Published online May 7, 2020. doi: 10.3748/wjg.v26.i17.1987
Peer-review started: December 31, 2019
First decision: February 19, 2020
Revised: March 31, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 7, 2020
Processing time: 127 Days and 14.9 Hours
This article reviews the current evidence and knowledge of progressive liver fibrosis after pediatric liver transplantation. This often-silent histologic finding is common in long-term survivors and may lead to allograft dysfunction in advanced stages. Surveillance through protocolized liver allograft biopsy remains the gold standard for diagnosis, and recent evidence suggests that chronic inflammation precedes fibrosis.
Core tip: Progressive liver allograft fibrosis is a common finding after liver transplantation in children and may lead to allograft failure in the long-term. Recent data from centers performing sequential protocol biopsies after pediatric liver transplantation demonstrate that chronic inflammation precedes fibrosis. In this review, we provide an update on pathogenesis, diagnosis and management of progressive liver fibrosis in pediatric liver transplant recipients.
- Citation: George M, Paci P, Taner T. Significance of progressive liver fibrosis in pediatric liver transplants: A review of current evidence. World J Gastroenterol 2020; 26(17): 1987-1992
- URL: https://www.wjgnet.com/1007-9327/full/v26/i17/1987.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i17.1987
Improvements in organ preservation, perioperative care and immunosuppression have increased patient and graft survival after pediatric liver transplantation over the past three decades. As such, currently, the 10-year patient and graft survival are 83% and 73%, respectively[1]. Despite these good early outcomes, most pediatric liver transplant recipients fail to meet the goal of “one graft for lifetime”. Progressive liver fibrosis, a common cause of liver allograft failure in pediatric liver transplant recipients, remains highly prevalent in late post-transplant liver biopsies; reported in 69% to 97% of all cases[2-7] (Table 1). Here, we review the current evidence on pathogenesis, etiology, diagnosis and management of progressive liver fibrosis in pediatric liver transplant recipients.
While a detailed overview of the liver fibrosis is beyond the scope of this review, understanding the main instigators of this process is of paramount importance in the context of progressive liver fibrosis. Fibrosis in the liver is a wound healing response to chronic injury, secondary to infections (e.g., viral hepatitis), immune-mediated mechanisms (e.g., auto-immune hepatitis) or chemicals (e.g., alcoholic hepatitis). Fibrosis can occur both in the native and transplanted liver. Studies have shown that the central event in liver fibrosis is activation of hepatic stellate cells (HSC) in response to chronic injury. HSC are located in the subendothelial space of Disse, between sinusoidal epithelium and hepatocytes. Activated HSC increase expression of cytoplasmic alpha-smooth muscle actin. This differentiation is followed by secretion of collagen Type 1 and 3[8,9]. In addition, HSC activate other fibrogenic mechanisms through paracrine stimuli.
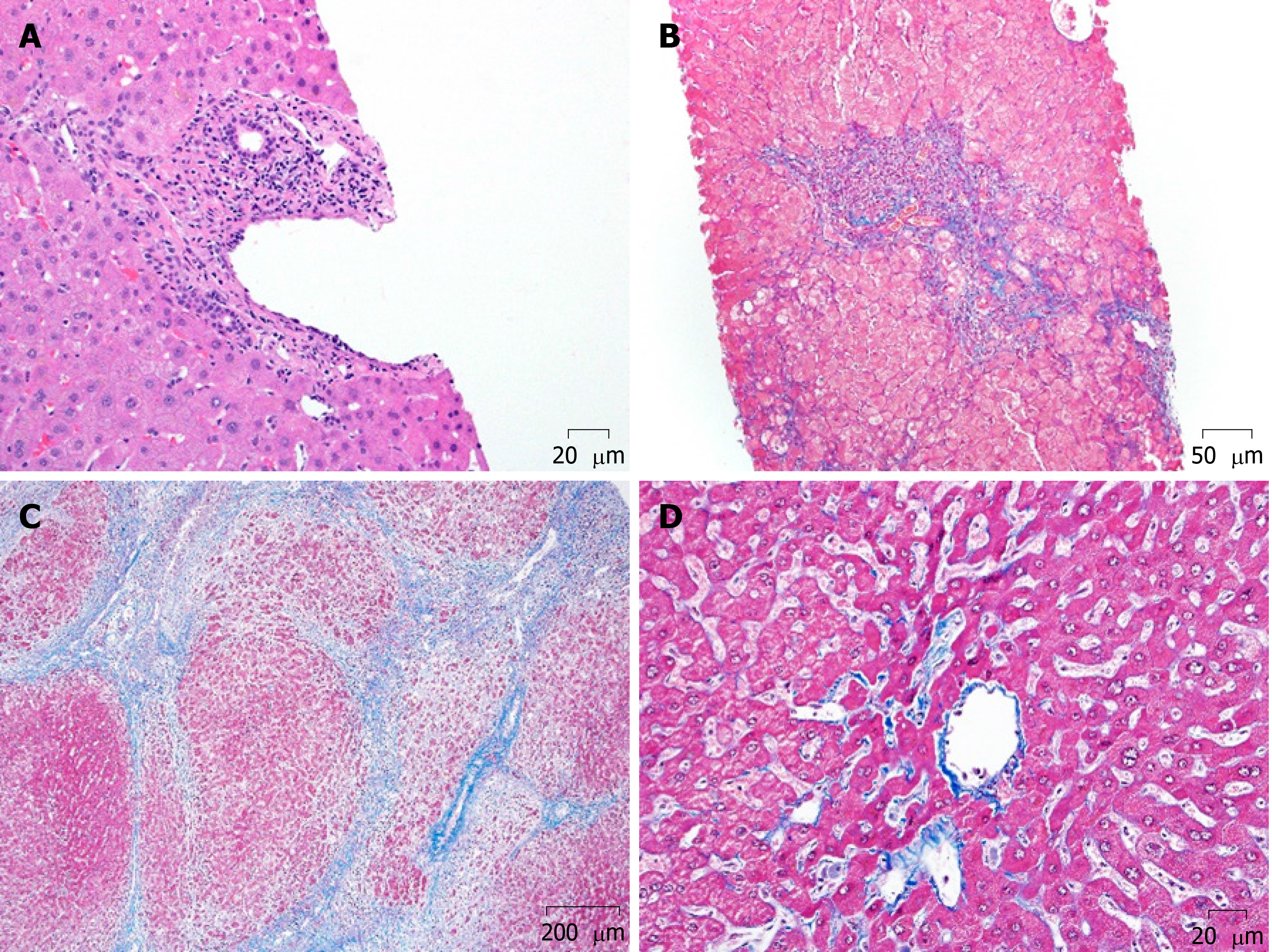
Two additional mechanisms of chronic injury can occur in transplanted livers; alloimmune inflammation (Figure 1A and B) and biliary outflow obstruction (Figure 1C). The association between allograft inflammation and progressive fibrosis has been demonstrated in multiple studies. Based on 1-, 5- and 10-year protocol liver allograft biopsies in pediatric liver transplant recipients, Evans et al[3] showed that the incidence of chronic, silent inflammation exceeds 40% at 5 years, and 60% at 10 years. The group from Belgium, Varma et al[10] subsequently investigated the temporal relationship of inflammation and fibrosis, using sequential allograft biopsies (a total of 5 biopsies in 10 years). Their analysis demonstrated that the biggest predictor of graft fibrosis is portal inflammation (Figure 1A) seen in the preceding biopsy. Furthermore, the severity of the inflammation correlated with the risk of fibrosis (Figure 1C and D) in the consecutive biopsy.
The role of donor-specific HLA antibodies (DSA) in development of allograft fibrosis after pediatric liver transplantation has been investigated in Kyoto, Japan[11]. Based on 5-20-year protocol liver biopsies, the investigators found a significant correlation with circulating DSA and stage 3 and 4 fibrosis. More in-depth evaluation of the biopsies, however, demonstrated that the incidence of inflammation in the preceding liver biopsy of the recipients with DSA was significantly higher, raising the question of whether the DSA is a consequence of inflammation, rather that the cause of fibrosis. Previous studies from our group at the Mayo Clinic have shown that the inflammation in patients with DSA is not different than that of patients without DSA[12], and the alloreactivity of T cells in liver patients, regardless of the DSA status, is reduced[13].
One of the theories that explain immune mediated injury and subsequent fibrosis is “antigenic mimicry”. HLA-DRB1*03/04 allele in liver recipients predisposes to such injury. The mechanism of injury is similar to autoimmune hepatitis as explained by Montano-Loza et al[14] and Liberal et al[15]. This allele favors faulty antigen processing causing immune mediated hepatocyte injury. Varma et al[10] in their observational study of 89 stable liver recipients noted that HLA-DRB1*03/04 is a risk factor for high grade fibrosis and these recipients need to be monitored closely.
Liver function tests are usually normal at early stages of liver allograft fibrosis after pediatric liver transplantation. Therefore, liver biopsy remains the current gold standard for diagnosis of fibrosis though with some limitations which include sampling errors. Although it is an invasive diagnostic tool, liver biopsy in pediatric liver transplant recipients has a low complication rate and provides invaluable information regarding the health of the allograft. Thus, most pediatric liver transplant programs have adapted protocolized liver biopsy at various time points. These biopsies are evaluated for fibrosis by using either the METAVIR or the Liver Allograft Fibrosis Score systems (Table 2). As the more commonly used METAVIR score was not designed for evaluating allograft biopsies, the latter may be better suited for transplant patients. Regardless, both score systems have advantages and disadvantages, detailed in Table 2. In order to decrease the odds of sampling error and observer variability, it is recommended that the biopsy specimen to be at least 20-25 mm[16,17].
| METAVIR score[25] | Liver allograft fibrosis score – LAFSc[26] |
| Portal and periportal (Figure 1B) tract-based fibrosis scoring systems | Periportal (Figure 1B), Perisinusoidal and perivenular (Figure 1C) fibrosis-based staging |
| Used for assessing fibrosis in post viral hepatitis, may not accurately quantify LAF in pediatric population. Assesses fibrosis located in portal tracts, underestimating LAF in the other areas | LAFSc system stages fibrosis adding portal tracts, sinusoids and centrilobular areas. Advantage of LAFSc is the individual assessment of fibrosis in portal tracts, sinusoids and centrilobular veins providing good representation of the whole hepatic acinus |
| The stage of fibrosis was assessed on a five-point scale: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = few septa, F3 = numerous septa without cirrhosis, F4 = cirrhosis; Activity was graded according to the intensity of necro inflammatory lesions: A0 = no histological activity, A1 = mild activity, A2 = moderate activity, A3 = severe activity | Fibrosis deposition was classified in three main areas of the liver parenchyma: Portal tracts, sinusoids (zones 1 and 2) and centrilobular veins (zone 3); in each area, fibrosis was staged from 0 to 3 (0 = absent, 1 = mild, 2 = moderate and 3 = severe fibrosis), with a total score of 9. Equal score weight was assigned to each area to accurately reflect fibrosis distribution in liver allograft specimens |
| A histopathological abnormality is a Metavir score of ≥ A1 or ≥ F1 and such scores indicate the need for treatment because liver fibrosis is reversible if early treatment is initiated | LAFSc: ≤ 3 – mild fibrosis; 4-5 – moderate fibrosis; ≥6 – severe fibrosis |
| In a sample of PLB specimens METAVIR detected LAF in 81.6% specimens[26] | LAFSc showed fibrosis in 93.5% of specimens[26] |
In addition to the routine histological evaluation, recently the utility of the assessment of alpha smooth muscle actin in biopsies was tested. As discussed above, HSC express cytoplasmic alpha smooth muscle actin prior to secreting collagen. Therefore, theoretically, quantification of alpha smooth muscle actin could predict severity of future fibrosis in the allograft. In fact, in a recent study, alpha smooth muscle actin -positive area percentage > 1.05 predicted increased fibrosis in the subsequent biopsy with a 90% specificity[18].
Fibro test: Fibro test in pediatric liver transplant patients is calculated from six biochemical markers (gamma glutamyl transpeptidase, alanine aminotransferase, haptoglobin, alpha-2-macroglobulin, apolipoprotein-A1, and total bilirubin) as well as age and sex of the patient. The test was devised by Bio Predictive, France. The score lies between 0 and 1. There was, however, no correlation of FT with the degree of fibrosis[19].
Enhanced liver fibrosis test: The enhanced liver fibrosis test (ELF test, Siemens) combines Hyaluronic Acid, amino-terminal propeptide of type III collagen, and tissue inhibitor of metalloproteinase 1 in an algorithm. ELF values were significantly higher in healthy transplant children compared with healthy controls. Values < 7.7 mild or no fibrosis, 7.7-9.8 moderate fibrosis, and > 9.8 severe fibrosis. There was however no correlation of ELF with the degree of fibrosis[19].
Transient elastography: Uses the propagation velocity of an ultrasound wave to calculate liver stiffness. It is useful to detect advanced fibrosis rather than milder forms. The technique is generally applicable in children and has been shown to be of some diagnostic value. However, it is not a test for screening fibrosis; although it can be used for follow up after establishing a base line for the patient[19]. Limitations of the elastography in children include the requirement of pediatric probes and distorted signals secondary to the midline position of the grafts. In addition, elastography in pediatric transplant recipients with no fibrosis (based on biopsy) reveals higher scores than that in children who have not had a transplant[19].
Acoustic radiation force impulse: This is another ultrasound based elastography method. The region of interest in the liver is located using a real time B mode ultrasound. Tissue in the region of interest is mechanically excited with an impulsive acoustic radiation force, which results in the generation of shear waves within the tissue; the velocity of these shear waves in meters per second indirectly measures the elasticity of the liver. Tomita et al[20] showed that acoustic radiation force impulse had good accuracy in detecting graft fibrosis after living donor liver transplantation.
Magnetic resonance imaging: Serai et al[21] in 2018 had used magnetic resonance imaging (MRI) derived liver stiffness in pediatric and young population for a wide variety of liver diseases. MRI was found to have moderate test performance for distinguishing stage 0-1 fibrosis from stage 2 or higher fibrosis in pediatric patients with liver disease. MRI has still not been used for detecting fibrosis in pediatric liver allografts but seems to be a modality for the future.
Because of the correlation between alloimmune injury and subsequent development of progressive allograft fibrosis after pediatric liver transplantation, intensifying immunosuppression has been the most common approach in management of patients with fibrosis. In a cross-sectional study of 60 pediatric liver transplant recipients from Hamburg, Germany, 14 patients were found to have inflammation and early fibrosis in protocol biopsies, despite normal laboratory findings[22]. Accordingly, immunosuppression was intensified by increasing the goal trough levels of Cyclosporin A and Tacrolimus. Five of the 14 patients were then biopsied, 12 to 18 mo later. Histologic evaluation demonstrated resolution of inflammation and improvement in the fibrosis score in 4 patients in whom the higher drug trough levels could be achieved. Similarly, Sanada et al[5] reported that in 11 pediatric liver transplant patients with 5 year protocol liver allograft biopsies demonstrating moderate inflammation and fibrosis, improvement in both the inflammation and fibrosis scores was achieved in all patients after intensifying the immunosuppression. In the most recent report from Sheikh et al[23], 44 liver transplant recipients in New Zealand were maintained on stable, Tacrolimus-based immunosuppression for the first 5 years, with a median trough level of 5.8 µg/L. With that, the incidence of fibrosis in liver allografts was as low as 2% at 5 years, notable for the lowest such incidences in the literature.
The role of steroids in preventing fibrosis was investigated by Venturi et al[17] in 2014. In a cohort of 38 pediatric liver transplant recipients, the authors found that the incidence of allograft fibrosis within the first year was higher among the group that was maintained on a steroid-free immunosuppression regimen. However, long term progression of the fibrosis was similar in the steroid-free and steroid-inclusive immunosuppression groups, indicating that the potentially protective impact of steroids on liver allograft fibrosis may not be sustained in the long-term.
Given these findings, it is reasonable to suggest that detection of early reversible graft fibrosis warrants increasing immunosuppression. It should be noted, however, that none of the above-mentioned studies are randomized.
As outlined above, much remains to be investigated to elucidate the mechanisms that trigger graft fibrosis in pediatric liver transplant recipients. A promising development is the establishment of the Graft Injury Group Observing Long-term Outcome group in early 2015[24]. This international multicenter, multidisciplinary collaboration aims to follow a population of children post liver transplant in order to identify mechanisms of allograft injury, and to determine predictive factors for progression of disease and treatment response, with the ultimate goal of improving long-term graft survival.
Preliminary data from 6 European transplant centers shows increasing incidence of chronic graft hepatitis (43% at 5 years, 53% at 10 years) and fibrosis (54% at 5 years, 79% at 10 years) over time. It appears that children treated with long-term low dose steroids are significantly less likely to have graft hepatitis compared to those not on steroids both at 5 years and 10 years. This improvement, however, was not associated with decreased graft fibrosis at 10 years.
As long-term survivors of pediatric liver transplantation continue to increase, so does the incidence of progressive graft fibrosis. The definitive way of diagnosis of graft fibrosis is still through a liver biopsy. Even though studies show early post-transplant biopsies are not needed (1 and 2 year) most of the centers do regular protocol biopsies at 1, 5, 10, 15 and 20 years. There is also evidence that some of the fibrosis could be driven by genetic predisposition, allo-immunity and inflammation. There has been evidence to suggest that preceding inflammation and its intensity correlated with subsequent fibrosis. Portal inflammation has been the strongest predictor of portal fibrosis in subsequent protocol biopsies and this is not seen in other areas[10]. The current understanding is early fibrosis is reversible with increasing immuno-suppression emphasizing the need for constant surveillance of pediatric liver allograft recipients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mandorfer M S-Editor: Dou Y L-Editor: A E-Editor: Liu MY
| 1. | Martinelli J, Habes D, Majed L, Guettier C, Gonzalès E, Linglart A, Larue C, Furlan V, Pariente D, Baujard C, Branchereau S, Gauthier F, Jacquemin E, Bernard O. Long-term outcome of liver transplantation in childhood: A study of 20-year survivors. Am J Transplant. 2018;18:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, Alonso EM. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Evans HM, Kelly DA, McKiernan PJ, Hübscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M, Pariente D, Chardot C, Dousset B, Massault PP, Bernard D, Houssin D, Bernard O, Gauthier F, Soubrane O. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Sanada Y, Matsumoto K, Urahashi T, Ihara Y, Wakiya T, Okada N, Yamada N, Hirata Y, Mizuta K. Protocol liver biopsy is the only examination that can detect mid-term graft fibrosis after pediatric liver transplantation. World J Gastroenterol. 2014;20:6638-6650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009;49:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Ueno T, Tanaka N, Ihara Y, Takama Y, Yamada H, Mushiake S, Fukuzawa M. Graft fibrosis in patients with biliary atresia after pediatric living-related liver transplantation. Pediatr Transplant. 2011;15:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 737] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 9. | Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 701] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 10. | Varma S, Ambroise J, Komuta M, Latinne D, Baldin P, Reding R, Smets F, Stephenne X, Sokal EM. Progressive Fibrosis Is Driven by Genetic Predisposition, Allo-immunity, and Inflammation in Pediatric Liver Transplant Recipients. EBioMedicine. 2016;9:346-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Egawa H, Yurugi K, Masuda S, Minamiguchi S, Maekawa T, Uemoto S, Haga H. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts. Liver Transpl. 2012;18:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Taner T, Heimbach JK, Rosen CB, Nyberg SL, Park WD, Stegall MD. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int. 2016;89:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Taner T, Gustafson MP, Hansen MJ, Park WD, Bornschlegl S, Dietz AB, Stegall MD. Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year. Kidney Int. 2018;93:1465-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Clinical significance of HLA DRB103-DRB104 in type 1 autoimmune hepatitis. Liver Int. 2006;26:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Liberal R, Vergani D, Mieli-Vergani G. Update on Autoimmune Hepatitis. J Clin Transl Hepatol. 2015;3:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 17. | Venturi C, Sempoux C, Quinones JA, Bourdeaux C, Hoyos SP, Sokal E, Reding R. Dynamics of allograft fibrosis in pediatric liver transplantation. Am J Transplant. 2014;14:1648-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Varma S, Stéphenne X, Komuta M, Bouzin C, Ambroise J, Smets F, Reding R, Sokal EM. The histological quantification of alpha-smooth muscle actin predicts future graft fibrosis in pediatric liver transplant recipients. Pediatr Transplant. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Goldschmidt I, Stieghorst H, Munteanu M, Poynard T, Schlue J, Streckenbach C, Baumann U. The use of transient elastography and non-invasive serum markers of fibrosis in pediatric liver transplant recipients. Pediatr Transplant. 2013;17:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Tomita H, Hoshino K, Fuchimoto Y, Ebinuma H, Ohkuma K, Tanami Y, Du W, Masugi Y, Shimojima N, Fujino A, Kano M, Fujimura T, Ishihama H, Shimizu T, Tanabe M, Saito H, Sakamoto M, Hibi T, Kitagawa Y, Kuroda T. Acoustic radiation force impulse imaging for assessing graft fibrosis after pediatric living donor liver transplantation: a pilot study. Liver Transpl. 2013;19:1202-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Serai SD, Trout AT, Sirlin CB. Elastography to assess the stage of liver fibrosis in children: Concepts, opportunities, and challenges. Clin Liver Dis (Hoboken). 2017;9:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Briem-Richter A, Ganschow R, Sornsakrin M, Brinkert F, Schirmer J, Schaefer H, Grabhorn E. Liver allograft pathology in healthy pediatric liver transplant recipients. Pediatr Transplant. 2013;17:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Sheikh A, Chau KY, Evans HM. Histological findings in protocol biopsies following pediatric liver transplant: Low incidence of abnormalities at 5 years. Pediatr Transplant. 2018;22:e13212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Group GI. Graft Injury Group Observing Long-term Outcome. 2015. [accessed 2019 December 1] Available from: http://www.graftinjurygroup.org/. |
| 25. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2160] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 26. | Venturi C, Sempoux C, Bueno J, Ferreres Pinas JC, Bourdeaux C, Abarca-Quinones J, Rahier J, Reding R. Novel histologic scoring system for long-term allograft fibrosis after liver transplantation in children. Am J Transplant. 2012;12:2986-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |