows true full-thickness closure. However, it is a complex procedure and it is required a high level of expertise and a proper training. Hence, its use is still limited to referral center. Moreover, even though most studies so far show a high technical success rate, further prospective studies are needed to determine its long-term efficacy and safety.
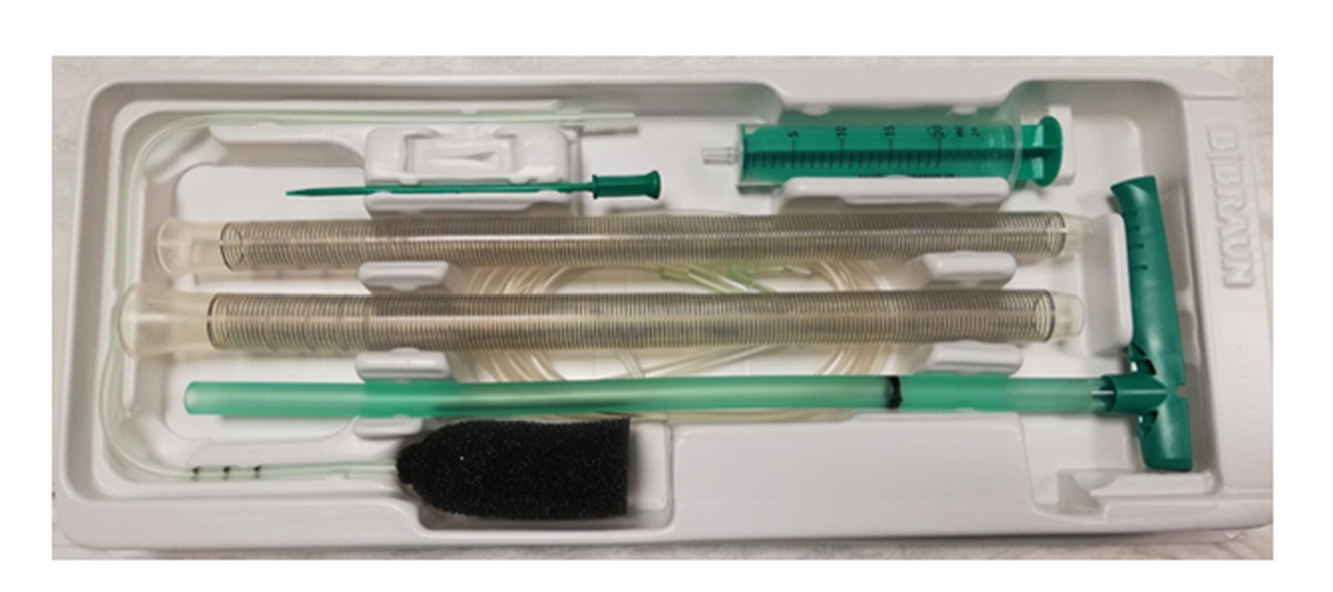
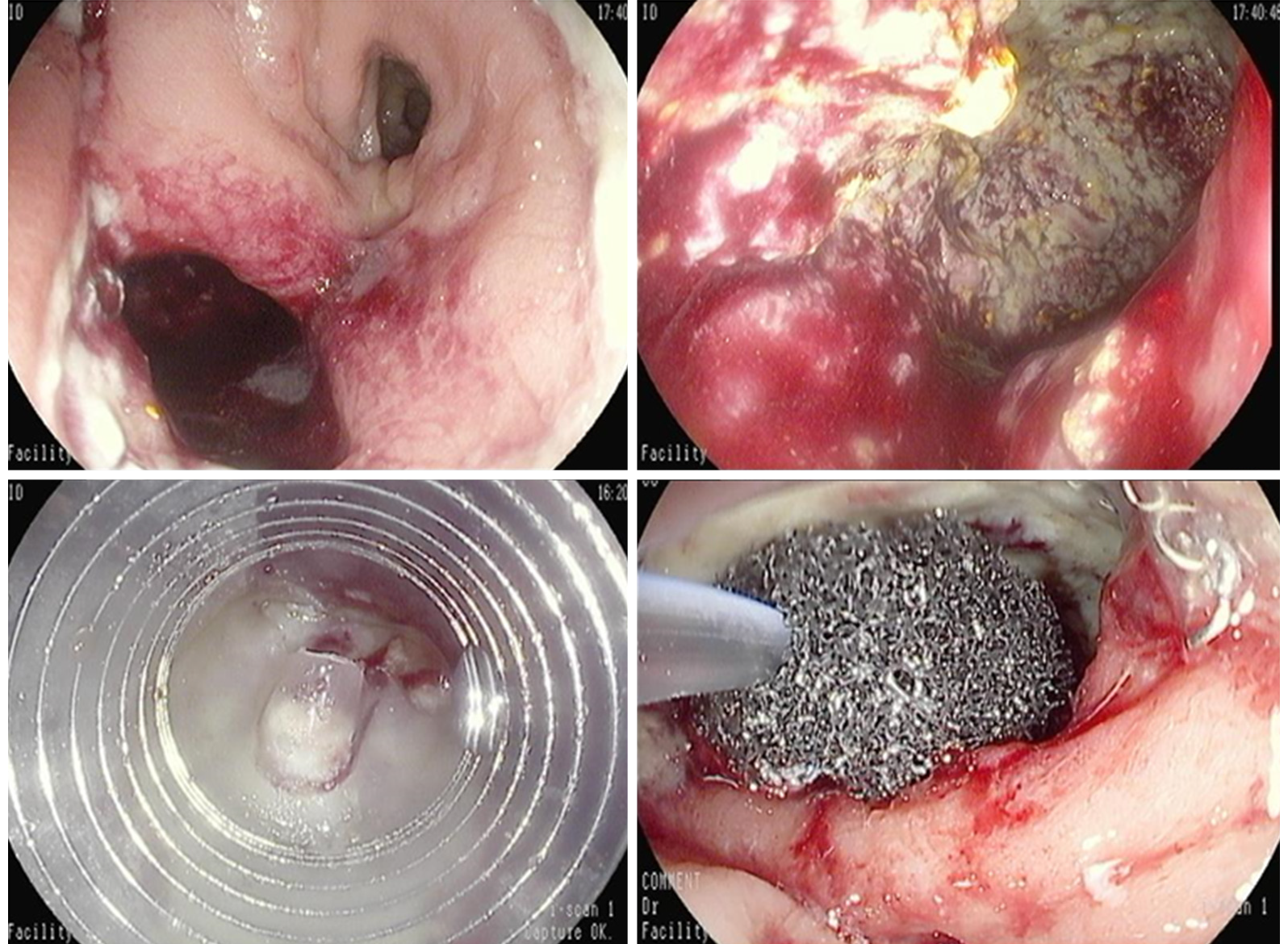
EVT is a minimally invasive technique for the management of anastomotic leakage, especially following rectal and esophageal surgery. EVT is an open-pored polyurethane foam connected by a suction tube to a wound drainage system producing a continuous endo-luminal vacuum therapy (Figure 9). It ensures continuous drainage, promotes granulation tissue formation and re-epithelialization, thus inducing second intention closure of the defect/cavity. Negative pressure within the defect allows mechanical cleaning of the wound from microorganism and interstitial edema reduction by improvement of microcirculation. The system needs to be changed every 3-4 d until wound cavity is healed.
In colon-rectum, the ultimate goal of EVT is to allow early closure of defunctioning ileostomy and to avoid Hartmann’s procedure. It has shown to be effective, well tolerated and safe, especially if offered at early stage in case of distal leakages in patients with a de-functioning stoma and without sepsis (Figure 10).
In detail, the first use of EVT for leaks following colorectal surgery has been proposed in 2004 but it was only until 2008 that the first large series was published by Weidenhagen et al[83]. The authors described EVT in 29 patients achieving definitive closure in 97% of cases. In a recent systematic