Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2852
Peer-review started: February 6, 2020
First decision: March 21, 2020
Revised: May 8, 2020
Accepted: May 23, 2020
Article in press: May 23, 2020
Published online: June 7, 2020
Processing time: 121 Days and 4.8 Hours
The introduction of biologics has revolutionized the management of the chronic inflammatory bowel disease, ulcerative colitis (UC), with many patients experiencing significant improvements not only in their symptoms but in other outcomes relevant to individuals and society as a whole. In Germany, there are no prospective data > 3 mo that assess the work productivity, daily activities and quality of life (QoL) of patients with moderate-to-severe UC treated with golimumab.
To assess change in work productivity, capacity for daily activities and QoL in UC patients treated with golimumab in Germany.
The validated Work Productivity Activity Impairment (WPAI) Questionnaire was used to analyze the change in work productivity, the capacity for daily activities after three months (primary endpoint) and disease specific and health related QoL (HRQoL) up to 1 year (secondary endpoints). The changes in work productivity and activity impairment were evaluated every three months until month twelve compared to baseline. Disease-specific and health-related QoL were assessed with the inflammatory bowel disease questionnaire and with the short-form 12 health survey questionnaire (SF-12).
This prospective non-interventional study included 287 patients. The analysis population was comprised of 282 patients who had completed at least two visits. At baseline, 61% of patients had moderate UC and 18% had severe UC. Furthermore, 75% of patients worked full-time or part-time at baseline. A total of 212 patients who were employed at the start of the study (employed population) were evaluated for the primary endpoint. Golimumab significantly reduced all WPAI sub-scores compared to baseline after three, six, nine and twelve months after the start of treatment (P < 0.0001). In addition, disease-specific QoL and HRQoL, as measured by the SF-12 questionnaire, improved significantly with golimumab at all evaluation times (P < 0.0001 in each case vs baseline).
Treatment of moderate-to-severe UC with golimumab leads to significant improvements in patient’s work productivity, daily activity and QoL over twelve months.
Core tip: Systematic reviews indicate that ulcerative colitis (UC) causes a significant socioeconomic burden, but prospective studies are scarce. The aim of this study is to evaluate the changes in work productivity and quality of life (QoL) of UC patients in Germany treated with the human monoclonal tumor necrosis factor alpha antibody golimumab in order to assess the specific benefit of this treatment option. This prospective non-interventional study included 287 patients. Based on these data we were able to show that treatment of moderate-to-severe UC with golimumab leads to significant improvements in patient´s work productivity, daily activity and QoL over twelve months.
- Citation: Teich N, Grümmer H, Jörgensen E, Liceni T, Holtkamp-Endemann F, Fischer T, Hohenberger S. Golimumab in real-world practice in patients with ulcerative colitis: Twelve-month results. World J Gastroenterol 2020; 26(21): 2852-2863
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2852
Ulcerative colitis (UC) is a chronic inflammatory disease of the gastrointestinal tract [inflammatory bowel disease (IBD)] with a peak onset between ages 15 and 30 years[1,2]. The main features are mucosal inflammation, which spreads proximally from the rectum, and the development of extensive superficial ulcerations[3]. The symptoms include recurrent episodes of bloody diarrhea with excretion of liquid bloody stools several times daily, a strong urge to defecate, abdominal pain, incontinence, weight loss and general malaise. In about 50%-80% of patients, UC follows a relapsing course with variable disease activity. In a further 15%-30% of patients it is difficult to achieve permanent remission[4]. Health-related quality of life (HRQoL) is severely impaired in moderate-to-severe UC. In addition, a negative association between HRQoL on the one hand and unemployment, sick days and claiming of disability pensions on the other, is described in patients with IBD[5]. Most perturbing is that the risk of work disability is highest among the youngest patients, when pioneering career steps generally need to be taken[6].
The early disease onset of UC with frequent hospitalizations as well as ex-traintestinal manifestations is associated with high utilization of health services[7]. UC patients often suffer from a significant impairment in their quality of life (QoL) and an overall poor general condition due to chronic recurrent disease. In addition to gastrointestinal symptoms, this also includes disturbances in social interaction, sleep and emotional behavior. The majority of patients also suffer from concentration problems; the lower working speed and lower productivity can lead to problems in the workplace. Incapacity for work is in turn associated with lower QoL and a higher rate of depressive and anxious symptoms, which further worsen functional status[8]. Many UC patients require continuous or intermittent treatment. Achieving and sustaining at least partial remission might help to improve the patient's functional status and ability to work[9].
Systematic reviews indicate that UC causes significant socioeconomic burden[10,11]. Long-term analyses by the European Collaborative Study on IBD over a 10-year period show large differences in the annual direct costs per patient for Europe depending, among other things, on geographical location, therapy practice, hospitalization duration and disease duration[12]. It is therefore necessary, not only for quality assurance of medical interventions, to evaluate the use of medical services in a health care system specific, naturalistic setting, including health economic endpoints[13]. Several studies show that biologic treatment of patients with IBD affects both the direct and indirect costs of healthcare[14,15]. For example, a profound assessment of the healthcare costs and productivity losses in a large cohort of IBD patients revealed that productivity losses accounted for 39% of the total costs in patients with UC[15].
The primary aim of UC therapy is to rapidly achieve clinical remission and maintain long-term steroid-free clinical and endoscopic remission[16]. Anti-tumor necrosis factor alpha (anti-TNFα) therapy offers a way of escalating treatment to induce and gradually maintain remission in UC. The current S3 UC guideline of the German Society for Gastroenterology, Digestive and Metabolic Diseases recommends the use of thiopurines or anti-TNFα antibodies (in the case of infliximab in combination with thiopurine where appropriate), vedolizumab or tofacitinib in patients with steroid dependent UC[16]. The human monoclonal TNFα antibody golimumab is indicated for the treatment of moderately to severely active UC in adult patients who have responded insufficiently to conventional therapy or who have an intolerance of or contraindication to such therapies[17].
Until now, there have been few data on how anti-TNFα therapy affects work productivity in patients with moderate-to-severe UC. In particular, there are no systematic data on the use of golimumab in patients with moderate-to-severe UC in Germany, containing insight on outcome parameters, QoL and health economics.
The aim of the GO-CUTE study is to evaluate the changes in work productivity and QoL of UC patients treated with golimumab in Germany in order to assess the specific benefit of this treatment option. The analysis is presented after a treatment period of three months (primary endpoint), six, nine and twelve months.
The non-interventional, multicenter, prospective study GO-CUTE (project ID: MK8259-031) is conducted in fifty gastroenterological practices in Germany. Patients will be observed for 2 years. The presented analysis is based on full one-year data from the third interim analysis [March 21, 2014 (first patient first visit) to August 16, 2019 (last patient last visit)].
GO-CUTE complies with all legal and regulatory requirements for non-interventional studies, including EU Directive 2001/20/EC, and in accordance with Section 67(6) of the German Medicinal Products Act (AMG), it was notified to the Paul Ehrlich Institute, the National Association of Statutory Health Insurance Physicians, the Central Federal Association of Health Insurance Funds and the Association of Private Health Insurance Companies. In accordance with AMG Section 4 No.23, the treatment of the patients presented here followed exclusively the individual medical decision in daily practice. Informed consent was obtained from all individual participants prior to study enrollment.
The observation plan and patient information of the GO-CUTE study were approved by the Institutional Review Board Ethics Committee of the Bavarian State Medical Association (Bayrische Landesärztekammer).
The primary endpoint was evaluation of the changes in work productivity or activity impairment (WPAI) in month three (from baseline) compared to baseline in UC patients treated in Germany with golimumab in clinical practice using the WPAI questionnaire.
Secondary endpoints of the study were as follows: (1) Evaluation of the change in the short-form 12 health survey questionnaire (SF-12) in months three, six, nine, twelve, eighteen and twenty-four after baseline compared to baseline in UC patients treated in Germany with golimumab in clinical practice; and (2) Evaluation of the change in the IBD questionnaire (IBDQ) in months three, six, nine, twelve, eighteen and twenty-fourafter baseline compared to baseline in UC patients treated in Germany with golimumab in clinical practice.
The study included 287 patients aged ≥ 18 years with UC, diagnosed by a gas-troenterologist, who were suitable for golimumab therapy in accordance with the approved product information and clinical standards.
The following patients were excluded: (1) Patients with a contraindication according to the current Simponi® Summary of Product Characteristics (SmPC)[17]; (2) Patients previously treated with golimumab; (3) Patients with previous biologic treatment whose treatment was changed due to a serious adverse event (SAE), an opportunistic infection or hypersensitivity reaction; and (4) Patients currently participating in another clinical trial (with the exception of register studies).
Treatment was carried out in accordance with the current Simponi® (golimumab) SmPC. Simponi® is approved in the European and is indicated for the treatment of moderately to severely active UC in adult patients who have had an inadequate response to conventional therapy, including corticosteroids and 6-mercaptopurine or azathioprine, or who are intolerant of or have medical contraindications to such therapies. It is administered subcutaneously: (1) In the induction phase: golimumab 200 mg in week 0 and golimumab 100 mg in week 2; and (2) In the maintenance phase: in patients with body weight < 80 kg: 50 mg every 4 weeks; in patients with body weight ≥ 80 kg: 100 mg every four weeks. The dosages and times of subcutaneous administration were documented throughout the study.
Work productivity: The validated WPAI questionnaire was used to analyze the primary endpoint. The WPAI is considered to be the psychometrically best validated instrument for determining health-related work productivity and is widely used[18]. The WPAI has previously been studied and validated in various chronic inflammatory diseases, including in patients with IBD[19,20]. In UC, it has proved its worth in randomized, controlled trials as well as in non-interventional observational studies[21]. Changes in work productivity and the capacity for daily activities were evaluated in months three (Visit 1, V1), six (V2), nine (V3), and twelve (V4) compared to baseline. All four WPAI subscores[22] were assessed: (1) Absence from work due to illness (absenteeism); (2) Reduced performance due to health problems (presenteeism); (3) Total work productivity impairment (TWPI); and (4) Daily activity based on general health problems or specific health problems. Patients were asked about a recall period of seven days.
SF-12: The HRQoL in UC patients was assessed using the SF-12 in months three, six, nine, and twelve after baseline. The SF-12 was developed as a shorter alternative to the more extensive SF-36, and can be used regardless of the patient’s disease and age[23]. The SF-12 includes twelve questions on physical and mental status [physical component score (PCS-12), and mental component score (MCS-12)] to assess the overall state of health and evaluate the ability to engage in moderate activities. Patients were asked about a recall period of four weeks.
IBDQ: Disease-specific QoL was assessed by the IBDQ[24]. Systemic symptoms such as sleep disorders and fatigue (systemic symptoms), specific bowel functions [frequency of pain and cramps (bowel systems)], impairment in social activities (social function) and emotional consequences of the disease (emotional health) are recorded in thirty-two items. Patients were asked about a recall period of two weeks.
Data were analyzed using SAS version 9.4 (NC, United States). All data were expressed as mean ± SD or as n and %. The primary analyses were carried out in the full analysis population (mITT) comprised of patients having data for at least two visits (n = 282). All patients who started treatment with golimumab were considered for analyses, regardless of whether they remained on golimumab at the time of evaluation.
For the WPAI, a one-sample t test for the change from baseline was used to compare the values obtained at months three, six, nine, and twelve (V1-V4), or the Wilcoxon signed rank test was used if the assumption of a normal distribution was doubtful. All differences with a P value less than 0.05 were considered as statistically significant and should be interpreted in an explorative manner. The statistical methods of this study were reviewed by Ulrich Elsasser, MedPharmTec GmbH, Munich, Germany.
A total of 287 patients were included in the study. The mITT was comprised of 282 patients who had data from at least two visits. A total of 212 patients who were employed at the start of the study (mITTe) were evaluated for the primary endpoint. The mITT and mITTe populations were well balanced in terms of their demographic and disease-specific characteristics (Table 1). Table 2 shows the occupational status in the analysis and employed populations. Table 3 summarizes immune-modulating medications concomitant to golimumab in the analysis and employed populations.
| mITT (n = 282) | mITTe (n = 212) | |
| Gender (male/female), % | 47.2/52.8 | 47.6/52.4 |
| Age (mean ± SD) | 40.8 (± 13.9) | 39.7 (± 12.1) |
| Marital status | ||
| Single | 116 (41.1) | 88 (41.5) |
| Married/living together | 156 (55.3) | 117 (55.2) |
| Divorced | 8 (2.8) | 7 (3.3) |
| Widowed | 2 (0.7) | - |
| Rectal bleeding | ||
| No blood seen | 95 (33.7) | 68 (32.1) |
| Streaks of blood with stool less than half the time | 81 (28.7) | 55 (25.9) |
| Obvious blood with stool most of the time | 81 (28.7) | 69 (32.5) |
| Blood alone passes | 25 (8.9) | 20 (9.4) |
| Physician global assessment | ||
| Normal | 8 (2.8) | 7 (3.3) |
| Mild disease | 54 (19.1) | 39 (18.4) |
| Moderate disease | 173 (61.3) | 131 (61.8) |
| Severe disease | 47 (16.7) | 35 (16.5) |
| Alcohol consumption (actual) | ||
| Yes/no | 142 (50.4)/140 (49.6) | 114 (53.8)/98 (46.2) |
| Concomitant disease | ||
| Yes/no | 121 (42.9)/161 (57.1) | 82 (38.7)/130 (61.3) |
| Frequency of alcohol consumption | ||
| Occasional | 117 (82.4) | 95 (83.3) |
| Once a week | 11 (7.7) | 10 (8.8) |
| Several times a week | 9 (6.3) | 5 (4.4) |
| Daily | 5 (3.5) | 4 (3.5) |
| Nicotine consumption (actual) | ||
| Yes/no | 28 (9.9)/254 (90.1) | 22 (10.4)/190 (89.6) |
| Abuse of other substances | ||
| Yes/no | 1 (0.4)/281 (99.6) | 1 (0.5)/281 (99.5) |
| Cannabis | 1 (100) | 1 (100.0) |
| mITT (n = 282) | mITTe (n = 212) | |
| Occupational status | ||
| Employed (full time) | 172 (61.0) | 172 (81.1) |
| Employed (part time) | 39 (13.8) | 39 (18.4) |
| Retired | 13 (4.6) | 1 (0.5)1 |
| Full reduced | 12 (4.3) | |
| Not working | 46 (16.3) | |
| Part time employment due to | ||
| Ulcerative colitis | 7 (17.9) | 7 (17.9) |
| Other reason | 32 (82.1) | 32 (82.1) |
| Full reduced due to | ||
| Ulcerative colitis | 8 (66.7) | N/A |
| Other reason | 4 (33.3) | N/A |
| mITT (n = 282) | mITTe (n = 212) | |
| Any medication | 125 (44.3) | 90 (42.5) |
| Corticosteroids | 123 (43.6) | 88 (41.5) |
| Thiopurines | ||
| Azathioprin | 4 (1.4) | 4 (1.9) |
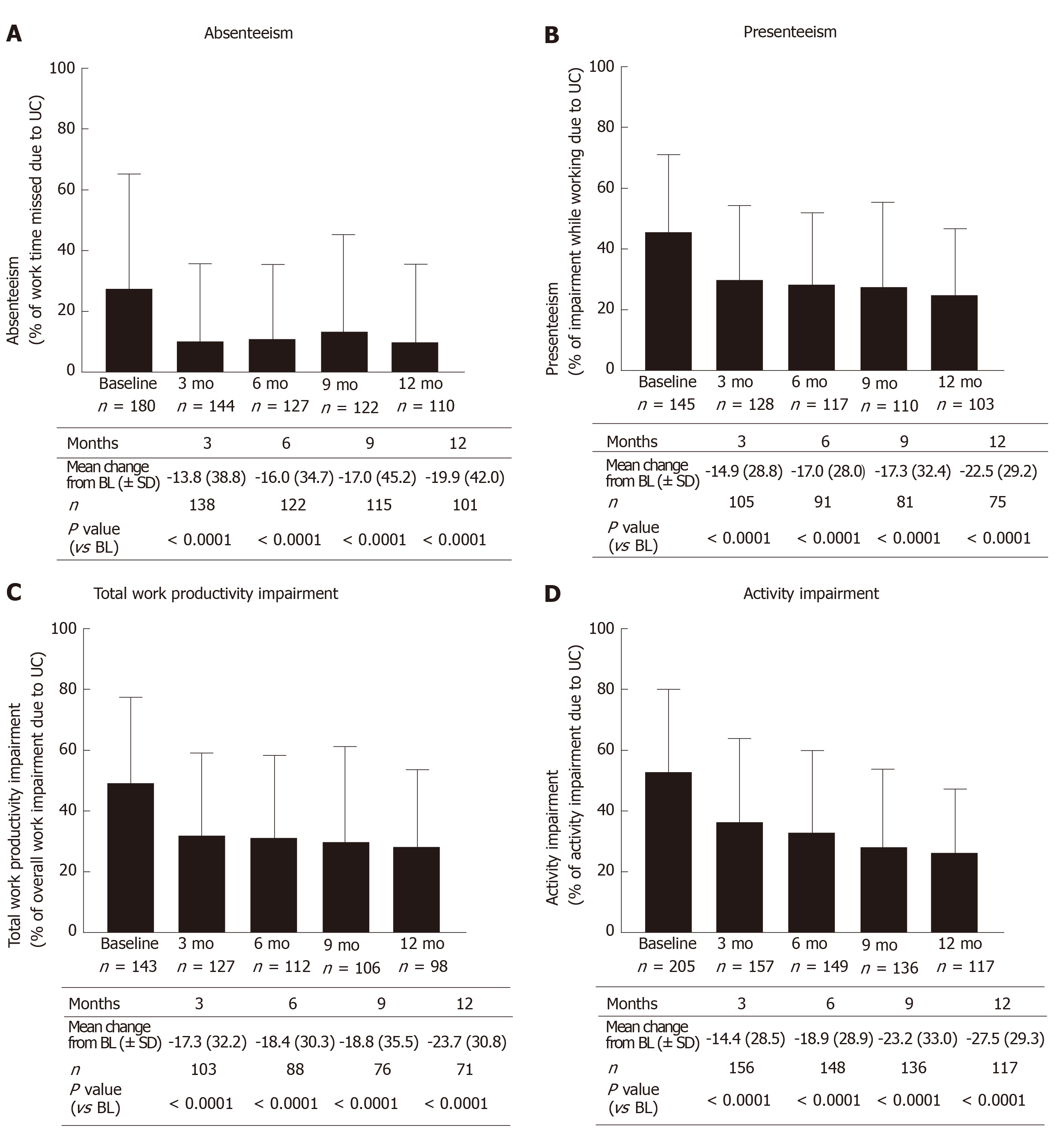
From three months after the start of treatment up to twelve months after the start of treatment there was a significant reduction in all WPAI sub-scores compared to baseline (P < 0.0001; Wilcoxon signed rank test) with golimumab (Figure 1).
The mean value in the absenteeism sub-score as observed decreased after three months (V1) from 27.6% ± 37.7% to 10.3% ± 25.3% (mean change from baseline: -13.8% ± 38.8%; P < 0.0001; Wilcoxon signed rank test, Figure 1A). A quarter of patients achieved a reduction of at least 25%; 15% of patients did not record any improvement. After twelve months (V4), the mean difference compared to baseline was 19.9% ± 42.0% (P < 0.0001; Wilcoxon signed rank test). About 35% of patients achieved a reduction of at least 40%, and there was no improvement in 20% of patients.
The mean value in the presenteeism sub-score as observed decreased after three months (V1) from 45.3% ± 26.0% to 29.4% ± 25.1% (mean change from baseline: -14.9% ± 28.8%; P < 0.0001; Wilcoxon signed rank test, Figure 1B). A quarter of the patients achieved a reduction of at least 40%; 20% of patients did not record any improvement. After twelve months (V4), the mean difference compared to baseline was 22.5% ± 29.2% (P < 0.0001; Wilcoxon signed rank test). A quarter of the patients achieved a reduction of at least 50%; there was no improvement in 15% of patients.
The mean value in the TWPI sub-score as observed decreased after three months (V1) from 49.7% ± 27.7% to 31.9% ± 26.9% (mean change from baseline: -17.3 ± 32.3; P < 0.0001; Wilcoxon signed rank test, Figure 1C). About one fifth of the subjects reached a reduction of at least 45%, 30% of the subjects had no improvement. After twelve months (V4), the mean difference compared to baseline was 23.7% ± 30.8% (P < 0.0001; Wilcoxon signed rank test). About 35% of patients achieved a reduction of at least 40%, and there was no improvement in 20% of patients.
The mean value in the activity impairment sub-score as observed decreased after three months (V1) from 52.8% ± 26.9% to 36.3% ± 27.8% (mean change from baseline: 14.4 ± 28.5; P < 0.0001; Wilcoxon signed rank test, Figure 1D). A quarter of the patients achieved a reduction of at least 40%; 25% of patients did not record any improvement. After twelve months (V4), the mean difference compared to baseline was 27.5% ± 29.3% (P < 0.0001; Wilcoxon signed rank test). A quarter of the patients achieved a reduction of at least 55%; there was no improvement in 10% of patients.
Significant improvements were also obtained in the secondary endpoints for disease-specific QoL, as measured by the IBDQ, as well as the HRQoL as measured by the SF-12, during treatment with golimumab (P < 0.0001 vs baseline; Wilcoxon signed rank test).
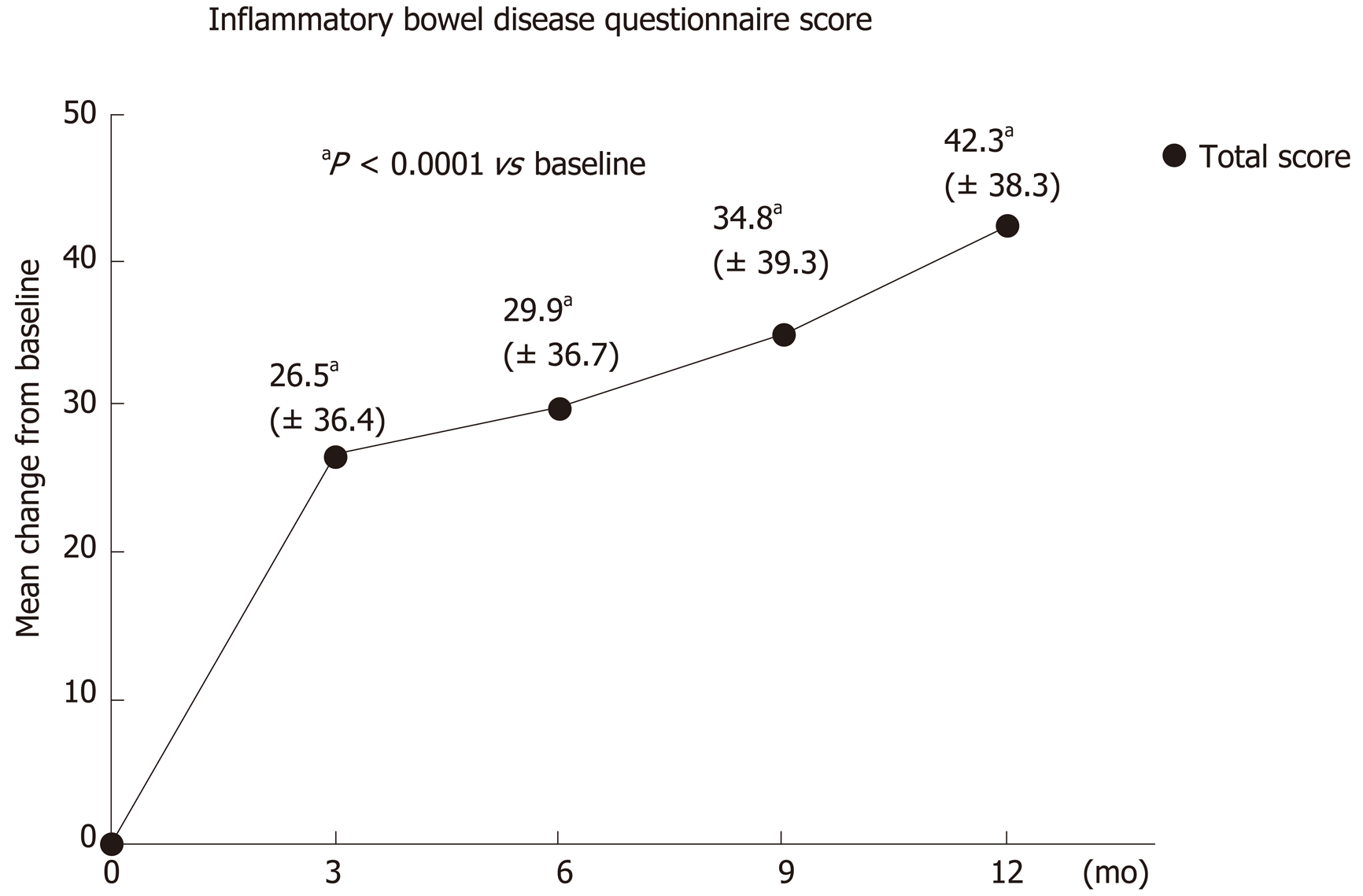
IBDQ: The average IBDQ score was 124.8 ± 35.8 points at baseline and 168.0 ± 32.3 points after 1 year (V4). This was equivalent to an increase of 42.3 ± 38.3 points. About 85% of participants achieved an improvement in their average IBDQ total score compared to baseline. The IBDQ total score improved by more than 50 points in 60% of participants. The significant improvement in the average IBDQ total score was already seen after three months (V1) with an increase of 26.5 ± 36.4 points, and in subsequent visits after six months (V2: 29.9 ± 36.7 points), nine months (V3: 34.8 ± 39.2 points) and after twelve months (V4: 43.3 ± 38.3 points). The difference from baseline in the total IBDQ score was statistically significant for all visits (P < 0.0001; Wilcoxon signed rank test, Figure 2).
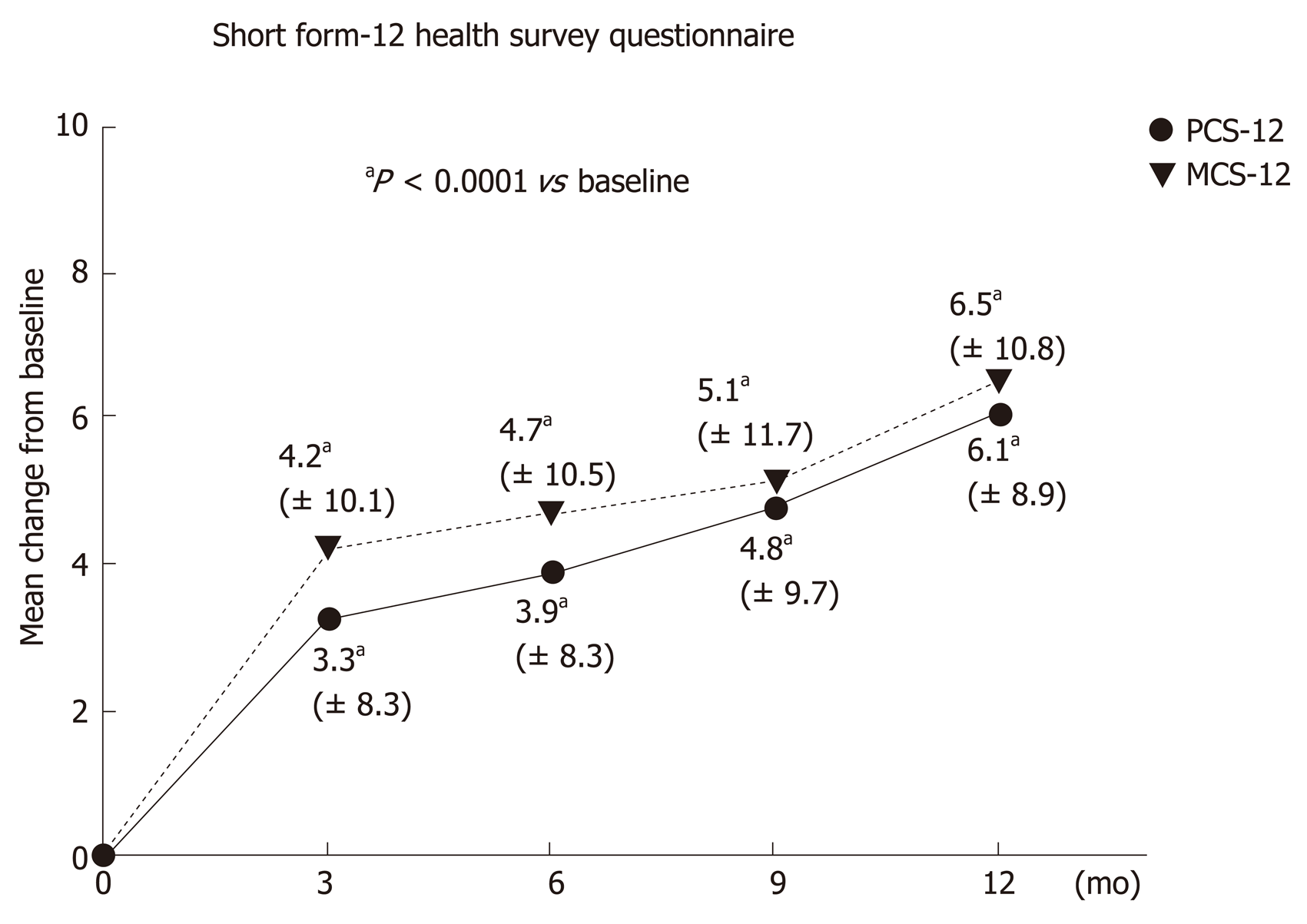
SF-12: The mean SF-12 physical component score (PCS-12) increased continuously from 43.1 ± 8.7 points at baseline (V0) to 48.7 ± 7.2 points after 1 year (V4). This corresponded to an average difference of 6.1 ± 8.9 points (median 5.9, range -13.6 to 36.5). The significant improvement in the mean PCS-12 was firstly seen after three months (V1) with an increase of 3.3 ± 8.3 points, and in subsequent visits after six months (V2: 3.9 ± 8.3 points) and nine months (V3: 4.8 ± 9.7 points). The difference from baseline in the PCS12 score was statistically significant for all visits (P < 0.0001; Wilcoxon signed rank test, Figure 3).
The mean SF-12 mental component score (MCS-12) increased continuously from 39.7 ± 10.1 points at baseline (V0) to 46.4 ± 9.0 points after 1 year (V4). This corresponded to an average difference of 6.5 ± 10.8 points (median 6.3, range -24.1 to 31.9). The significant improvement in the mean MCS-12 was firstly seen after three months (V1) with an increase of 4.2 ± 10.1 points, and in subsequent visits after six months (V2: 4.7 ± 10.5 points) and nine months (V3: 5.1 ± 11.7 points). The difference from baseline in the MCS-12 score was statistically significant for all visits (P < 0.0001; Wilcoxon signed rank test, Figure 3).
In this prospective study, we showed that golimumab leads to a significant improvement in work productivity, daily activity and QoL in UC patients after three months of golimumab induction. These benefits persisted for twelve months. Our data contribute to fulfilling the recent recommendation from a systematic literature review that WPAI should be used for measuring work outcomes in UC patients[21].
Our results may illustrate that golimumab is able to restore patients’ QoL; a well-accepted therapeutic goal beyond achieving induction and maintenance of remission[25,26]. Furthermore, our study provides correlative data on QoL in a real world setting which were limited to date[27,28]. Comparable to our data, a prospective multicenter study of golimumab effectiveness and QoL in a real-life population showed a marked improvement of QoL measured by IBDQ[29]: From baseline (start of induction) to week eight and week thirty-two a significant IBDQ mean increase (32.9; mean value: 172; and 25.2, mean value 170; respectively) was observed (P < 0.05), respectively[29]. In an interim analysis of a prospective cohort study from Sweden, QoL improved in golimumab treated patients, with a significant reduction in the overall short health scale score (P = 0.04)[30]. To date, there have been no systematic data on subjectively-assessed outcome parameters, QoL and health economics regarding the use of golimumab in patients with moderate-to-severe UC in Germany.
Our data compare well to the non-interventional QUO-VADIS study that evaluated the health-economic aspects of anti-TNFα therapy for ankylosing spondylitis[31]. This study, reporting on 963 ankylosing spondylitis patients, showed a gain in work productivity and activity and fewer disease-related absences in patients newly treated with golimumab or infliximab within six months of treatment[31]. Evidence from randomized controlled trials is needed to directly evaluate WPAI’s responsiveness to treatment.
To conclude, our study shows a strong WPAI's responsiveness of the treatment of moderate-to-severe UC with golimumab. Golimumab induction resulted in significant improvements in the work productivity, daily activity and QoL of patients over full treatment duration of 12 mo.
Ulcerative colitis (UC) represents a chronic inflammatory bowel disease with recurrent episodes of debilitating symptoms leading to an impaired health-related quality of life (HRQoL), especially in those patients with moderate-to severe UC. Besides HRQoL, in most of the patients, work productivity is negatively affected and an increased incapacity to work is reported due to UC. Therefore, UC causes additionally a substantial socioeconomic burden. Consequently, it is a considerable necessity to evaluate the impact of treatment options on work productivity and work life impairment. Golimumab, a human monoclonal tumor necrosis factor alpha (TNFα) antibody is indicated to treat moderate-to-severe UC in adult patients without effective response to conventional therapies and its use has led to significant decrease of symptom burden in treated subjects.
Until now, it is rarely evaluated how anti-TNFα therapy affected work life in patients with moderate-to-severe UC. In particular, there are no systematic data on the use of golimumab in patients with moderate-to-severe UC in Germany with regard to work life impairment, quality of life (QoL) and health economics.
The GO-CUTE study aimed to evaluate the changes in work productivity and HRQoL in UC patients treated with golimumab in Germany in order to assess the specific benefit of this treatment option. Changes in work productivity and the capacity for daily activities after three months represented the primary endpoint. The changes in HRQoL and disease specific QoL up to 1 year during golimumab treatment were defined as secondary endpoints.
This non-interventional, observational, prospective study was conducted in fifty gas-troenterological practices in Germany. Work productivity and activity impairment were analyzed using the validated Work Productivity Activity Impairment (WPAI) Questionnaire. Short-form 12 health survey questionnaire (SF-12) and inflammatory bowel disease questionnaire (IBDQ) were used to complete HRQoL and disease-specific QoL assessment.
Our results showed a significant reduction in all WPAI sub-scores after the start of treatment with golimumab for each time point (month three, six, nine and twelve) when compared to baseline data (for each visit P < 0.0001, Wilcoxon signed rank test). A quarter of patients achieved a reduction of at least 25% in the absenteeism sub-score and a reduction of at least 40% in the presenteeism sub-score after three months of golimumab treatment. After twelve months, in 80% of the subjects the absenteeism sub-score and in 85% of the patients the presenteeism sub-score was enhanced. Significant improvements were also detected for disease-specific QoL as well as for HRQoL during treatment with golimumab (P < 0.0001 vs baseline, Wilcoxon signed rank test) assessed by IBDQ and SF-12, respectively.
The results of the GO CUTE study demonstrated that golimumab treatment in patients suffering from moderate-to-severe UC significantly improves both patient´s work productivity and daily activity as well as HRQoL and disease-specific QoL. Furthermore, our data revealed that these benefits persisted over twelve months of treatment.
We were able to show a strong responsiveness of the WPAI to the treatment of moderate-to severe UC with golimumab, but evidence from randomized controlled trials is additionally needed for final conclusions.
The authors would like to thank the study participants and site staff who collaborated in the study. For medical writing assistance the authors thank Dr. Michael Wenzel, MCG Medical Consulting Group, Düsseldorf, Germany and Dr. Katharina Bakhaus, Alcedis GmbH, Giessen, Germany.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The Corresponding Author is the member of the German Society for Digestive and Metabolic Diseases (DGVS), No. 03216.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sitkin S S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 304] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (4)] |
| 3. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3353] [Article Influence: 186.3] [Reference Citation Analysis (11)] |
| 4. | Holtmann MH, Galle PR. Current concept of pathophysiological understanding and natural course of ulcerative colitis. Langenbecks Arch Surg. 2004;389:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Bernklev T, Jahnsen J, Henriksen M, Lygren I, Aadland E, Sauar J, Schulz T, Stray N, Vatn M, Moum B. Relationship between sick leave, unemployment, disability, and health-related quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Høivik ML, Moum B, Solberg IC, Henriksen M, Cvancarova M, Bernklev T; IBSEN Group. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut. 2013;62:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 7. | De Boer AG, Bennebroek Evertsz' F, Stokkers PC, Bockting CL, Sanderman R, Hommes DW, Sprangers MA, Frings-Dresen MH. Employment status, difficulties at work and quality of life in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2016;28:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Filipovic BR, Filipovic BF. Psychiatric comorbidity in the treatment of patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3552-3563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 9. | Yarlas A, D'Haens G, Willian MK, Teynor M. Health-Related Quality of Life and Work-Related Outcomes for Patients With Mild-to-Moderate Ulcerative Colitis and Remission Status Following Short-Term and Long-Term Treatment With Multimatrix Mesalamine: A Prospective, Open-Label Study. Inflamm Bowel Dis. 2018;24:450-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31:693-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Wolters FL, Russel MG, Sijbrandij J, Schouten LJ, Odes S, Riis L, Munkholm P, Langholz E, Bodini P, O'Morain C, Katsanos K, Tsianos E, Vermeire S, Van Zeijl G, Limonard C, Hoie O, Vatn M, Moum B, Stockbrügger RW; European Collaborative Study Group On Inflammatory Bowel Disease. Disease outcome of inflammatory bowel disease patients: general outline of a Europe-wide population-based 10-year clinical follow-up study. Scand J Gastroenterol Suppl. 2006;46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Odes S, Vardi H, Friger M, Wolters F, Russel MG, Riis L, Munkholm P, Politi P, Tsianos E, Clofent J, Vermeire S, Monteiro E, Mouzas I, Fornaciari G, Sijbrandij J, Limonard C, Van Zeijl G, O'morain C, Moum B, Vatn M, Stockbrugger R; European Collaborative Study on Inflammatory Bowel Disease. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology. 2006;131:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Büsch K, da Silva SA, Holton M, Rabacow FM, Khalili H, Ludvigsson JF. Sick leave and disability pension in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2014;8:1362-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Louis E, Löfberg R, Reinisch W, Camez A, Yang M, Pollack PF, Chen N, Chao J, Mulani PM. Adalimumab improves patient-reported outcomes and reduces indirect costs in patients with moderate to severe Crohn's disease: results from the CARE trial. J Crohns Colitis. 2013;7:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, Mahmmod N, van de Meeberg PC, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk CJ, Vermeijden JR, Siersema PD, van Oijen MG, Oldenburg B; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 16. | Kucharzik T, Dignass AU, Atreya R, Bokemeyer B, Esters P, Herrlinger K, Kannengießer K, Kienle P, Langhorst J, Lügering A, Schreiber S, Stallmach A, Stein J, Sturm A, Teich N, Siegmund B. Updated S3-Guideline Ulcerative Colitis. German Society for Digestive and Metabolic Diseases (DGVS). Z Gastroenterol. 2019;57:162-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | European Medicines Agency. Summary of product characteristics Simponi®. [cited 20 January 2020]. Available from: https://www.ema.europa.eu/en/documents/product-information/simponi-epar-product-information_en.pdf . |
| 18. | Prasad M, Wahlqvist P, Shikiar R, Shih YC. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics. 2004;22:225-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Reilly MC, Gooch KL, Wong RL, Kupper H, van der Heijde D. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology (Oxford). 2010;49:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Reilly MC, Gerlier L, Brabant Y, Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn's disease. Clin Ther. 2008;30:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Yarlas A, Maher SM, Bayliss MS, Lovley A, Cappelleri JC, DiBonaventura MD. Psychometric validation of the work productivity and activity impairment questionnaire in ulcerative colitis: results from a systematic literature review. J Patient Rep Outcomes. 2018;2:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Reilly MA. Reilly Associates: WPAI General Information. 2002 [cited 20 January 2020]. Available from: http://www.reillyassociates.net/WPAI_General.html . |
| 23. | Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11319] [Cited by in RCA: 12811] [Article Influence: 441.8] [Reference Citation Analysis (0)] |
| 24. | Janke KH, Steder-Neukamm U, Bauer M, Raible A, Meisner C, Hoffmann JC, Gregor M, Klump B, Häuser W. [Quality of life assessment in Inflammatory Bowel Disease (IBD): German version of the Inflammatory Bowel Disease Questionnaire (IBDQ-D; disease-specific instrument for quality of life assessment) -- first application and comparison with international investigations]. Gesundheitswesen. 2005;67:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1408] [Article Influence: 140.8] [Reference Citation Analysis (115)] |
| 26. | Ho EY, Cominelli F, Katz J. Ulcerative Colitis: What is the Optimal Treatment Goal and How Do We Achieve It? Curr Treat Options Gastroenterol. 2015;13:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Olivera P, Danese S, Pouillon L, Bonovas S, Peyrin-Biroulet L. Effectiveness of golimumab in ulcerative colitis: A review of the real world evidence. Dig Liver Dis. 2019;51:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Bressler B, Williamson M, Sattin B, Camacho F, Steinhart AH. Real World Effectiveness of Golimumab Therapy in Ulcerative Colitis Regardless of Prior TNF Exposure. J Can Assoc Gastroenterol. 2018;1:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Armuzzi A, Marchi S, Gasbarrini A, Saibeni S, Geccherle A, Principi M, Germano V, Bossa F, Privitera AC. GO-CARE: a prospective multi-centre observational study of golimumab effectiveness and quality of life in a real-life UC patient population in Italy. J Crohns Colitis. 2018;12:S496-S497. [DOI] [Full Text] |
| 30. | Eriksson C, Bergemalm D, Vigren L, Nilsson L, Visuri I ,Hjortswang H, Udumyan R, Almer S, Seddighzadeh M, Hertervig E, Karlen P, Strid H, Halvarson J; The GO-SWIBREG study group. Clinical effectiveness of golimumab: interim analysis of the observational study of patients with ulcerative colitis on golimumab in the Swedish National Qualty Registry for IBD-GO-SWIBREG. J Crohns Colitis. 2018;12:S409-S410. [DOI] [Full Text] |
| 31. | Claudepierre P, Van den Bosch F, Sarzi-Puttini P, Vastesaeger N, Govoni M, Kachroo S. Treatment with golimumab or infliximab reduces health resource utilization and increases work productivity in patients with ankylosing spondylitis in the QUO-VADIS study, a large, prospective real-life cohort. Int J Rheum Dis. 2019;22:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |