Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.2902
Peer-review started: January 24, 2020
First decision: February 27, 2020
Revised: March 27, 2020
Accepted: May 28, 2020
Article in press: May 28, 2020
Published online: June 14, 2020
Processing time: 141 Days and 20.7 Hours
With over 100000 hospital admissions per annum, acute pancreatitis remains the leading gastrointestinal cause of hospitalization in the United States and has far-reaching impact well beyond. It has become increasingly recognized that drug-induced pancreatitis (DIP), despite accounting for less than 3% of all cases, represents an important and growing though often inconspicuous cause of acute pancreatitis. Nevertheless, knowledge of DIP is often curtailed by the limited availability of evidence needed to implicate given agents, especially for non-prescription medications. Indeed, the majority of available data is derived from case reports, case series, or case control studies. Furthermore, the mechanism of injury and causality for many of these drugs remain elusive as a definitive correlation is generally not established (< 10% of cases). Several classification systems have been proposed, but no single system has been widely adopted, and periodic updates are required in light of ongoing pharmacologic expansion. Moreover, infrequently prescribed medications or those available over-the-counter (including herbal and other alternative remedies) are often overlooked as a potential culprit of acute pancreatitis. Herein, we review the ever-increasing diversity of DIP and the potential mechanisms of injury with the goal of raising awareness regarding the nature and magnitude of this entity. We believe this manuscript will aid in increasing both primary and secondary prevention of DIP, thus ultimately facilitating more expedient diagnosis and a decrease in DIP-related morbidity.
Core tip: Despite living in an era of pharmacologic expansion, our knowledge of drug-induced pancreatitis (DIP) is often curtailed by evidence needed to implicate particular medications. Several causative agent classification systems (with medication lists) have been reported and their mechanisms proposed. Nonetheless, they require regular updates and a complete review of this topic is warranted. In addition, infrequently prescribed medications or those available over-the-counter are often omitted from those summarized lists. We review the ever-increasing diversity of DIP and their potential mechanisms of injury to aid in increasing both primary and secondary prevention of DIP.
- Citation: Weissman S, Aziz M, Perumpail RB, Mehta TI, Patel R, Tabibian JH. Ever-increasing diversity of drug-induced pancreatitis. World J Gastroenterol 2020; 26(22): 2902-2915
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/2902.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.2902
Acute pancreatitis is an acute, inflammatory, potentially life-threatening condition of the pancreas. With over 100000 hospital admissions per annum, acute pancreatitis is the leading gastrointestinal cause of hospitalization in the United States and the 10th most common non-malignant cause of death among all gastrointestinal, pancreatic, and liver diseases[1-3]. It is a major cause of morbidity and healthcare expenditure not only in the United States, but worldwide. There are numerous established etiologies of acute pancreatitis, among which gallstones and alcohol are the most common (40%-70% and 25%-35%, respectively)[4]. The remaining cases are primarily attributable to the following etiologic factors: Hypertriglyceridemia, autoimmune, infection, hyper/hypocalcemia, malignancy, genetics, endoscopic retrograde cholan-giopancreatography, and trauma. Despite accounting for approximately only 1%-2% of cases overall, drug-induced pancreatitis (DIP) has become increasingly recognized as an additional and vitally important, albeit often inconspicuous, etiology of acute pancreatitis[5,6].
The World Health Organization database lists 525 different medications associated with acute pancreatitis (i.e. DIP)[7]. Many of these medications are widely used to treat highly prevalent medical conditions. Unfortunately, few population-based studies on the true incidence of DIP exist, limiting knowledge of true incidence and prevalence. In this setting, we review the ever-increasing diversity of DIP, with emphasis on the wide range of drug classes reported and their respective pathophysiologic mechanisms - in an attempt to raise awareness of the true and underestimated prevalence of DIP. We hope this manuscript will aid in increasing secondary prevention of DIP ultimately leading to a decrease in overall acute pancreatitis-related hospitalizations and economic burden on the health care system.
As there is no standardized approach to stratifying patients to determine their risk of developing acute pancreatitis, primary prevention for the majority of etiologies cannot be fully implemented. Secondary prevention of acute pancreatitis, on the other hand, can more easily be executed. For example, abstinence from alcohol reduces the risk of alcoholic pancreatitis, cholecystectomy reduces the risk of gallstone pancreatitis, and tight control of triglycerides reduces the risk of recurrent episodes of pancreatitis secondary to hypertriglyceridemia. On this notion, unique to DIP, is the fact that it can be prevented in both the primary and secondary fashion. Unfortunately, however, most of the available data in reference to DIP is derived from case reports, case series, or case control studies. In this vein, the causality between specific medications and acute pancreatitis has been established in only a minority of cases (< 10%)[7]. In addition, oftentimes, lack of a known etiology for acute pancreatitis directly increases length of hospitalization due to delayed diagnosis and subsequent treatment[8,9]. Moreover, patients unaware of an adverse drug reaction to a prior medication may continue taking that medication leading to repeat hospitalizations[8,9]. Finally, with the rapid expansion of pharmacologic agents, widespread legalization of cannabis, increase in recognized medications, supplements, and alternative medications reported to induce pancreatitis, the need to become familiar with this esoteric group remains imperative, and knowledge in the form of awareness regarding certain medications is warranted[10-12].
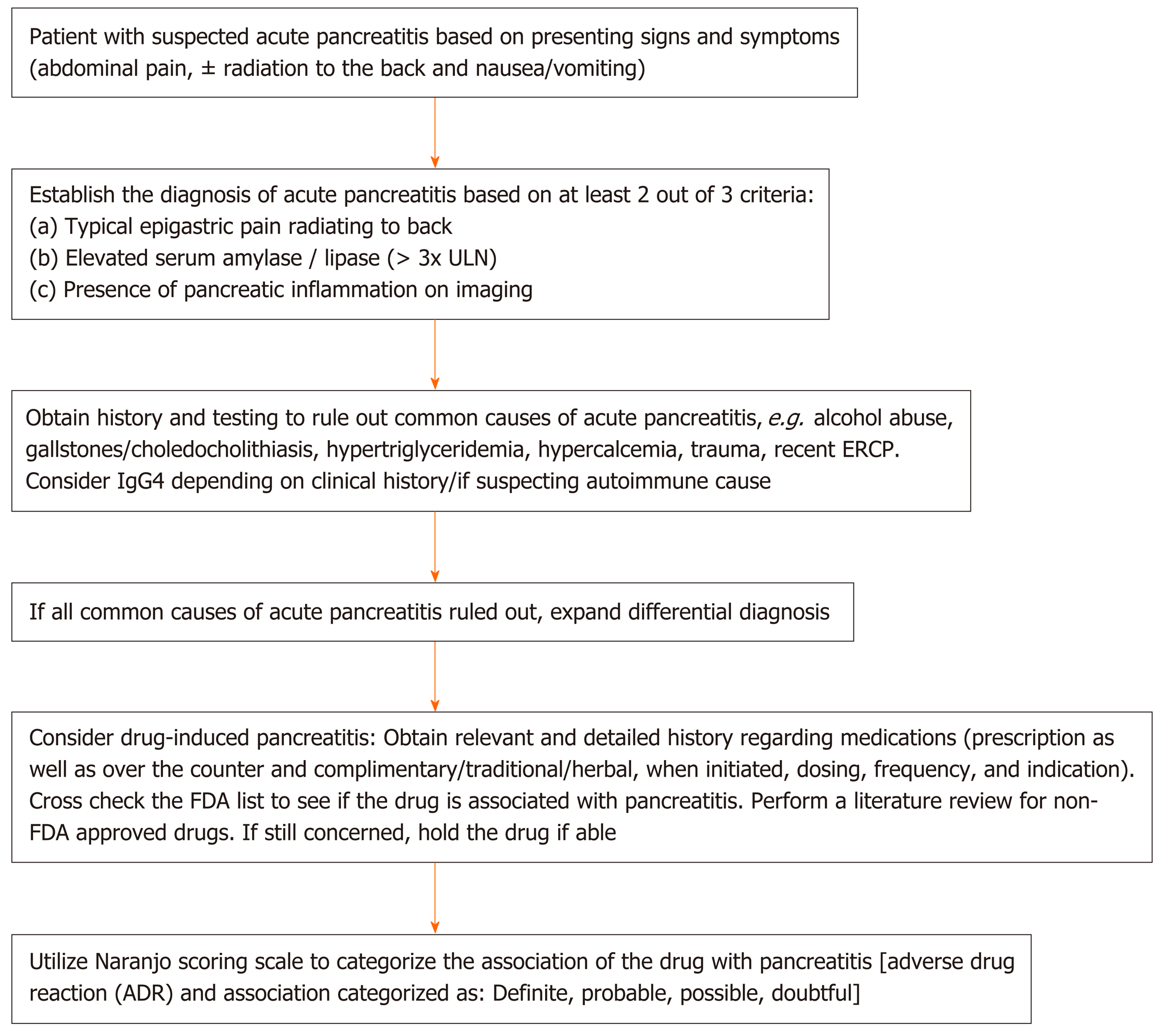
Numerous factors limit the ability of clinicians to causally link acute pancreatitis with medications. First, the lack of mandatory adverse drug reporting systems allow many cases to go unreported[6]. Second, bias exists, in the sense that clinicians tend to forgo linking unusual medication suspects to a rare adverse event[6]. Third, it is often difficult to rule out other, more common, causes of drug-induced pancreatitis, especially in patients who have multiple comorbidities and underlying risk factors[6]. Fourth, many cases lack a re-challenge test or drug latency period to definitively link acute pancreatitis to a particular drug[13]. Finally, evidence is lacking to support the use of any serial monitoring technique - namely, imaging or pancreatic enzymes to help detect cases of drug-induced pancreatitis[14]. Despite these limitations, as illustrated in Figure 1, following a thorough algorithm can aid in detecting cases of drug-induced pancreatitis that would otherwise have been difficult to diagnose (Figure 1).
In accordance with the aforementioned limitations, evidence implicating numerous medications is inconsistent and, at times, even contradictory. Hence, although not uniform, nor universally accepted, official tier systems exist to help quantify the likelihood of a drug to be established as a culprit of acute pancreatitis. The earliest classification system was developed in 1980 and was designed to include three classes; Class I: Included drugs that were implicated to induce pancreatitis in a minimum of 20 cases of which at least one case documented drug re-exposure, Class II: Included drugs that were implicated to induce pancreatitis in 10-20 cases with or without documented drug re-exposure, and Class III: Included all drugs implicated in pancreatitis[15] (Table 1). Trivedi et al[16] reviewed the top 100 prescription medications in the United States for their association with acute pancreatitis using this three-tier classification system. They noted that, of the top 100 most frequently prescribed medications, 44 were Class III pancreatitis medications. Additionally, 14 of these medications were Class I or II[16].
| Class I | Class II | Class III |
| ≥ 1 case documenting a positive re-challenge | With or without drug re-challenge | All drugs associated with drug induced pancreatitis |
| ≥ 20 case reports | 10-20 case reports | < 10 case reports |
The most recent classification system was developed by Badalov et al[13], in which the authors categorized implicated drugs into four classes (Table 2). Class I drugs are medications in which a re-challenge was established in at least one case report. This class is further divided into whether other causes of acute pancreatitis were ruled out (Ia) or not (Ib). Class II drugs are medications in which there is a latency period in 75% of at least four reported cases, all with no evidence of re-challenge. Class III drugs are medications that neither a re-challenge nor a consistent latency period was established but had two or more case reports published. Class IV drugs are medications that neither a re-challenge nor a consistent latency period was established, and only 1 case report had been published[13].
| Minimum No. of case reports | Re-challenge required | Latency established | Alternative causes of pancreatitis excluded | |
| Class Ia | 1 | Yes | N/A | Yes |
| Class Ib | 1 | Yes | N/A | No |
| Class II | 4 | No | Yes1 | N/A |
| Class III | 2 | No | No | N/A |
| Class IV | 1 | No | No | N/A |
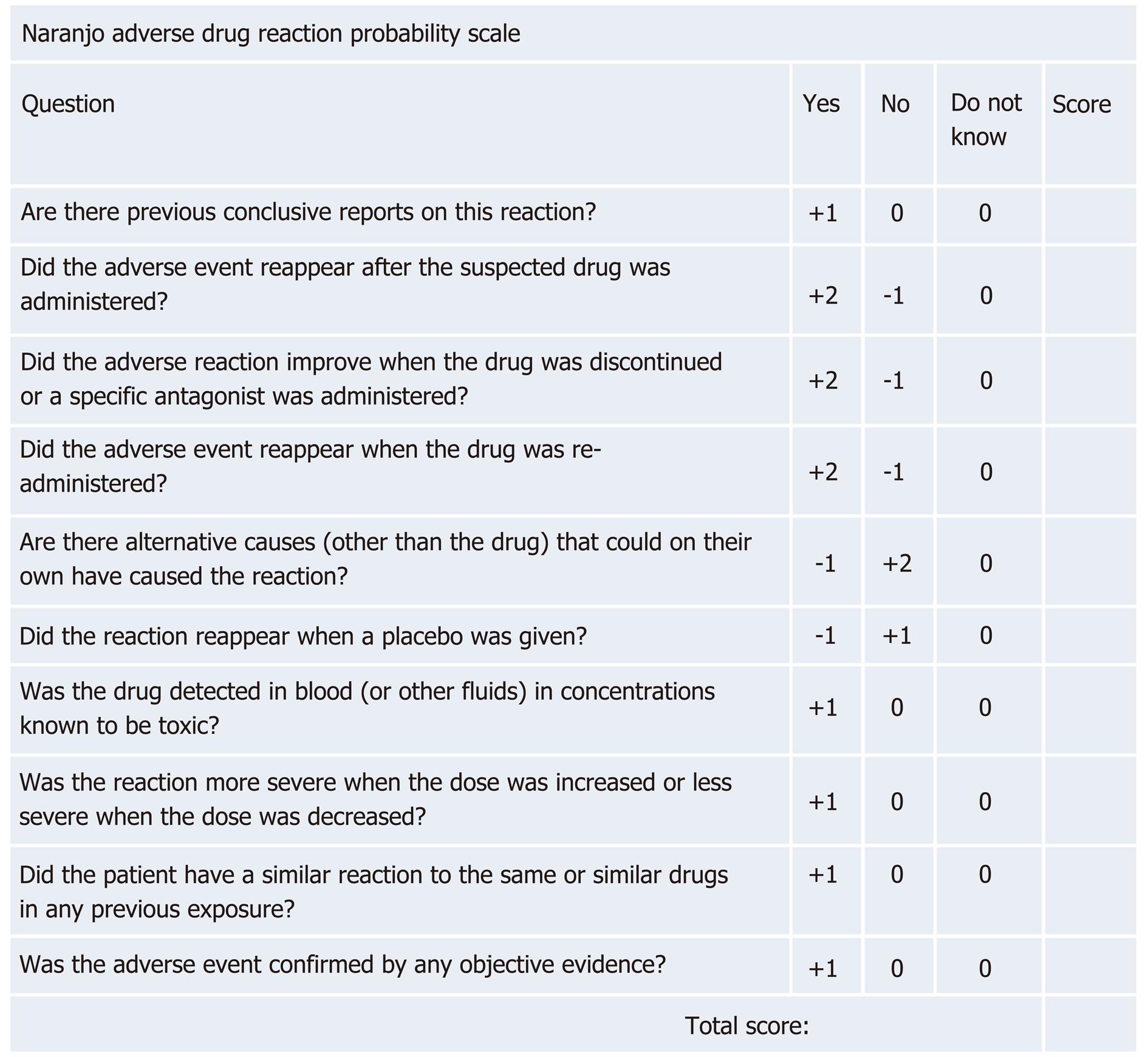
Additionally, the Naranjo adverse drug reaction probability scale can be helpful in establishing the degree of association between a drug and an adverse reaction[17]. This tool determines the likelihood of an adverse drug reaction based on the cumulative score on 10 questions. A score of < 1 signifies a doubtful drug reaction, 1-4 a possible drug reaction, 5-8 a probable drug reaction, and > 9 a definitive drug reaction (Figure 2).
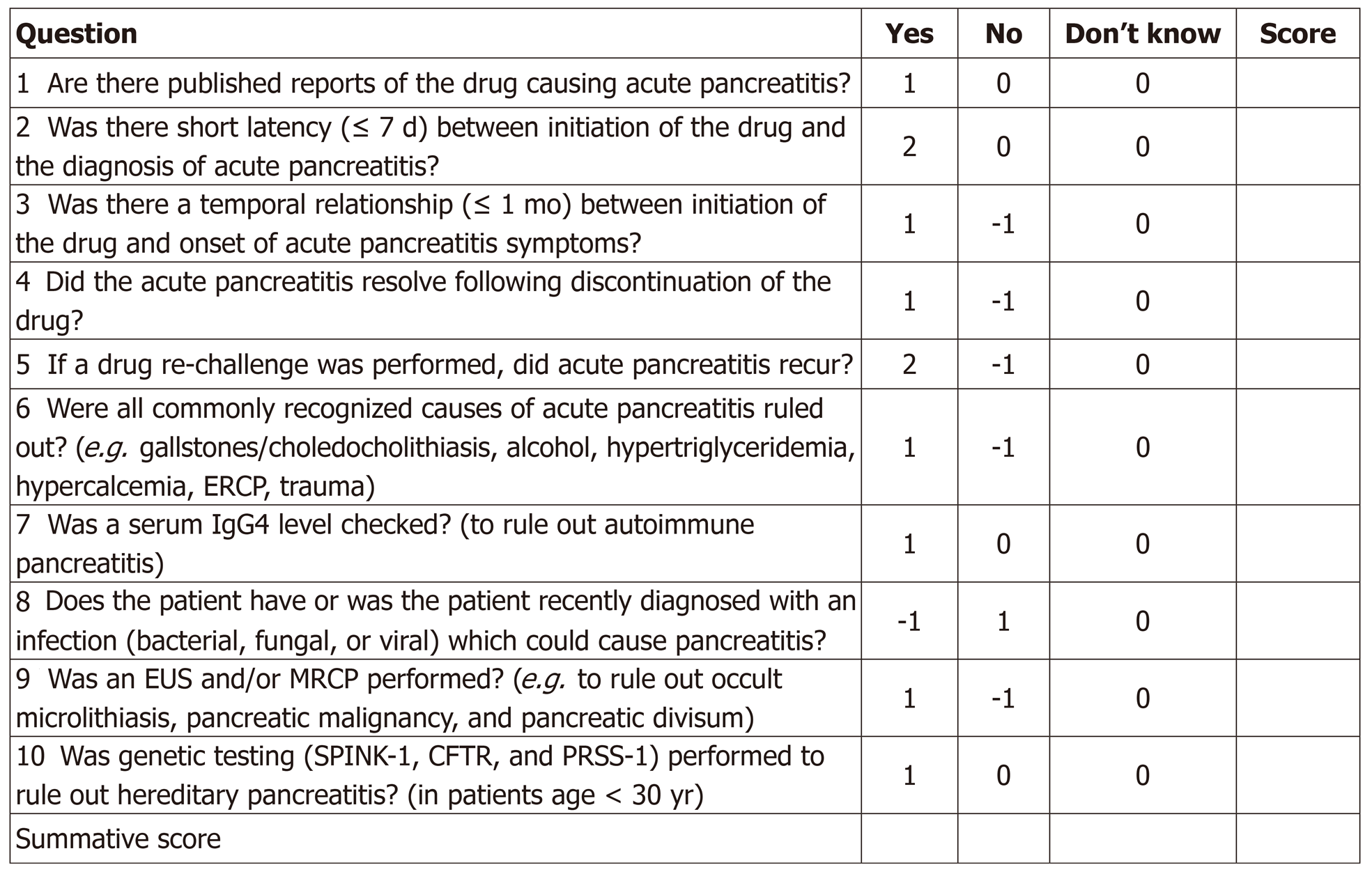
Finally, and most recently, our proposed specific drug-induced pancreatitis probability scale (modified from the Naranjo scale to be more pancreatitis-specific) can serve as a standardized tool for determining the likelihood of drug-induced pancreatitis based on the aggregate score from a series of 10 questions. A score of < 2 suggests doubtful DIP, 3-5 possible DIP, 6-8 probable DIP, and > 9 highly probable DIP (Figure 3). We believe this tool, in particular, enhances one’s ability to accurately identify and implicate potential acute pancreatitis-causing drugs.
While consensus has yet to be reached regarding the cause of drug-induced pancreatitis in many cases, numerous potential mechanisms have been speculated. These include, pancreatic/biliary duct constriction, cytotoxic effects, metabolic effects, accumulation of a toxic metabolite or intermediary, and idiosyncratic and/or hypersensitivity reaction, with idiosyncratic response or direct toxic effect likely accounting for the majority of cases[18,19] (Figure 4).
Studies concerning the incidence of drug-induced pancreatitis have established a range of 0.3% to 1.4% of all acute pancreatitis cases being due to drugs[5,20-22]. Certain medications such as azathioprine/mercaptopurine and didanosine are well-known culprits of drug-induced pancreatitis with incidences of 5% and 23% respectively[23]. As illustrated in Table 3, compiling a list of drug classes implicated in pancreatitis may yield clinical use owning to increased clinician awareness of other medications in these classes.
| Class I | Class II | Class III |
| Aminosalicylates | Alkylating antineoplastics | Aminosalicylates |
| Anticonvulsants | Angiotensin-converting enzyme inhibitors | Antacids |
| Antimetabolite antineoplastics | Anticonvulsants | Antiarrhythmics |
| Antimicrobials | Antimicrobials | Antibacterials |
| Hormone replacement therapies | Antitubercular agents | Anticholinesterases |
| Loop diuretics | Interferons | Anticonvulsants |
| Non-biologic immunosuppressives | Nonopioid analgesics | Antidepressants |
| Nonsteroidal anti-inflammatories | Reverse transcriptase inhibitors | Antifungals |
| Opiates | Somatostatin analogs | Antihypertensives |
| Reverse transcriptase inhibitors | Thiazides | Antimetabolite antineoplastics |
| Steroids | Antineoplastics | |
| Antiplatelets | ||
| Antivirals | ||
| Atypical antipsychotics | ||
| Cholesterol lowering agents | ||
| Cyclooxygenase II inhibitors | ||
| Estrogens | ||
| Immunomodulators | ||
| Nonsteroidal anti-inflammatories | ||
| Parasympathetic agents | ||
| Proton pump inhibitors | ||
| Selective serotonin agonists | ||
| Somatostatin analogs | ||
| Steroids | ||
| TNF-alpha inhibitors | ||
| Vitamins |
Among the many drugs that have been associated with pancreatitis, statins have been increasingly reported as a cause of acute pancreatitis[19,24]. In fact, as numerous members of this class (atorvastatin, fluvastatin, rosuvastatin, simvastatin, and pravastatin) have been implicated in acute pancreatitis, statin-induced pancreatitis may indeed be a class-effect[19,24]. Mechanisms of action of statin-induced acute pancreatitis are associated with rhabdomyolytic and cytochrome P-450 interactions leading to an immune-mediated inflammatory response, direct cellular toxicity, or perhaps a metabolic effect[25]. As with many other drugs, its true prevalence in acute pancreatitis remains unknown, as the onset of statin-induced pancreatitis has been observed from hours to years after treatment[25]. Interestingly, the degree of P-450 CYPA4 inhibition correlates with individual statin safety profiles[26].
Although rare, several 5-aminosalicylic acid (5-ASA)-induced acute pancreatitis cases have been published in the literature. Interestingly, both oral and enema mesalamine preparations have been implicated in causing pancreatitis within days[27,28]. In addition, sulfasalazine has been implicated in inducing pancreatitis perhaps through an immune-mediated mechanism[29]. In general, however, a hypersensitivity mechanism seems to be involved and pancreatitis can occur from days to years after starting mesalamine therapy[27,29].
Metronidazole has been reported in association with acute pancreatitis, although the mechanism is not fully known[30,31]. Free-radical production, immune-mediated, direct toxic affect, and metabolic effects have been suggested as possible pathophysiological mechanisms[30,31]. Notably, a study showed that patients receiving metronidazole as part of Helicobacter pylori triple-therapy have an approximate eight-fold increased risk of acute pancreatitis[32]. The tetracycline class (tetracycline, minocycline, and oxytetracycline) has also been associated with acute pancreatitis, with the mechanism believed to be a direct toxic-effect, or hypersensitivity reaction[33-36]. In addition, numerous cases of erythromycin-induced pancreatitis have been reported to date[37,38]. Although less established, other antibiotics such as ampicillin, ceftriaxone, clarithromycin, trimethoprim-sulfamethoxazole, and nitrofurantoin have been implicated in pancreatitis as well[39-43].
Numerous steroids (dexamethasone, prednisone, prednisolone, cortisone acetate, and adrenocorticotropic hormone) have been associated with inducing acute pancreatitis nearly all with a short latency period[44-47]. As a large proportion of these cases resulted in death, it has been suggested that this drug class may be linked to a more severe disease course[13,46]. The most common non-steroidal anti-inflammatory drugs (NSAIDs) that have been reported to cause pancreatitis are sulindac and salicylates, with latency ranging from weeks to years, however others have been implicated as well[48-55]. A clear limitation that exists is the fact that NSAIDs may be initiated in response to early symptoms of unrecognized pancreatitis leading to erroneously attributing the pancreatitis to this class of medication[56,57]. Interestingly, naproxen has been recommended as the preferred analgesic in this scenario owning to its limited risk of inducing acute pancreatitis[58]. The mechanism being, a structural (compression or obstruction) effect on the sphincter of Oddi leading to acute pancreatitis. Of note, both diclofenac and indomethacin may significantly reduce the risk of acute pancreatitis post-endoscopic retrograde cholangiopancreatography[59,60].
Immunotherapy agents have long been associated with acute pancreatitis, however their increased use in recent decades has led to a concomitant increase in im-munotherapy-associated pancreatitis. Interleukin-2 immunotherapy-associated pancreatitis in particular has been reported[61]. The mechanism of injury is believed to be either immune-mediated or a direct drug toxicity. Newer programmed cell death protein 1 blockers (i.e. nivolumab) and anti-cytotoxic T-lymphocyte-associated protein 4 agents (i.e. ipilimumab) have been associated with acute-pancreatitis as well[62,63]. The exact mechanism is currently unknown, but it is speculated to be associated with T-lymphocyte mediated inflammation[62,63].
There have been many well-documented case reports of acute pancreatitis due to Angiotensin-converting-enzyme inhibitors (ACE-Is)[64-70]. One case-control study suggested a dose-dependent correlation with an odds ratio of 1.5[71]. While enalapril has been the most extensively reported culprit in this class[64,69], other agents (such as lisinopril, captopril, ramipril, benazepril, quinapril, and perindopril) have been described in the literature as well[65-68,70-72]. Similar to ACE-Is, angiotensin receptor blockers (such as telmisartan and losartan) has also been implicated in acute pancreatitis[73,74]. Interestingly, the latency period between ACE-I initiation and an associated pancreatitis event may range from days to years and may be associated with severe disease[64-66,69]. The proposed mechanism involved is due to decreased bradykinin degradation, increasing pancreatic vascularity and edema, and pancreatic enzyme trapping causing local tissue damage secondary to pancreatic duct obstruction[75].
Although proven to be relatively safe for the management of type 2 diabetes mellitus, numerous classes of oral anti-glycemic agents including biguanides (metformin)[76], dipeptidyl peptidase-4 inhibitors[77-79], glucagon-like peptide-1 (GLP-1) analogues[80-86], and sodium glucose co-transporter-2 inhibitors[87,88] have been associated with acute pancreatitis. The highest incidence is reported with GLP-1 analogues[81,83-85], of which exenatide has the highest association with an up to 6-fold increase in the rate noted in post-marketing surveillance[80,82,86]. The proposed mechanism is pancreatic acinar cell hypertrophy, the subsequent release of proinflammatory cytokines, leading to increased vascular permeability, resulting in pancreatic inflammation[89]. Another hypothesis suggests an obstructive type phenomenon, as an increased incidence of gallstones with the use of GLP-1 analogues has been recorded[90].
Marijuana (cannabis) is the most common illicit drug globally with over 4% of the population using it per annum[91,92]. As such, many case reports suggesting the association between cannabis and pancreatitis have been published in the literature, and may even suggest a dose-dependent phenomenon[91-95]. While cannabinoid receptors are found in the islet of Langerhans cells, the exact pathophysiology of cannabis-induced pancreatitis is currently not well-understood[93-95]. Cocaine-induced pancreatitis has also been reported, and is believed to be due to splanchnic vasoconstriction and thrombotic microangiopathy leading to ductal obstruction[96-98]. Similarly, codeine has also been described in the literature with regards to inducing acute pancreatitis with a mechanism believed to relate to dysfunctional sphincter of Oddi contraction as well[99-101].
The highly active anti-retroviral therapy therapy drugs have long thought to be associated with the development of acute pancreatitis[102,103]. The most common offenders include nucleoside reverse transcriptase inhibitors (NRTIs), non-NRTIs (NNRTIs), and protease inhibitors (PIs)[104]. In a large retrospective cohort study including nearly 5000 patients who received antiretroviral therapy, 3.2% developed AP[103]. Furthermore, 5.2% of patients who received didanosine, 4.2% of patients who received a PI plus either an NRTI or a NNRTI, and 3.5% of patients who received NRTIs combined with NNRTIs—developed acute pancreatitis[25,103]. The exact mechanism by which NRTIs and NNRTIs cause pancreatitis is unidentified, but it is thought to be related to direct drug toxicity or ductal obstruction leading to mitochondrial damage resulting in cellular death and organ damage[18]. PIs are directly related to induction of hypertriglyceridemia which is a well-established cause of pancreatitis in the literature[105,106].
Diuretics [e.g. furosemide, chlorothiazide, hydrochlorothiazide (HCTZ), and others] have long been implicated in the development of acute pancreatitis, with the majority of cases suggesting a short latency period and a more mild disease course[107-113]. Notably, HCTZ has well-established side effects of causing hypercalcemia and hyperlipidemia, both of which are well known to lead to acute pancreatitis[25,114]. HCTZ can further cause hyperparathyroidism, which can also lead to hypercalcemia-induced pancreatitis[25]. It has been postulated that furosemide affects the pancreas by causing a hyper-stimulation of secretions leading to a direct toxic injury and/or ischemia[25,114].
Numerous cases have been reported in which estrogen-containing products were thought to induce acute pancreatitis[115-119]. Hypercoagulability and hyper-triglyceridemia have been speculated as the main cause of inducing pancreatitis in patients taking hormone replacement therapy and oral contraceptives[115-119]. Nonetheless, patients with existing hypertriglyceridemia and familial hy-perlipoproteinemia can have an exacerbation of their underlying condition leading to pancreatitis[18,25,115,120,121].
Although both H2-blockers and proton-pump inhibitors have been reported in the literature to cause acute pancreatitis, the evidence regarding this relationship is controversial[122]. A retrospective study conducted by Eland et al[56] failed to identify any association of pancreatitis with the use of ranitidine (RR: 1.3; 95%CI: 0.4-4.1), cimetidine (RR: 2.1; 95%CI: 0.6-7.2), and/or omeprazole (RR: 1.1; 95%CI: 0.3-4.6). Case reports in the literature have generally linked pancreatitis in these cases to excessive consumption of antacids which likely was directly related to hypercalcemia[123,124].
Many cases have linked antidepressants (e.g. mirtazapine and sertraline) to acute pancreatitis[125-128]; nonetheless, a population-based study by Nørgaard et al[129] failed to demonstrate a significant association between selective serotonin reuptake inhibitors (SSRIs) and acute pancreatitis. Only a mild increase in the risk of pancreatitis was seen with first-time users of SSRI (aOR: 2.8, 95%CI: 1.1-7.0); however, the results are limited due to confounding variables[129]. A recent meta-analysis demonstrated a significant association between SSRIs and acute pancreatitis (aOR: 1.26, 95%CI: 1.13-1.40)[130]. The risk was much higher in the first 2 weeks of following initiation of SSRIs[130]. The exact mechanism by which SSRIs can lead to pancreatitis is unknown, though it is speculated that SSRIs can cause cellular apoptosis, insulin secretion inhibition, and further development of diabetes and chronic pancreatitis as well[130]. Roberge et al[131] additionally reported a case of a patient who developed acute pancreatitis due to an acute overdose of clomipramine.
Numerous anti-seizure medications (clozapine, olanzapine, and valproic acid) have been associated with inducing pancreatitis, especially in the pediatric population[132-142]. Interestingly, this class seems to be associated with a more severe disease course that may result in pancreatic necrosis and death[136,138,142]. The mechanism has been postulated to involve a direct toxic effect on pancreatic cells causing depletion of superoxide dismutase, catalase, and glutathione peroxidase on a biochemical level[25,136,142].
To our knowledge, two cases of vitamin-induced acute pancreatitis have been reported, both involving vitamin D. One involved oral vitamin D, wherein the injury was seemingly related to the hypercalcemic effect of vitamin D[143]. The other case involved tacalcitol (a vitamin D-analog) ointment as the inciting agent[144]. In addition, we recently encountered a second (suspected) case of oral vitamin D-induced acute pancreatitis (unpublished data), which we are currently examining.
Although seldom in nature, several herbal medications have been reported the in literature as being associated with DIP. These including: Sambucol (black elderberry extract), “Immune factors” [combination of Echinacea, Goldenseal (Hydrastis Canadensis), and Shiitake, Maitake, and Reishi mushrooms], saw palmetto (Serenoa repens), and mangosteen (Garcinia cambogia)[145-148]. The mechanism of injury underlying these rare cases is unclear[145,147,148]. Some reports, however, believe these cases to be due to an induced hypercoagulable state via estrogen receptor activation[6].
In the setting of an ever-increasing armamentarium of pharmacological agents, drug-induced adverse effects including acute pancreatitis are increasingly encountered. DIP is a difficult diagnosis to establish and is thus likely underreported, owing in part to its often unsuspected nature as well as the technical difficulty in causally linking a drug to acute pancreatitis. Criteria for definite DIP are many and generally include requiring that the drug cause acute pancreatitis during or predictably after initiating treatment with the drug, resolution of pancreatitis upon discontinuation of the drug, and reoccurrence of pancreatitis upon re-administration of the drug, granted that other likely causes of acute pancreatitis have been ruled out. With these caveats in mind, the current list of drugs associated with DIP is by no means complete nor fully understood, and further research is needed.
As cases of DIP are associated with higher morbidity, extended hospital stays, and increased healthcare costs, in large part due to delays in diagnosis, patients presenting with pancreatitis of unknown etiology should be carefully questioned regarding drugs that could be linked to DIP[8,9]. Notably, as new medications with known severe side effects are usually more closely monitored, drugs which are infrequently prescribed, or considered relatively harmless, such as over-the-counter medications and herbal supplements, may remain illusory and inadequately considered in this context. Hence, following a streamlined diagnostic approach for cases of possible DIP (Figure 1-3) and expeditiously identifying early-on the responsible agent are critical.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hauser G, Lei JJ, Rodrigo L S-Editor: Wang JL L-Editor: A E-Editor: Zhang YL
| 1. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1079] [Article Influence: 179.8] [Reference Citation Analysis (1)] |
| 2. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1384] [Article Influence: 115.3] [Reference Citation Analysis (3)] |
| 3. | Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA. Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol. 2007;17:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Lankisch PG, Dröge M, Gottesleben F. Drug induced acute pancreatitis: incidence and severity. Gut. 1995;37:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 161] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 6. | Hung WY, Abreu Lanfranco O. Contemporary review of drug-induced pancreatitis: A different perspective. World J Gastrointest Pathophysiol. 2014;5:405-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 7. | Nitsche C, Maertin S, Scheiber J, Ritter CA, Lerch MM, Mayerle J. Drug-induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 8. | Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Hirota M, Kimura Y, Takeda K, Isaji S, Koizumi M, Otsuki M, Matsuno S; JPN. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Wong SW, Lin HC. Medical marijuana legalization and associated illicit drug use and prescription medication misuse among adolescents in the U.S. Addict Behav. 2019;90:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Sansgiry SS, Bhansali AH, Bapat SS, Xu Q. Abuse of over-the-counter medicines: a pharmacist's perspective. Integr Pharm Res Pract. 2017;6:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Donnelly J, Young M. The Legalization of Medical/Recreational Marijuana: Implications for School Health Drug Education Programs. J Sch Health. 2018;88:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648-61; quiz 644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 366] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Wolfe D, Kanji S, Yazdi F, Skidmore B, Moher D, Hutton B. Methods for the early detection of drug-induced pancreatitis: a systematic review of the literature. BMJ Open. 2019;9:e027451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Mallory A, Kern F. Drug-induced pancreatitis: a critical review. Gastroenterology. 1980;78:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 232] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8202] [Article Influence: 186.4] [Reference Citation Analysis (0)] |
| 18. | Underwood TW, Frye CB. Drug-induced pancreatitis. Clin Pharm. 1993;12:440-448. [PubMed] |
| 19. | Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-induced acute pancreatitis: a review. Ochsner J. 2015;15:45-51. [PubMed] |
| 20. | Rünzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Werth B, Kuhn M, Hartmann K, Reinhart WH. [Drug-induced pancreatitis: experience of the Swiss Drug Adverse Effects Center (SANZ) 1981-1993]. Schweiz Med Wochenschr. 1995;125:731-734. [PubMed] |
| 22. | Haber CJ, Meltzer SJ, Present DH, Korelitz BI. Nature and course of pancreatitis caused by 6-mercaptopurine in the treatment of inflammatory bowel disease. Gastroenterology. 1986;91:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Maxson CJ, Greenfield SM, Turner JL. Acute pancreatitis as a common complication of 2',3'-dideoxyinosine therapy in the acquired immunodeficiency syndrome. Am J Gastroenterol. 1992;87:708-713. [PubMed] |
| 24. | Etienne D, Reda Y. Statins and their role in acute pancreatitis: Case report and literature review. World J Gastrointest Pharmacol Ther. 2014;5:191-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kaurich T. Drug-induced acute pancreatitis. Proc (Bayl Univ Med Cent). 2008;21:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Singh S, Loke YK. Statins and pancreatitis: a systematic review of observational studies and spontaneous case reports. Drug Saf. 2006;29:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Fiorentini MT, Fracchia M, Galatola G, Barlotta A, de la Pierre M. Acute pancreatitis during oral 5-aminosalicylic acid therapy. Dig Dis Sci. 1990;35:1180-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Isaacs KL, Murphy D. Pancreatitis after rectal administration of 5-aminosalicylic acid. J Clin Gastroenterol. 1990;12:198-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Fernández J, Sala M, Panés J, Feu F, Navarro S, Terés J. Acute pancreatitis after long-term 5-aminosalicylic acid therapy. Am J Gastroenterol. 1997;92:2302-2303. [PubMed] |
| 30. | Bellocchi MC, Campagnola P, Frulloni L. Drug-induced acute pancreatitis. Pancreatitis. 2016;217. |
| 31. | Sura ME, Heinrich KA, Suseno M. Metronidazole-associated pancreatitis. Ann Pharmacother. 2000;34:1152-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Nørgaard M, Ratanajamit C, Jacobsen J, Skriver MV, Pedersen L, Sørensen HT. Metronidazole and risk of acute pancreatitis: a population-based case-control study. Aliment Pharmacol Ther. 2005;21:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Nicolau DP, Mengedoht DE, Kline JJ. Tetracycline-induced pancreatitis. Am J Gastroenterol. 1991;86:1669-1671. [PubMed] |
| 34. | Elmore MF, Rogge JD. Tetracycline-induced pancreatitis. Gastroenterology. 1981;81:1134-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Kunelis CT, Peters JL, Edmondson HA. Fatty liver of pregnancy and its relationship to tetracycline therapy. Am J Med. 1965;38:359-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 108] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Steinberg WM. Acute drug and toxin induced pancreatitis. Hosp Pract (Off Ed). 1985;20:95-102. [PubMed] [DOI] [Full Text] |
| 37. | Hawksworth CR. Acute pancreatitis associated with infusion of erythromycin lactobionate. BMJ. 1989;298:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Tenenbein MS, Tenenbein M. Acute pancreatitis due to erythromycin overdose. Pediatr Emerg Care. 2005;21:675-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Antonow DR. Acute pancreatitis associated with trimethoprim-sulfamethoxazole. Ann Intern Med. 1986;104:363-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Hanline MH. Acute pancreatitis caused by ampicillin. South Med J. 1987;80:1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Nelis G. Nitrofurantoin-induced Pancreatitis: Report of a Case. Gastroenterology. 1983;84:1032-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Zimmermann AE, Katona BG, Jodhka JS, Williams RB. Ceftriaxone-induced acute pancreatitis. Ann Pharmacother. 1993;27:36-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Liviu L, Yair L, Yehuda S. Pancreatitis induced by clarithromycin. Ann Intern Med. 1996;125:701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 44. | Levine RA, McGuire RF. Corticosteroid-induced pancreatitis: a case report demonstrating recurrence with rechallenge. Am J Gastroenterol. 1988;83:1161-1164. [PubMed] |
| 45. | Nelp WB. Acute pancreatitis associated with steroid therapy. Arch Intern Med. 1961;108:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Sash L. Relationship of cortisone therapy to pancreatic necrosis. Br Med J. 1959;2:867-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Steinberg WM, Lewis JH. Steroid-induced pancreatitis: does it really exist? Gastroenterology. 1981;81:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Amaravadi RK, Jacobson BC, Solomon DH, Fischer MA. Acute pancreatitis associated with rofecoxib. Am J Gastroenterol. 2002;97:1077-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Goldstein J, Laskin DA, Ginsberg GH. Sulindac associated with pancreatitis. Ann Intern Med. 1980;93:151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Memis D, Akalin E, Yücel T. Indomethacin-induced pancreatitis: a case report. JOP. 2005;6:344-347. [PubMed] |
| 51. | Nind G, Selby W. Acute pancreatitis: a rare complication of celecoxib. Intern Med J. 2002;32:624-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Siefkin AD. Sulindac and pancreatitis. Ann Intern Med. 1980;93:932-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Stevenson DD, White AA, Simon RA. Aspirin as a cause of pancreatitis in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2012;129:1687-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Zygmunt DJ, Williams HJ, Bienz SR. Acute pancreatitis associated with long-term sulindac therapy. West J Med. 1986;144:461-462. [PubMed] |
| 56. | Eland IA, Alvarez CH, Stricker BH, Rodríguez LA. The risk of acute pancreatitis associated with acid-suppressing drugs. Br J Clin Pharmacol. 2000;49:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Evans JM, McMahon AD, Steinke DT, McAlpine RR, MacDonald TM. Do H2-receptor antagonists cause acute pancreatitis? Pharmacoepidemiol Drug Saf. 1998;7:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Pezzilli R, Morselli-Labate AM, Corinaldesi R. NSAIDs and Acute Pancreatitis: A Systematic Review. Pharmaceuticals (Basel). 2010;3:558-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 59. | Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Birchfield GR, Ward JH, Redman BG, Flaherty L, Samlowski WE. Acute pancreatitis associated with high-dose interleukin-2 immunotherapy for malignant melanoma. West J Med. 1990;152:714-716. [PubMed] |
| 62. | Alabed YZ, Aghayev A, Sakellis C, Van den Abbeele AD. Pancreatitis Secondary to Anti-Programmed Death Receptor 1 Immunotherapy Diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-e529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer. 2016;99:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Carnovale A, Esposito P, Bassano P, Russo L, Uomo G. Enalapril-induced acute recurrent pancreatitis. Dig Liver Dis. 2003;35:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Gershon T, Olshaker JS. Acute pancreatitis following lisinopril rechallenge. Am J Emerg Med. 1998;16:523-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Jeandidier N, Klewansky M, Pinget M. Captopril-induced acute pancreatitis. Diabetes Care. 1995;18:410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Kanbay M, Korkmaz M, Yilmaz U, Gur G, Boyacioglu S. Acute pancreatitis due to ramipril therapy. Postgrad Med J. 2004;80:617-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Muchnick JS, Mehta JL. Angiotensin-converting enzyme inhibitor-induced pancreatitis. Clin Cardiol. 1999;22:50-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Maringhini A, Termini A, Patti R, Ciambra M, Biffarella P, Pagliaro L. Enalapril-associated acute pancreatitis: recurrence after rechallenge. Am J Gastroenterol. 1997;92:166-167. [PubMed] |
| 70. | Gallego-Rojo FJ, Gonzalez-Calvin JL, Guilarte J, Casado-Caballero FJ, Bellot V. Perindopril-induced acute pancreatitis. Dig Dis Sci. 1997;42:1789-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Eland IA, van Puijenbroek EP, Sturkenboom MJ, Wilson JH, Stricker BH. Drug-associated acute pancreatitis: twenty-one years of spontaneous reporting in The Netherlands. Am J Gastroenterol. 1999;94:2417-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Arjomand H, Kemp DG. Quinapril and pancreatitis. Am J Gastroenterol. 1999;94:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 73. | Bosch X. Losartan-induced acute pancreatitis. Ann Intern Med. 1997;127:1043-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Baffoni L, Durante V, Grossi M. Acute pancreatitis induced by telmisartan overdose. Ann Pharmacother. 2004;38:1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Griesbacher T. Kallikrein-kinin system in acute pancreatitis: potential of B(2)-bradykinin antagonists and kallikrein inhibitors. Pharmacology. 2000;60:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Fimognari FL, Corsonello A, Pastorell R, Antonelli-Incalzi R. Metformin-induced pancreatitis: A possible adverse drug effect during acute renal failure. Diabetes Care. 2006;29:1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Girgis CM, Champion BL. Vildagliptin-induced acute pancreatitis. Endocr Pract. 2011;17:e48-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Kunjathaya P, Ramaswami PK, Krishnamurthy AN, Bhat N. Acute necrotizing pancreatitis associated with vildagliptin. JOP. 2013;14:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 79. | Mosquera JE, Torres N, Restrepo J, Ruz-Pau C, Suryanarayanan S. Linagliptin-induced pancreatitis: A case report. AACE Clin Case Rep. 2019;6:e37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Ayoub WA, Kumar AA, Naguib HS, Taylor HC. Exenatide-induced acute pancreatitis. Endocr Pract. 2010;16:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Chis BA, Fodor D. Acute pancreatitis during GLP-1 receptor agonist treatment. A case report. Clujul Med. 2018;91:117-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | U.S. Food and Drug Administration. FDA alert: Information for healthcare professionals: Exenatide (marketed as Byetta). 2008. |
| 83. | Franks AS, Lee PH, George CM. Pancreatitis: a potential complication of liraglutide? Ann Pharmacother. 2012;46:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Knezevich E, Crnic T, Kershaw S, Drincic A. Liraglutide-associated acute pancreatitis. Am J Health Syst Pharm. 2012;69:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Lee PH, Stockton MD, Franks AS. Acute pancreatitis associated with liraglutide. Ann Pharmacother. 2011;45:e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 86. | Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 87. | Verma R. Canagliflozin-Associated Acute Pancreatitis. Am J Ther. 2016;23:e972-e973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Gutch M, Bhattacharya A, Kumar S, Pahan RK, Singh RS. Dapagliflozin Induced Pancreatitis. Int J Med Public Health. 2018;8:10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Rouse R, Xu L, Stewart S, Zhang J. High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicol Appl Pharmacol. 2014;276:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Koehler JA, Baggio LL, Cao X, Abdulla T, Campbell JE, Secher T, Jelsing J, Larsen B, Drucker DJ. Glucagon-like peptide-1 receptor agonists increase pancreatic mass by induction of protein synthesis. Diabetes. 2015;64:1046-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Wargo KA, Geveden BN, McConnell VJ. Cannabinoid-induced pancreatitis: a case series. JOP. 2007;8:579-583. [PubMed] |
| 93. | Fatma H, Mouna B, Leila M, Radhouane D, Taoufik N. Cannabis: a rare cause of acute pancreatitis. Clin Res Hepatol Gastroenterol. 2013;37:e24-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Belze O, Legras A, Ehrmann S, Garot D, Perrotin D. Cannabis-induced acute pancreatitis. Am J Emerg Med. 2011;29:131.e3-131.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Akkucuk MH, Erbayrak M. A Rare and Unexpected Side-Effect of Cannabis Use: Abdominal Pain due to Acute Pancreatitis. Case Rep Emerg Med. 2015;2015:463836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Cerezo-Ruiz A, Lozano Rodríguez-Mancheño A, Cortés-Rodríguez B, de Paula Rosa-Jiménez F. [Mild acute pancreatitis associated with cocaine consumption]. Gastroenterol Hepatol. 2012;35:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Carlin N, Nguyen N, DePasquale JR. Multiple gastrointestinal complications of crack cocaine abuse. Case Rep Med. 2014;2014:512939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Chapela SP, Paz SLA, Ballestero FM. Pancreatitis Induced by Cocaine. Case Rep Gastroenterol. 2017;11:212-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 99. | Hastier P, Buckley MJ, Peten EP, Demuth N, Dumas R, Demarquay JF, Caroli-Bosc FX, Delmont JP. A new source of drug-induced acute pancreatitis: codeine. Am J Gastroenterol. 2000;95:3295-3298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Hastier P, Demarquay JF, Maes B, Caroli-Bosc FX, Dumas R, Delmont J, Chichmanian RM. Acute pancreatitis induced by codeine-acetaminophen association: a case report with positive rechallenge. Pancreas. 1996;13:324-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Hastier P, Longo F, Buckley M, Chichmanian RM, Delmont JP. Pancreatitis induced by codeine: a case report with positive rechallenge. Gut. 1997;41:705-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Di Martino V, Ezenfis J, Benhamou Y, Bernard B, Opolon P, Bricaire F, Poynard T. Severe acute pancreatitis related to the use of nelfinavir in HIV infection: report of a case with positive rechallenge. AIDS. 1999;13:1421-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 103. | Guo JJ, Jang R, Louder A, Cluxton RJ. Acute pancreatitis associated with different combination therapies in patients infected with human immunodeficiency virus. Pharmacotherapy. 2005;25:1044-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Oliveira NM, Ferreira FA, Yonamine RY, Chehter EZ. Antiretroviral drugs and acute pancreatitis in HIV/AIDS patients: is there any association? A literature review. Einstein (Sao Paulo). 2014;12:112-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Dragovic G. Acute pancreatitis in HIV/AIDS patients: an issue of concern. Asian Pac J Trop Biomed. 2013;3:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 106. | Bush ZM, Kosmiski LA. Acute pancreatitis in HIV-infected patients: are etiologies changing since the introduction of protease inhibitor therapy? Pancreas. 2003;27:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Jones PE, Oelbaum MH. Frusemide-induced pancreatitis. Br Med J. 1975;1:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Eckhauser ML, Dokler M, Imbembo AL. Diuretic-associated pancreatitis: a collective review and illustrative cases. Am J Gastroenterol. 1987;82:865-870. [PubMed] |
| 109. | Pickleman J, Straus FH 2nd, Paloyan E. Pancreatitis associated with thiazide administration. A role for the parathyroid glands? Arch Surg. 1979;114:1013-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Juang P, Page RL 2nd, Zolty R. Probable loop diuretic-induced pancreatitis in a sulfonamide-allergic patient. Ann Pharmacother. 2006;40:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Chao CT, Chao JY. Case report: furosemide and pancreatitis: Importance of dose and latency period before reaction. Can Fam Physician. 2013;59:43-45. [PubMed] |
| 112. | Ghatak R, Masso L, Kapadia D, Kulairi ZI. Medication as a Cause of Acute Pancreatitis. Am J Case Rep. 2017;18:839-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Jones MF, Caldwell JR. Acute hemorrhagic pancreatitis associated with administration of chlorthalidone. Report of a case. N Engl J Med. 1962;267:1029-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Frick TW, Speiser DE, Bimmler D, Largiadèr F. Drug-induced acute pancreatitis: further criticism. Dig Dis. 1993;11:113-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 115. | Perego E, Scaini A, Romano F, Franciosi C, Uggeri F. Estrogen-induced severe acute pancreatitis in a male. JOP. 2004;5:353-356. [PubMed] |
| 116. | Zorrilla E, Hulse M, Hernandez A, Gershberg H. Severe endogenous hypertriglyceridemia during treatment with estrogen and oral contraceptives. J Clin Endocrinol Metab. 1968;28:1793-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Seo D, Suh H, Lee JK, Jang DK, Kwon HY, Lee CH, Yoon SH, Roh JW, Park HS. Estrogen-induced acute pancreatitis: A case report and literature review. Obstet Gynecol Sci. 2017;60:485-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 118. | Mungall IP, Hague RV. Pancreatitis and the pill. Postgrad Med J. 1975;51:855-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Abraham M, Mitchell J, Simsovits D, Gasperino J. Hypertriglyceridemic Pancreatitis Caused by the Oral Contraceptive Agent Estrostep. J Intensive Care Med. 2015;30:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Davidoff F, Tishler S, Rosoff C. Marked hyperlipidemia and pancreatitis associated with oral contraceptive therapy. N Engl J Med. 1973;289:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 93] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 121. | Stone NJ. Estrogen-induced pancreatitis: a caveat worth remembering. J Lab Clin Med. 1994;123:18-19. [PubMed] |
| 122. | Youssef SS, Iskandar SB, Scruggs J, Roy TM. Acute pancreatitis associated with omeprazole. Int J Clin Pharmacol Ther. 2005;43:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Nykamp D, Kraus EJ. Antacid-induced acute pancreatitis. Consult Pharm. 2013;28:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 124. | Vassallo P, Green N, Courtney E. Hypercalcemia secondary to excessive self-medication with antacids causing acute pancreatitis: a case report. Croat Med J. 2019;60:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Chen JL, Spinowitz N, Karwa M. Hypertriglyceridemia, acute pancreatitis, and diabetic ketoacidosis possibly associated with mirtazapine therapy: a case report. Pharmacotherapy. 2003;23:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Malbergier A, de Oliveira Júnior HP. [Sertraline and acute pancreatitis: a case-report]. Braz J Psychiatry. 2004;26:39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Lankisch PG, Werner HM. Mirtazapine: another drug responsible for drug-induced acute pancreatitis? A letter of warning. Pancreas. 2003;26:211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 128. | Bowers RD, Valanejad SM, Holombo AA. Mirtazapine-Induced Pancreatitis-A Case Report. J Pharm Pract. 2019;32:586-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Nørgaard M, Jacobsen J, Gasse C, Pedersen L, Mortensen PB, Sørensen HT. Selective serotonin reuptake inhibitors and risk of acute pancreatitis: a population-based case-control study. J Clin Psychopharmacol. 2007;27:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Yao S, Li J, Fan X, Liu Q, Lian J. The effect of selective serotonin re-uptake inhibitors on risk of type II diabetes mellitus and acute pancreatitis: a meta-analysis. Biosci Rep. 2018;38:BSR20180967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Roberge RJ, Martin TG, Hodgman M, Benitez JG. Acute chemical pancreatitis associated with a tricyclic antidepressant (clomipramine) overdose. J Toxicol Clin Toxicol. 1994;32:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Houben ML, Wilting I, Stroink H, van Dijken PJ. Pancreatitis, complicated by a pancreatic pseudocyst associated with the use of valproic acid. Eur J Paediatr Neurol. 2005;9:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 133. | Asconapé JJ, Penry JK, Dreifuss FE, Riela A, Mirza W. Valproate-associated pancreatitis. Epilepsia. 1993;34:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Rosenberg HK, Ortega W. Hemorrhagic pancreatitis in a young child following valproic acid therapy. Clinical and ultrasonic assessment. Clin Pediatr (Phila). 1987;26:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Coulter DL, Allen RJ. Pancreatitis associated with valproic acid therapy for epilepsy. Ann Neurol. 1980;7:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Fecik SE, Stoner SC, Raphael J, Lindsey C. Recurrent acute pancreatitis associated with valproic acid use for mood stabilization. J Clin Psychopharmacol. 1999;19:483-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 137. | Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry. 1999;32:78-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Evans RJ, Miranda RN, Jordan J, Krolikowski FJ. Fatal acute pancreatitis caused by valproic acid. Am J Forensic Med Pathol. 1995;16:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 139. | Mileusnic D, Donoghue ER, Lifschultz BD. Pathological case of the month: sudden death in a child as a result of pancreatitis during valproic acid therapy. Pediatr Pathol Mol Med. 2002;21:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 140. | Camfield PR, Bagnell P, Camfield CS, Tibbles JA. Pancreatitis due to valproic acid. Lancet. 1979;1:1198-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Kerr TA, Jonnalagadda S, Prakash C, Azar R. Pancreatitis following Olanzapine Therapy: A Report of Three Cases. Case Rep Gastroenterol. 2007;1:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 142. | Parker PH, Helinek GL, Ghishan FK, Greene HL. Recurrent pancreatitis induced by valproic acid. A case report and review of the literature. Gastroenterology. 1981;80:826-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 143. | Waele BD, Smitz J, Willems G. Recurrent pancreatitis secondary to hypercalcemia following vitamin D poisoning. Pancreas. 1989;4:378-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 144. | Knackstedt C, Winograd R, Koch A, Abuzahra F, Trautwein C, Wasmuth HE. Acute necrotic pancreatitis induced by severe hypercalcaemia due to tacalcitol ointment. Br J Dermatol. 2007;156:576-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 145. | Weissman S, Lo A, Patel R, Mehta TI, Singh V, Aziz M, Belyayeva A, Cherian J, Amrutiya V, Atoot A, Hassan A, Sotiriadis J, Atoot A, Tabibian JH. An Unusual Culprit of Drug-Induced Pancreatitis. Dig Dis Sci. 2020;65:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 146. | Iqbal U, Anwar H, Siddiqui HU, Mehmood A. Acute Pancreatitis Secondary to Use of Appetite Suppressant: Garcinia cambogia. Cureus. 2019;11:e4676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 147. | Weissman S, Amrutiya V, Saleem S, Mehta TI, Aziz M, Lo A, Elias S, Sotiriadis J, Takakura K, Pandol SJ, Tabibian JH. Herbal supplement-induced acute pancreatitis: An unfamiliar culprit. Pancreatology. 2020;20:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 148. | Bruminhent J, Carrera P, Li Z, Amankona R, Roberts IM. Acute pancreatitis with saw palmetto use: a case report. J Med Case Rep. 2011;5:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (1)] |