review, analyzing 17 studies for a total of 276 patients treated with EVT for various colorectal pathologies (209/276 anastomotic leakage), a weighted mean success rate of 85.3 was highlighted with 25 patients (9.1%) requiring additional treatment and 38 (13.8%) developing procedure related AEs[21]. Similar results were confirmed by Popivanov et al[84] reporting in their review a success rate of 85.4% (range 80%-91%) with ileostomy closure achieved in 72.6% of cases. A median of 7 sponges (2-34) were required for a median period of treatment of 31 d (14-217). AEs were observed in 19% of cases with abscess being the most frequent (11.5%) followed by anastomotic stenosis (4.4%). From literature, factors associated with EVT failure are late start of EVT, neoadjuvant therapy, lack of protective stoma, age over 60 years and male sex. Interestingly most aforementioned conditions are also known risk factors for anastomotic leakage after surgery[85].
A study compared 21 patients, treated with EVT for anastomotic colorectal leakage, and a historical cohort of 41 patients, receiving conventional treatment. EVT showed, at intention-to-treat analysis, a significantly higher success rate over the conventional treatment (95.2% vs 65.9%; P = 0.011). Moreover, EVT was associated with preservation of intestinal continuity in a significant higher percentage of patients (86.7% vs 37.5%; P = 0.001)[86]. In a study from 2014 analyzing management of 103 leaks after colorectal surgery, non-operative management (drainage and antibiotics) was successful in 57% of patients with extra-peritoneal leak, whereas surgical revision (diverting ileostomy, Hartmann’s procedure and redo anastomosis) was successful in 41% of patients[87].
EVT has been subsequently proposed as a viable treatment for UGI defects as well. In UGI the use EVT has been described both inside the cavity (intra-cavitary) in case of large sized leaks or within the esophageal lumen (intra-luminal) in case of small defects.
Yim et al[25] reported their experience of EVT in 77 patients. 59 of these patients presented post-operative leakages (36 after Ivor-Lewis esophagectomy, 15 after gastrectomy and 8 other procedures). In most cases, EVT was placed intraluminal (68/77) rather than intra-cavitary (12/77). Considering the leakage subgroup only, the authors reported a success rate of 77.9% (46/59) and a median treatment period of 11 d (1-65) with a median of 2.75 (1-9) sponges per patient. In 2017, Kuehn et al[88] published a systematic review comprising more than 200 patients treated with EVT for management of UGI defects. Analyzing all published series with more than 5 patients, the study highlighted a success rate of 90% (range 70%-100%), with low incidence of AE: Stricture (7.6%) and anecdotally bleeding after intra-cavitary sponge deployment. Although RCT are not available, the authors evaluated 4 retrospective studies[89-92] and reported higher success rate, lower mortality and lower incidence of AEs for EVT compared to stent therapy.
Presumed advantages of EVT over SEMS are continuous drainage of septic locus, ability of a regular endoscopic evaluation of the defect and the possibility to deploy the sponge in all esophageal region (e.g., cricopharyngeal).
Low quality evidence (retrospective studies)[88-91,93-97] showed advantages of EVT over surgical revisions for patients with sepsis or major esophageal defects in particular.
Tissue sealants have been successfully used in the management of anastomotic leak and low output fistula[102]. The 2 most common tissue sealants are fibrin glue and cyanoacrylate.
Fibrin glue consists of two components: Human fibrinogen reconstituted with aprotronin and human thrombin reconstituted with calcium chloride. The glue is applied with a double lumen catheter forming an absorbable flexible fibrin cloth mimicking the early stage of blood coagulation and wound healing. Fibrin glue acts more efficiently in dry areas; therefore, it is advisable to remove all purulent material and to ablate the surrounding mucosa before its application.
Ramón Rábago et al[103] reported their experience in fistula closure with fibrin glue in a case series of 30 patients, refractory to standard conservative treatment. Complete sealing of fistulas was achieved in 75% of cases (80% in low-output, 25% in high-output and 55.5% in internal fistulas). Healing time was 17 d (4-90) with a mean of 2.8 sessions per patient (1-5). Lippert et al[104] published in 2011 the largest series on fibrin glue management of GI leak and fistulae. The author reported in their retrospective study on 52 patients a durable closure with fibrin glue as sole endoscopic option in 36.5% of cases (n = 19) and in 55.7% of patients (n = 29) when fibrin glue was coupled with others endoscopic techniques (cyanoacrylate, clip or stent). From 2 to 81 mL fibrin glue (median 8.5) was used in 1-40 sessions (median 4). Nonetheless endoscopic treatment, surgical intervention became necessary in 23.1% (n = 12).
Cyanoacrylate (N-butyl-2-cyanoacrylate) is a synthetic glue that polymerizes after contact with moisture, causing tissue necrosis and inflammatory reaction acting as a foreign body, thus inducing tissue healing. Cyanoacrylate presents high adhesive properties that are not affected by gastric or pancreatic juice. Moreover, its antibacterial properties make its use suitable for infected areas[105]. The efficacy of cyanoacrylate was summarized in a systematic review in 2015 comprising 13 studies (prospective and retrospective case series) for a total of 203 patients, which presented foregut, midgut and hindgut fistulae. Cumulative success rate was 81% (range 0% to 100%) and 3 out of 203 patients (1%) developed minor AEs[106].
Although data from literature shows satisfactory results, the use of tissue sealants as the sole endoscopic treatment, it should be limited to small low-output leaks or fistulae only. Fibrin glue and cyanoacrylate may play a useful role for the management of GI defects in combination with other endoscopic techniques[107].
Darrien et al[108] proposed an interesting approach for closure of refractory entero-cutaneous fistulae with Surgisis® anal fistula plug (Cook Surgical, Bloomington, United States). The Surgisis® anal fistula plug is an advanced tissue repair graft made from porcine submucosa developed for the management of perineal fistula. It serves as a scaffold for host cells to replace and repair damaged tissue. The acellular matrix promotes fistula closure without foreign body inflammatory reaction. Surgisis® has been used for management of fistulae after bariatric surgery as well. In a case series of 25 patients with gastro-cutaneous fistula after Roux-en-Y-gastric Bypass strip-shaped Surgisis was used for 20 patients and cone-shaped Surgisis in 5 patients[109]. Using the strip-shaped biomaterial, success rates were approximately 75% after two or three sessions, whereas using cone-shaped matrix fistula closure was accomplished after a single session in all patients.
Endoscopy is emerging as first line approach over surgery for the management of Gastrointestinal leaks and fistulae. The steadfast advancements of interventional endoscopy in the last decades allowed for new endoscopic closure devices and techniques, which provide a minimally invasive and more effective therapeutic option than surgery. A single therapy, or even a combination of different techniques, can integrate the use of different endoscopic options (Table 2). Comparison between different approaches is difficult due to heterogeneous populations, prevalence of retrospective studies, lack of uniform definitions and lack of comparative studies. Therefore, it is difficult to establish a standardized therapeutic algorithm. Each treatment should be tailored to the single patient, by taking into account the several variables that may at the end influence the outcome. Endoscopic management of leaks and fistulae requires a personalized and multidisciplinary approach, comprising a close collaboration between surgeon, interventional radiologist and endoscopist, allowing Gastrointestinal wall defect management with high clinical success rate and low rate of morbidity and mortality.
| Mechanism ofaction | Advantages | Disadvantages | Cost | N° of sessions | Expertise needed | |
| Endoclips | Direct closure | More effective in acute setting | Less effective in chronic setting; Need of external drainage | + | + | + |
| Stent | Defect sealing | Early oral intake; Reduce stricture formation | Stent migration; Tissue ingrowth/overgrowth pain; Need of externaldrainage | ++ | + | ++ |
| EID | Second intention closure | Early oral intake; Internal drainage; More effective in acute setting | Stricture | + | ++ | ++ |
| Suturing system | Direct closure | True full-thickness closure; Single operator (Overstich®) | On healthy tissue; More difficult in tight endoluminal space and tangential suturing site | +++ | + | +++ |
| EVT | Second intention closure | Continuous drainage; More effective in early stage | Limited to rectal/esophageal site; Need of de-functioning stoma; Less effective if late diagnosis | + | +++ | + |
| Tissue sealant | Miscellaneous | Antibacterial (cyanoacrylate); Used in combination; No inflammatory reaction (Surgisis®) | On dry areas (fibrin glue); Inflammatory reaction (cyanoacrylate/fibrin glue) | + | ++ | + |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martini F, Marwah S S-Editor: Zhang L L-Editor: A E-Editor: Ma YJ
| 1. | Kumar N, Thompson CC. Endoscopic therapy for postoperative leaks and fistulae. Gastrointest Endosc Clin N Am. 2013;23:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 2. | Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 396] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Blencowe NS, Strong S, McNair AG, Brookes ST, Crosby T, Griffin SM, Blazeby JM. Reporting of short-term clinical outcomes after esophagectomy: a systematic review. Ann Surg. 2012;255:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 4. | Lang H, Piso P, Stukenborg C, Raab R, Jähne J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol. 2000;26:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Morales MP, Miedema BW, Scott JS, de la Torre RA. Management of postsurgical leaks in the bariatric patient. Gastrointest Endosc Clin N Am. 2011;21:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, Rosenthal R, Murr MM. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Vermeer TA, Orsini RG, Daams F, Nieuwenhuijzen GA, Rutten HJ. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: Incidence, risk factors and treatment. Eur J Surg Oncol. 2014;40:1502-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Bemelman WA, Baron TH. Endoscopic Management of Transmural Defects, Including Leaks, Perforations, and Fistulae. Gastroenterology. 2018;154:1938-1946.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am. 2020;30:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I, Shimonov M, Assalia A. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 11. | Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH; Yonsei Gastric Cancer Clinic. Complications requiring reoperation after gastrectomy for gastric cancer: 17-year experience in a single institute. J Gastrointest Surg. 2009;13:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Rodrigues-Pinto E, Repici A, Donatelli G, Macedo G, Devière J, van Hooft JE, Campos JM, Galvao Neto M, Silva M, Eisendrath P, Kumbhari V, Khashab MA. International multicenter expert survey on endoscopic treatment of upper gastrointestinal anastomotic leaks. Endosc Int Open. 2019;7:E1671-E1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Lee S, Ahn JY, Jung HY, Lee JH, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, Kim JH, Kim BS, Yook JH, Oh ST, Kim BS, Han S. Clinical outcomes of endoscopic and surgical management for postoperative upper gastrointestinal leakage. Surg Endosc. 2013;27:4232-4240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Law R, Wong Kee Song LM, Irani S, Baron TH. Immediate technical and delayed clinical outcome of fistula closure using an over-the-scope clip device. Surg Endosc. 2015;29:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Donatelli G, Cereatti F, Dhumane P, Vergeau BM, Tuszynski T, Marie C, Dumont JL, Meduri B. Closure of gastrointestinal defects with Ovesco clip: long-term results and clinical implications. Therap Adv Gastroenterol. 2016;9:713-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Haito-Chavez Y, Law JK, Kratt T, Arezzo A, Verra M, Morino M, Sharaiha RZ, Poley JW, Kahaleh M, Thompson CC, Ryan MB, Choksi N, Elmunzer BJ, Gosain S, Goldberg EM, Modayil RJ, Stavropoulos SN, Schembre DB, DiMaio CJ, Chandrasekhara V, Hasan MK, Varadarajulu S, Hawes R, Gomez V, Woodward TA, Rubel-Cohen S, Fluxa F, Vleggaar FP, Akshintala VS, Raju GS, Khashab MA. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc. 2014;80:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Morrell DJ, Winder JS, Johri A, Docimo S, Juza RM, Witte SR, Alli VV, Pauli EM. Over-the-scope clip management of non-acute, full-thickness gastrointestinal defects. Surg Endosc. 2020;34:2690-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Kobara H, Mori H, Nishiyama N, Fujihara S, Okano K, Suzuki Y, Masaki T. Over-the-scope clip system: A review of 1517 cases over 9 years. J Gastroenterol Hepatol. 2019;34:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Freeman RK, Ascioti AJ, Wozniak TC. Postoperative esophageal leak management with the Polyflex esophageal stent. J Thorac Cardiovasc Surg. 2007;133:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg. 2007;83:2003-7; discussion 2007-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | van Boeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, Vleggaar FP, Siersema PD. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Saxena P, Khashab MA. Endoscopic Management of Esophageal Perforations: Who, When, and How? Curr Treat Options Gastroenterol. 2017;15:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Yim HB. Self-expanding metallic stents and self-expanding plastic stents in the palliation of malignant oesophageal dysphagia. Ann Palliat Med. 2014;3:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Ott C, Ratiu N, Endlicher E, Rath HC, Gelbmann CM, Schölmerich J, Kullmann F. Self-expanding Polyflex plastic stents in esophageal disease: various indications, complications, and outcomes. Surg Endosc. 2007;21:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Anikhindi SA, Ranjan P, Sachdeva M, Kumar M. Self-expanding plastic stent for esophageal leaks and fistulae. Indian J Gastroenterol. 2016;35:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Dai YY, Gretschel S, Dudeck O, Rau B, Schlag PM, Hünerbein M. Treatment of oesophageal anastomotic leaks by temporary stenting with self-expanding plastic stents. Br J Surg. 2009;96:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Dabizzi E, Arcidiacono PG. Update on Enteral Stents. Curr Treat Options Gastroenterol. 2016;14:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Fischer A, Thomusch O, Benz S, von Dobschuetz E, Baier P, Hopt UT. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg. 2006;81:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Babor R, Talbot M, Tyndal A. Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Surg Laparosc Endosc Percutan Tech. 2009;19:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Zhou JH, Gong TQ, Jiang YG, Wang RW, Zhao YP, Tan QY, Ma Z, Lin YD, Deng B. Management of delayed intrathoracic esophageal perforation with modified intraluminal esophageal stent. Dis Esophagus. 2009;22:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | van Halsema EE, van Hooft JE. Clinical outcomes of self-expandable stent placement for benign esophageal diseases: A pooled analysis of the literature. World J Gastrointest Endosc. 2015;7:135-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 35. | Berry SM, Fischer JE. Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am. 1996;76:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | di Costanzo J, Cano N, Martin J, Richieri JP, Mercier R, Lafille C, Lepeuch D. Treatment of external gastrointestinal fistulas by a combination of total parenteral nutrition and somatostatin. JPEN J Parenter Enteral Nutr. 1987;11:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Bakken JC, Wong Kee Song LM, de Groen PC, Baron TH. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc. 2010;72:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | El Hajj II, Imperiale TF, Rex DK, Ballard D, Kesler KA, Birdas TJ, Fatima H, Kessler WR, DeWitt JM. Treatment of esophageal leaks, fistulae, and perforations with temporary stents: evaluation of efficacy, adverse events, and factors associated with successful outcomes. Gastrointest Endosc. 2014;79:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Freeman RK, Ascioti AJ, Giannini T, Mahidhara RJ. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg. 2012;94:959-64; discussion 964-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Takimoto Y, Nakamura T, Yamamoto Y, Kiyotani T, Teramachi M, Shimizu Y. The experimental replacement of a cervical esophageal segment with an artificial prosthesis with the use of collagen matrix and a silicone stent. J Thorac Cardiovasc Surg. 1998;116:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Hünerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg. 2004;240:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, Eder F, Lippert H, Pross M. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005;61:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg. 2008;12:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. Esophageal stent placement for the treatment of spontaneous esophageal perforations. Ann Thorac Surg. 2009;88:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Ko HK, Song HY, Shin JH, Lee GH, Jung HY, Park SI. Fate of migrated esophageal and gastroduodenal stents: experience in 70 patients. J Vasc Interv Radiol. 2007;18:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Law R, Prabhu A, Fujii-Lau L, Shannon C, Singh S. Stent migration following endoscopic suture fixation of esophageal self-expandable metal stents: a systematic review and meta-analysis. Surg Endosc. 2018;32:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Wright A, Chang A, Bedi AO, Wamsteker EJ, Elta G, Kwon RS, Carrott P, Elmunzer BJ, Law R. Endoscopic suture fixation is associated with reduced migration of esophageal fully covered self-expandable metal stents (FCSEMS). Surg Endosc. 2017;31:3489-3494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Ngamruengphong S, Sharaiha RZ, Sethi A, Siddiqui AA, DiMaio CJ, Gonzalez S, Im J, Rogart JN, Jagroop S, Widmer J, Hasan RA, Laique S, Gonda T, Poneros J, Desai A, Tyberg A, Kumbhari V, El Zein M, Abdelgelil A, Besharati S, Hernaez R, Okolo PI, Singh V, Kalloo AN, Kahaleh M, Khashab MA. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy. 2016;48:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Diana M, Swanström LL, Halvax P, Lègner A, Liu YY, Alzaga A, D'Urso A, Marescaux J. Esophageal covered stent fixation using an endoscopic over-the-scope clip. Mechanical proof of the concept and first clinical experience. Surg Endosc. 2015;29:3367-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Irani S, Baron TH, Gluck M, Gan I, Ross AS, Kozarek RA. Preventing migration of fully covered esophageal stents with an over-the-scope clip device (with videos). Gastrointest Endosc. 2014;79:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Ngamruengphong S, Sharaiha R, Sethi A, Siddiqui A, DiMaio CJ, Gonzalez S, Rogart J, Jagroop S, Widmer J, Im J, Hasan RA, Laique S, Gonda T, Poneros J, Desai A, Wong K, Villgran V, Brewer Gutierrez O, Bukhari M, Chen YI, Hernaez R, Hanada Y, Sanaei O, Agarwal A, Kalloo AN, Kumbhari V, Singh V, Khashab MA. Fully-covered metal stents with endoscopic suturing vs. partially-covered metal stents for benign upper gastrointestinal diseases: a comparative study. Endosc Int Open. 2018;6:E217-E223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Seven G, Irani S, Ross AS, Gan SI, Gluck M, Low D, Kozarek RA. Partially versus fully covered self-expanding metal stents for benign and malignant esophageal conditions: a single center experience. Surg Endosc. 2013;27:2185-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Sandha GS, Marcon NE. Expandable metal stents for benign esophageal obstruction. Gastrointest Endosc Clin N Am. 1999;9:437-446. [PubMed] |
| 54. | Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, Devière J. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011;73:890-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 55. | Fiocca F, Cereatti F, Antypas P, Donatelli G. Argon plasma coagulation: a less-expensive alternative to the "stent-in-stent" technique for removal of embedded partially covered esophageal stents. Gastrointest Endosc. 2016;83:453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Speer E, Dunst CM, Shada A, Reavis KM, Swanström LL. Covered stents in cervical anastomoses following esophagectomy. Surg Endosc. 2016;30:3297-3303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Boerlage TCC, Houben GPM, Groenen MJM, van der Linde K, van de Laar AWJM, Emous M, Fockens P, Voermans RP. A novel fully covered double-bump stent for staple line leaks after bariatric surgery: a retrospective analysis. Surg Endosc. 2018;32:3174-3180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Shehab HM, Hakky SM, Gawdat KA. An Endoscopic Strategy Combining Mega Stents and Over-The-Scope Clips for the Management of Post-Bariatric Surgery Leaks and Fistulas (with video). Obes Surg. 2016;26:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Arezzo A, Bini R, Lo Secco G, Verra M, Passera R. The role of stents in the management of colorectal complications: a systematic review. Surg Endosc. 2017;31:2720-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Lamazza A, Sterpetti AV, De Cesare A, Schillaci A, Antoniozzi A, Fiori E. Endoscopic placement of self-expanding stents in patients with symptomatic anastomotic leakage after colorectal resection for cancer: long-term results. Endoscopy. 2015;47:270-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Cereatti F, Fiocca F, Dumont JL, Ceci V, Vergeau BM, Tuszynski T, Meduri B, Donatelli G. Fully covered self-expandable metal stent in the treatment of postsurgical colorectal diseases: outcome in 29 patients. Therap Adv Gastroenterol. 2016;9:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Venezia L, Michielan A, Condino G, Sinagra E, Stasi E, Galeazzi M, Fabbri C, Anderloni A. Feasibility and safety of self-expandable metal stent in nonmalignant disease of the lower gastrointestinal tract. World J Gastrointest Endosc. 2020;12:60-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 63. | Eisendrath P, Cremer M, Himpens J, Cadière GB, Le Moine O, Devière J. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 64. | Gonzalez JM, Garces Duran R, Vanbiervliet G, Lestelle V, Gomercic C, Gasmi M, Desjeux A, Grimaud JC, Barthet M. Double-type metallic stents efficacy for the management of post-operative fistulas, leakages, and perforations of the upper gastrointestinal tract. Surg Endosc. 2015;29:2013-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Pequignot A, Fuks D, Verhaeghe P, Dhahri A, Brehant O, Bartoli E, Delcenserie R, Yzet T, Regimbeau JM. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Gonzalez JM, Lorenzo D, Guilbaud T, Bège T, Barthet M. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc Int Open. 2018;6:E745-E750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Donatelli G, Dumont JL, Cereatti F, Ferretti S, Vergeau BM, Tuszynski T, Pourcher G, Tranchart H, Mariani P, Meduri A, Catheline JM, Dagher I, Fiocca F, Marmuse JP, Meduri B. Treatment of Leaks Following Sleeve Gastrectomy by Endoscopic Internal Drainage (EID). Obes Surg. 2015;25:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Donatelli G, Ferretti S, Vergeau BM, Dhumane P, Dumont JL, Derhy S, Tuszynski T, Dritsas S, Carloni A, Catheline JM, Pourcher G, Dagher I, Meduri B. Endoscopic Internal Drainage with Enteral Nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Lorenzo D, Guilbaud T, Gonzalez JM, Benezech A, Dutour A, Boullu S, Berdah S, Bège T, Barthet M. Endoscopic treatment of fistulas after sleeve gastrectomy: a comparison of internal drainage versus closure. Gastrointest Endosc. 2018;87:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 70. | Donatelli G, Dumont JL, Cereatti F, Dhumane P, Tuszynski T, Vergeau BM, Meduri B. Endoscopic internal drainage as first-line treatment for fistula following gastrointestinal surgery: a case series. Endosc Int Open. 2016;4:E647-E651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Muniraj T, Aslanian HR. The use of OverStitch for the treatment of intestinal perforation. fistulas and leaks. Gastrointest Interv. 2017;6:151–156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Granata A, Amata M, Martino A, De Monte L, Bertani A, Ligresti D, Traina M. Full-thickness gastric plication with Overstitch endoscopic suturing device for postsurgical chronic gastroparesis. Endoscopy. 2020;52:E235-E236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 74. | Vargas EJ, Bazerbachi F, Rizk M, Rustagi T, Acosta A, Wilson EB, Wilson T, Neto MG, Zundel N, Mundi MS, Collazo-Clavell ML, Meera S, Abu-Lebdeh HS, Lorentz PA, Grothe KB, Clark MM, Kellogg TA, McKenzie TJ, Kendrick ML, Topazian MD, Gostout CJ, Abu Dayyeh BK. Transoral outlet reduction with full thickness endoscopic suturing for weight regain after gastric bypass: a large multicenter international experience and meta-analysis. Surg Endosc. 2018;32:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 75. | Fujii LL, Bonin EA, Baron TH, Gostout CJ, Wong Kee Song LM. Utility of an endoscopic suturing system for prevention of covered luminal stent migration in the upper GI tract. Gastrointest Endosc. 2013;78:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Kantsevoy SV, Bitner M, Mitrakov AA, Thuluvath PJ. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc. 2014;79:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 77. | Sharaiha RZ, Kumta NA, DeFilippis EM, Dimaio CJ, Gonzalez S, Gonda T, Rogart J, Siddiqui A, Berg PS, Samuels P, Miller L, Khashab MA, Saxena P, Gaidhane MR, Tyberg A, Teixeira J, Widmer J, Kedia P, Loren D, Kahaleh M, Sethi A. A Large Multicenter Experience With Endoscopic Suturing for Management of Gastrointestinal Defects and Stent Anchorage in 122 Patients: A Retrospective Review. J Clin Gastroenterol. 2016;50:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Mukewar S, Kumar N, Catalano M, Thompson C, Abidi W, Harmsen W, Enders F, Gostout C. Safety and efficacy of fistula closure by endoscopic suturing: a multi-center study. Endoscopy. 2016;48:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am. 2020;30:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Abidi WM, Thompson CC. Endoscopic treatment of a chronic fistula by resection and sutured closure. Gastrointest Endosc. 2016;83:1031-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Pang M, Mousa O, Werlang M, Brahmbhatt B, Woodward T. A hybrid endoscopic technique to close tracheoesophageal fistula. VideoGIE. 2018;3:15-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Granata A, Amata M, Ligresti D, Martino A, Tarantino I, Barresi L, Traina M. Endoscopic management of post-surgical GI wall defects with the overstitch endosuturing system: a single-center experience. Surg Endosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 84. | Popivanov GI, Mutafchiyski VM, Cirocchi R, Chipeva SD, Vasilev VV, Kjossev KT, Tabakov MS. Endoluminal negative pressure therapy in colorectal anastomotic leaks. Colorectal Dis. 2020;22:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 591] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 86. | Kühn F, Janisch F, Schwandner F, Gock M, Wedermann N, Witte M, Klar E, Schiffmann L. Comparison Between Endoscopic Vacuum Therapy and Conventional Treatment for Leakage After Rectal Resection. World J Surg. 2020;44:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Blumetti J, Chaudhry V, Cintron JR, Park JJ, Marecik S, Harrison JL, Prasad LM, Abcarian H. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg. 2014;38:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Kuehn F, Loske G, Schiffmann L, Gock M, Klar E. Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc. 2017;31:3449-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 89. | Hwang JJ, Jeong YS, Park YS, Yoon H, Shin CM, Kim N, Lee DH. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine (Baltimore). 2016;95:e3416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, Schreiber S, Becker T, Hampe J. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc. 2013;27:3883-3890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 91. | Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy. 2013;45:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 92. | Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes D, Senninger N, Laukoetter MG. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointest Surg. 2015;19:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 93. | Smallwood NR, Fleshman JW, Leeds SG, Burdick JS. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc. 2016;30:2473-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 94. | Maus MK, Leers J, Herbold T, Bludau M, Chon SH, Kleinert R, Hescheler DA, Bollschweiler E, Hölscher AH, Schäfer H, Alakus H. Gastric Outlet Obstruction After Esophagectomy: Retrospective Analysis of the Effectiveness and Safety of Postoperative Endoscopic Pyloric Dilatation. World J Surg. 2016;40:2405-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Loske G, Schorsch T, Schmidt-Seithe H, Müller C. Intraluminal endoscopic vacuum therapy in a case of ischemia of the blind end of the jejunal loop after Roux-en-Y gastrectomy. Endoscopy. 2014;46 Suppl 1 UCTN:E575-E576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Schröder W, Leers JM, Bludau M, Herbold T, Hölscher AH. [Perforations near the cardia in benign diseases]. Chirurg. 2014;85:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Loske G, Schorsch T, van Ackeren V, Schulze W, Müller CT. Endoscopic vacuum therapy in Boerhaave's syndrome with open-pore polyurethane foam and a new open-pore film drainage. Endoscopy. 2015;47 Suppl 1 UCTN:E410-E411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Loske G, Lang U, Schorsch T, Müller CT. [Complex vacuum therapy of an abdominal abscess from gastric perforation: case report of innovative operative endoscopic management]. Chirurg. 2015;86:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Wedemeyer J, Kubicka S, Lankisch TO, Wirth T, Patecki M, Hiss M, Manns MP, Schneider AS. Transgastrically placed endoscopic vacuum-assisted closure system as an addition to transgastric necrosectomy in necrotizing pancreatitis (with video). Gastrointest Endosc. 2012;76:1238-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Wallstabe I, Tiedemann A, Schiefke I. Endoscopic vacuum-assisted therapy of an infected pancreatic pseudocyst. Endoscopy. 2011;43 Suppl 2 UCTN:E312-E313. [PubMed] |
| 101. | Schorsch T, Müller C, Loske G. Pancreatico-gastric anastomotic insufficiency successfully treated with endoscopic vacuum therapy. Endoscopy. 2013;45 Suppl 2 UCTN:E141-E142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Kotzampassi K, Eleftheriadis E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery. 2015;157:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Ramón Rábago L, Moral I, Delgado M, Guerra I, Quintanilla E, Castro JL, Llorente R, Martínez Veiga JL, Gea F. [Endoscopic treatment of gastrointestinal fistulas with biological fibrin glue]. Gastroenterol Hepatol. 2006;29:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Lippert E, Klebl FH, Schweller F, Ott C, Gelbmann CM, Schölmerich J, Endlicher E, Kullmann F. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis. 2011;26:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 105. | Jackson MR. Fibrin sealants in surgical practice: An overview. Am J Surg. 2001;182:1S-7S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 106. | López J, Rodriguez K, Targarona EM, Guzman H, Corral I, Gameros R, Reyes A. Systematic review of cyanoacrylate embolization for refractory gastrointestinal fistulae: a promising therapy. Surg Innov. 2015;22:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Böhm G, Mossdorf A, Klink C, Klinge U, Jansen M, Schumpelick V, Truong S. Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined vicryl plug and fibrin glue. Endoscopy. 2010;42:599-602. [PubMed] [DOI] [Full Text] |
| 108. | Darrien JH, Kasem H. Successful closure of gastrocutaneous fistulas using the Surgisis(®) anal fistula plug. Ann R Coll Surg Engl. 2014;96:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | Maluf-Filho F, Hondo F, Halwan B, de Lima MS, Giordano-Nappi JH, Sakai P. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc. 2009;23:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |