Published online Jun 21, 2020. doi: 10.3748/wjg.v26.i23.3236
Peer-review started: December 30, 2019
First decision: January 19, 2020
Revised: April 20, 2020
Accepted: May 30, 2020
Article in press: May 30, 2020
Published online: June 21, 2020
Processing time: 174 Days and 0.7 Hours
Locally advanced adenocarcinoma of the esophagus (EAC) and squamous cell carcinoma (ESCC) result in a worse prognosis. Neoadjuvant treatment improves survival, however, only for responders. The transmembrane glycoprotein podoplanin is overexpressed in squamous cell carcinomas, miRNA-363 is associated to its regulation in head and neck cancer.
To predict therapy response and prognosis markers, and targets for novel therapies would individualize treatments leading to more favourable outcomes.
Expression of podoplanin protein has been visualized by immunohistochemistry in surgical specimens of 195 esophageal cancer patients who underwent transthoracic esophagectomy: 90 ESCC and 105 EAC with clinical T2-3, Nx, M0. One hundred and six patients received neoadjuvant chemoradiation. RNA was extracted from paraffin-embedded tissue, and miRNA-363 quantified by real-time TaqMan-real-time-PCR. D2-40 mab staining of > 5% was scored as high podoplanin expression (HPE). We related podoplanin and miRNA-363 expression to histopathologic response after neoadjuvant treatment and clinicopathological characteristics, such as histological tumor type, survival rate or clinical tumor category.
We confirmed expression of membrane-bound podoplanin in 90 ESCC patients. 26% showed HPE of > 5%. In addition, absence in EAC patients (only 2% with HPE) was shown. Lower podoplanin expression has been detected in resection-specimen of 58 ESCC patients after neoadjuvant (RTx/CTx) treatment, only 11% with HPE, compared to 50% HPE of 32 non-pretreated primary surgery patients, P = 0.0001. This difference of podoplanin expression was confirmed comparing pre-treatment biopsies with matching post-treatment surgical specimens, P < 0.001. Podoplanin has been identified as a prognostic marker in 32 patients that underwent primary surgery without neoadjuvant treatment. Low (0-5%) podoplanin expression was associated with better prognosis compared to patients with HPE, P = 0.013. Podoplanin expression has been associated with post-transcriptional regulation by miRNA-363. At a cut-off value of miR-363 < 7, lower miR-363 expression correlated with HPE in surgical tissue specimens of primary surgery patients, P = 0.013. Therefore, ESCC patients with miRNA-363 expression < 7 had a worse prognosis than patients expressing miRNA-363 ≥ 7, P = 0.049.
Analysis of the molecular process that leads to decrease in podoplanin expression during neoadjuvant treatment and its regulation may provide novel markers and targets to improve targeted therapy of ESCC.
Core tip: Podoplanin is an oncofetal membrane protein, re-expressed in squamous cell carcinoma of the esophagus. Podoplanin expression seems to be, among others, controlled by miR-363. Chemoradiation results in reduction of podoplanin protein expression, and furthermore, podoplanin is a prognostic factor for survival. This implicates that a decrease of podoplanin might become a therapeutic option.
- Citation: Warnecke-Eberz U, Plum P, Schweinsberg V, Drebber U, Bruns CJ, Müller DT, Hölscher AH, Bollschweiler E. Neoadjuvant chemoradiation changes podoplanin expression in esophageal cancer patients. World J Gastroenterol 2020; 26(23): 3236-3248
- URL: https://www.wjgnet.com/1007-9327/full/v26/i23/3236.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i23.3236
Esophageal squamous cell- (ESCC) and adenocarcinoma (EAC) are common malignancies worldwide[1]. EAC is the most rapidly increasing cancer in the Western world, thus ESCC no longer represents the most prevalent histological subtype[2]. These two tumor types differ in risk factors and biological characteristics[3,4].
The poor prognosis of patients with locally advanced cancer encouraged the assessment of neoadjuvant treatment strategies to improve patients’ survival[5]. Since only responders, 40% to 50% of all patients profit by better survival, after going through the burden of chemoradiation, we urgently need markers predicting response prior to therapy[6,7]. The molecular predictive factors previously identified, failed translation to clinical application[8-10]. There is an additional need for markers indicating tumor progression and prognosis. Putative markers include growth-factor receptors, enzymes of angiogenesis, tumor suppressor genes, cell cycle regulators, enzymes involved in DNA repair, apoptosis and in degradation of extracellular matrix[11-13]. In addition to predictive and prognostic markers, novel targets for therapy are imperative for the future approach of a tailored multimodality treatment and its surveillance.
Podoplanin is a mucin-type transmembrane glycoprotein, thought to be one of the cancer stem cell markers for squamous-cell carcinoma in several tumor entities, including esophageal cancer[14]. Podoplanin has been identified as a platelet aggregation-inducing factor and a specific marker for lymphatic vessels[15,16]. It has been shown to stimulate invasion and migration of tumor cells and was correlated to lymph node metastasis, disease stage, lymphatic and vascular invasion, recurrence and poor prognosis[17].
MicroRNAs are small non-coding RNAs that act post-transcriptionally as master regulators for a variety of mRNA targets by sequence homology[18,19]. miR-363 has been reported to be deregulated in several tumors and has been associated with podoplanin expression[20]. Knowledge about regulation of podoplanin expression in esophageal cancer is scarse. The mechanism of re-expression of podoplanin in tumor cells of ESCC still has to be elucidated.
The aim of the present study was to determine expression of podoplanin protein in esophageal cancer patients with regard to a potential benefit for personalization of neoadjuvant treatment of esophageal carcinoma. We evaluated its predictive impact for therapy response and prognosis and a potential association of post-transcriptional regulation by miR-363 as one mechanism of deregulation of podoplanin expression in cancer.
One hundred and ninety-five patients with advanced esophageal cancer (clinical T2-3, Nx, M0) who received transthoracic esophagectomy are characterized in Table 1. Matching endoscopic biopsies and resection tissue were available for 56 primary surgery ESCC patients. Paraffin-embedded tissue for RNA extraction and quantification of miR-363 expression in addition to podoplanin protein were available for 29 ESCC patients and 19 EAC patients who received primary surgery.
| Total | ESCC | EAC | ||||
| n | 5Y-SR (%) | n | 5Y-SR (%) | n | 5Y-SR (%) | |
| Total study cohort | 195 | 27 | 90 | 27 | 105 | 26 |
| Median age (yr) | 60.4 | - | 60 | - | 62 | - |
| Gender | ||||||
| Male | 155 | 28 | 61 | 25 | 94 | 31 |
| Female | 40 | 26 | 29 | 32 | 11 | 12 |
| Therapy | ||||||
| Surgery only | 89 | 29 | 32 | 27 | 57 | 31 |
| RTx/CTx + surgery | 106 | 27 | 58 | 27 | 48 | 28 |
| T-category | ||||||
| pT2 | 30 | 46 | 6 | 62 | 24 | 43 |
| cT/pT3 | 165 | 25 | 84 | 24 | 81 | 26 |
| Endoscopic biopsy | 104 | 25 | 58 | 22 | 46 | 29 |
Endoscopic biopsies were obtained during routine staging esophagogastroduo-denoscopy prior to therapy. None of the patients had received prior radio- and /or chemotherapy. One hundred and six patients of the study population with an advanced tumor stage (cT3, Nx, M0) received preoperative chemoradiation. Briefly, cisplatin (20 mg/m2 per day) was administered as a short-term infusion on days 1-5 and 5-fluorouracil (5-FU) (1000mg/m2 per day) as a continuous infusion over 24 h on days 1-5. Radiation was delivered in daily fractions of 1.8 Gy to a total dose of 40 Gy using a multiple field technique. Standardized trans-thoracic en bloc esophagectomy with 2-field lymphadenectomy was performed 4-5 wk after completion of chemoradiation.
Informed consent was obtained from each patient and the scientific protocol was approved by the local ethics committee.
The degree of histomorphologic regression of the primary tumor was classified into four categories (Cologne Regression Scale): Grade 1, complete response; Grade 2, nearly complete response with less than 10% vital residual tumor cells (VRTCs) classified as major response; Grade 3, 10% to 50% VRTCs; and Grade 4, more than 50% VRTCs, categorized as minor histomorphologic response[21]. Classification has been performed by experienced staff pathologists.
Podoplanin protein was detected by mouse anti-human D2-40 monoclonal antibody (DakoCytomation,Hamburg, Germany) raised against 40 kDa O-linked sialogly-coprotein. Paraffin embedded endoscopic biopsies and surgical specimens have been analyzed. Five µm sections were cut and deparaffinized according to standard histological techniques. A high sensitivity immunohistochemical staining was performed applying Dako EnVision System (DakoCytomation, Hamburg,) following the manufacturer’s instructions. In brief, sections were covered with citrate buffer (pH 6.0) for antigen retrieval. Endogenous peroxidase was blocked by 0.3% hydrogen peroxide for 20 min. Sections were covered by 100 μL mouse monoclonal D2-40 primary antibody (D2-40 mouse monoclonal antibody, Lot.Nr:10066658; DakoCytomation, Hamburg) at a dilution of 1:100 and incubated at 4°C overnight. The nuclei have been counterstained with hematoxylin. The staining procedure without a primary antibody was used as a negative control. For quantification of podoplanin expresson a scoring system was applied by a pathologist. Score 1: 0-5% tumor cells stained by D2-40 mab; Score 2: 6%-35% of tumor cells stained; Score 3: 36%-65%; Score 4: > 65%.
Paraffin embedded resection specimen of EAC and ESCC have been selected from the Institute of Pathology, University Hospital of Cologne, Germany. Tissues were fixed in 10% buffered formalin prior to embedding in paraffin. Histological sections of 5-μm thickness were cut from each tissue block using a microtome. About 60 μm tissues per patient have been applied for total RNA extraction after macrodissection of the tumor area and purified by miRNeasy FFPE kit (Qiagen, Hilden), according to the protocol of the manufacturer. Samples have been lysed by Proteinase-K and treated with DNase-I. Concentrated RNA was purified using RNeasy MinElute spin columns and eluted by 12 μL of nuclease-free H2O.
Reverse transcription of miR-363 from total RNA was performed using miR-363 specific reverse primer Assay-ID hsa-miR-363 001271 and TaqMan MicroRNA reverse transcription kit, Thermo Fisher Scientific, Darmstadt.
Quantification has been performed by TaqMan7900HT real-time PCR system Thermo Fisher Scientific, Darmstadt, RNU6 was used as a calibrator. The 15 μL RT-reaction included 5 μL RNA, 3 μL reverse primer, 1 μL MultiScribe™ Reverse Transcriptase, 0.15 μL dNTP mixture, 0.19 μL RNase inhibitor, 1.5 μL RT-buffer, 4.16 μL nuclease-free H2O: 2 min 50 °C, 10 min 95 °C, 15 s 95 °C, and 1 min 60°C. TaqMan PCR analysis provided relative quantification of miR-363 expression related to expression of snoU6 as an endogenous control.
Fisher's exact test or Chi-Square test have been used to evaluate the correlation between scored podoplanin expression and histological tumor category (ESCC vs EAC). Significance of correlation between podoplanin expression and clinicopathological characteristics (pTNM-category), or different therapies was determined. Categorial data of paired samples has been compared by McNemer test. Survival curves based on post-therapeutic popdoplanin-expression in ESCC have been estimated according to Kaplan-Meier and compared with the Log-Rank test.
TaqMan PCR analysis provided relative quantification of miR-363 expression /expression of reference RNA snoU6. Receiver operating characteristic curve analysis has been used to define an optimal cut-off value for miR-363 in relation to podoplanin expression. Statistical calculations of prognosis were performed using MedCalc Statistical Software version 17.9.6 (MedCalc Software bvba, Ostend, Belgium); http://www.medcalc.org; 2017. Other statistical analyses were carried out using SPSS version 25 (Chicago, IL, United States).
Podoplanin staining of resected specimen derived from patients without preoperative chemoradiation revealed 56 of 57 EAC patients (98%) with none or nearly no protein expression (0-5%). 16 of 32 ESCC patients (50%) have been identified to express score 1 0-5% detectable protein. Comparision of podoplanin expression between ESCC and EAC, P < 0.001 is depicted in Table 2.
| Total | ESCC | EAC | ||||
| n | Podoplanin > 5% (HPE, %) | n | Podoplanin > 5% (HPE, %) | n | Podoplanin > 5% (HPE, %) | |
| Total study cohort | 195 | 13 | 90 | 26 | 105 | 2 |
| Gender | ||||||
| Male | 155 | 10 | 61 | 23 | 94 | 1 |
| Female | 40 | 25 | 29 | 32 | 11 | 9 |
| Therapy | ||||||
| Surgery only | 89 | 19 | 32 | 50 | 57 | 2 |
| RTx/CTx + surgery | 106 | 7 | 58 | 11 | 48 | 2 |
| T-category | ||||||
| pT2 | 30 | 3 | 6 | 17 | 24 | 0 |
| cT/pT3 | 165 | 14 | 84 | 27 | 81 | 3 |
| Endoscopic biopsy | 104 | 7 | 58 | 38 | 46 | 2 |
Different expression levels of podoplanin have been stained in EAC and ESCC patients in both, resected tissues of patients with primary surgery and endoscopic biopsies. One hundred and four endoscopic biopsies of 46 EAC and 58 ESCC patients confirmed podoplanin expression in ESCC, and its absence in EAC. Fourty-four/fourty-six EAC patients (96%) were scored for expression of 0-5% protein compared to 36 of 58 ESCC patients (63%), P = 0.002, Table 2.
Podoplanin expression in resected tissues of 32 ESCC patients without chemoradiation did not show any significant relation to pT-category (P = 0.085, P = 0.051 for pT2 to pT4) or lymph node metastases (P = 0.457), however, results are limited by the small number of patients in the subgroups (Table 3).
| Resected tissue | Score | Podoplanin, % | Patients, n | Patients, % |
| pT2, n = 6 | 1 | 0-5 | 5 | 83 |
| 2 | 6-35 | 1 | 17 | |
| 3 | 36-65 | 0 | 0 | |
| pT3/4, n = 26 | 1 | 0-5 | 11 | 43 |
| 2 | 6-35 | 10 | 38 | |
| 3 | 36-65 | 5 | 19 | |
| pN0, n = 11 | 1 | 0-5 | 7 | 64 |
| 2 | 6-35 | 2 | 18 | |
| 3 | 36-65 | 2 | 18 | |
| pN+, n = 21 | 1 | 0-5 | 9 | 43 |
| 2 | 6-35 | 9 | 43 | |
| 3 | 36-65 | 3 | 14 |
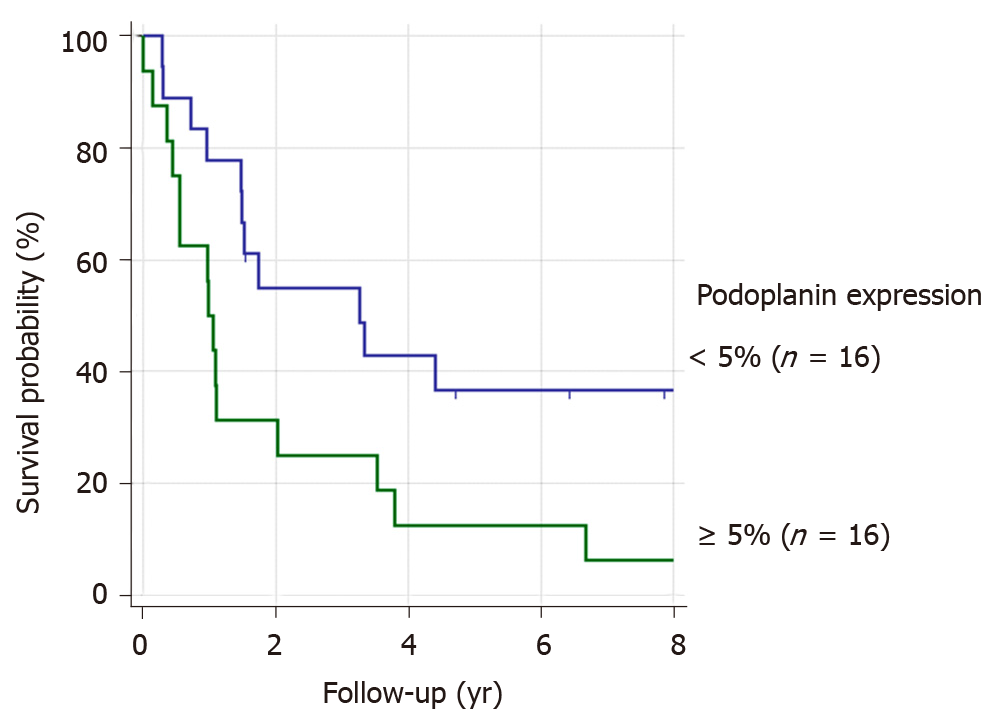
We analyzed a potential association of podoplanin expression and survival of ESCC-patients without preoperative chemotherapy. Patients with a stronger podoplanin protein expression > 5% [high podoplanin expression (HPE)] had a worse prognosis compared to the patient group with low expression (P = 0.013), Figure 1.
Sixteen patients showing a lower podoplanin expression of 0-5%, had a better survival rate of 34% than the patient group with HPE, resulting in a five-year survival of 12%.
Fifty-eight ESCC and 48 EAC patients received preoperative chemoradiation therapy due to an advanced tumor stage (cT3NxM0).
Only one of these 48 EAC patients expressed HPE in resection specimen, 47 patients showed none or low expression. Staining of 57 primary surgery EAC patients provided the same result of absence of podoplanin, Table 2.
Seven of 58 ESCC patients expressed HPE in resected tissue after chemoradiation, 51 patients had a lower expression < 5%. We compared the protein expression of this group of 58 ESCC patients who received chemoradiation to the non-pretreated primary surgery group of 32 ESCC patients. Podoplanin expression has been identified to be significantly lower in resected specimen of neoadjuvantly pre-treated patients compared to patients receiving only surgery, P < 0.001 (Table 4).
| Resected tissue | Podoplanin | Patients | ||
| Score | % | n | % | |
| RTx/CTx + surgery | 1 | 0-5 | 51 | 89 |
| 2 | 6-35 | 5 | 9 | |
| 3 | 36-65 | 2 | 2 | |
| Primary surgery non-pretreated | 1 | 0-5 | 16 | 50 |
| 2 | 6-35 | 11 | 34 | |
| 3 | 36-65 | 5 | 16 | |
To confirm the different podoplanin expression levels between neoadjuvantly treated and non-pretreated primary surgery patient groups, we compared the protein expression in endoscopic biopsies with the expression after CTx/RTx of the individual matching resected tissue specimen of 56 ESCC patients.
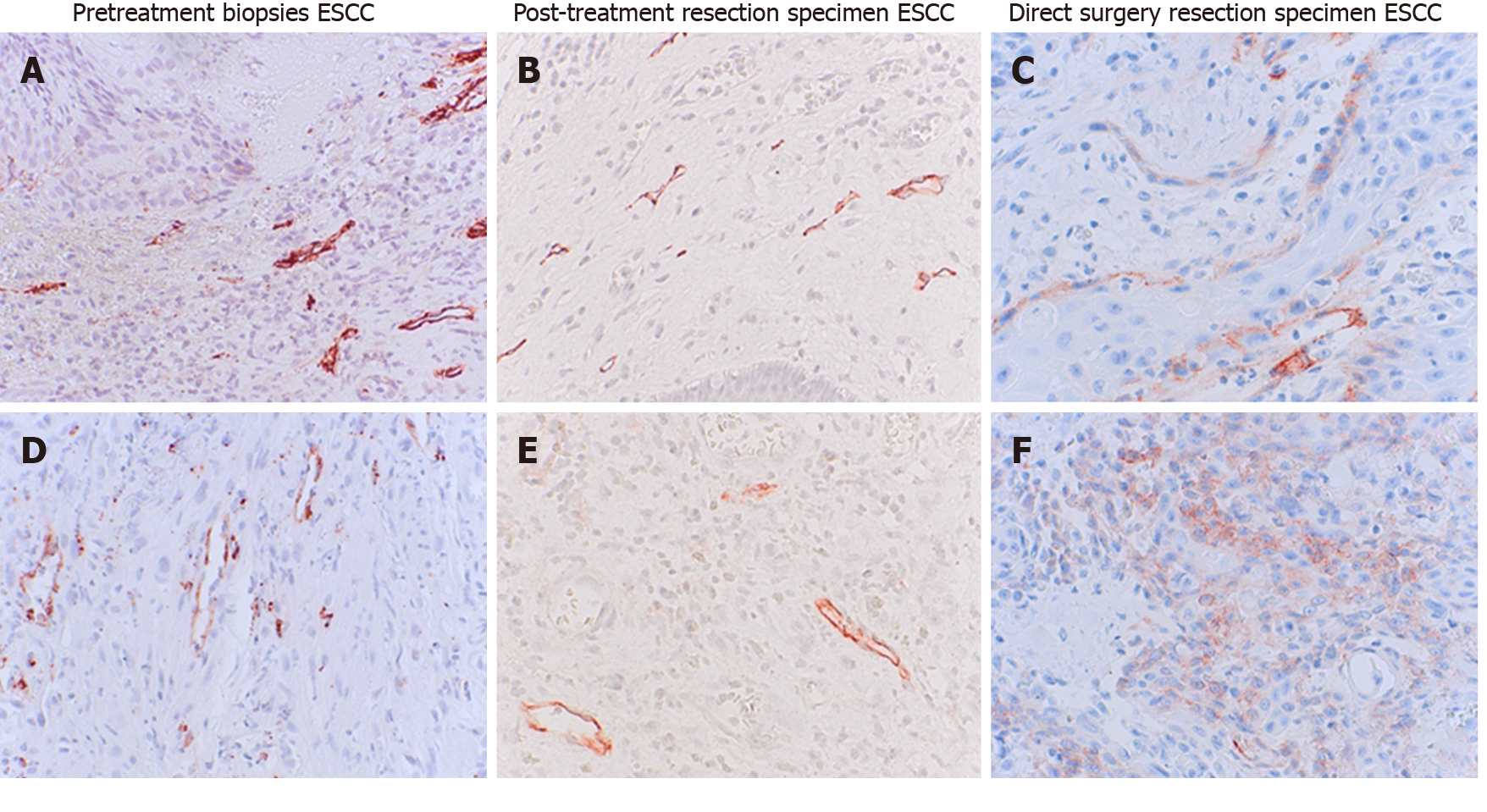
Thirty-five pretreatment biopsies did not express podoplanin 0-5%, score 1. Thirty-four of 35 neoadjuvantly treated patients (97%) did not express podoplanin in resected tissues. Twenty-one patients showed a higher podoplanin expression of > 5% (Score 2-4) in their biopsies before chemoradiation. In resection specimen of 16 of these 21 patients (76%) podoplanin was not detectable after neoadjuvant treatment. The matching resected specimen of these patients showed a decreased podoplanin expression after CTx/RTx, P < 0.001, examples in Figure 2, survey in Table 5.
| Endoscopic biopsies pre RTx/CTx | Post RTx/CTx resected tissue | ||||
| Podoplanin expression | Patients, n | 0-5% | 6%-35% | 36%-65% | > 65% |
| 0-5% | 35 | 34 | 1 | 0 | 0 |
| 6%-35% | 14 | 10 | 3 | 1 | 0 |
| 36%-65% | 3 | 3 | 0 | 0 | 0 |
| > 65% | 4 | 3 | 1 | 0 | 0 |
There was no significant difference detectable in podoplanin expression between 35 minor and 23 majors responders among 58 ESCC patients (P = 0.879). Thirty-one of 35 minors responders showed a score 1, 4 of 35 Score 2 and 3 (> 5% podoplanin expression). Twenty-one of 23 majors responders showed Score 1, 2 of 23 Score 2.
To examine a potential role of miR-363 for post-transcriptional control of podoplanin up-regulation in ESCC, miR-363 expression has been quantified and compared to the expression level of podoplanin protein in resected tissues of 29 ESCC and 19 EAC patients – all patients without neoadjuvant therapy. There was no significant difference in miR-363 expression between EAC n = 19 and ESCC n = 29 patient groups. miR-363 expression was not associated to histological tumor types. Median miRNA-363 expression was 8.4 (min-max: 1.05-303) in ESCC and 7.07 (min-max: 1.7-42.5) in EAC patients.
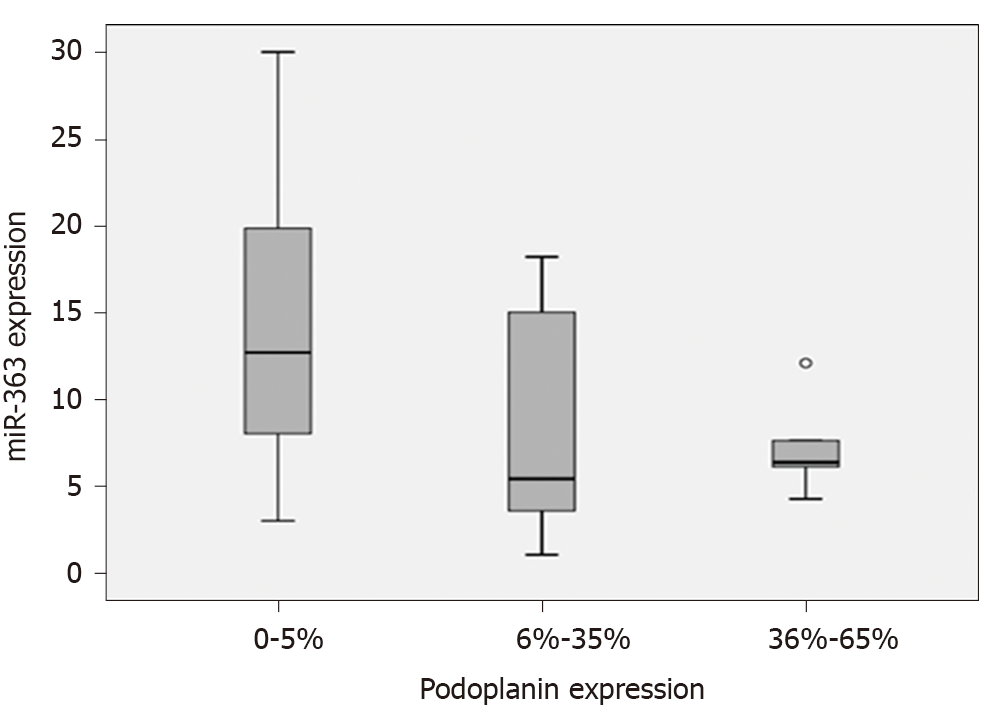
The association between miR-363 and podoplanin expression in the study group of ESCC patients without neoadjuvant treatment is shown as boxplots, Figure 3. An up-regulated podoplanin expression has been detected to be associated with a lower miR-363 expression. Subgrouping of ESCC patients by podoplanin expression of 0-5% and HPE resulted in a trend of a correlation between expression of miR-363 and podoplanin protein: 0-5% n = 14: median miR-363 12.7 (min-max: 3.0-303), > 5%: n = 15: median miR-363 6.1 (min-max: 1.05-131).
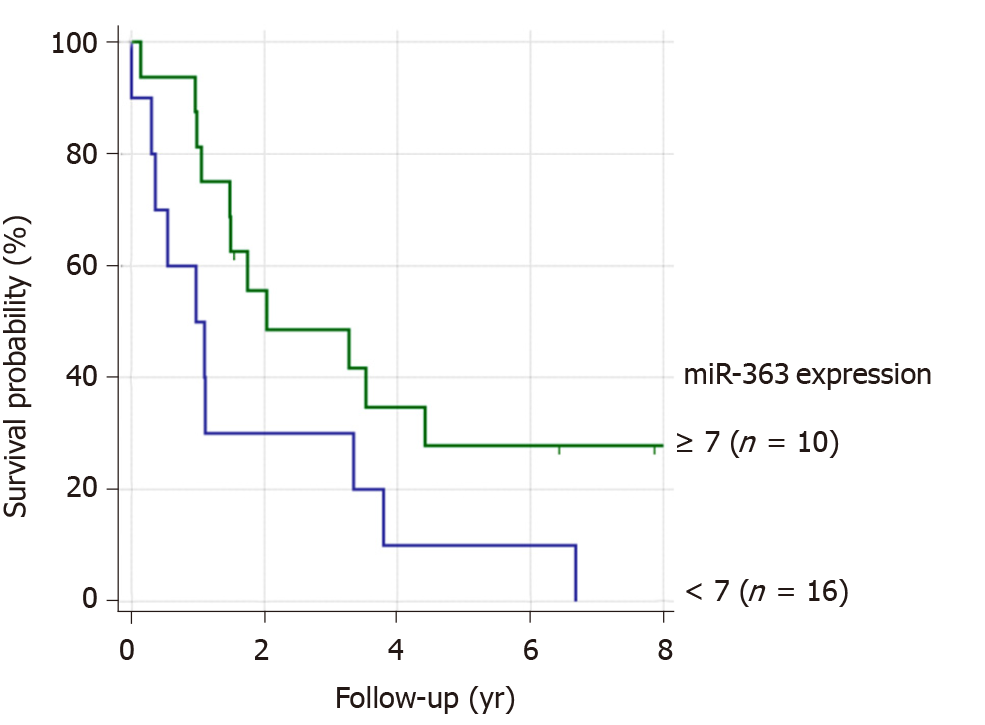
Patients with lower miR-363 expression had a worse prognosis compared to those with higher miR-363 expression. We identified a prognostic relevant cut-off value for miR-363. Patients without distant metastases with miR-363 expression < 7 (n = 10) had a significantly worse prognosis than patients with miR expression ≥ 7 (n = 16), P = 0.049. (Figure 4). Based on this cut-off value for miR-363 expression of 7, the lower miR-363 expression (miR-363 < 7: n = 9) was associated with HPE (n = 15). Six of these 15 non-pretreated ESCC patients showed miR-363 expression ≥ 7 in resected tissue specimen. miR-363 expression ≥ 7 (n = 12) was associated with a low podoplanin expression of 0-5% n = 14, (P = 0.011). Only two of these 12 patients with low podoplanin expression expressed miR-363 < 7.
The histologically different tumor types EAC and ESCC depend on different risk factors, show biologically different characteristics, and respond with different rates to chemoradiotherapy[3,4]. Podoplanin expression is one example for the biological diversity between these most frequent histological subtypes of esophageal cancer. Our study confirms data of none to nearly no expression of the transmembrane protein podoplanin in EAC patients, and its expression in ESCC patients[22]. The oncofetal protein podoplanin has been described to be re-expressed in squamous cell carcinoma of other tumor entities, as well[23-25].
The most important result of our study is the change of podoplanin expression during neoadjuvant chemoradiation. This is a novel finding.
Our study has demonstrated a change of podoplanin protein expression after neoadjuvant treatment in resected tissue compared to pretreatment biopsies of the same individual patients. The comparison of resection specimen of a patient cohort neoadjuvantly pretreated with an only surgery study cohort provided the same result of lower podoplanin expression associated with chemoradiation.
The present study also revealed that podoplanin expression in pretreatment biopsies is not predictive for therapy response to chemoradiation. A low expression level did not seem to have the consequence of a major response, neither did a high podoplanin level with therapy resistance or minor response. Therefore, the thesis that podoplanin is expressed specifically by a differentiated tumor cell population with a stem cell like phenotype conferring therapy resistance as described by Lynam-Lennon et al[26] does not seem to be the fact for ESCC. There was no significant difference in podoplanin expression between 35 minor and 23 majors responding ESCC patients. Due to the changed podoplanin expression after neoadjuvant treatment this protein might rather be a target for therapy than a response predictive marker.
Upregulation of podoplanin expression was associated with ESCC. A reason for high podoplanin expression in squamous cell carcinoma in contrast to adenocarcinoma might be that podoplanin is expressed in some adult normal cells like lung alveolar cells, glomerular podocytes, as well as in basal epithelium of cervix and esophagus. ESCC develops from squamous epithelial cells, whereas in EAC these cells are replaced by columnar intestinal-type mucosa. EAC develops from Barrett esophagus[27].
Additionally, we were able to demonstrate high expression of podoplanin to be a marker for worse prognosis in accordance with data reviewed by Wang et al[22]. Therefore, there may be a therapeutic effect by inhibiting this target. Monoclonal antibodies against podoplanin have already been produced for a potential target-based therapy[28-30].
The biological function of podoplanin does support this therapeutic option. Podoplanin is a mucin-like transmembrane glycoprotein associated with cancer cell invasion and migration[31] and has been identified as a marker for early infiltrative carcinoma[27]. Podoplanin has been suggested to be involved in lymphangiogenesis, since podoplanin deficient mice had dilated malfunctioning lymphatic vessels and lymphedema[32]. The most studied physiological role of podoplanin is its ability to bind and activate the C-type lectin receptor (CLEC) that is highly expressed by platelets and immunocompetent cells[33,34]. Podoplanin has been identified as a key factor in tumor-induced platelet aggregation enhancing metastasis by secreting growth factors and forming tumor emboli in the microvasculature, reviewed by Hisada et al[35]. Therefore, the podoplanin-mediated platelet aggregation might be an effective target for anti-tumor therapies. Platelet aggregation-inducing domains have been detected[15]. Aggregated platelets are coating tumor cells during their transit through the bloodstream, are mediating adherence to vascular endothelium, evasion from immune molecules and facilitate growth at metastatic sites. Podoplanin elicits platelet aggregation as the ligand of CLEC-2[36,37]. The strong upregulation of podoplanin expression supports its potential role as a therapeutic target reviewed by Takemoto et al[38]. An anti-human podoplanin antibody (NZ-1) inhibiting podoplanin-induced platelet aggregation, abrogated experimental metastasis has already been formed, however with strong toxic side effects. A further novel chimeric humanized anti-human podoplanin antibody inhibiting podoplanin-induced platelet aggregation has been developed as a potential novel anticancer agent[39]. Since there is toxicity due to interferences with endogenous podoplanin in other cell types like type I lung alveolar cells, kidney podocytes, choroid plexus epithelium, a less interfering antigen has been developed[40]. However, the knowledge about the function of podoplanin in these organs is scarce.
Podoplanin has been reported to be involved in tumor progression[41] correlating with lymph node metastasis[42-46]. We were unable to demonstrate a significant association of podoplanin expression and T-category by staining of resection tissue of primary surgery patients, although a trend of a non-significant increase of podoplanin expression in T3 category compared to T2 has been observed. The lack of association between podoplanin expression and lymph node metastases might be due to the small number of patients in this subgroup, a weakness of our study.
Despite this limitation we were able to detect an association of podoplanin expression with prognosis. Our data complements that of Tanaka et al[17] reporting on increased podoplanin expression as a predictor of mortality. Therefore, podoplanin could be applied as a prognostic marker for identification of patients with high risk for tumor progression.
Podoplanin represents an oncofetal antigen not expressed in most adult cells and re-expressed in squamous cell carcinoma. The transcriptional regulation of podoplanin expression has been reviewed by Astarita et al[47]. Podoplanin transcription is regulated by AP-1 transcription factor comprised of Fos and Jun proteins. PTEN expression, a negative regulator of PI3K-AKT-AP-1 pathway, has inversely been correlated with podoplanin expression. The podoplanin promoter is heavily methylated keeping it repressed. Upregulation during malignant conditions seems to depend on the activity of Fos and Jun (AP-1) transcription factors. miRNAs are a major tool of posttranscriptional regulation. As a kind of master regulator these small molecules inhibit protein expression binding or degrading mRNAs[25]. Circulating microRNAs have been associated with unfavorable response to neoadjuvant chemoradiotherapy[48]. A potential impact of post-transcriptional regulation of podoplanin protein expression by miR-363 has been described for head and neck squamous cell carcinoma[20]. Anti-proliferative properties have also been reported for miR-363[44], in gastric cancer[49]. We correlated miR-363 quantification with podoplanin protein expression and identified a cut-off value associated with a significant correlation. Patients with high miR-363 expression and down-regulated podoplanin expression had a better prognosis. This association and the potential role of miR-363 for up-regulation of podoplanin expression in ESCC also is a novel finding.
This is the first study showing a significant down-regulation of podoplanin protein expression during neoadjuvant chemoradiation in patients with ESCC. Podoplanin as well as its post-transcriptional regulator miR-363 might be important targets for a tailored therapy of locally advanced ESCC, as well as markers for prognosis.
Patients with locally advanced esophageal carcinoma have a poor prognosis. Additionally, only 40%-50% of the patients profit by improved survival from neoadjuvant therapies after the burden of chemoradiation.
We urgently need markers for diagnosis of earlier tumor stages, for prediction of therapy response and prognosis, as well as targets for novel therapies.
Our aim was to evaluate the predictive impact of podoplanin expression for therapy response and prognosis and a potential association with post-transcriptional regulation by miR-363 as one mechanism of deregulation in cancer. Podoplanin protein expression has been related to clinical parameters (histological tumor type, histopathologic response classification, survival rate, clinical tumor category), with regard to a potential benefit for personalization of neoadjuvant treatment.
Podoplanin has been visualized by immunohistochemistry in resection-specimen of 195 patients: 90 squamous cell carcinomas of the esophagus (ESCC), 105 adenocarcinomas of the esophagus (EAC) with clinical T2-3, Nx, M0. RNA was extracted from paraffin-embedded tissue, miRNA-363 quantified by real-time TaqMan-real-time-PCR.
We confirmed high podoplanin expression (HPE) in ESCC patients and its absence in EAC. We detected lower podoplanin expression in resection-specimen of 58 ESCC patients after neoadjuvant (RTx/CTx) treatment, only 11% with HPE of > 5%, compared to 32 non-pretreated primary surgery patients with 50% HPE, P = 0.0001. This novel finding of a lower podoplanin expression in the pretreated patient cohort was confirmed by the comparison of corresponding surgical specimens after neoadjuvant treatment with the individual matching pretherapeutic biopsies of 56 patients, P < 0.001. Podoplanin, however, is no predictive marker for response to neoadjuvant chemoradiation. Due to the small number of cT1-T2 patients we were only able to show a trend of association with podoplanin protein expression. We were able to demonstrate a prognostic impact of podoplanin, as well as for miR-336, a posttranscriptional regulator of this protein.
Direct surgery ESCC patients with a low podoplanin expression have a better prognosis. Chemoradiation results in reduction of expression of podoplanin protein in patients with ESCC. Podoplanin expression seems to be, among others, controlled by miR-363.
The decrease of podoplanin expression might become a therapeutic option.
We thank Michaela Heitmann, Susanne Neiss and Anke Wienand-Dorweiler for their excellent technical assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hashimoto N, Surucu E S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2486] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 2. | Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 3. | Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 499] [Cited by in RCA: 595] [Article Influence: 54.1] [Reference Citation Analysis (4)] |
| 4. | Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia--the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Hölscher AH, Bollschweiler E, Bogoevski D, Schmidt H, Semrau R, Izbicki JR. Prognostic impact of neoadjuvant chemoradiation in cT3 oesophageal cancer - A propensity score matched analysis. Eur J Cancer. 2014;50:2950-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Bollschweiler E, Metzger R, Drebber U, Baldus S, Vallböhmer D, Kocher M, Hölscher AH. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol. 2009;20:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Hölscher AH, Drebber U, Schmidt H, Bollschweiler E. Prognostic classification of histopathologic response to neoadjuvant therapy in esophageal adenocarcinoma. Ann Surg. 2014;260:779-84; discussion 784-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Metzger R, Schneider PM, Warnecke-Eberz U, Brabender J, Hölscher AH. Molecular biology of esophageal cancer. Onkologie. 2004;27:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Miyazono F, Metzger R, Warnecke-Eberz U, Baldus SE, Brabender J, Bollschweiler E, Doerfler W, Mueller RP, Dienes HP, Aikou T, Hoelscher AH, Schneider PM. Quantitative c-erbB-2 but not c-erbB-1 mRNA expression is a promising marker to predict minor histopathologic response to neoadjuvant radiochemotherapy in oesophageal cancer. Br J Cancer. 2004;91:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Warnecke-Eberz U, Metzger R, Miyazono F, Baldus SE, Neiss S, Brabender J, Schaefer H, Doerfler W, Bollschweiler E, Dienes HP, Mueller RP, Danenberg PV, Hoelscher AH, Schneider PM. High specificity of quantitative excision repair cross-complementing 1 messenger RNA expression for prediction of minor histopathological response to neoadjuvant radiochemotherapy in esophageal cancer. Clin Cancer Res. 2004;10:3794-3799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Plum PS, Bollschweiler E, Hölscher AH, Warnecke-Eberz U. Novel diagnostic and prognostic biomarkers in esophageal cancer. Expert Opin Med Diagn. 2013;7:557-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Warnecke-Eberz U, Metzger R, Bollschweiler E, Baldus SE, Mueller RP, Dienes HP, Hoelscher AH, Schneider PM. TaqMan low-density arrays and analysis by artificial neuronal networks predict response to neoadjuvant chemoradiation in esophageal cancer. Pharmacogenomics. 2010;11:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Metzger R, Heukamp L, Drebber U, Bollschweiler E, Zander T, Hoelscher AH, Warnecke-Eberz U. CUL2 and STK11 as novel response-predictive genes for neoadjuvant radiochemotherapy in esophageal cancer. Pharmacogenomics. 2010;11:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Rahadiani N, Ikeda J, Makino T, Tian T, Qiu Y, Mamat S, Wang Y, Doki Y, Aozasa K, Morii E. Tumorigenic role of podoplanin in esophageal squamous-cell carcinoma. Ann Surg Oncol. 2010;17:1311-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Sekiguchi T, Takemoto A, Takagi S, Takatori K, Sato S, Takami M, Fujita N. Targeting a novel domain in podoplanin for inhibiting platelet-mediated tumor metastasis. Oncotarget. 2016;7:3934-3946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Nakashima Y, Yoshinaga K, Kitao H, Ando K, Kimura Y, Saeki H, Oki E, Morita M, Kakeji Y, Hirahashi M, Oda Y, Maehara Y. Podoplanin is expressed at the invasive front of esophageal squamous cell carcinomas and is involved in collective cell invasion. Cancer Sci. 2013;104:1718-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Kijima H, Shimada H, Makuuchi H, Ozawa S, Inokuchi S. Expression of podoplanin and vimentin is correlated with prognosis in esophageal squamous cell carcinoma. Mol Med Rep. 2015;12:4029-4036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Ng SB, Yan J, Huang G, Selvarajan V, Tay JL, Lin B, Bi C, Tan J, Kwong YL, Shimizu N, Aozasa K, Chng WJ. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood. 2011;118:4919-4929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Qiao J, Lee S, Paul P, Theiss L, Tiao J, Qiao L, Kong A, Chung DH. miR-335 and miR-363 regulation of neuroblastoma tumorigenesis and metastasis. Surgery. 2013;154:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Sun Q, Zhang J, Cao W, Wang X, Xu Q, Yan M, Wu X, Chen W. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. Int J Biochem Cell Biol. 2013;45:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Puetz K, Bollschweiler E, Semrau R, Mönig SP, Hölscher AH, Drebber U. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer - which response grading system best impacts prognostic discrimination? Histopathology. 2019;74:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Wang C, Wang J, Chen Z, Gao Y, He J. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: a systematic review. Chin J Cancer. 2017;36:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Pula B, Jethon A, Piotrowska A, Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J, Ugorski M, Dziegiel P, Podhorska-Okolow M. Podoplanin expression by cancer-associated fibroblasts predicts poor outcome in invasive ductal breast carcinoma. Histopathology. 2011;59:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Toll A, Gimeno-Beltrán J, Ferrandiz-Pulido C, Masferrer E, Yébenes M, Jucglà A, Abal L, Martí RM, Sanmartín O, Baró T, Casado B, Gandarillas A, Barranco C, Costa I, Mojal S, García-Patos V, Pujol RM. D2-40 immunohistochemical overexpression in cutaneous squamous cell carcinomas: a marker of metastatic risk. J Am Acad Dermatol. 2012;67:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Funayama A, Cheng J, Maruyama S, Yamazaki M, Kobayashi T, Syafriadi M, Kundu S, Shingaki S, Saito C, Saku T. Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology. 2011;78:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos Soc. 2009;84:55-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Chen G, Xu R, Yue B, Mei X, Li P, Zhou X, Huang S, Gong L, Zhang S. The expression of podoplanin protein is a diagnostic marker to distinguish the early infiltration of esophageal squamous cell carcinoma. Oncotarget. 2017;8:19013-19020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kato Y, Kaneko MK. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep. 2014;4:5924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Kato Y, Kaneko MK, Kuno A, Uchiyama N, Amano K, Chiba Y, Hasegawa Y, Hirabayashi J, Narimatsu H, Mishima K, Osawa M. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem Biophys Res Commun. 2006;349:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Nakazawa Y, Takagi S, Sato S, Oh-hara T, Koike S, Takami M, Arai H, Fujita N. Prevention of hematogenous metastasis by neutralizing mice and its chimeric anti-Aggrus/podoplanin antibodies. Cancer Sci. 2011;102:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Li JC, Li Y, Ai JY, Chen K, Zhu YH, Fu L, Qin YR, Wang LJ, Guan XY. Podoplanin‑positive cancer cells at the edge of esophageal squamous cell carcinomas are involved in invasion. Mol Med Rep. 2014;10:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546-3556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 515] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 33. | Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 34. | Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 35. | Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 36. | Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res. 2012;129 Suppl 1:S30-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Suzuki-Inoue K. CLEC-2/podoplanin and thromboinflammation. Blood. 2017;129:1896-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Takemoto A, Miyata K, Fujita N. Platelet-activating factor podoplanin: from discovery to drug development. Cancer Metastasis Rev. 2017;36:225-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Ogasawara S, Kaneko MK, Honma R, Oki H, Fujii Y, Takagi M, Suzuki H, Kato Y. Establishment of Mouse Monoclonal Antibody LpMab-13 Against Human Podoplanin. Monoclon Antib Immunodiagn Immunother. 2016;35:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Yamada S, Ogasawara S, Kaneko MK, Kato Y. LpMab-23: A Cancer-Specific Monoclonal Antibody Against Human Podoplanin. Monoclon Antib Immunodiagn Immunother. 2017;36:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Suchanski J, Tejchman A, Zacharski M, Piotrowska A, Grzegrzolka J, Chodaczek G, Nowinska K, Rys J, Dziegiel P, Kieda C, Ugorski M. Podoplanin increases the migration of human fibroblasts and affects the endothelial cell network formation: A possible role for cancer-associated fibroblasts in breast cancer progression. PLoS One. 2017;12:e0184970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Cueni LN, Hegyi I, Shin JW, Albinger-Hegyi A, Gruber S, Kunstfeld R, Moch H, Detmar M. Tumor lymphangiogenesis and metastasis to lymph nodes induced by cancer cell expression of podoplanin. Am J Pathol. 2010;177:1004-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Huber GF, Fritzsche FR, Züllig L, Storz M, Graf N, Haerle SK, Jochum W, Stoeckli SJ, Moch H. Podoplanin expression correlates with sentinel lymph node metastasis in early squamous cell carcinomas of the oral cavity and oropharynx. Int J Cancer. 2011;129:1404-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Tong L, Yuan S, Feng F, Zhang H. Role of podoplanin expression in esophageal squamous cell carcinoma: a retrospective study. Dis Esophagus. 2012;25:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Khuu C, Sehic A, Eide L, Osmundsen H. Anti-proliferative Properties of miR-20b and miR-363 from the miR-106a-363 Cluster on Human Carcinoma Cells. Microrna. 2016;5:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Chuang WY, Yeh CJ, Chao YK, Liu YH, Chang YS, Tseng CK, Chang HK, Wan YL, Hsueh C. Concordant podoplanin expression in cancer-associated fibroblasts and tumor cells is an adverse prognostic factor in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:4847-4856. [PubMed] |
| 47. | Astarita JL, Acton SE, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 288] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 48. | Yu J, Li N, Wang X, Ren H, Wang W, Wang S, Song Y, Liu Y, Li Y, Zhou X, Luo A, Liu Z, Jin J. Circulating serum microRNA-345 correlates with unfavorable pathological response to preoperative chemoradiotherapy in locally advanced rectal cancer. Oncotarget. 2016;7:64233-64243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Song B, Yan J, Liu C, Zhou H, Zheng Y. Tumor Suppressor Role of miR-363-3p in Gastric Cancer. Med Sci Monit. 2015;21:4074-4080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |