Published online Apr 21, 2020. doi: 10.3748/wjg.v26.i15.1841
Peer-review started: January 6, 2020
First decision: January 19, 2020
Revised: March 26, 2020
Accepted: April 1, 2020
Article in press: April 1, 2020
Published online: April 21, 2020
Processing time: 105 Days and 12.9 Hours
Aminoacyl tRNA synthetases/ligases (ARSs) are highly conserved enzymes involved in attaching amino acids to tRNA promoting protein synthesis. Although deficiencies of ARSs localized to the mitochondria classically present with neuropathology, the clinical features of cytosolic ARS deficiencies are more variable. They have previously been associated with neonatal hepatitis, but never with early-onset inflammatory bowel disease.
A nine-year-old Bangladeshi boy presented with neonatal liver failure and deranged clotting, transaminitis and cholestasis. His parents were first cousins. Two older brothers and a sister were well. The patient suffered from loose stools from early infancy which became more troublesome and persistent from five years old with ten bloody motions a day. Repeated endoscopies showed persistent pancolitis, which was refractory to mesalazine, corticosteroids, azathioprine, sirolimus and anti-TNF (adalimumab) therapy, but has improved recently with subcutaneous methotrexate.Whole Genome Sequencing revealed a novel pathogenic missense variant (c.290A > G) in the cytosolic isoleucyl-tRNA synthetase gene, leading to an amino acid substitution (p.Asp97Gly). Pathogenic variants in other genes associated with inflammatory bowel disease (IBD) (ADAM17, EGFR, FOXP3, IL10RA, IL10RB, IL21R, NCF4, STAT3) were excluded. Cytokine assays demonstrated markedly elevated IL-2, IL-5, IL-13, IL-9 and IL-10 by the patient’s CD4+ T-cells, while IL-17A, IL-17F, IFNβ were lower, and TNFα not significantly different when compared to healthy controls.
This case report provides evidence that recessive mutations in cytosolic isoleucyl-tRNA synthetase are a novel monogenic cause of IBD, which should be considered, particularly in infants and children with a history of neonatal hepatitis and very early-onset IBD poorly responsive to treatment.
Core tip: Consider cytosolic isoleucyl-tRNA synthetase in children presenting with neonatal hepatitis and refractory very early-onset inflammatory bowel disease. This case report provides evidence for a novel monogenic cause of inflammatory bowel disease that should be considered, particularly in patients with very early-onset, poor response to treatment and a history of neonatal hepatitis.
- Citation: Fagbemi A, Newman WG, Tangye SG, Hughes SM, Cheesman E, Arkwright PD. Refractory very early-onset inflammatory bowel disease associated with cytosolic isoleucyl-tRNA synthetase deficiency: A case report. World J Gastroenterol 2020; 26(15): 1841-1846
- URL: https://www.wjgnet.com/1007-9327/full/v26/i15/1841.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i15.1841
Aminoacyl tRNA synthetases/ligases (ARSs) are a group of 20 ubiquitously expressed, highly conserved enzymes involved in attaching each specific amino acid to a tRNA to promote protein synthesis. ARS deficiencies localized to the mitochondria have been known for 40 years and classically present with lactic acidosis, encephalopathy, failure to thrive and global developmental delay. Clinical features of cytosolic ARS deficiency are more variable[1,2]. In 2016, three unrelated individuals harboring compound homozygous variants in cytosolic isoleucyl-tRNA synthetase (IARS) [p.(Arg418)(Ile1174Asn), p.(Arg254)(Pro437Leu) and p.(Val370Gly)(Asn992Asp) (MIM: 600709)] were reported[3]. The patients suffered growth and developmental delay, sensorineural hearing loss, muscular hypotonia, diabetes mellitus, hepatic dysfunction with steatosis and fibrosis, and zinc deficiency. A further individual with compound heterozygous for the missense substitutions p.(Arg739Cys)(Phe556Ser) with microcephaly, growth and developmental delay and neonatal cholestasis was described in 2017[4] and a seven year old boy compound heterozygous for p.(Gln671fs)(Thr69Ile) has also been reported with hepatopathy, hypotonia, intellectual disability and growth retardation[5,6]. Here, we present a child with cytosolic IARS deficiency, whose main clinical feature was refractory inflammatory bowel disease (IBD), hitherto not previously described in other patients with IARS deficiency.
A nine-year-old Bangladeshi boy born in the United Kingdom presented with poor feeding, weight loss, drowsiness due to neonatal liver failure with deranged clotting, transaminitis and cholestasis.
The patient suffered from loose stools from early infancy and was commenced on an amino acid formula from the age of two months with initial improvement. His diarrhea has been much more troublesome since the age of five years old with ten bloody stools a day.
The patient was free from other medical history.
His parents were first cousins. Two older brothers and a sister were well.
On examination, he was microcephalic, with normal weight, height and gross motor development. He was clubbed with mild hepatomegaly.
Serum orosomucoid concentration was persistently raised [1736–2685 mg/L (normal range 300–1200 mg/L)], as was his ESR [28–140 mm/h (normal range 4–10 mm/h)]. C-reactive protein was normal. Since the neonatal period, his liver function tests, including gamma-glutamyl transferase have been normal. Fecal calprotectin is intermittently elevated (peak 393 mcg/g), although the latest level is normal at 50 mcg/g. Plasma zinc concentration was normal at 17.2 mol/L (10–18 mol/L).
Abdominal ultrasound showed no signs of hepatosplenomegaly, but the liver had diffusely increased echogenicity consistent with fatty change, possibly related to weight gain associated with chronic corticosteroid use.
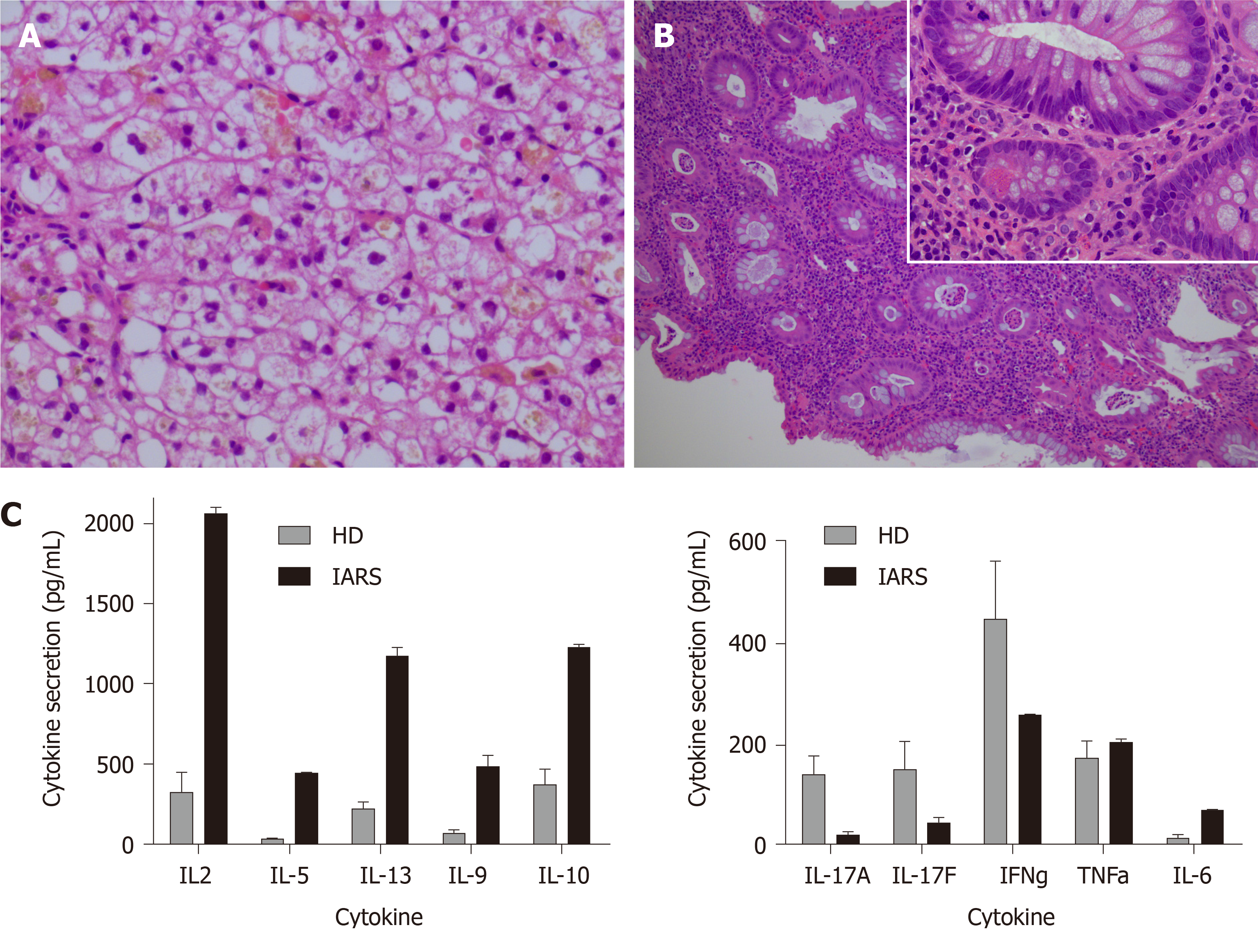
Liver biopsy showed hydropic degeneration of hepatocytes (Figure 1A). There was no evidence of mitochondrial DNA depletion and plasma lactate was normal. Repeated endoscopy at 6, 7 and 8 years old showed persistent chronic inflammation in the cecum, and pancolitis with diffuse inflammation, superficial ulceration, distortion of the crypt architecture and crypt abscess formation, in a distribution similar to that found in ulcerative colitis (Figure 1B). There were no inflammatory changes in the esophagus, stomach and duodenum, nor were there any granuloma to suggest Crohn’s disease.
In view of the very early-onset of the disease and its poor response to immunosuppres-sive medication, an underlying genetic cause was considered. Whole genome sequencing performed as part of the United Kingdom 100000 Genome Project revealed a homozygous c.290A > G, p.(Asp97Gly) variant in cytosolic IARS (encoded by IARS1, NM_013417). Parents were both heterozygous as were two unaffected siblings with the other unaffected sibling wild type. The variant was absent in the gnomAD database of about 140000 controls and scored by in silico predictors (MutationTaster 0.99; CADD score 32) to be pathogenic. The aspartic acid residue at this position is conserved to Caenorhabditis elegans and the variant lies in the IleRS core domain, where other disease causing variants have been identified[7]. Variants in other genes associated with IBD (ADAM17, EGFR, FOXP3, IL10RA, IL10RB, IL21R, NCF4, STAT3) were screened and no potentially pathogenic variants were found.
Immune phenotyping was determined by flow cytometry. Mucosal-associated invariant T-cells cell and natural killer cell frequencies were reduced, comprising < 0.2 and 1% of lymphocytes, respectively (range: 1%-15%). Greater than 60% of the patient’s CD4+ and CD8+ T cells were of a naïve phenotype, reflecting the young age of the patient. T-cells, total B-cells as well as B-cell subsets (transitional, naïve, memory, class switching) were within normal range. Release of Th1, Th2, Th17 and Treg pathway cytokines (IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, IL-17A, IL-17F, TNFα and IFNβ) that may be deranged in IBD were assayed by cytometric bead array (Becton Dickinson) after 5-d stimulation of purified memory CD4+ T cells with anti-CD2/CD3/CD28 monoclonal antibodies. Secretion of some inflammatory (IL-2, IL-5, IL-13, IL-9) and immune regulatory (IL-10) cytokines by the patient memory CD4+ T-cells was markedly elevated, while IL-17A, IL-17F, IFNβ were lower and TNFα not significantly different compared to healthy donors (Figure 1C).
Transient neonatal hepatitis and refractory very early-onset IBD due to cytosolic isoleucyl-tRNA synthetase.
His IBD was refractory to additional zinc supplementation, as well as immune modulation with mesalazine, corticosteroids, azathioprine, sirolimus and anti-TNF therapy (adalimumab). Recent treatment with subcutaneous methotrexate has led to reduction in his stool frequency to 4 – 5 stools/d with no blood or mucous.
Nine-year-old boy with chronic IBD (ten bloody watery stools/day and abdominal pain) despite normal plasma zinc concentration and most standard immune modulatory therapy. His symptoms have recently improved with the addition of subcutaneous methotrexate. Vedolizumab infusions are being considered as a further steroid-sparing immunomodulator. Informed written consent was obtained to report on this case from the child’s parents.
This is the first report of a homozygous variant in cytosolic IARS associated with refractory very early-onset IBD. The fact that this variant is novel, is predicted to result in an amino acid substitution from aspartate to glycine at a highly conserved residue, and segregates with the phenotype in the family, indicates that it is disease causing. Supportive evidence from additional cases and functional enzyme assays would be helpful in definitively confirming the role of the IARS gene in very early-onset IBD. Assays are unfortunately not currently available. This case report will hopefully lead to additional patients with very early-onset IBD due to variants in the IARS gene being reported by other clinicians.
ARSs may be associated with T-cell dysfunction[8,9] as seen in our patient, but the lack of improvement with most immunosuppressant drugs suggests that other inflammatory pathways are involved. The high IL-10 level is in keeping with an ineffectual compensatory Treg response rather than a defect in this pathway. There is some suggestion that sirolimus, which blocks mammalian target of rapamycin complex 1 may ameliorate the disease, but use of sirolimus in our patient made no difference to his clinical course[10].
Maintaining optimal zinc levels may also help optimize aminoacyl-tRNA synthetase activity, but our patient had normal plasma zinc concentrations and zinc supplements did not help ameliorate his IBD. It is possible that there are defects in cell turnover and resultant barrier dysfunction of gut mucosa leading to microbe-induced inflammation particularly in the colon. Broad-spectrum antibiotics may help for severe exacerbations.
This case report provides evidence for a novel monogenic cause of IBD that should be considered, particularly in patients with very early-onset, poor response to treatment and a history of neonatal hepatitis.
This research was made possible through access to data generated by the 100000 Genomes Project; http://www.genomicsengland.co.uk. We thank Geetha Rao for performing the immune functional assays presented in this report.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: ESPGHAN, No. 481.
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Day A, Sorrentino D S-Editor: Zhang L L-Editor: A E-Editor: Liu MY
| 1. | Fuchs SA, Schene IF, Kok G, Jansen JM, Nikkels PGJ, van Gassen KLI, Terheggen-Lagro SWJ, van der Crabben SN, Hoeks SE, Niers LEM, Wolf NI, de Vries MC, Koolen DA, Houwen RHJ, Mulder MF, van Hasselt PM. Aminoacyl-tRNA synthetase deficiencies in search of common themes. Genet Med. 2019;21:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Schwartzentruber J, Buhas D, Majewski J, Sasarman F, Papillon-Cavanagh S, Thiffault I, Sheldon KM, Massicotte C, Patry L, Simon M, Zare AS, McKernan KJ; FORGE Canada Consortium, Michaud J, Boles RG, Deal CL, Desilets V, Shoubridge EA, Samuels ME. Mutation in the nuclear-encoded mitochondrial isoleucyl-tRNA synthetase IARS2 in patients with cataracts, growth hormone deficiency with short stature, partial sensorineural deafness, and peripheral neuropathy or with Leigh syndrome. Hum Mutat. 2014;35:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Kopajtich R, Murayama K, Janecke AR, Haack TB, Breuer M, Knisely AS, Harting I, Ohashi T, Okazaki Y, Watanabe D, Tokuzawa Y, Kotzaeridou U, Kölker S, Sauer S, Carl M, Straub S, Entenmann A, Gizewski E, Feichtinger RG, Mayr JA, Lackner K, Strom TM, Meitinger T, Müller T, Ohtake A, Hoffmann GF, Prokisch H, Staufner C. Biallelic IARS Mutations Cause Growth Retardation with Prenatal Onset, Intellectual Disability, Muscular Hypotonia, and Infantile Hepatopathy. Am J Hum Genet. 2016;99:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Orenstein N, Weiss K, Oprescu SN, Shapira R, Kidron D, Vanagaite-Basel L, Antonellis A, Muenke M. Bi-allelic IARS mutations in a child with intra-uterine growth retardation, neonatal cholestasis, and mild developmental delay. Clin Genet. 2017;91:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Casey JP, McGettigan P, Lynam-Lennon N, McDermott M, Regan R, Conroy J, Bourke B, O'Sullivan J, Crushell E, Lynch S, Ennis S. Identification of a mutation in LARS as a novel cause of infantile hepatopathy. Mol Genet Metab. 2012;106:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Smigiel R, Biela M, Biernacka A, Stembalska A, Sasiadek M, Kosinska J, Rydzanicz M, Ploski R. New evidence for association of recessive IARS gene mutations with hepatopathy, hypotonia, intellectual disability and growth retardation. Clin Genet. 2017;92:671-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Lu J, Bergert M, Walther A, Suter B. Double-sieving-defective aminoacyl-tRNA synthetase causes protein mistranslation and affects cellular physiology and development. Nat Commun. 2014;5:5650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Burastero SE, Fabbri M. Aminoacyl-tRNA synthetase-interacting multifunctional protein-1 (AIMP1): the member of a molecular hub with unexpected functions, including CD4 T cell homeostasis. Clin Immunol. 2012;143:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Hong HJ, Kim E, Jung MY, Kim S, Kim TS. AIMP1 deficiency enhances airway hyperreactivity in mice via increased TH2 immune responses. Clin Immunol. 2012;143:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Song J, Luo L, Ma J. Loss of Leucyl-tRNA synthetase b leads to ILFS1-like symptoms in zebrafish. Biochem Biophys Res Commun. 2018;505:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |