Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2729
Peer-review started: January 30, 2020
First decision: March 15, 2020
Revised: March 18, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: June 7, 2020
Processing time: 128 Days and 9.2 Hours
High-risk human papillomavirus has been suggested as a risk factor for esophageal adenocarcinoma. Tumor human papillomavirus status has been reported to confer a favorable prognosis in esophageal adenocarcinoma. The size of the primary tumor and degree of lymphatic spread determines the prognosis of esophageal carcinomas. Lymph node status has been found to be a predictor of recurrent disease as well as 5-year survival in esophageal malignancies. In human papillomavirus driven cancers, e.g. cervical, anogenital, head and neck cancers, associated lymph nodes with a high viral load suggest metastatic lymph node involvement. Thus, human papillomavirus could potentially be useful as a marker of micro-metastases. To date, there have been no reported studies regarding human papillomavirus involvement in lymph nodes of metastatic esophageal adenocarcinoma. This review highlights the importance of investigating human papillomavirus in lymph node metastasis of esophageal adenocarcinoma based on data derived from other human papillomavirus driven cancers.
Core tip: Esophageal adenocarcinoma (EAC) is one of the fastest growing cancers in the western world. EAC has been associated with high-risk human papillomavirus and has been shown to grant a positive prognosis in EAC. In some human papillomavirus (HPV) driven malignancies (e.g., cervical and head and neck tumors), associated lymph nodes with a high viral load suggest metastatic lymph node involvement. Therefore, HPV is a potential marker of micro-metastases. This review highlights the importance of investigating HPV in lymph node metastasis of EAC based on data derived from other HPV driven malignancies.
- Citation: Sharma P, Gautam SD, Rajendra S. Importance of investigating high-risk human papillomavirus in lymph node metastasis of esophageal adenocarcinoma. World J Gastroenterol 2020; 26(21): 2729-2739
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2729
The incidence rates for esophageal adenocarcinoma (EAC) has rapidly risen in the western world[1-7]. EAC has a high mortality rate with a 5-year survival of less than 15%[8,9]. Even patients (< 50%) considered fit for surgery (with neo-adjuvant therapy) and a curative intent, the outcome remains poor with a 5-year survival rate of < 45% [10,11]. This is predominantly because esophageal cancer often proliferates through the lymphatic system. Lymph node metastasis (LNM) is the most crucial prognostic factor in esophageal carcinoma, whereby the number of lymph nodes (LN) is directly correlated with disease progression[12-21]. The extent of the primary tumor and lymphatic spread determines the prognosis of esophageal carcinoma. In particular, it has been found that LN status is a predictor of recurrent disease as well as 5-year survival[19,20,22].
Over 200 years ago, LeDran et al[23] first observed the difference in prognosis in breast cancer patients with metastatic spread of tumor cells to regional lymph nodes (RLNs) and patients without spread. Ever since, several studies have focused on the mechanisms involved in this phenomenon. Tumor cells migrate to regional lymph nodes through systematic circulation. Nearly all types of cancers involve circulating tumor cells[24]. Few studies supporting the intravasation of these tumor cells into blood vessels have been reported. LNM is a well-established prognostic factor in breast cancer, head and neck cancer, colorectal cancer and prostate cancer. However, the methods of vascular invasions (lymphatic invasion, hematogenous dissemination, etc.) vary in different cancers[25-29]. Hosch et al[30] classified esophageal carcinoma as an immunogenic tumor. Mechanisms reported include immune evasion due to functional suppression of cytotoxic T-lymphocytes by DNA methylation in MHC class I phenotype esophageal cancers and hypercoagulation[30-33].
Human papillomavirus (HPV) has been associated with tumorigenesis of several cancers including cervical, anal, head and neck, oral and oropharyngeal squamous cell carcinomas[34-37]. The presence of HPV DNA in the RLNs has been associated with disease recurrence and early signs of metastasis[20,38-43].
HPV is a non-enveloped, double-stranded DNA virus from the papillomavirus family. It is approximately 8kB in size with its genome encoding for at least 8 proteins of which 2 oncoproteins E6 and E7 are of importance. The E6 oncogene causes disruption to the p53 pathway with ultimate degradation of the cell. The E7 oncogene binds and inactivates the retinoblastoma protein (pRb) which is responsible for the major G1 checkpoint that blocks the S-phase entry and cell growth phase transition, thereby upregulating p16 expression[44,45].
HPV can be classified into high risk type i.e. HPV type 16 and 18 that causes cervical malignancy and head and neck cancers and low-risk type e.g. HPV type 6 and 11 which are linked to genital warts and benign changes in the cervix. The causal link between high-risk human papillomavirus (hr-HPV) infection and cervical malignancy, anogenital, and a subset of oropharyngeal squamous cell carcinoma has been well established by molecular and epidemiological investigations[46-50].
HPV detection in these cancers has been undertaken using a combination of techniques: Polymerase chain reaction (PCR), in-situ hybridization (ISH) and p16INK4A expression. Detection of both high-risk and low-risk HPV types in tumor samples has been performed by nested PCR. The PCR amplifies the L1 gene of HPV using MY09 and MY11 and GP5+ and GP6+ primers. PCR products were then separated on 2% agarose gel and sequencing performed to determine the genotypes. Viral load has been calculated by real-time PCR assay method. It measures E6 and E7 copy numbers using genotype-specific HPV-16 and HPV-18 primers. Hr-HPV16 and 18 E6/E7 mRNA has been determined by RT-PCR and/or RNA ISH, which are considered the gold standard for detection of transcriptionally active hr-HPV. p16INK4A expression has been assessed by immunohistochemical staining on FFPE (formalin fixed paraffin-embedded) tumor biopsies and controls. These techniques have been widely used in all the HPV driven cancers[50-53].
Currently, Barrett’s esophagus (BE) is the only recognized visible precursor lesion for EAC[54,55]. It has been proposed that a metaplasia – dysplasia – cancer sequence exists[56,57], whereby patients with BE (no dysplasia) undergo dysplastic transformation (low- and high-grade dysplasia) before cancer development. Although, the cancer risk in BE gets progressively downgraded, the exponential rise in EAC continues unabated[58,59].
Syrjanen reported the association of HPV with esophageal squamous cancer for the first time in 1982[60,61]. In 2010, Rajendra et al[62] hypothesized that HPV could be a common denominator in the pathogenesis of EAC due to similar immunogenetics of BE and cervical neoplasia. Later in 2013, they provided world first evidence for a strong association of transcriptionally active hr-HPV with EAC[52,63]. Hr-HPV has now been proposed as one of the risk factors for esophageal adenocarcinoma[64].
Despite the suggested association of HPV with Barrett's dysplasia and EAC, a few studies found contradictory results. A study by Antonsson et al[65] found no evidence of HPV in 233 formalin-fixed EAC tissue specimens > 10 years old. In another case-control study by Serag et al[66], no viral presence was detected in 127 patients with BE in a US cohort, as the virus is not associated with metaplastic tissue. Rai et al[67] again confirmed the virtual absence of HPV infection in Barrett's metaplasia in the UK population. Iyer et al[68]. found no statistical difference in the HPV prevalence between BE (27.4%), EAC (31%) and controls (20.7%) and thus concluded that it was of no etiologic significance. Cross-contamination may explain their results. Plausible reasons for negative studies in EAC include limited sample size, geographical location of patient cohorts, racial disparity, methods of HPV detection especially as the viral load in esophageal tissue is low, sample collection, storage, age of specimens and poor tissue classification. HPV detection by PCR is an extremely sensitive assay. Thus, quality of tissue samples, storage conditions and contamination can all adversely affect the assay. For example, the PCR primers MY09/MY11 might not amplify the 450 bp target area on the biopsy tissues collected over 10 years ago. Also, HPV detection is significantly superior in fresh-frozen samples than in the paraffin embedded tissues[34,69].
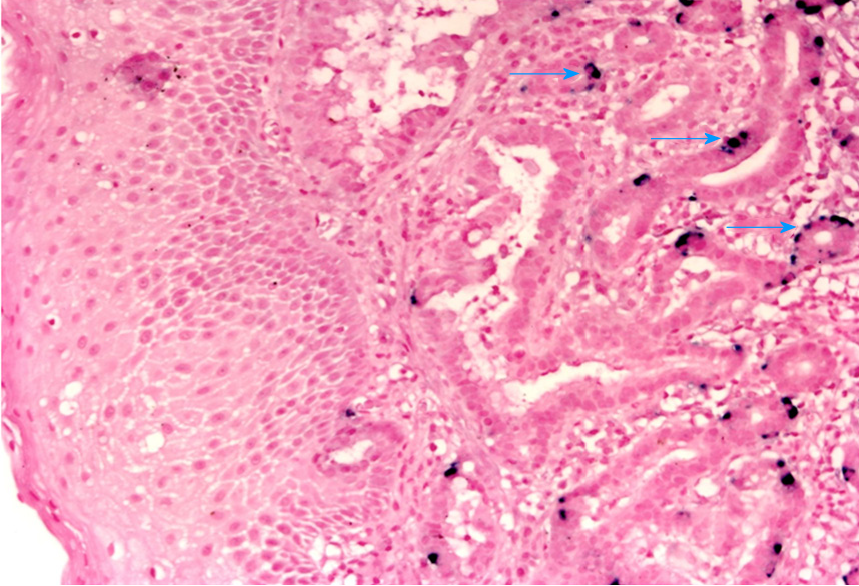
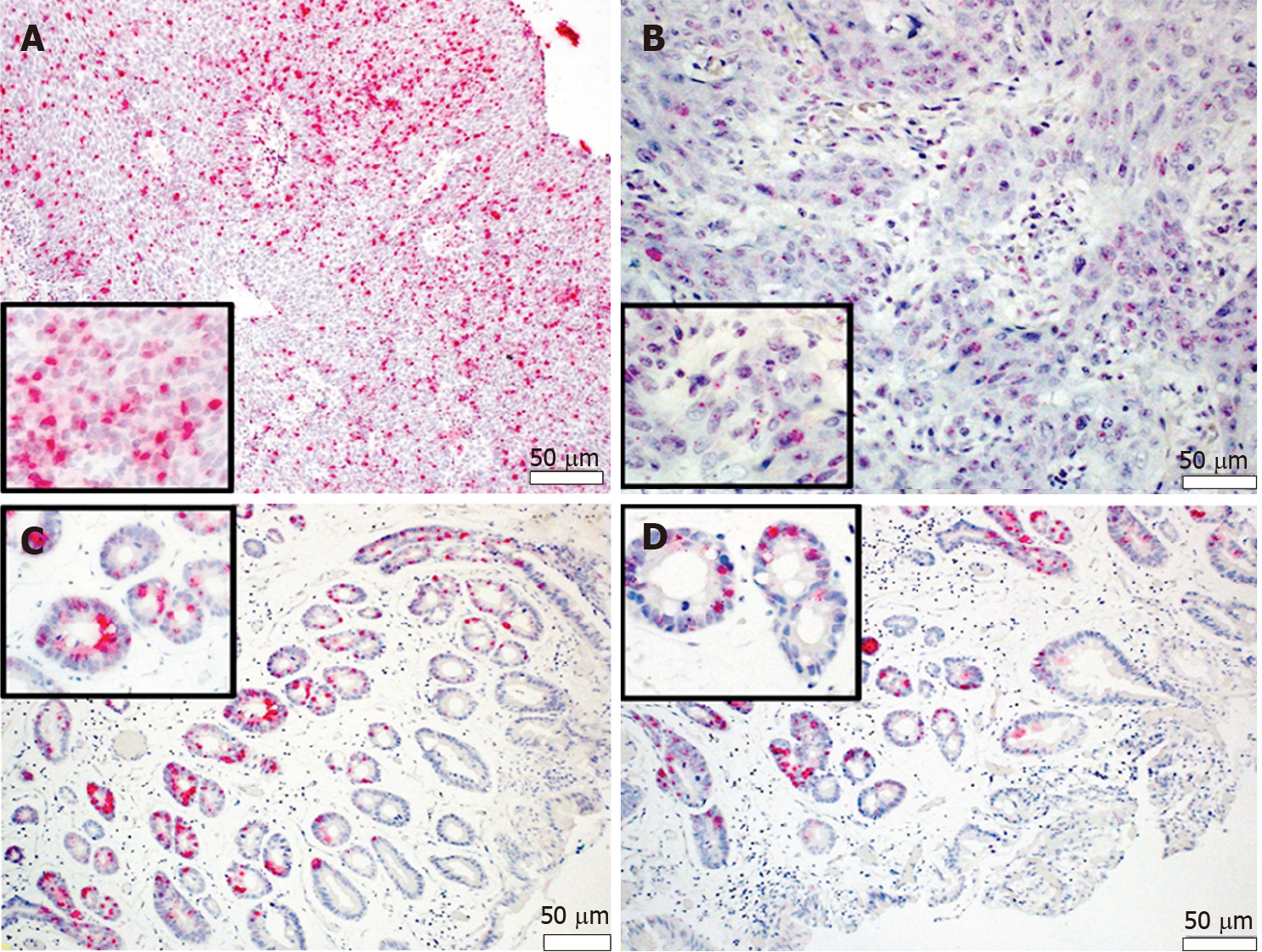
A systematic review has reported HPV prevalence rates of 35% in EAC (n = 174), which is not much different to our findings. Another systematic review which included 19 studies concluded that the pooled prevalence of HPV in EAC was 13%. The authors suggested that the low prevalence rate may have been caused by small sample sizes and compromised detection methods[34,69]. Increasing hr-HPV viral load and integration status has been linked with more severe disease along the Barrett metaplasia-dysplasia-adenocarcinoma sequence. In situ-hybridization (ISH) demonstrated for the first time that the hr-HPV genome is present in BE, BD and EAC cells but not the squamous epithelium (Figure 1)[70]. The presence of HPV oncogene E6/7 mRNA was also observed in the BD and EAC (Figure 2)[51].
Moreover, treatment failure after endoscopic ablation of Barrett's dysplasia (BD) or EAC is predicted by persistent hr-HPV infection and overexpression of the p53 gene (TP53)[71]. Epidemiological data indicate various other risk factors for EAC including Caucasian race, gender, age, obesity and smoking[72-74].
HPV-16 DNA has been detected in the cervical lymph nodes of esophageal squamous cell carcinoma (ESCC)[42]. Currently no studies have been reported regarding HPV involvement with LNM in EAC. This review will therefore emphasize the significance of conducting such an investigation.
Cervical cancer is the archetypal HPV driven malignancy. This has led investigators to examine HPV involvement in other less common cancers which involve the transformation zone and/or the genital area. Examples are head and neck tumors which have a physiological transformation zone rather an anatomical one, esophageal adenocarcinoma (anatomical transformation zone), penile, vulvar and anal carcinomas. Summarized below, we review LNM in the HPV driven cancers.
Cervical cancer: More than 90% of cervical cancer cases are caused by untreated HPV infection[75-77]. The presence of HPV DNA in lymph nodes ranges between 40%-99.7% and has been linked to early signs of metastasis with poor prognosis. The disparity in results can be due to technical differences and variations in patient cohorts[20,38,43,78-84]. Although, there is a more apparent correlation of HPV-16 and LNM, both hr-HPV types 16 and 18 have been reported to be predictive of LNM in cervical cancer thus affecting prognosis[82,85,86].
Early detection of epithelial cells containing transcriptionally active HPV in lymph nodes has been proposed as a marker for metastases[87,88]. In 2012, Zhang et al[89] reported hr-HPV positivity in 95.8% metastatic lymph nodes with same HPV type as in the primary lesion. Thus, testing for HPV DNA in pathologically negative lymph nodes could be a crucial marker of micro-metastases[89]. About 15% of cervical cancer cases have non-metastatic lymph nodes and detection of HPV in negative lymph nodes has been indicated as a marker of micro-metastasis[87,90]. HPV positive DNA in pelvic lymph nodes has been found in 25%-80% of women in early stages cervical cancer[83,91]. In contrast to the above findings, few studies reported no correlation between HPV positivity and LNM[92-94].
While, some studies associate HPV-positive lymph nodes with higher risk of disease recurrence and lower overall survival[38,81], others find no notable differences in these parameters between HPV positive and negative lymph node status[93,94]. Coutant et al[91] reported no correlation between the HPV status in pelvic lymph nodes and overall prognosis. These disparities could be due to limited sample size and DNA detection methods. A study by Lukaszuk et al[41] concluded that LNM detection is significantly more accurate by ascertaining HPV status than normal histopathological testing.
Anal, penile and vulvar cancers: The incidence of penile and vulvar cancers is highest in some developing countries. HPV has been linked with the pathogenesis of a subset of these cancers[95-98]. Studies have reported the overall prevalence of HPV DNA in other anogenital cancers as: Penile cancer (15%-71%); anal (80%) and vulvar (40.1%) cancers have been reported to be HPV-associated[95,99]. These prevalence rates are obviously dependent on the methods of DNA detection, patient cohort selection as well as sample handling and processing.
Hr-HPV 16 and 18 has been detected in penile and vulvar cancer tissues using polymerase chain reaction, Southern blot and in-situ hybridization techniques[100,101]. The prevalence of HPV DNA has been reported higher (> 55%) in basaloid and warty penile cancers as compared to verrucous and keratinizing cancers (< 10%)[95]. Involvement of inguinal lymph node in penile cancer is an unfavorable prognostic indicator[102]. In a study by Picconi et al[103], 71% of cases were positive for HPV DNA. The positivity was consistent in both the primary tumor and lymph node metastasis. Also, HPV positivity was found in histologically normal lymph nodes which could indicate early metastasis and disease recurrence[103].
In regard to the prevalence of HPV and location in the transformation zone, anal cancer mimics cervical malignancy better than other HPV associated anogenital cancers. Using the PCR detection technique, HPV positivity has been found in up to 89% of the squamous cell anal carcinoma cases. HPV 16 is the most prevalent high-risk type[104,105].
Head and neck squamous cell carcinoma (HNSCC) is the fifth most common cancer worldwide[106]. Most of the primary tumors detected in HNSCC are of unknown primary origin and are found in the oropharynx. Cervical LNM in oropharyngeal squamous cell carcinoma (OSCC) has been linked to regional recurrence and decreased overall survival[106-109]. Lymph node metastasis has been described as an independent prognostic factor in head and neck cancers[108,110,111].
Around 80-90% oropharyngeal tumors are associated with hr- HPV[112-115]. Of all high-risk types, HPV-16 is the most frequent genotype. HPV driven HNSCCs have considerably superior prognosis and superior chemo- and radio-sensitivity than the typical non-HPV related HNSCC. Normally, HNSCC is keratinizing and is usually caused by genetic alterations; whereas, HPV-associated HNSCC is non-keratinizing and is triggered by inactivation of tumor suppressor genes (e.g. TP53 and RB) by viral oncogenes E6 and E7[116]. However, a subset of patients with HPV related HNSCC develop metastasis similar to non-HPV related HNSCC[117].
Furthermore, transcriptionally active HPV has been detected in metastatic lymph nodes in these cancers[118,119]. Takes et al[120] proposed that hr-HPV positive DNA in metastatic cervical lymph nodes, could identify the primary origin of head and neck cancers. 70% of OSCC patients were diagnosed with LNM at early stages and 20-30% have occult metastases. Cancer free lymph nodes with high viral load could indicate occult metastasis[42].
HPV driven head and neck cancer especially in oropharyngeal and tonsillar regions have an increased overall survival. Schwartz et al[121] and Nicolas et al[122] found that patients with HPV-positive tumors have an increased disease free, overall survival and reduced disease specific mortality rates compared to viral negative head and neck cancers. Fujita et al[121] concluded that LNM was more prevalent in HPV linked HNSCC and HPV negative tumors were associated with disease recurrence.
Gastric cancers (GC) have been caused by various microbial infections. Major pathogens linked to GC are Helicobacter pylori (H. pylori) and Epstein Barr virus (EBV)[122-124]. EBV infection raises the risk of gastric cancer. It is present in 9% of gastric carcinomas[125]. Very few studies have been reported on the prevalence of EBV in lymph nodes in GC. These studies found that EBV positive gastric cancer was negatively associated with lymph node metastasis, resulting in better prognosis[126-128].
The association of HPV and gastric cancers is still poorly understood. de Souza et al[128] and Fakhraei et al[129] observed only 3% and 5% of samples infected with HPV. Although, hr-HPV 16 and 18 were found in some tumors, E6 and E7 expression was negative implying the absence of biologically active virus. These studies therefore concluded that HPV had no role in initiation or progression of GC. Where some studies reported higher frequencies (37.5%-52%), other publications documented complete absence of the virus in GC tumor samples[130-135]. This discrepancy can be attributed to DNA detection and tumor samples processing methods. In a meta-analysis of 1917 patients, Zeng et al[136] reported that the HPV positivity was greater in the Chinese population (31%) than in non-Chinese regions (9%). Also, higher rates of HPV infection were obtained using the ISH (36%) method than PCR technique (26%).
HPV associated LNM has been well established as a major prognostic factor in oropharyngeal, head and neck squamous cell carcinoma and other anogenital cancers. Studies have demonstrated an association of transcriptionally active HPV with esophageal adenocarcinoma. The viral involvement in LNM in EAC is still unexplored and unidentified. Through this review, we propose that HPV detection should be undertaken in LNM of EAC tissue samples which test positive for HPV infection in the primary tumor, using PCR, DNA ISH, RT-PCR, E6-E7 mRNA ISH and p16INK4A. We shall correlate HPV status of the primary tumor and lymph nodes with clinical outcomes including overall survival, disease-free survival micro-metastasis and diseases-specific death. The results of these studies we hope, shall be keenly anticipated in the coming years.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Fellow of the Royal Australian College of Physicians; Fellow of the Royal College of Physicians of Edinburgh.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bratlie SO, Iwaya Y S-Editor: Zhang H L-Editor: A E-Editor: Ma YJ
| 1. | Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LAG, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual Report to the Nation on the Status of Cancer, 1975–2007, Featuring Tumors of the Brain and Other Nervous System. J Natl Cancer Inst. 2011;103:714-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 2. | Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [PubMed] |
| 3. | Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol. 2017;112:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (2)] |
| 5. | Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology. 2015;149:302-17.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 7. | Xie SH, Mattsson F, Lagergren J. Incidence trends in oesophageal cancer by histological type: An updated analysis in Sweden. Cancer Epidemiol. 2017;47:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 9. | Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 835] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 10. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1958] [Article Influence: 163.2] [Reference Citation Analysis (5)] |
| 11. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2218] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 12. | Akutsu Y, Matsubara H. The significance of lymph node status as a prognostic factor for esophageal cancer. Surg Today. 2011;41:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Cho JW, Choi SC, Jang JY, Shin SK, Choi KD, Lee JH, Kim SG, Sung JK, Jeon SW, Choi IJ, Kim GH, Jee SR, Lee WS, Jung HY; Korean ESD Study Group. Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist's View. Clin Endosc. 2014;47:523-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Zhang HL, Chen LQ, Liu RL, Shi YT, He M, Meng XL, Bai SX, Ping YM. The number of lymph node metastases influences survival and International Union against Cancer tumor–node–metastasis classification for esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Kunisaki C, Makino H, Kimura J, Oshima T, Fujii S, Takagawa R, Kosaka T, Ono H, Akiyama H. Impact of Lymph-Node Metastasis Site in Patients With Thoracic Esophageal Cancer. J Surg Oncol. 2010;101:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Lin CS, Chang SC, Wei YH, Chou TY, Wu YC, Lin HC, Wang LS, Hsu WH. Prognostic Variables in Thoracic Esophageal Squamous Cell Carcinoma. Ann Thorac Surg. 2009;87:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Natsugoe S, Matsumoto M, Okumura H, Ikeda M, Ishigami S, Owaki T, Takao S, Aikou T. Prognostic factors in patients with submucosal esophageal cancer. J Gastrointest Surg. 2004;8:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hsu WH, Hsu PK, Hsieh CC, Huang CS, Wu YC. The metastatic lymph node number and ratio are independent prognostic factors in esophageal cancer. J Gastrointest Surg. 2009;13:1913-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Cense HA, van Eijck CH, Tilanus HW. New insights in the lymphatic spread of oesophageal cancer and its implications for the extent of surgical resection. Best Pract Res Clin Gastroenterol. 2006;20:893-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Lukaszuk K, Liss J, Gulczynski J, Nowaczyk M, Nakonieczny M, Piatkowski M, Sliwinski W, Baay M, Wozniak I, Maj B, Lukaszuk M. Predictive value of HPV DNA in lymph nodes in surgically treated cervical carcinoma patients--a prospective study. Gynecol Oncol. 2007;104:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Carow K, Read C, Häfner N, Runnebaum IB, Corner A, Dürst M. A comparative study of digital PCR and real-time qPCR for the detection and quantification of HPV mRNA in sentinel lymph nodes of cervical cancer patients. BMC Res Notes. 2017;10:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Daly JM, Fry WA, Little AG, Winchester DP, McKee RF, Stewart AK, Fremgen AM. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562-572; discussion 572-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 362] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 23. | LeDran H. Traite des operations de chirurgie (English translation Gataker). Hitch, C and Dosley, R London 1752. |
| 24. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1950] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 25. | Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 544] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 26. | Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 538] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 27. | Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1901] [Cited by in RCA: 1899] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 28. | Gujam FJ, Going JJ, Edwards J, Mohammed ZM, McMillan DC. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol. 2014;89:231-41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Hosch SB, Izbicki JR, Pichlmeier U, Stoecklein N, Niendorf A, Knoefel WT, Broelsch CE, Pantel K. Expression and prognostic significance of immunoregulatory molecules in esophageal cancer. Int J Cancer. 1997;74:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Algarra I, García-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Tomimaru Y, Yano M, Takachi K, Kishi K, Miyashiro I, Ohue M, Ohigashi H, Sasaki Y, Ishikawa O, Imaoka S. Plasma D-dimer levels show correlation with number of lymph node metastases in patients with esophageal cancer. J Am Coll Surg. 2006;202:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Seliger B. Strategies of tumor immune evasion. BioDrugs. 2005;19:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Li X, Gao C, Yang Y, Zhou F, Li M, Jin Q, Gao L. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther. 2014;39:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 36. | Li X, Gao L, Li H, Gao J, Yang Y, Zhou F, Gao C, Li M, Jin Q. Human Papillomavirus Infection and Laryngeal Cancer Risk: A Systematic Review and Meta-Analysis. J Infect Dis. 2013;207:479-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Beckmann AM, Daling JR, Sherman KJ, Maden C, Miller BA, Coates RJ, Kiviat NB, Myerson D, Weiss NS, Hislop TG, Beagrie M, McDougall JK. Human papillomavirus infection and anal cancer. Int J Cancer. 1989;43:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Kobayashi Y, Yoshinouchi M, Tianqi G, Nakamura K, Hongo A, Kamimura S, Mizutani Y, Kodama J, Miyagi Y, Kudo T. Presence of human papilloma virus DNA in pelvic lymph nodes can predict unexpected recurrence of cervical cancer in patients with histologically negative lymph nodes. Clin Cancer Res. 1998;4:979-983. [PubMed] |
| 39. | Nawa A, Nishiyama Y, Kikkawa F, Kawai M, Mano H, Goto S, Suganuma N, Tomoda Y, Nakashima N. Detection of human papillomaviruses from histologically normal lymph nodes of Japanese cervical cancer patients by nested polymerase chain-reaction assay. Int J Cancer. 1993;53:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Burnett AF, Barnes WA, Johnson JC, Grendys E, Willett GD, Barter JF, Doniger J. Prognostic significance of polymerase chain reaction detected human papillomavirus of tumors and lymph nodes in surgically treated stage IB cervical cancer. Gynecol Oncol. 1992;47:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Lukaszuk K, Liss J, Wozniak I, Sliwinski W, Emerich J, Wojcikowski C. HPV and histological status of pelvic lymph node metastases in cervical cancer: a prospective study. J Clin Pathol. 2004;57:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Mirghani H, Moreau F, Lefèvre M, Tam C, Périé S, Soussan P, St Guily JL. Human Papillomavirus Type 16 Oropharyngeal Cancers in Lymph Nodes as a Marker of Metastases. Arch Otolaryngol Head Neck Surg. 2011;137:910-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Lancaster WD, Castellano C, Santos C, Delgado G, Kurman RJ, Jenson AB. Human papillomavirus deoxyribonucleic acid in cervical carcinoma from primary and metastatic sites. Am J Obstet Gynecol. 1986;154:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Deng Z, Hasegawa M, Kiyuna A, Matayoshi S, Uehara T, Agena S, Yamashita Y, Ogawa K, Maeda H, Suzuki M. Viral load, physical status, and E6/E7 mRNA expression of human papillomavirus in head and neck squamous cell carcinoma. Head Neck. 2013;35:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220-5227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 869] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 46. | Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24:S3/11‐S3/25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 813] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 47. | Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1829] [Cited by in RCA: 1879] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 48. | de Sanjosé S, Bruni L, Alemany L. HPV in genital cancers (at the exception of cervical cancer) and anal cancers. Presse Med. 2014;43:e423-e428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Parikh F, Duluc D, Imai N, Clark A, Misiukiewicz K, Bonomi M, Gupta V, Patsias A, Parides M, Demicco EG, Zhang DY, Kim-Schulze S, Kao J, Gnjatic S, Oh S, Posner MR, Sikora AG. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res. 2014;74:7205-7216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Rajendra S, Xuan W, Merrett N, Sharma P, Sharma P, Pavey D, Yang T, Santos LD, Sharaiha O, Pande G. Survival Rates for Patients With Barrett High-grade Dysplasia and Esophageal Adenocarcinoma With or Without Human Papillomavirus Infection. JAMA Netw Open. 2018;1:e181054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Rajendra S, Wang B, Pavey D, Sharma P, Yang T, Lee CS, Gupta N, Ball MJ, Gill RS, Wu X. Persistence of Human Papillomavirus, Overexpression of p53, and Outcomes of Patients After Endoscopic Ablation of Barrett's Esophagus. Clin Gastroenterol Hepatol. 2015;13:1364-1368.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Wang B, Rajendra S, Pavey D, Sharma P, Merrett N, Wu X, Snow ET, Kumbhari V, Ball MJ, Robertson IK. Viral load and integration status of high-risk human papillomaviruses in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2013;108:1814-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 54. | Altaf K, Xiong JJ, Iglesia DDl, Hickey L, Kaul A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett's oesophagus. Br J Surg. 2017;104:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 56. | Miros M, Kerlin P, Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett's oesophagus. Gut. 1991;32:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Poehlmann A, Kuester D, Malfertheiner P, Guenther T, Roessner A. Inflammation and Barrett's carcinogenesis. Pathol Res Pract. 2012;208:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Rajendra S, Sharma P. Management of Barrett's oesophagus and intramucosal oesophageal cancer: a review of recent development. Therap Adv Gastroenterol. 2012;5:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 978] [Article Influence: 69.9] [Reference Citation Analysis (1)] |
| 60. | Syrjänen K, Pyrhönen S, Aukee S, Koskela E. Squamous cell papilloma of the esophagus: a tumour probably caused by human papilloma virus (HPV). Diagn Histopathol. 1982;5:291-296. [PubMed] |
| 61. | Syrjänen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch. 1982;52:283-292. [PubMed] |
| 62. | Rajendra S, Robertson IK. Similar immunogenetics of Barrett's oesophagus and cervical neoplasia: is HPV the common denominator? J Clin Pathol. 2010;63:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Rajendra S, Wang B, Snow ET, Sharma P, Pavey D, Merrett N, Ball MJ, Brain T, Fernando R, Robertson IK. Transcriptionally active human papillomavirus is strongly associated with Barrett's dysplasia and esophageal adenocarcinoma. Am J Gastroenterol. 2013;108:1082-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 65. | Antonsson A, Knight L, Whiteman DC; Australian Cancer Study. Human papillomavirus not detected in esophageal adenocarcinoma tumor specimens. Cancer Epidemiol. 2016;41:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | El-Serag HB, Hollier JM, Gravitt P, Alsarraj A, Younes M. Human papillomavirus and the risk of Barrett's esophagus. Dis Esophagus. 2013;26:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Rai N, Jenkins GJ, McAdam E, Hibbitts SJ, Fiander AN, Powell NG. Human papillomavirus infection in Barrett's oesophagus in the UK: an infrequent event. J Clin Virol. 2008;43:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Iyer A, Rajendran V, Adamson CS, Peng Z, Cooper K, Evans MF. Human papillomavirus is detectable in Barrett's esophagus and esophageal carcinoma but is unlikely to be of any etiologic significance. J Clin Virol. 2011;50:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Kunzmann AT, Graham S, McShane CM, Doyle J, Tommasino M, Johnston B, Jamison J, James JA, McManus D, Anderson LA. The prevalence of viral agents in esophageal adenocarcinoma and Barrett's esophagus: a systematic review. Eur J Gastroenterol Hepatol. 2017;29:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Al-Haddad S, El-Zimaity H, Hafezi-Bakhtiari S, Rajendra S, Streutker CJ, Vajpeyi R, Wang B. Infection and esophageal cancer. Ann N Y Acad Sci. 2014;1325:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Rajendra S, Wang B, Pavey D, Sharma P, Yang T, Lee CS, Gupta N, Ball MJ, Gill RS, Wu X. Persistence of Human Papillomavirus, Overexpression of p53, and Outcomes of Patients After Endoscopic Ablation of Barrett's Esophagus. Clin Gastroenterol Hepatol. 2015;13:1364-1368.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, Brown LM, Risch HA, Ye W, Sharp L, Wu AH, Ward MH, Casson AG, Murray LJ, Corley DA, Nyrén O, Pandeya N, Vaughan TL, Chow WH, Gammon MD. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 73. | Cook MB, Shaheen NJ, Anderson LA, Giffen C, Chow WH, Vaughan TL, Whiteman DC, Corley DA. Cigarette smoking increases risk of Barrett's esophagus: an analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 75. | Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 76. | Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2332] [Cited by in RCA: 2224] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 77. | zur Hausen H, Schneider A. The Role of Papillomaviruses in Human Anogenital Cancer. In: Salzman NP, Howley PM. The Papovaviridae: The Papillomaviruses. Boston, MA: Springer US, 1987: 245-263. |
| 78. | Fuchs P, Girardi F, Pfister H. Human papillomavirus 16 DNA in cervical cancers and in lymph nodes of cervical cancer patients: a diagnostic marker for early metastases? Int J Cancer. 1989;43:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Claas E, Melchers W, van der Linden HC, Lindeman J, Quint W. Human papillomavirus detection in paraffin-embedded cervical carcinomas and metastases of the carcinomas by the polymerase chain reaction. Am J Pathol. 1989;135:703. [PubMed] |
| 80. | Burger RA, Monk BJ, Kurosaki T, Anton-Culver H, Vasilev SA, Berman ML, Wilczynski SP. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J Natl Cancer Inst. 1996;88:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 81. | Pilch H, Günzel S, Schäffer U, Tanner B, Brockerhoff P, Maeurer M, Höckel M, Hommel G, Knapstein PG. Human papillomavirus (HPV) DNA in primary cervical cancer and in cancer free pelvic lymph nodes--correlation with clinico-pathological parameters and prognostic significance. Zentralbl Gynakol. 2001;123:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Baser E, Can F, Unlukaplan M, Ayhan A. Lymph node human papillomavirus DNA positivity in uterine cervical cancers and its relationship with prognostic factors. Int J Gynecol Cancer. 2011;21:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Lee YS, Rhim CC, Lee HN, Lee KH, Park JS, Namkoong SE. HPV status in sentinel nodes might be a prognostic factor in cervical cancer. Gynecol Oncol. 2007;105:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Clifford GM, Shin HR, Oh JK, Waterboer T, Ju YH, Vaccarella S, Quint W, Pawlita M, Franceschi S. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16:1874-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 85. | Cavuslu S, Goodlad J, Hobbs C, Connor AM, Raju KS, Best JM, Cason J. Relationship between human papillomavirus infection and overexpression of p53 protein in cervical carcinomas and lymph node metastases. J Med Virol. 1997;53:111-117. [PubMed] |
| 86. | Häfner N, Gajda M, Altgassen C, Hertel H, Greinke C, Hillemanns P, Schneider A, Dürst M. HPV16-E6 mRNA is superior to cytokeratin 19 mRNA as a molecular marker for the detection of disseminated tumour cells in sentinel lymph nodes of patients with cervical cancer by quantitative reverse-transcription PCR. Int J Cancer. 2007;120:1842-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Tortora M, Annunziata C, Liguori G, Losito S, Botti G, Greggi S, Buonaguro L, Buonaguro FM, Tornesello ML. Detection of human papillomavirus DNA in peri-tumor tissues and pelvic lymph nodes as potential molecular marker of micrometastasis in cervical cancer. Infect Agent Cancer. 2016;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Vande Pol SB, Klingelhutz AJ. Papillomavirus E6 oncoproteins. Virology. 2013;445:115-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 89. | Zhang F, Liu D, Lin B, Hao Y, Zhou D, Qi Y, Zhang S. Expression of high-risk HPV DNA and CK19 in pelvic lymph nodes in stage Ia-IIa cervical cancer and their clinical value. Oncol Rep. 2012;27:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Slama J, Drazdakova M, Dundr P, Fischerova D, Zikan M, Pinkavova I, Freitag P, Pavlista D, Zima T, Cibula D. High-risk human papillomavirus DNA in the primary tumor, sentinel, and nonsentinel pelvic lymph nodes in patients with early-stage cervical cancer: a correlation with histopathology. Int J Gynecol Cancer. 2009;19:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Coutant C, Barranger E, Cortez A, Dabit D, Uzan S, Bernaudin J, Darai E. Frequency and prognostic significance of HPV DNA in sentinel lymph nodes of patients with cervical cancer. Ann Oncol. 2007;18:1513-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Hording U, Ravn V, Knudsen J, Visfeldt J. The use of polymerase chain reaction to detect metastatic cancer cells within lymph nodes in stage I cervical carcinoma. Int J Gynecol Pathol. 1995;14:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Baay MF, Koudstaal J, Hollema H, Duk JM, Burger MP, Quint WG, Stolz E, Herbrink P. Detection of HPV-16 DNA by PCR in histologically cancer free lymph nodes from patients with cervical cancer. J Clin Pathol. 1997;50:960-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Füle T, Csapó Z, Máthé M, Tátrai P, László V, Papp Z, Kovalszky I. Prognostic significance of high-risk HPV status in advanced cervical cancers and pelvic lymph nodes. Gynecol Oncol. 2006;100:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WG, Pirog EC. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 96. | Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Guerrero D, Guarch R, Ojer A, Casas JM, Ropero S, Mancha A, Pesce C, Lloveras B, Garcia-Bragado F, Puras A. Hypermethylation of the thrombospondin-1 gene is associated with poor prognosis in penile squamous cell carcinoma. BJU Int. 2008;102:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Schiffman M, Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 99. | Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 100. | Villa LL, Lopes A. Human papillomavirus DNA sequences in penile carcinomas in Brazil. Int J Cancer. 1986;37:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Chan KW, Lam KY, Chan AC, Lau P, Srivastava G. Prevalence of human papillomavirus types 16 and 18 in penile carcinoma: a study of 41 cases using PCR. J Clin Pathol. 1994;47:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Ornellas AA, Nóbrega BL, Wei Kin Chin E, Wisnescky A, da Silva PC, de Santos Schwindt AB. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Picconi MA, Eiján AM, Distéfano AL, Pueyo S, Alonio LV, Gorostidi S, Teyssié AR, Casabé A. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol. 2000;61:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 104. | Frisch M, Fenger C, van den Brule AJ, Sørensen P, Meijer CJ, Walboomers JM, Adami H-O, Melbye M, Glimelius B. Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res. 1999;59:753-757. [PubMed] |
| 105. | Gilbert DC, Williams A, Allan K, Stokoe J, Jackson T, Linsdall S, Bailey CM, Summers J. p16INK4A, p53, EGFR expression and KRAS mutation status in squamous cell cancers of the anus: correlation with outcomes following chemo-radiotherapy. Radiother Oncol. 2013;109:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 106. | Vokes EE, Agrawal N, Seiwert TY. HPV-Associated Head and Neck Cancer. J. Natl Cancer Inst. 2015;107:djv344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 107. | Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1201] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 108. | Xing Y, Zhang J, Lin H, Gold KA, Sturgis EM, Garden AS, Lee JJ, William Jr WN. Relation between the level of lymph node metastasis and survival in locally advanced head and neck squamous cell carcinoma. Cancer. 2016;122:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Jacobi C, Rauch J, Hagemann J, Lautz T, Reiter M, Baumeister P. Prognostic value of the lymph node ratio in oropharyngeal carcinoma stratified for HPV-status. Eur Arch Otorhinolaryngol. 2018;275:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, Califano JA, Tufano RP, Koch WM. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 111. | Sivars L, Tani E, Näsman A, Ramqvist T, Munck-Wikland E, Dalianis T. Human Papillomavirus as a Diagnostic and Prognostic Tool in Cancer of Unknown Primary in the Head and Neck Region. Anticancer Res. 2016;36:487-493. [PubMed] |
| 112. | Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2814] [Cited by in RCA: 2762] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 113. | Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2091] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 114. | Gillison ML. Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: implications for clinical research in head and neck cancers. J Clin Oncol. 2006;24:5623-5625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Paver EC, Currie AM, Gupta R, Dahlstrom JE. Human papilloma virus related squamous cell carcinomas of the head and neck: diagnosis, clinical implications and detection of HPV. Pathology. 2020;52:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 116. | Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1352] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 117. | Huang SH, Perez-Ordonez B, Liu F-F, Waldron J, Ringash J, Irish J, Cummings B, Siu LL, Kim J, Weinreb I, Hope A, Gullane P, Brown D, Shi W, O’Sullivan B. Atypical Clinical Behavior of p16-Confirmed HPV-Related Oropharyngeal Squamous Cell Carcinoma Treated With Radical Radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:276-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 118. | Shimura E, Hama T, Suda T, Ikegami M, Urashima M, Kojima H. The Presence of HPV DNA in Neck Lymph Node Metastasis Correlates with Improved Overall Survival of Patients with Oropharyngeal Cancer Undergoing Surgical Treatment. Oncology. 2017;92:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 119. | Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469-6475. [PubMed] |
| 120. | Takes RP, Kaanders JHAM, van Herpen CML, Merkx MAW, Slootweg PJ, Melchers WJG. Human papillomavirus detection in fine needle aspiration cytology of lymph node metastasis of head and neck squamous cell cancer. J Clin Virol. 2016;85:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Fujita A, Buch K, Truong MT, Qureshi MM, Mercier G, Jalisi S, Sugimoto H, Sakai O. Imaging characteristics of metastatic nodes and outcomes by HPV status in head and neck cancers. Laryngoscope. 2016;126:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Shah MA. Gastric Cancer: The Gastric Microbiota-Bacterial Diversity and Implications. Nat Rev Gastroenterol Hepatol. 2017;14:692-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1009] [Article Influence: 67.3] [Reference Citation Analysis (1)] |
| 124. | Zhang C, Powell SE, Betel D, Shah MA. The gastric microbiome and its influence on gastric carcinogenesis: current knowledge and ongoing research. Hematol Oncol Clin North Am. 2017;31:389-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Bae JM, Kim EH. Epstein-Barr Virus and Gastric Cancer Risk: A Meta-analysis With Meta-regression of Case-control Studies. J Prev Med Public Health. 2016;49:97-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 126. | Tokunaga M, Land CE. Epstein-Barr virus involvement in gastric cancer: biomarker for lymph node metastasis. Cancer Epidemiol Biomarkers Prev. 1998;7:449-450. [PubMed] |
| 127. | Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K, Suehiro Y, Yamasaki T, Sakaida I. Clinical Importance of Epstein⁻Barr Virus-Associated Gastric Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 128. | de Souza CRT, Almeida MCA, Khayat AS, da Silva EL, Soares PC, Chaves LC, Burbano RMR. Association between Helicobacter pylori, Epstein-Barr virus, human papillomavirus and gastric adenocarcinomas. World J Gastroenterol. 2018;24:4928-4938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 129. | Fakhraei F, Haghshenas MR, Hosseini V, Rafiei A, Naghshvar F, AlizadehNavaei R. Detection of human papillomavirus DNA in gastric carcinoma specimens in a high-risk region of Iran. Biomed Rep. 2016;5:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Ma TY, Liu WK, Chu YL, Jiang XY, An Y, Zhang MP, Zheng JW. Detection of human papillomavirus type 16 DNA in formalin-fixed, paraffin-embedded tissue specimens of gastric carcinoma. Eur J Gastroenterol Hepatol. 2007;19:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 131. | Xu WG, Zhang LJ, Lu ZM, Li JY, Ke Y, Xu GW. [Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract]. Zhonghua Yi Xue Za Zhi. 2003;83:1910-1914. [PubMed] |
| 132. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4829] [Article Influence: 439.0] [Reference Citation Analysis (2)] |
| 133. | Yuan XY, Wang MY, Wang XY, Chang AY, Li J. Non-detection of Epstein-Barr virus and Human Papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genet Mol Biol. 2013;36:183-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Koshiol J, Wei WQ, Kreimer AR, Ren JS, Gravitt P, Chen W, Kim E, Abnet CC, Zhang Y, Kamangar F, Lin DM, Wang GQ, Roth MJ, Dong ZW, Taylor PR, Qiao YL, Dawsey SM. The gastric cardia is not a target for human papillomavirus-induced carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2010;19:1137-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Snietura M, Waniczek D, Piglowski W, Kopec A, Nowakowska-Zajdel E, Lorenc Z, Muc-Wierzgon M. Potential role of human papilloma virus in the pathogenesis of gastric cancer. World J Gastroenterol. 2014;20:6632-6637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 136. | Zeng ZM, Luo FF, Zou LX, He RQ, Pan DH, Chen X, Xie TT, Li YQ, Peng ZG, Chen G. Human papillomavirus as a potential risk factor for gastric cancer: a meta-analysis of 1,917 cases. Onco Targets Ther. 2016;9:7105-7114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |