Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2740
Peer-review started: February 22, 2020
First decision: April 12, 2020
Revised: April 24, 2020
Accepted: May 14, 2020
Article in press: May 14, 2020
Published online: June 7, 2020
Processing time: 105 Days and 5.5 Hours
Liver cirrhosis and diabetes mellitus (DM) are both common conditions with significant socioeconomic burden and impact on morbidity and mortality. A bidirectional relationship exists between DM and liver cirrhosis regarding both etiology and disease-related complications. Type 2 DM (T2DM) is a well-recognized risk factor for chronic liver disease and vice-versa, DM may develop as a complication of cirrhosis, irrespective of its etiology. Liver transplantation (LT) represents an important treatment option for patients with end-stage liver disease due to non-alcoholic fatty liver disease (NAFLD), which represents a hepatic manifestation of metabolic syndrome and a common complication of T2DM. The metabolic risk factors including immunosuppressive drugs, can contribute to persistent or de novo development of DM and NAFLD after LT. T2DM, obesity, cardiovascular morbidities and renal impairment, frequently associated with metabolic syndrome and NAFLD, may have negative impact on short and long-term outcomes following LT. The treatment of DM in the context of chronic liver disease and post-transplant is challenging, but new emerging therapies such as glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) targeting multiple mechanisms in the shared pathophysiology of disorders such as oxidative stress and chronic inflammation are a promising tool in future patient management.
Core tip: This review explores complex relationships and mechanisms involved in the interplay between diabetes mellitus and liver disease before and after liver transplantation, especially in the term of non-alcoholic fatty liver disease, which then relate to management issues, newer treatment options and patient outcomes.
- Citation: Cigrovski Berkovic M, Virovic-Jukic L, Bilic-Curcic I, Mrzljak A. Post-transplant diabetes mellitus and preexisting liver disease - a bidirectional relationship affecting treatment and management. World J Gastroenterol 2020; 26(21): 2740-2757
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2740.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2740
Diabetes mellitus (DM) represents a group of heterogeneous diseases caused by an impaired insulin secretion and/or action. It is characterized by hyperglycemia and derangements in carbohydrate, lipid and protein metabolism, causing the development of numerous complications resulting in increased morbidity and mortality of patients[1]. According to the World Health Organization data, 422 million adults live with DM worldwide and global prevalence is estimated to rise to 592 million by 2035[2,3].
The liver has a key role in glucose metabolism. It is responsible for the maintenance of total carbohydrate stores and for gluconeogenesis. Regulation of the complex and interdependent glycolytic-gluconeogenic pathways is dependent on multiple factors, including hormonal status and the relative availability of nutrient substrates[4].
The association between DM and liver disease is well established and complex in nature. Insulin resistance (IR) and type 2 DM (T2DM) within the metabolic syndrome are important risk factors for the development of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC)[5]. On the other hand, liver cirrhosis of any etiology can cause impaired glucose regulation due to diminished liver function, and the term hepatogenous diabetes has been proposed for this entity[6,7]. However, it is sometimes difficult to establish this diagnosis, due to the aforementioned bidirectional relationship between impaired glucose metabolism and chronic liver disease.
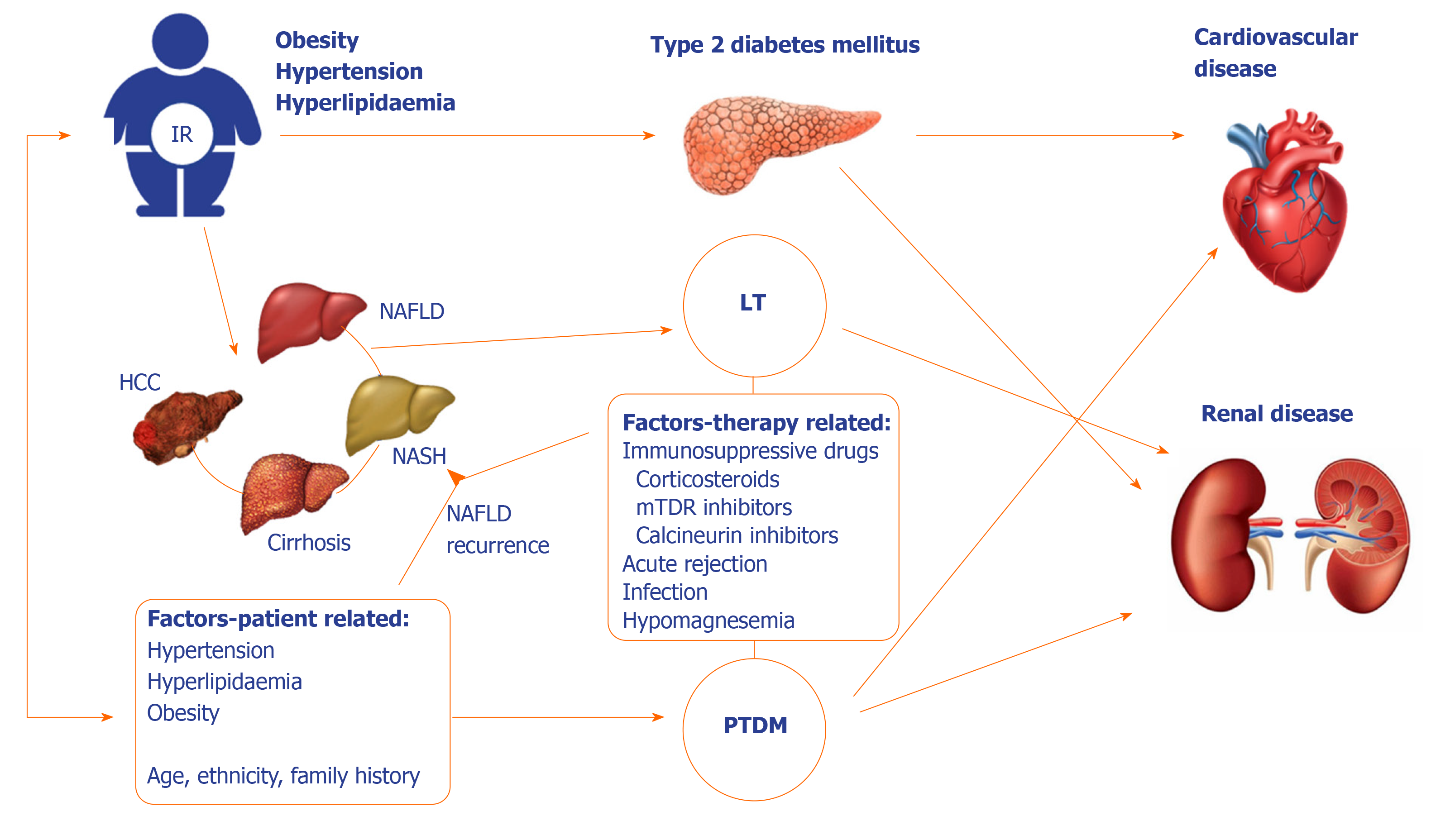
Liver transplantation (LT) is a well-established treatment option for end-stage liver disease of any etiology. Following LT, glucose metabolism can improve and DM resolve, especially in patients with diabetes following liver cirrhosis development. However, this may not be true for patients with pre-existing diabetes, and to further complicate this relationship, DM may develop post-transplant, due to genetic factors and lifestyle changes of the patient, immunosuppressive treatment, donor-dependent and procedure-related factors (Figure 1).
The complex relationship and mechanisms involved in the interplay between DM and liver disease before and after LT will be explored, and related to management issues, newer treatment options and patient outcomes.
The global prevalence of metabolic syndrome characterized by the morbidity cluster of obesity, T2DM, hypertension and dyslipidemia is rapidly rising, reflecting the changes in eating habits and inclination towards sedentary lifestyle. In parallel, its liver manifestation NAFLD is becoming the most common cause of chronic liver disease and the leading cause of liver-related mortality[8]. The global prevalence of NAFLD is now estimated around 25%, the highest being in South America and in the Middle East (30%-31%) and the lowest in Africa (13%)[9].
NAFLD encompasses a wide spectrum of histological changes in the liver, ranging from simple steatosis or non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis, fibrosis and finally cirrhosis[10]. Approximately 25% of NAFLD patients develop NASH, and 5% progress to cirrhosis. Severe liver-related events, such as death or need for LT are relatively uncommon, and occur in 1-2% of patients with NAFLD. However, given its high global prevalence, NAFLD has become the fastest growing indication for LT[11].
The pathogenesis of NAFLD is multifactorial and complex, with multiple mechanisms involved in the development and progression of the disease[12,13]. Genetic factors, dietary habits and other environmental factors lead to obesity and insulin resistance (IR), which is the key mechanism responsible for the NAFLD forming cascade of events. Impaired inhibition of adipose tissue lipolysis with consequent increased influx of free fatty acids into the liver together with increased hepatic de novo lipogenesis outweigh fatty acid oxidation and triglyceride secretion, resulting in the accumulation of triglycerides in hepatocytes[12,13]. Increased lipotoxicity causes mitochondrial dysfunction, oxidative stress and production of reactive oxygen species; these changes result in a more severe form of NASH[14]. Obesity and insulin resistance are associated with alterations in gut microbiota and changes in intestinal permeability with consequent activation of inflammatory pathways and release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin 1 and 6[12,13]. Changes in the secretion of adipokines, hormones derived from adipose tissue, such as adiponectin, leptin, resistin or ghrelin, contribute to chronic inflammatory state which consequently leads to further liver injury, where long-standing liver damage and repair responses result in activation of hepatic stellate cells and deposition of fibrous matrix, leading to cirrhosis and development of HCC[15,16]. However, although steatosis in NAFLD usually precedes inflammation, it has been recognized that hepatic inflammation and fibrosis can exist without steatosis[12,17]. Numerous and diverse processes which contribute to the development of steatosis and liver inflammation occur in parallel, and it is possible that different risk of progression of simple steatosis or NASH to more advanced liver disease may actually allude that these two are separate entities in terms of pathogenesis[12,17].
Given the high burden of NAFLD and its consequences, end-stage liver disease and HCC, NAFLD represents a growing indication for LT both in Europe and the United States. In the near future, the prevalence of NAFLD as indication for LT is expected to rise due to increasing prevalence of obesity, T2DM and metabolic syndrome, but also because of lack of symptoms and reliable non-invasive tests for the timely and accurate diagnosis, as well as lack of effective treatment options that could significantly alter the course of the disease. The problem is augmented by the reportedly high rates of NAFLD recurrence after LT, ranging between 30%-100% of NAFLD liver transplant recipients, and de novo occurrence of NAFLD in 20%-30% of patients undergoing LT for other indications[18-20].
T2DM is an important risk factor for the development of NAFLD, and vice versa. NAFLD is associated with up to five-fold increased risk of T2DM[21]. The prevalence of DM is higher (48.4%) in NASH than in other indications waitlisted for LT. Moreover, NASH patients are more likely to be white, female, older, and with higher body mass index[22]. Concomitant NAFLD in patients with diabetes often compromises achievement of desired glycemic targets and moreover aggravates complications such as chronic kidney disease by almost 2-fold; these factors later on contribute to overall poor treatment outcomes[23,24].
Available evidence suggests an intimate relationship between NAFLD/NASH, T2DM, cardiovascular disease (CVD) and chronic kidney disease[25]. This is not a surprise since NAFLD/NASH and T2DM share many metabolic risk factors with cardiovascular and chronic kidney disease, such as insulin resistance, atherogenic dyslipidaemia, hypertension and obesity. Different pathophysiological mechanisms have been described that contribute to CVD and chronic kidney disease development in patients with NAFLD/NASH and T2DM and this topic has been extensively reviewed elsewhere[25-27]. Although epidemiological evidence from many primarily cross-sectional, case-control and retrospective studies involving NAFLD patients with and without diabetes have shown an increased prevalence of CVD and chronic kidney disease, the complex interplay between the traditional cardiometabolic risk factors makes a causal relationship between NAFLD, T2DM, and cardiovascular and chronic kidney disease sometimes difficult to establish[28,29]. In the Multi-Ethnic Study of Atherosclerosis (MESA), NAFLD proved as independent risk factor for latent atherosclerosis, chronic inflammation and coronary artery calcium scores[30]. Similarly in the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD), the fatty liver index was a predictor of incident CVD in the middle-aged men over a median of 17-year follow-up[31]. In addition, data coming from observational studies showed the association between NAFLD and significant increase in the risk of incident chronic kidney disease[32].
Liver cirrhosis represents the end-stage liver disease caused by various etiologies and mechanisms of liver injury that lead to inflammation, necrosis, and consequent fibrogenesis. It is an important cause of morbidity and mortality worldwide, with significant regional differences in both the prevalence and etiology of the disease. Cirrhosis represents the 14th most common cause of death in adults and results in 1.03 million deaths per year[33].
T2DM and IR are important risk factors for the development of NAFLD and NASH, which can progress to liver cirrhosis. On the other hand, IR and hyperinsulinemia are common features in cirrhosis[6,34]. Recent studies using current criteria for DM diagnosis have shown that, based on fasting plasma glucose levels, alone or associated with measurements of hemoglobin (Hb) A1c, prevalence of DM in cirrhotic individuals is approximately 30%-40%, but if individuals were subjected to an oral glucose tolerance test, the prevalence of abnormal glucose tolerance and DM would rise up to 96%[35].
Although it has been recognized that liver cirrhosis of any etiology represents a risk factor for the development of DM, the mechanisms responsible for the development of cirrhosis-associated DM are multiple and not completely understood[6,7]. A body of evidence suggests that liver dysfunction in cirrhosis directly affects insulin secretion, clearance and insulin sensitivity, but certain etiological agents may cause IR in the earlier, pre-cirrhotic stages, through various mechanisms[6,36-39].
Numerous epidemiological, clinical and experimental studies have reported hepatitis C virus to be strongly associated with hepatic steatosis, IR and T2DM, even before cirrhosis development[40]. HCV induces IR in the liver and peripheral tissues through multiple mechanisms. A large meta-analysis of 34 studies confirmed a positive correlation between HCV infection and increased risk of T2DM in comparison to the general population[41,42]. The prevalence of T2DM was also reported to be higher in patients with chronic hepatitis caused by HCV compared to subjects with chronic liver diseases from other etiologies[6,43]. However, prevalence of T2DM was higher in HCV infected patients following cirrhosis development, compared to earlier stages of fibrosis, and was similar to patients with cirrhosis of other etiologies, thus suggesting that diabetogenic potential of liver dysfunction was even higher than that of hepatitis C virus itself[6]. HCV related IR and steatosis appear to be associated with progression of fibrosis and development of hepatocellular carcinoma[40].
Alcohol is known to reduce insulin-mediated glucose uptake upon acute ingestion, although the effects of chronic alcohol intake are less well understood[44]. Patients with chronic consumption of alcohol frequently suffer from chronic pancreatitis and damage to pancreatic islet β-cells can result in diabetes development[44]. The risk of developing DM seems to be related to the amount of alcohol ingested and increased twofold in persons with high alcohol intake compared to those with moderate intake[45]. Prevalence of diabetes among patients with alcohol-related liver cirrhosis according to studies ranges between 10% and 40%, with an average prevalence of around 27%[46].
Hereditary haemochromatosis is a metabolic disorder characterized by iron accumulation and deposition in several organs. Haemochromatosis as well as alcohol consumption induce iron deposition in the liver which could interfere with the ability of insulin to suppress hepatic gluconeogenesis. However, the pathogenesis of diabetes due to haemochromatosis has also been related to concomitant injury of pancreatic β-cells caused by iron deposits[6]. For this reason DM represents a clinical manifestation of haemochromatosis, and can be found in 40% do 85% of patients in advanced stages[44,47,48].
Unlike chronic HCV infection, the relationship between hepatitis B virus infection (HBV) and insulin resistance has not been completely understood as the studies produced conflicting results[49]. However, most studies concur that the incidence of DM in patients with HBV infection is not increased compared to patients without infection indicating that HBV may not influence the development of DM[49]. However, a meta-analysis comprising results of 15 studies found that the prevalence of T2DM among HBV infected patients was 1.3 times higher and concluded that chronic HBV infection does increase the risk of T2DM development[50]. The prevalence of DM is higher among patients with HBV who developed cirrhosis (22.2%), compared to those without cirrhosis (12.8%)[51]. The pooled prevalence of DM in patients with HBV-related cirrhosis was around 22.2% (ranging from 9.4% to 35.0% in various studies)[46]. This was significantly lower than in patients with cirrhosis related to NAFLD which had the highest prevalence of DM (a pooled estimate of 56.1%), or alcohol-related, HCV or cryptogenic cirrhosis (estimated prevalence ranging from 27.3% to 50.8%). The lowest prevalence of DM was found in patients with cirrhosis secondary to cholestatic liver disease (overall 7.1%, ranging from 1.3% to 15.2%)[46].
Regardless of the etiology of cirrhosis, and irrespective of which developed first, when cirrhosis and DM coexist, they interfere with each other with reflection on course of the disease. In a population-based Italian study, the risk of death from cirrhosis was significantly higher among patients with DM[52]. A number of prospective and retrospective studies indicated that complications of liver disease (spontaneous bacterial peritonitis and other bacterial infections, ascites, hepatic encephalopathy, and gastrointestinal hemorrhage) as well as death rates are higher in cirrhotic patients with DM compared to non-diabetic patients with advanced liver disease[6]. Moreover, DM associated with poor glycemic control (HbA1c > 9%) is an independent risk factor for development of HCC in patients with chronic liver disease[53] although poor glycemic control in cirrhotic patients is not necessarily reflected through high HbA1c, but rather higher glucovariability[54]. Therefore, both uncontrolled and controlled T2DM as well as glucose intolerance can negatively impact survival of patients with liver cirrhosis[52,55]. The mechanisms involved are still not clear. Plausible explanation is that insulin resistance alone, even without the element of hyperglycemia increases adipokine production through chronic inflammation, such as leptin and TNF alpha leading to activation of TGF beta1, a profibrotic cytokine in turn activating hepatic stellate cells to produce high quantities of collagen and extracelullar matrix proteins. Of course, all these unfavorable effects of chronic inflammation mediated through IR are then largely potentiated by chronic hyperglycemia[56-61].
Post-transplant DM (PTDM) has been recognized as a major complication after LT with the potential for serious consequences and ultimately worse patient and graft survival[62]. It seems that in case of LT, the onset of PTDM is rapid; 50% of cases occurring within first 6 months and 75% after 12 mo following procedure[63]. Although IR and DM related to liver dysfunction and cirrhosis can improve following LT, the presence of metabolic syndrome and extrahepatic comorbidities, as well as immunosuppressive drugs can contribute to persistent or de novo DM following LT. It occurs in 7%-45% of liver transplant recipients and is associated with increased morbidity, health care costs and impairs both patient and graft survival[62-64]. So far, parameters such as advanced age, ethnicity, family history, body mass index, hepatitis C virus infection and immunosuppressive drugs (mainly corticosteroids and tacrolimus) have all been reported as risk factors for PTDM after LT[65,66]. Moreover, obesity, either predating or gained after transplantation, is associated with an increased risk for PTDM but also post-transplant NAFLD[67]. According to 2014 International Consensus Meeting diagnosis of PTDM can be established in patients after achieving stable doses of maintenance immunosuppressants, with stable allograft function, once they have been discharged from the hospital to avoid misdiagnosis of transient hyperglycemia[64]. Preferred criteria to diagnose PTDM (either fasting plasma glucose > 7 mmol/L, random plasma glucose > 11.1 mmol/L accompanied by symptoms or 2-hour post 75g oral glucose test plasma glucose > 11.1 mmol/L) are those of American Diabetes Association/World Health Organization criteria for the diagnosis of diabetes, and HbA1c > 6.5% should not be used alone to screen for PTDM within the first year after transplant.
As we enter an era where recurrent hepatitis C after LT becomes a rare entity and de novo or recurrent NAFLD increases in incidence, we are likely to see changes in the patterns of incidence and impact of PTDM. Current data suggest that PTDM increases the risk of infection, chronic renal failure, biliary complications and decreases survival[68,69]. Poor LT outcomes seem not only to be related to PTDM, but also to much earlier derangements in the pathophysiology of hyperglycemia, during IR stage[70]. Still, nearly 30% of deaths in LT patients are caused by cardiovascular or cerebrovascular diseases[71].
After LT, NAFLD/NASH may re-occur or develop de novo. Histological features of recurrent or de novo NAFLD/NASH are the same as in native livers, and there are no features to differentiate recurrent from de novo NAFLD/NASH in the graft. The diagnosis is based on correctly identifying preexisting liver disease – NASH-related or in some cases cryptogenic cirrhosis[72]. The studies assessing NAFLD recurrence after LT report a wide range from 10 to 100%[19,20,73-75]. However, regardless of indication for LT, risk factors for metabolic syndrome and NAFLD increase over time in the post-transplant setting[76,77]. Side-effects of immunosuppressive medications and rapid weight gain in the post-transplant period can lead to impaired glucose control, dyslipidemia, development of metabolic syndrome and de novo NAFLD. In fact, it has been reported in 20%-30% of patients following LT for non-NAFLD/NASH etiologies[18,19]. Weight gain is common in patients following LT and 30%-60% of patients become overweight or obese, with a mean weight gain of 2–9 kg usually within the first year after LT. After one year post-LT, weight gain typically slows down[18,78-81]. Obesity appears to be one of the strongest risk factors for the development of NAFLD in the post-transplant period[18,20]. Moreover obese patients after LT have a 2-fold higher risk of mortality compared to normal weight LT recipients[82]. The mechanisms leading to a post-transplant weight gain are driven by a complex interaction of genetic, physiological, behavioral, and environmental factors[83,84]. Dyslipidemia and hypertension occur in 40%–70% of patients, usually related to use of mammalian target of rapamycin (mTOR) inhibitors and calcineurin inhibitors. Metabolic syndrome has been reported in 40%–50% of patients after LT, both in patients with previous NAFLD as well as other etiologies of chronic liver disease. In a recent report from Mayo Clinic, 84.6% of patients had de novo allograft steatosis and 15.4% recurrent NAFLD after 2 months post-liver transplant. After 10 years, 48% of liver transplant recipients had evidence of allograft steatosis, including 77.6% of patients transplanted for NASH and 44.7% of individuals transplanted for other indications[20]. Interestingly, in this study allograft steatosis was not associated with worse outcomes regarding graft and patient survival. However, the incidence of cardiovascular events 5 years post-transplant was approximately 40% in patients transplanted for NASH compared to 5%-10% incidence in LT recipients transplanted for non-NASH etiologies, regardless of graft steatosis[20]. This could suggest that impaired metabolic profile before LT could have more significant impact on CV events and outcomes than the post-transplant NAFLD itself. However, the long-term outcomes and consequences of complex interplay between T2DM, obesity, metabolic syndrome, pre- and post-liver transplant NAFLD with CV events require further investigation and explanation.
Treating type 2 diabetes, although now with available wide range of armamentarium, still remains challenging, especially in the presence of coexisting NAFLD and obesity. Acknowledging the fact that NAFLD and T2DM act synergistically in driving adverse outcomes, in the way that the NAFLD accelerates the development of diabetic chronic micro- and macrovascular complications, while the diabetes increases the likelihood of more severe forms of NAFLD such as steatohepatitis, cirrhosis, and HCC could be an important issue in choosing the treatment modality and pharmacological agent[24,85-89]. Therefore, when taking into account pathophysiology, treating NAFLD/NASH could prevent T2DM development and/or progression, but, also the other way around[90].
As there is no specific pharmacotherapy yet available for treating NAFLD, anti-diabetic drugs with pleiotropic effects, targeting multiple metabolic disorders, seem promising. Among them, sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) are recognized by American Diabetes Association (ADA) and European Association for Study of Diabetes (EASD) as the best treatment option for patients with T2DM and cardiovascular disease, heart failure and/or chronic kidney disease. These two classes of drugs both exert weight loosing effect, making them especially interesting for patients with associated obesity, and according to results of both randomized controlled trials (RCTs) and open-label studies, offer promising effects in reducing liver fat content[91].
Previously used agents such as metformin and thiazolidinediones (TZDs), affecting insulin resistance, did show benefit on biochemical and metabolic features of NAFLD, but improvement of patients’ histological response or fibrosis was modest and studies were usually short-term therefore lacking information on liver-related long-term outcomes. Moreover, side-effects usually override their wider use in NAFLD patients[92-95]. Especially interesting results came from studies with NASH patients receiving TZDs. Mentioned agents activate the nuclear receptor of peroxisome proliferator-activated receptor gamma (PPARγ) located in adipose, liver and muscle tissue and exert insulin-sensitizing activity. Additionally, TZDs increase adiponectin, reduce liver fat content and modulate intra hepatic inflammation[94]. Recently published large cohort study in Taiwan using propensity score matching suggested patients with newly diagnosed T2DM receiving either rosiglitazone (not widely used anymore due to associated increased CV risk) or pioglitazone for mean of 3.84 years, compared to newly diagnosed T2DM patients that were non-TZD users, had lower risk of hepatic cirrhosis (incidence rate: 0.77 vs 1.95 per 1000 person-y)[96].
One of the newer therapeutic modalities that needs to be addressed is certainly pre- transplant bariatric surgery in the obese patients with or without diabetes. So far, available data for this particular subset of patients are scarce and data from randomized clinical trials are lacking. However two major points should be considered regarding bariatric procedure in liver transplant (LT) – timing and type of procedure. In a study including 37 patients who underwent LT, and 7 who underwent LT combined with sleeve gastrectomy (SG), majority of patients with LT alone had weight gain to BMI > 35, post-LT diabetes and steatosis with 3 deaths and 3 grafts losses as compared to patients undergoing the combined procedure, who had no graft losses and there were no developed post-LT DM or steatosis, with significant weight loss (mean BMI = 29) after 12 months of follow up. One patient developed a leak from the gastric staple line, and one had excess weight loss. Average time from SG to LT was 17 months[97]. This was confirmed in other study performed in liver and kidney transplant patients who underwent SG 16 months before transplant[98]. There are also some small studies available investigating SG after solid organ transplant showing favorable outcomes regarding weight loss and PTDM incidence[99-101].
In a large cohort study comparing in-hospital mortality and length of stay for patients with no cirrhosis, compensated cirrhosis, and decompensated cirrhosis, who underwent bariatric surgery, patients with decompensated cirrhosis had highest postoperative mortality rate compared to those with compensated and non-cirrhosis patients (16.3% vs 0.9% and 0.3%, respectively, P = 0.0002)[102].
Therefore, we can conclude that sleeve gastrectomy is procedure of choice in liver transplant patients with compensated liver cirrhosis since it does not affect absorption compared to other operative modalities and provides beneficial effects in terms of weight reduction, development of PTDM and liver steatosis.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a newer class of antidiabetic medications with a spectrum of beneficial metabolic effects. Besides improving glycaemia by promoting glucose-dependent insulin secretion and inhibition of glucagon secretion, these agents promote weight loss by increasing satiety through central nervous system, delaying gastric emptying and potentially increasing thermogenesis in brown fat tissue. Moreover, GLP-1RAs have proven cardiovascular safety, while some representatives of the class have shown additional cardiovascular benefits/protection in large cardiovascular outcome trials[103-106].
Although receptors for the GLP-1 on the hepatocytes are still a matter of scientific dispute, they appear to have a direct effect on the lipid metabolism of hepatocytes, which leads to reduction of hepatic steatosis and their potential in NAFLD treatment[107-110]. Of additional interest in NAFLD treatment would be their lipid-lowering and anti-inflammatory potential[111,112].
In patients with NAFLD intrahepatic triglyceride accumulates, while hepatic as well as muscle insulin sensitivity declines, which correlates with the IR at the adipose tissue level and its dysfunction. This was a scientific context for assessing the potential of GLP-1Ras in the treatment of NAFLD/NASH from which optimistic results showing reduction in liver enzymes and intrahepatic triglycerides content in patients with T2DM first came[113-116]. It seems that GLP-1RA inhibit dysfunctional endoplasmic reticulum stress response to fatty-acids in hepatocytes and therefore provide protective effects on hepatocytes[110]. In human studies including exenatide (5-10 μg/d) improvements in weight, body mass index, glycemic control, liver enzymes, hsCRP (high-sensitivity C-reactive protein) and adiponectin were seen in patients with ultrasound diagnosed NAFLD treated with GLP-1RA compared to metformin[117], Table 1.
| Therapy effect | NAFLD | T2DM | PTDM | Post-LT liver disease |
| GLP-1RA proven | Improvement in liver enzymes and intrahepatic triglycerides content[113-116,130,131] | Improvement of glycemia; increase insulin secretion in a glucose-dependent manner; inhibit glucagon secretion; weight loosing effect; cardiovascular protection[103-106] | Improvement of insulin and normalization of glucagon concentrations[150] | None metabolized by liver, no dose adjustments needed[156] |
| Resistance against toxicity of IS drugs in vitro[154] | ||||
| Weight loss during first weeks after LT[158] | ||||
| Improvements in weight, body mass index, glycemic control, liver enzymes, hsCRP[117] | ||||
| Prevention of steroid diabetes[155] | ||||
| Improvement in liver histology[118-120] | ||||
| Resolution of NASH[122,127-129] | ||||
| GLP-1RA possible | Reduction of hepatic steatosis; anti-inflammatory effect[107-112] | Improvement of cardiovascular outcomes[103-106], GI side effects potentiating GI disturbances caused by IS drugs[157] | ||
| SGLT-2i proven | Reduction of liver enzymes[65,135-142] | Improvement of glycemia; weight loosing effect; decrease in systolic and diastolic blood pressures; cardiovascular benefit[132-134] | Reduction of weight and blood pressure[162] | |
| Improved glycemia[163] | ||||
| Reduction of body weight and body fat[143-147] | ||||
| SGLT2i possible | Reduction of oxydative stress and inflammation[143-147] | Genitourinary infections[164] | Reduction in fat mass and visceral adipose tissue[165] |
Moreover, smaller studies suggested beneficial effects of exenatide and liraglutide on liver histology[118-120]. Regarding short acting GLP-1RA lixisenatide, studies in obese or overweight T2DM patients reported increase in the proportion of patients achieving normalization of ALT[121], but there is no data on lixisenatide effects on hepatic steatosis or fibrosis.
The first RCT confirming the efficacy of GLP-1RA in the resolution of NASH in patients with biopsy-proven diagnosis was the LEAN trial (Liraglutide Efficacy and Action in NASH) which followed patients for 48 weeks while taking liraglutide 1.8 mg daily[122]. Resolution was achieved in 39% of patients in the active arm compared with 2/22 (9%) participants in the placebo group. It seems that the improvements in liver fat were greater with prolonged duration of liraglutide treatment[119,123-126].
Similar results were suggested in the recently published large scale RCT with semaglutide, a once weekly GLP-1RA including patients with biopsy-proven NASH[127]. There is currently no published data available on oral form of semaglutide regarding the effect on NAFLD/NASH, although it shows superiority over liraglutide in terms of weight loss[128,129].
Another long acting GLP-1RA with a once weekly dosing, dulaglutide, according to results of a Japanese retrospective case series study, and results from the AWARD (Assessment of Weekly Administration of LY2189265 (dulaglutide) in Diabetes) development program seems promising for NASH patients in terms of reduction of liver enzymes and liver stiffness (measured by transient elastography)[130,131]. So far, no data is available for albiglutide (another once weekly administered GLP-1 RA) on NAFLD/NASH features.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are widely used oral anti diabetic agents targeting hyperglycemia in an untraditional way, by enhancing glycosuria (with the daily glucose loss ranging between 60 and 80 g). In addition to blood glucose lowering effect, glycosuria also enhances calorie loss (240–320 kcal) and thus gliflozins exert a clinically significant weight-lowering effect. Moreover, their osmotic diuretic and natriuretic effects contribute to plasma volume contraction, and decrease in both systolic and diastolic blood pressures. In the large, recently published cardiovascular outcome trials (EMPA-REG OUTCOME trial with empagliflozin, CANVAS Program with canagliflozin, DECLARE-TIMI 58 with dapagliflozin) SGLT2i proved cardiovascular benefits, primarily seen through the reduction of heart failure risk and studies assessing their renal protective effects are on their way[132-134].
SGLT2 inhibitors as a class have shown promising results in open-label studies and RTCs in reducing liver fat[92,135,136]. Majority of mentioned studies used biochemical markers, but some also employed different imaging techniques for assessing fatty liver content and fibrosis, while one small pilot study conducted liver biopsy to determine the hepatic effects of SGLT2 use[137-140]. Gliflozins were effective in reducing liver enzymes in diabetic patients, and more pronounced results were seen in those with accompanying NAFLD[141]. The mechanisms underlying the improvement of NAFLD with SGLT2 inhibitors remain unknown and can only be speculated, Table 1.
It seems that SGLT2 inhibitors, compared to other oral antidiabetic agents with comparable glycemic control, offer more pronounced reductions in serum liver enzymes[142]. Mentioned effect, exceeding glucose-lowering activity, relies on other mechanisms including reduction of body weight and body fat, reduction of serum uric acid, oxidative stress and inflammation which might prove important in NAFLD treatment[143-147].
There are no specific guidelines for treatment of PTDM thus general re-commendations for diabetes management are followed due to paucity of data regarding usage of antihyperglycemic drugs in transplanted patients.
The main challenge in treatment of post-transplant diabetes is increased cardiovascular risk observed in patients after solid organ transplantation[148]. Due to severe metabolic disturbances caused by weight gain after transplantation and immunosuppressant drugs incidence of cardiovascular events is higher than in non-transplant patients and are the leading cause of mortality[149]. Given that recent data from large cardiovascular outcome trials demonstrated cardiovascular benefits for majority of long acting GLP-1 RA (liraglutide, semaglutide, albiglutide and dulaglutide[103-106]) in terms of reducing 3 point major adverse cardiovascular endpoints comprised of non-fatal myocardial infarction, non-fatal stroke and cardiovascular mortality this therapeutic option has its merits in treatment of PTDM.
Presently, there are no clinical trials published regarding GLP 1RA therapy in PTDM. The only studies available are conducted in case series of patients after kidney and pancreas transplant providing enough scientific rationale for this treatment concept in liver PTDM[150-153]. In a study performed by Halden et al[150] in patients with and without PTDM after kidney transplant GLP infusion in hyperglycemic clamp led to improvement of insulin and normalization of glucagon concentrations. Furthermore, GLP 1RAs also exhibit resistance against toxicity of immunosuppressive drugs in vitro[154] and seem to prevent steroid diabetes by diminishing glucocorticoid-induced glucose metabolism impairment and beta-cell dysfunction in healthy humans[155].
Since long acting GLP 1RA agonists are eliminated through enzymatic degradation and renaly cleared, drug-drug interactions are avoided which is extremely important in terms of immunosuppressant drug metabolism[156]. However, by delaying gastric emptying, absorption of other drugs could be affected. In a two studies of case series of kidney transplant recipients with PTDM safe co-administration of liraglutide and tacrolimus was reported mitigating, at least partially, concerns regarding absorption interference[152,153].
One of the major downsides of GLP 1RA therapy are gastrointestinal (GI) side effects which could potentiate a GI disturbances caused by immunosuppressant drugs leading to limited usage of GLP 1 RA in spite of their numerous beneficial effects[157], Table 1.
Pharmacokinetic and pharmacodynamic properties of GLP 1 RA seems to be of extreme convenience in LT since none are metabolized by the liver and no dose adjustment is needed[156]. As already mentioned, there is growing body of evidence suggesting beneficial effects of GLP 1RA therapy in patients with NAFLD/NASH affecting not only liver enzymes normalization but also reducing inflammation and progression of fibrosis[122,127,130,131]. There are two different aspects of potential mechanisms of action involving GLP 1RA in post-transplant NAFLD. For one, improvement of metabolic disturbances through induction of weight loss, especially seen during first few weeks after transplantation[158]. Thus, lipogenesis is decreased leading to a reduction in free fatty acid and triglyceride toxic metabolites positively affecting insulin resistance, oxidative stress and inflammation, while increasing insulin secretion initiating changes in gut liver axis which are not completely understood yet[4]. The second relates to direct anti-fibrotic effects independent of metabolic changes recently shown in animal and human studies[159]. Unfortunately there are no clinical studies performed in post-transplant NAFLD/NASH patients, although based on present knowledge this therapeutic approach would be completely justified, Table 1.
SGLT2 inhibitors have recently been recommended as a treatment of choice for patients with diabetes and high cardiovascular risk, which is also a characteristic of patients with PTDM[160]. The evidence of the use of gliflozins in PTDM in LT patients is scarce, but results from animal studies are promising. Rats with tacrolimus-induced diabetes that received empagliflozin had lower glycemia and increased plasma insulin levels[161]. In a case series of patients with post heart transplant diabetes mellitus, empagliflozin reduced weight, and blood pressure[162]. In a small group of kidney recipients, canagliflozin treatment improved glycemia, reduced weight and blood pressure[163]. Recently published study using empagliflozin in a larger population of PTDM patients after kidney transplantation showed improvement of glycemic control compared to placebo, and a concomitant reduction of body weight[164]. Major potential problem with SGLT2 inhibitors in PTDM might be genitourinary infections, considering concurrent immunosuppressive therapy and glycosuria. Up to date collected data, mainly in the kidney transplant patients with PTDM suggests incidence of the GU infections is not higher than that seen in T2DM patients and resolves with usual antibiotics/antifungal treatment[164], Table 1.
Currently, SGLT2 inhibitors have not the indication of improving NAFLD and dedicated research is mandatory in this evolving field. In several RCTs SGLT2 inhibitors have shown positive effects on NAFLD in patients with T2DM, but the exact mechanisms are still speculative, as is their role in treating post-transplant NAFLD in patients with and without diabetes[165]. SGLT2 inhibitors are cleared by the liver, but pharmacokinetic trials involving patients with mild to moderate hepatic impairment demonstrated their safety and no need for dose adjustments in the setting of liver disease[166]. Besides safety, by reducing liver fat content and hepatocyte injury biomarkers which was shown in several gliflozin RCTs they might prove liver protective and improve NAFLD-associated endoplasmatic reticulum stress and mitochondrial function[91]. Additionally, SGLT2 inhibitors are associated with significant weight loss, especially reduction in fat mass and visceral adipose tissue, which makes them interesting agents in treating post-transplant NAFLD in LT patients. Additionally, they attenuate the renin-angiotensin-aldosteron pathway by inhibition of sodium reabsorption which in theory makes the SGLT2 inhibitors ideal for patients with cirrhosis[165], Table 1.
In conclusion, pharmacological management of PTDM is further complicated given there are no published randomized clinical trials regarding effectiveness and safety of antihyperglycemic agents. Particular characteristics of PTDM include prodiabetogenic potential of immunosuppressant drugs as well as increased cardiovascular risk due to higher incidence of metabolic disturbances present in post-transplant period both of which could be successfully resolved with GLP 1RA and SGLT2i therapy. Further prospective studies including larger number of patients are needed.
Considering that currently NASH is one of the main causes of LT and it is associated with metabolic disorders mainly IR, which is a particular problem in transplanted patients, GLP 1 RA and SGLT2i treatment certainly has the potential to influence precisely this bidirectional relationship between PTDM and preexisting liver disease.
The authors would like to thank Hrvojka Dolic for the help with the graphics.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koch T, Reggiani GM S-Editor: Gong ZM L-Editor: A E-Editor: Ma YJ
| 1. | Smirčić-Duvnjak L, Vučković-Rebrina S, Duvnjak M, Smirčić-Duvnjak L (editors): Gastrointestinal Complications of Diabetes. Duvnjak M, Smirčić-Duvnjak L (editors): Gastrointestinal Complications of Diabetes. A Comprehensive Guide. Switzerland: Springer International Publishing AG, 2018: 3-27. |
| 2. | World Health Organization. Global Report on Diabetes. WHO Press, World Health Organization, 2016. Available from: URL: http://www.who.int. |
| 3. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2956] [Article Influence: 268.7] [Reference Citation Analysis (1)] |
| 4. | Virović-Jukić L, Živković M. Liver Function. In: Duvnjak M, Smirčić-Duvnjak L (editors): Gastrointestinal Complications of Diabetes. A Comprehensive Guide. Switzerland: Springer International Publishing AG, 2018: 267-274. |
| 5. | Virović-Jukić L, Forgač J, Ogresta D, Filipec-Kanižaj T, Mrzljak A, Duvnjak M. Duvnjak M. Clinical Manifestations of Liver Disease in Diabetes Mellitus. In: Duvnjak M, Smirčić-Duvnjak L (editors): Gastrointestinal Complications of Diabetes. A Comprehensive Guide. Switzerland: Springer International Publishing AG, 2018: 275-315. |
| 6. | Orsi E, Grancini V, Menini S, Aghemo A, Pugliese G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. 2017;37:950-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (3)] |
| 7. | Grancini V, Trombetta M, Lunati ME, Boselli ML, Gatti S, Donato MF, Palmieri E, Resi V, Pugliese G, Bonadonna RC, Orsi E. Central role of the β-cell in driving regression of diabetes after liver transplantation in cirrhotic patients. J Hepatol. 2019;70:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 8. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 9. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8003] [Article Influence: 727.5] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3179] [Article Influence: 353.2] [Reference Citation Analysis (4)] |
| 11. | Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090-1099.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 12. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2116] [Article Influence: 235.1] [Reference Citation Analysis (1)] |
| 13. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 14. | Bedossa P. Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why liver biopsy is essential. Liver Int. 2018;38 Suppl 1:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights. Curr Pharm Des. 2013;19:5239-5249. [PubMed] |
| 16. | Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, Smirčić Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:18070-18091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 252] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 18. | Pais R, Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 328] [Article Influence: 36.4] [Reference Citation Analysis (1)] |
| 19. | Dureja P, Mellinger J, Agni R, Chang F, Avey G, Lucey M, Said A. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Narayanan P, Mara K, Izzy M, Dierkhising R, Heimbach J, Allen AM, Watt KD. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation. 2019;103:e14-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 22. | Cholankeril G, Wong RJ, Hu M, Perumpail RB, Yoo ER, Puri P, Younossi ZM, Harrison SA, Ahmed A. Liver Transplantation for Nonalcoholic Steatohepatitis in the US: Temporal Trends and Outcomes. Dig Dis Sci. 2017;62:2915-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Afolabi BI, Ibitoye BO, Ikem RT, Omisore AD, Idowu BM, Soyoye DO. The Relationship Between Glycaemic Control and Non-Alcoholic Fatty Liver Disease in Nigerian Type 2 Diabetic Patients. J Natl Med Assoc. 2018;110:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Mantovani A, Rigolon R, Mingolla L, Pichiri I, Cavalieri V, Salvotelli L, Stoico V, Zoppini G, Bonora E, Targher G. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J Diabetes Complications. 2017;31:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 803] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 26. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [PubMed] |
| 27. | Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638-652. [PubMed] |
| 28. | Marcuccilli M, Chonchol M. NAFLD and Chronic Kidney Disease. Int J Mol Sci. 2016;17:562. [PubMed] |
| 29. | Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, George J, Barrera F, Hafliðadóttir S, Björnsson ES, Armstrong MJ, Hopkins LJ, Gao X, Francque S, Verrijken A, Yilmaz Y, Lindor KD, Charlton M, Haring R, Lerch MM, Rettig R, Völzke H, Ryu S, Li G, Wong LL, Machado M, Cortez-Pinto H, Yasui K, Cassader M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. [PubMed] |
| 30. | Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, Katz R, Blumenthal RS, Blaha MJ. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Olubamwo OO, Virtanen JK, Voutilainen A, Kauhanen J, Pihlajamäki J, Tuomainen TP. Association of fatty liver index with the risk of incident cardiovascular disease and acute myocardial infarction. Eur J Gastroenterol Hepatol. 2018;30:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, Targher G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2018;79:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 33. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 34. | Kawaguchi T, Taniguchi E, Itou M, Sakata M, Sumie S, Sata M. Insulin resistance and chronic liver disease. World J Hepatol. 2011;3:99-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 35. | Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Letiexhe MR, Scheen AJ, Gérard PL, Bastens BH, Pirotte J, Belaiche J, Lefèbvre PJ. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J Clin Endocrinol Metab. 1993;77:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Petrides AS, Stanley T, Matthews DE, Vogt C, Bush AJ, Lambeth H. Insulin resistance in cirrhosis: prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology. 1998;28:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Kruszynska YT, Home PD, McIntyre N. Relationship between insulin sensitivity, insulin secretion and glucose tolerance in cirrhosis. Hepatology. 1991;14:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Greco AV, Mingrone G, Mari A, Capristo E, Manco M, Gasbarrini G. Mechanisms of hyperinsulinaemia in Child's disease grade B liver cirrhosis investigated in free living conditions. Gut. 2002;51:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 40. | Kralj D, Virović Jukić L, Stojsavljević S, Duvnjak M, Smolić M, Čurčić IB. Hepatitis C Virus, Insulin Resistance, and Steatosis. J Clin Transl Hepatol. 2016;4:66-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 479] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 42. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 43. | Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, Brun JM, Hillon P. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 236] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (6)] |
| 45. | Wei M, Gibbons LW, Mitchell TL, Kampert JB, Blair SN. Alcohol intake and incidence of type 2 diabetes in men. Diabetes Care. 2000;23:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Lee WG, Wells CI, McCall JL, Murphy R, Plank LD. Prevalence of diabetes in liver cirrhosis: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 47. | Adams PC, Kertesz AE, Valberg LS. Clinical presentation of hemochromatosis: a changing scene. Am J Med. 1991;90:445-449. [PubMed] |
| 48. | Milman N, Pedersen P, á Steig T, Byg KE, Graudal N, Fenger K. Clinically overt hereditary hemochromatosis in Denmark 1948-1985: epidemiology, factors of significance for long-term survival, and causes of death in 179 patients. Ann Hematol. 2001;80:737-744. [PubMed] |
| 49. | Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 50. | Cai C, Zeng J, Wu H, Shi R, Wei M, Gao Y, Ma W. Association between hepatitis B virus infection and diabetes mellitus: A meta-analysis. Exp Ther Med. 2015;10:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Xu C, Chen J, Zhang PA. Relationship Between Diabetes Mellitus and Cirrhosis Risk in Chronic Hepatitis B Patients in Wuhan, China. Med Sci Monit. 2019;25:8112-8119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 272] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 53. | Li CI, Chen HJ, Lai HC, Liu CS, Lin WY, Li TC, Lin CC. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes--National cohort of Taiwan Diabetes Study. Int J Cancer. 2015;136:2668-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Honda F, Hiramatsu A, Hyogo H, Aikata H, Daijo K, Teraoka Y, Inagaki Y, Morio K, Kobayashi T, Nakahara T, Nagaoki Y, Kawaoka T, Yoneda M, Tsuge M, Imamura M, Kawakami Y, Ochi H, Chayama K. Evaluation of glycemic variability in chronic liver disease patients with type 2 diabetes mellitus using continuous glucose monitoring. PLoS One. 2018;13:e0195028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, González-González JA, Muñoz-Espinosa LE, Villarreal-Pérez JZ, Maldonado-Garza HJ. Subclinical abnormal glucose tolerance is a predictor of death in liver cirrhosis. World J Gastroenterol. 2014;20:7011-7018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 57. | Flisiak R, Pytel-Krolczuk B, Prokopowicz D. Circulating transforming growth factor beta(1) as an indicator of hepatic function impairment in liver cirrhosis. Cytokine. 2000;12:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 445] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 59. | Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Jonsson JR, Moschen AR, Hickman IJ, Richardson MM, Kaser S, Clouston AD, Powell EE, Tilg H. Adiponectin and its receptors in patients with chronic hepatitis C. J Hepatol. 2005;43:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A, Folli F. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Galindo RJ, Wallia A. Hyperglycemia and Diabetes Mellitus Following Organ Transplantation. Curr Diab Rep. 2016;16:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Lieber SR, Lee RA, Jiang Y, Reuter C, Watkins R, Szempruch K, Gerber DA, Desai CS, DeCherney GS, Barritt AS 4th. The impact of post-transplant diabetes mellitus on liver transplant outcomes. Clin Transplant. 2019;33:e13554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, Berlakovich G, Krebs M, Kautzky-Willer A, Schernthaner G, Marchetti P, Pacini G, Ojo A, Takahara S, Larsen JL, Budde K, Eller K, Pascual J, Jardine A, Bakker SJ, Valderhaug TG, Jenssen TG, Cohney S, Säemann MD. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 65. | Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, Dumortier J, Salamé E, Calmus Y, Maugendre D; Diapason Study Group. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection : an observational multicenter study. Liver Transpl. 2007;13:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation. 2010;89:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Shivaswamy V, Boerner B, Larsen J. Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr Rev. 2016;37:37-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 68. | Lv C, Zhang Y, Chen X, Huang X, Xue M, Sun Q, Wang T, Liang J, He S, Gao J, Zhou J, Yu M, Fan J, Gao X. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 2015;7:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Morbitzer KA, Taber DJ, Pilch NA, Meadows HB, Fleming JN, Bratton CF, McGillicuddy JW, Baliga PK, Chavin KD. The impact of diabetes mellitus and glycemic control on clinical outcomes following liver transplant for hepatitis C. Clin Transplant. 2014;28:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Rosen CB, Heimbach JK, Janssen HL, Charlton MR. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Peláez-Jaramillo MJ, Cárdenas-Mojica AA, Gaete PV, Mendivil CO. Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment. Diabetes Ther. 2018;9:521-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Germani G, Laryea M, Rubbia-Brandt L, Egawa H, Burra P, OʼGrady J, Watt KD. Management of Recurrent and De Novo NAFLD/NASH After Liver Transplantation. Transplantation. 2019;103:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 73. | Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 74. | Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Vallin M, Guillaud O, Boillot O, Hervieu V, Scoazec JY, Dumortier J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl. 2014;20:1064-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Rubín A, Sánchez-Montes C, Aguilera V, Juan FS, Ferrer I, Moya A, Montalva E, Pareja E, López-Andujar R, Prieto M, Berenguer M. Long-term outcome of 'long-term liver transplant survivors'. Transpl Int. 2013;26:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Di Maira T, Rubin A, Puchades L, Aguilera V, Vinaixa C, Garcia M, De Maria N, Villa E, Lopez-Andujar R, San Juan F, Montalva E, Perez J, Prieto M, Berenguer M. Framingham score, renal dysfunction, and cardiovascular risk in liver transplant patients. Liver Transpl. 2015;21:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Rezende Anastácio L, García Ferreira L, Costa Liboredo J, de Sena Ribeiro H, Soares Lima A, García Vilela E, Correia MI. Overweight, obesity and weight gain up to three years after liver transplantation. Nutr Hosp. 2012;27:1351-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 79. | Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 81. | Beckmann S, Nikolic N, Denhaerynck K, Binet I, Koller M, Boely E, De Geest S; Psychosocial Interest Group, Swiss Transplant Cohort Study. Evolution of body weight parameters up to 3 years after solid organ transplantation: The prospective Swiss Transplant Cohort Study. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | van Son J, Stam SP, Gomes-Neto AW, Osté MCJ, Blokzijl H, van den Berg AP, Porte RJ, Bakker SJL, de Meijer VE. Post-transplant obesity impacts long-term survival after liver transplantation. Metabolism. 2020;106:154204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 84. | Beckmann S, Denhaerynck K, Stampf S, Saigi-Morgui N, Binet I, Koller M, Boely E, De Geest S; Psychosocial Interest Group; Swiss Transplant Cohort Study. New-onset obesity after liver transplantation-outcomes and risk factors: the Swiss Transplant Cohort Study. Transpl Int. 2018;31:1254-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 86. | Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 87. | Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 88. | Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 89. | Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 90. | Hu M, Phan F, Bourron O, Ferré P, Foufelle F. Steatosis and NASH in type 2 diabetes. Biochimie. 2017;143:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Scheen AJ. Effect of sodium-glucose cotransporter type 2 inhibitors on liver fat in patients with type 2 diabetes: hepatic beyond cardiovascular and renal protection? Ann Transl Med. 2018;6:S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 93. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2469] [Article Influence: 164.6] [Reference Citation Analysis (2)] |
| 94. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 95. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4946] [Article Influence: 706.6] [Reference Citation Analysis (9)] |
| 96. | Yen FS, Yang YC, Hwu CM, Wei JC, Huang YH, Hou MC, Hsu CC. Liver-related long-term outcomes of thiazolidinedione use in persons with type 2 diabetes. Liver Int. 2020;40:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, Hay JE, Wiesner RH, Sanchez W, Rosen CB, Swain JM. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 98. | Lin MY, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, Posselt AM. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis. 2013;9:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 99. | Takata MC, Campos GM, Ciovica R, Rabl C, Rogers SJ, Cello JP, Ascher NL, Posselt AM. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis. 2008;4:159-64; discussion 164-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 100. | Lin MY, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, Posselt AM. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc. 2013;27:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 101. | Khoraki J, Katz MG, Funk LM, Greenberg JA, Fernandez LA, Campos GM. Feasibility and outcomes of laparoscopic sleeve gastrectomy after solid organ transplantation. Surg Obes Relat Dis. 2016;12:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 102. | Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 103. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 104. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4071] [Article Influence: 452.3] [Reference Citation Analysis (1)] |
| 105. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1214] [Article Influence: 173.4] [Reference Citation Analysis (0)] |
| 106. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 1789] [Article Influence: 298.2] [Reference Citation Analysis (1)] |
| 107. | Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 408] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 108. | Bernsmeier C, Meyer-Gerspach AC, Blaser LS, Jeker L, Steinert RE, Heim MH, Beglinger C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS One. 2014;9:e87488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 109. | Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 110. | Kalogirou M, Sinakos E. Treating nonalcoholic steatohepatitis with antidiabetic drugs: Will GLP-1 agonists end the struggle? World J Hepatol. 2018;10:790-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Lee YS, Jun HS. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediators Inflamm. 2016;2016:3094642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 112. | Sun F, Wu S, Wang J, Guo S, Chai S, Yang Z, Li L, Zhang Y, Ji L, Zhan S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2015;37:225-241.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 113. | Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia. 2016;59:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 114. | Ohki T, Isogawa A, Iwamoto M, Ohsugi M, Yoshida H, Toda N, Tagawa K, Omata M, Koike K. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. ScientificWorldJournal. 2012;2012:496453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 115. | Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 116. | Petit JM, Cercueil JP, Loffroy R, Denimal D, Bouillet B, Fourmont C, Chevallier O, Duvillard L, Vergès B. Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J Clin Endocrinol Metab. 2017;102:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 117. | Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 118. | Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, Düring M, Zdravkovic M, Strauss BJ, Garber AJ; LEAD-2 and LEAD-3 Study Groups. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 119. | Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, Araki N, Tanaka K, Yamaguchi M, Matsuda Y, Ide Y, Otsuka T, Ozaki I, Ono N, Eguchi T, Anzai K; Japan Study Group for NAFLD (JSG-NAFLD). Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res. 2015;45:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 120. | Dong Y, Lv Q, Li S, Wu Y, Li L, Li J, Zhang F, Sun X, Tong N. Efficacy and safety of glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:284-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 121. | Gluud LL, Knop FK, Vilsbøll T. Effects of lixisenatide on elevated liver transaminases: systematic review with individual patient data meta-analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open. 2014;4:e005325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1469] [Article Influence: 163.2] [Reference Citation Analysis (1)] |
| 123. | Tang A, Rabasa-Lhoret R, Castel H, Wartelle-Bladou C, Gilbert G, Massicotte-Tisluck K, Chartrand G, Olivié D, Julien AS, de Guise J, Soulez G, Chiasson JL. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients With Type 2 Diabetes: A Randomized Trial. Diabetes Care. 2015;38:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 124. | Bouchi R, Nakano Y, Fukuda T, Takeuchi T, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J. 2017;64:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Feng WH, Bi Y, Li P, Yin TT, Gao CX, Shen SM, Gao LJ, Yang DH, Zhu DL. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial. J Diabetes Investig. 2019;10:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 126. | Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, Li Y, Dou J, Yang W, Qin G, Yuan H, Xiao X, Luo S, Shan Z, Deng H, Tan Y, Xu F, Xu W, Zeng L, Kang Z, Weng J. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:2414-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 127. | Newsome P, Francque S, Harrison S, Ratziu V, Van Gaal L, Calanna S, Hansen M, Linder M, Sanyal A. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther. 2019;50:193-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 128. | Hedrington MS, Davis SN. Oral semaglutide for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2019;20:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ; PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 368] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 130. | Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, Okajima A, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Kanemasa K, Yasui K, Imai S, Shimada K, Itoh Y. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 131. | Cusi K, Sattar N, García-Pérez LE, Pavo I, Yu M, Robertson KE, Karanikas CA, Haupt A. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabet Med. 2018;35:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 132. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8288] [Article Influence: 828.8] [Reference Citation Analysis (1)] |
| 133. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5394] [Article Influence: 674.3] [Reference Citation Analysis (0)] |
| 134. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4248] [Article Influence: 708.0] [Reference Citation Analysis (0)] |
| 135. | Snyder HS, Sakaan SA, March KL, Siddique O, Cholankeril R, Cummings CD, Gadiparthi C, Satapathy SK, Ahmed A, Cholankeril G. Non-alcoholic Fatty Liver Disease: A Review of Anti-diabetic Pharmacologic Therapies. J Clin Transl Hepatol. 2018;6:168-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 507] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 137. | Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 138. | Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 139. | Akuta N, Watanabe C, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Suzuki Y, Suzuki F, Ikeda K, Kumada H. Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: Preliminary prospective study based on serial liver biopsies. Hepatol Commun. 2017;1:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 140. | Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, Takekawa H, Usui I, Hiraishi H, Aso Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 141. | Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 142. | Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 143. | Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 144. | Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Biochem. 2016;49:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 145. | Darmawan G, Hamijoyo L, Hasan I. Association between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Acta Med Indones. 2017;49:136-147. [PubMed] |
| 146. | Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 147. | Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 148. | Rao NN, Coates PT. Cardiovascular Disease After Kidney Transplant. Semin Nephrol. 2018;38:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 149. | Kashyap R, Jain A, Reyes J, Demetris AJ, Elmagd KA, Dodson SF, Marsh W, Madariaga V, Mazariegos G, Geller D, Bonham CA, Cacciarelli T, Fontes P, Starzl TE, Fung JJ. Causes of death after liver transplantation in 4000 consecutive patients: 2 to 19 year follow-up. Transplant Proc. 2001;33:1482-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 150. | Halden TA, Egeland EJ, Åsberg A, Hartmann A, Midtvedt K, Khiabani HZ, Holst JJ, Knop FK, Hornum M, Feldt-Rasmussen B, Jenssen T. GLP-1 Restores Altered Insulin and Glucagon Secretion in Posttransplantation Diabetes. Diabetes Care. 2016;39:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 151. | Cariou B, Bernard C, Cantarovich D. Liraglutide in whole-pancreas transplant patients with impaired glucose homoeostasis: A case series. Diabetes Metab. 2015;41:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 152. | Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: a case series. Diabetes Care. 2013;36:e171-e172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 153. | Liou JH, Liu YM, Chen CH. Management of Diabetes Mellitus With Glucagonlike Peptide-1 Agonist Liraglutide in Renal Transplant Recipients: A Retrospective Study. Transplant Proc. 2018;50:2502-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 154. | D'Amico E, Hui H, Khoury N, Di Mario U, Perfetti R. Pancreatic beta-cells expressing GLP-1 are resistant to the toxic effects of immunosuppressive drugs. J Mol Endocrinol. 2005;34:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 155. | van Raalte DH, van Genugten RE, Linssen MM, Ouwens DM, Diamant M. Glucagon-like peptide-1 receptor agonist treatment prevents glucocorticoid-induced glucose intolerance and islet-cell dysfunction in humans. Diabetes Care. 2011;34:412-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 156. | Hurren KM, Pinelli NR. Drug-drug interactions with glucagon-like peptide-1 receptor agonists. Ann Pharmacother. 2012;46:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 157. | Helderman JH, Goral S. Gastrointestinal complications of transplant immunosuppression. J Am Soc Nephrol. 2002;13:277-287. [PubMed] |
| 158. | Munagala MR, Phancao A. Managing Cardiovascular Risk in the Post Solid Organ Transplant Recipient. Med Clin North Am. 2016;100:519-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 159. | de Mesquita FC, Guixé-Muntet S, Fernández-Iglesias A, Maeso-Díaz R, Vila S, Hide D, Ortega-Ribera M, Rosa JL, García-Pagán JC, Bosch J, de Oliveira JR, Gracia-Sancho J. Liraglutide improves liver microvascular dysfunction in cirrhosis: Evidence from translational studies. Sci Rep. 2017;7:3255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 160. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1917] [Article Influence: 319.5] [Reference Citation Analysis (0)] |
| 161. | Jin J, Jin L, Luo K, Lim SW, Chung BH, Yang CW. Effect of Empagliflozin on Tacrolimus-Induced Pancreas Islet Dysfunction and Renal Injury. Am J Transplant. 2017;17:2601-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 162. | Muir CA, Greenfield JR, MacDonald PS. Empagliflozin in the management of diabetes mellitus after cardiac transplantationResearch Correspondenceretain-->. J Heart Lung Transplant. 2017;36:914-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 163. | Rajasekeran H, Kim SJ, Cardella CJ, Schiff J, Cattral M, Cherney DZI, Singh SKS. Use of Canagliflozin in Kidney Transplant Recipients for the Treatment of Type 2 Diabetes: A Case Series. Diabetes Care. 2017;40:e75-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 164. | Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, Bollerslev J, Hartmann A, Åsberg A, Jenssen T. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care. 2019;42:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 165. | Saffo S, Taddei T. SGLT2 inhibitors and cirrhosis: A unique perspective on the comanagement of diabetes mellitus and ascites. Clin Liver Dis (Hoboken). 2018;11:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 166. | Heise T, Seewaldt-Becker E, Macha S, Hantel S, Pinnetti S, Seman L, Woerle HJ. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |