Published online Apr 21, 2020. doi: 10.3748/wjg.v26.i15.1691
Peer-review started: December 26, 2019
First decision: February 18, 2020
Revised: March 23, 2020
Accepted: March 27, 2020
Article in press: March 27, 2020
Published online: April 21, 2020
Processing time: 116 Days and 22.4 Hours
The review presents the data accumulated for more than 20 years of research of torque teno virus (TTV). Its molecular genetic structure, immunobiology, epidemiology, diagnostic methods, possible replication sites, and pathogenicity factors are described. TTV is a virus that is frequently detectable in patients with different viral hepatitides, in cases of hepatitis without an obvious viral agent, as well as in a healthy population. There is evidence suggesting that biochemical and histological changes occur in liver tissue and bile duct epithelium in TTV monoinfection. There are sufficient histological signs of liver damage, which confirm that the virus can undergo a replicative cycle in hepatocytes. Along with this, cytological hybridization in TTV-infected cells has shown no substantial cytopathic (cell-damaging) effects that are characteristic of pathogenic hepatotropic viruses. Studying TTV has led to the evolution of views on its role in the development of human pathology. The first ideas about the hepatotropism of the virus were gradually reformed as new data became available on the prevalence of the virus and its co-infection with other viruses, including the viruses of the known types of hepatitides. The high prevalence of TTV in the human population indicates its persistence in the body as a virome and a non-pathogenic virus. It has recently been proposed that the level of TTV DNA in the blood of patients undergoing organ transplantation should be used as an endogenous marker of the body’s immune status. The available data show the polytropism of the virus and deny the fact that TTV can be assigned exclusively to hepatitis viruses. Fortunately, the rare detection of the damaging effect of TTV on hepatic and bile duct epithelial cells may be indirect evidence of its conditionally pathogenic properties. The ubiquity of the virus and the variability of its existence in humans cannot put an end to its study.
Core tip: The review is dedicated to torque teno virus (TTV) discovered in the late 20th century. It presents data on the morphofunctional properties of the virus, its immunobiology, prevalence, transmission routes, possible replication sites, methods for diagnosis and co-infection in liver diseases. The authors tried to analyze the information accumulated in the literature on these issues and to show the evolution of views on the importance of the virus to humans: From its hepatotropism to virome and a marker for the human immune status. Along with this, the review gives data suggesting that there are biochemical and histological changes in the liver and bile duct epithelium during TTV monoinfection. On the one hand, this information points to the possible replication of the virus in the cells of the liver and the epithelium of the bile ducts. On the other hand, the high prevalence of TTV in the population indicates its persistence in the body as a virome and a non-pathogenic virus. Moreover, the fortunately rare identification of the damaging effect of the virus on the cells of the liver and the epithelium of the bile ducts may be indirect evidence of its conditionally pathogenic properties.
- Citation: Reshetnyak VI, Maev IV, Burmistrov AI, Chekmazov IA, Karlovich TI. Torque teno virus in liver diseases: On the way towards unity of view. World J Gastroenterol 2020; 26(15): 1691-1707
- URL: https://www.wjgnet.com/1007-9327/full/v26/i15/1691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i15.1691
For two decades, the problem of studying torque teno virus (TTV) has occupied the minds of many scientists around the world: TTV is a virus that is frequently detectable in patients with different viral hepatitides, in cases of hepatitis without an obvious viral agent, as well as among a healthy population. TTV was first found in a patient with acute post-transfusion hepatitis of unknown etiology and described by Japanese researchers in 1997[1]. The 58-year-old patient was observed to have an elevated level of alanine aminotransferase (ALT) at postoperative week 9-11[1,2]. At the same time, significant amounts of blood were transfused during surgery. A previously unknown DNA-containing virus was found by PCR assay using the patient’s serum[1].
Initially, the virus got its name from the first letters of the patient’s initials (TT). In 2009, the International Committee on Taxonomy of Viruses (ICTV) gave the virus the name that combines the most common transmission route (transfusion transmitted) and explains the main features of the genetic organization (torque teno) of the virus (the name deriving from the Latin terms “torque” meaning “necklace” and “tenuis” meaning “thin”)[3-5]. TTV belongs to the Circoviridae, genus of the Anelloviridae family[2-6]. After the TTV was discovered, its other isolates were discovered: TTV, torque teno mini virus (TTMV) and torque teno midi virus (TTMDV) (Figure 1)[7], which have significant genome variability[2,7,8].
Studying TTV has led to a significant evolution of views on its role in the development of pathology in humans. The idea of the predominant hepatotropism of the virus has been replaced by the data indicating its asymptomatic persistence in the body as one of the representatives of the human virome. Moreover, it has recently been proposed to use the blood TTV DNA level in patients undergoing organ transplantation as an endogenous marker of the body’s immune status.
However, the currently collected data on the prevalence of the virus in the population, tropism and the pathogenetic aspects of persistence are significantly contradictory. There is evidence suggesting that there may be biochemical and histological changes in liver tissue and bile duct epithelium during TTV monoinfection. There are sufficient histological signs of liver damage, which confirm that the virus can undergo a replicative cycle in the hepatocytes.
At the same time, a number of works indicate that the virus replicates outside the liver tissue, including in the cells of the immune system and lungs. This variety of facts does not allow an unambiguous assessment of the impact of TTV persistence on the development of pathology in the human body.
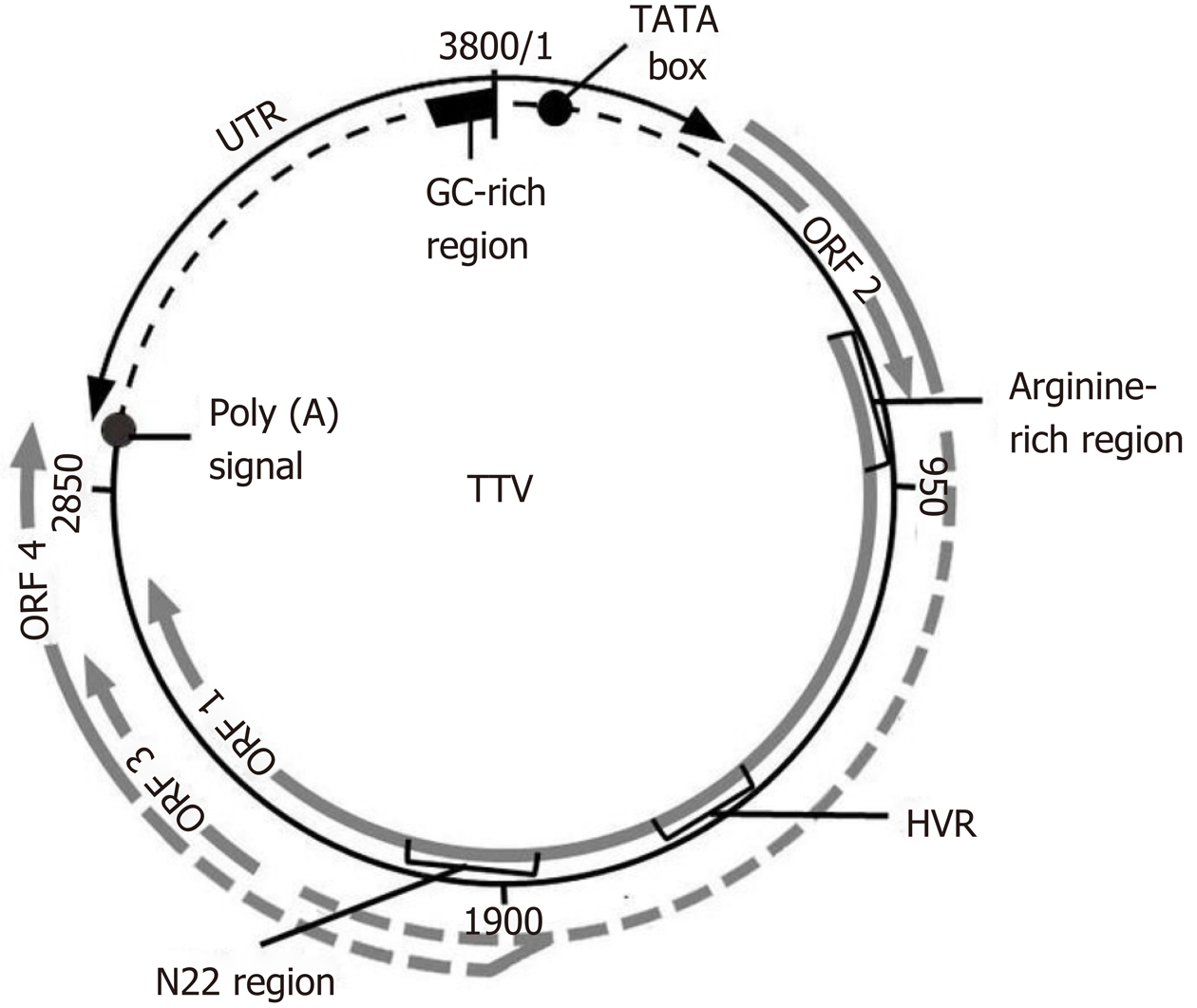
The TTV particles are spherical in shape, devoid of the outer shell, and measure 30-50 nm[9]. TTV is a small, non-enveloped, single stranded circular DNA virus with negative polarity[3,10,11] (Figure 2)[11]. The genome of TTV has a range of 3.8 kb in length and comprises 3739 bp[5,8,12]. The buoyant density in CsCl of TTV is 1.31–1.34 g/cm3[10].
The TTV genome has a wide range of nucleotide sequences and consists of an untranslated region (UTR) of 1.2 kb and a potential coding region of 2.6 kb[3,5]. Analysis of the DNA coding region revealed that there were at least four partially overlapping open reading frames (ORF1-4)[5], as well as a hypervariable region (HVR) and an N-terminal arginine fragment[7,10,11,13].
When the nucleotide sequences differ by more than 30% and by 11% to 15%, the isolate is considered as a genotype and its subtype, respectively[14]. Five genotypes (1-5) and more than 30 subtypes of TTV are identified based on the difference in their structure[2,3,11,15,16]. TTV genotype 1 was detected in both healthy carriers and patients with elevated liver enzymes and hepatitis of unknown etiology[2,8]. TTV genotype 4 is not found in healthy individuals, but it is seen in patients with rheumatoid arthritis and severe acute respiratory diseases[17].
Phylogenetic analysis of DNA establishes that genotype 1 is most common in Asian countries and genotype 3 in Africa. Hungary and the Middle East are dominated by genotype 3 (65.5%); and less by genotypes 5 (24%), 2 (5.8%), and 1 (4.7%)[7,16,18-25]. South America is characterized by genotypes 1, 2, and 3 in descending order[26]. TTV genotypes 2 and 1 are predominant in South Korea[27]. TTV isolates circulating in Russia and Kazakhstan belong to genotype 1b.
TTV produces three mRNA types, whereby the synthesis of at least 6 viral proteins takes place[13,28]. The UTR fragment is the most stable genomic element that preserves its nucleotide sequence in most TTV isolates. Focosi et al[5] point to its identity in 90% of virus isolates. This site is assumed to perform regulatory functions during viral replication[7].
On the contrary, the potential coding region is a more variable fragment than the UTR one and therefore has a specific organization in each genotype. Each element of the potentially coding fragment is responsible for the synthesis of certain proteins[8]. Thus, ORF1 encodes the synthesis of capsid proteins (ORF1 protein) that are necessary for viral persistence and avoidance of an immune response. The ORF2 protein is a suppressor of proinflammatory cytokines [interleukins (IL)-6, IL-8, and COX-2] and that of the transcription factor nuclear factor kappa-B. The ORF3 protein plays an important role in the cell cycle and in the synthesis of TTV-derived apoptosis-inducing protein, which impedes antiviral host defense[12,13,28].
TTV can be found in most tissues, cells, and body biological fluids, with the exception of red blood cells and platelets[5,8]. TTV replicates in mononucleocytes, liver, bone marrow, and peripheral blood cells, especially in T lymphocytes[5,29]. Kosulin et al[30] suggest that granulocytes are the primary site for TTV replication in children after hematopoietic stem cell transplantation. There is evidence that the virus is present in saliva, sweat, bile, semen, urine, feces, nasal and vaginal secretions, and na-sopharyngeal mucosa scrapes[2,7]. Biopsy specimens of the liver, lymph nodes, bone marrow, spleen, pancreas, lungs, and thyroid gland can be used for the diagnosis of TTV infection[7].
TTV can be detected by quantitative or qualitative polymerase chain reaction (PCR) assays in various clinical samples[31-34]. For this purpose, standard PCR, nested or semi-nested PCR, and real-time PCR are used[11,35,36].
Initially, primers for the N22-ORF1 region were used to diagnose TTV infection[11]. However, the potentially coding fragment is a variable genomic element. Therefore, its use for amplification is associated with a possible decrease in the detection rate of TTV. Unlike the N22-ORF1 fragment, the UTR fragment in most virus genotypes is more stable in detecting TTV infection[37].
Replication of TTV and activation of cellular immunity that is the basis of antiviral defense are the trigger mechanisms for production of immunoglobulins[38-40]. Immunoprecipitation and immunoblotting are used for the qualitative and quantitative determination of anti-TTV antibodies in biological samples[3,4,38,40,41].
Anti-TTV IgM antibodies appear in blood 10-21 wk after TTV infection[42]. At 5-11 wk after their appearance, they usually decrease and gradually disappear. Anti-TTV IgG antibodies emerge at around 16 wk of infection and reach maximum concentrations at 5 mo of virus persistence[40]. IgG can be detectable in the body of those infected for four or more years[43]. The detection rate of anti-TTV antibodies in DNA seropositive blood donors was 17% while that in seronegative ones was 29%, as reported by Tsuda et al[42]. The authors believe that the emergence of anti-TTV antibodies in serum can serve as a marker of prior infection.
A number of authors claim that the detection of TTV by immunoassays is of limited use, since despite detectable antibody titers, anti-TTV antibodies are neither protective nor neutralizing[40,41].
TTV is widespread in the population, reaching 95% in healthy people in some regions of the world[8,18,44-46]. TTV varies in different geographic regions and populations[44]. Gallian et al[18] report that the detection rate of TTV DNA in African descent populations is significantly higher than that in representatives of Europe’s indigenous people (42.8% vs 24.3%, P = 0.034). According to Jarkasi et al[11], the high prevalence of the virus is observed in Asian countries, such as China, Pakistan, Iran, and Qatar.
Studies by Russian and Belarusian scientists also confirm the high prevalence of the virus among both healthy individuals and patients with different diseases. Vasilyev et al[45] reported that the frequency of TTV DNA in the whole blood of Russian athletes was 94%. The maximum viral load was about 1010 copies/mL of blood; the median was 2.7 × 106. In this case, no correlation was found between age, sex, and TTV DNA level (r = 0.02, P > 0.054; t value = -1.943, P = 0.052)[45]. Morozov et al[47] also revealed the high prevalence of TTV among blood donors: TTV DNA was detected in 96.5% of cases among primary blood donors in the Moscow Region.
The Belarusian scientists investigated the detection rate of TTV, TTMDV, and TTMV in patients with chronic liver diseases (CLD) and in those without signs of liver disease with negative viral hepatitis findings[48]. The detection rate of mixed infection was 67% and 72% in the study and control groups, respectively[48].
The identification of TTV DNA depends greatly on used detection methods[33,49]. Factors, such as the type of a sample (e.g. plasma or whole blood) and different PCR assays or completeness of the primers used, can influence the detection of TTV DNA[37,50]. For example, in the work by Abraham et al[49], the prevalence of TTV-positive patients in kidney transplant recipients was about 33% as estimated by nested PCR with ORF1-specific primers, while that among the same patients was 92% when using primers derived from the untranslated region of the TTV genome.
The frequency of TTV in populations detected in the recent decade studies is higher than in the earlier studies, which probably can be explained by the application of more accurate TTV detection methods[45]. Vasilyev et al[45] suggests that the true frequency of TTV presence in the human population tends to be close to 100%. Due to its high prevalence in the population, TTV is called a ubiquitous virus, thereby emphasizing its polytropism.
An interesting fact is that infants and young children have low virus infection rates (5.1%–25%), which increase with age[43,51,52].
There are studies suggesting that viral DNA can be present in tap water[53,54].
TTV is transmitted from the infected organism by several routes. Jarkasi et al[11] identify horizontal and vertical TTV transmission routes. The horizontal route includes fecal-oral, parenteral, and sexual. Most cases of infection may occur non-parenterally, which explains the widespread prevalence of TTV among healthy people, predominantly in older age group[55,56].
Infection occurring through the gastrointestinal tract is shown by high viral DNA titers in the examinees’ bile, saliva, and feces, which exceed serum titers[57-59]. The virus is detectable in the feces of TT viremic individuals[9,60]. Experimental evidence that TTV is transmitted by the fecal-oral and parenteral routes has been obtained[61,62]. Fecal supernatant (1 mL) and serum (0.5 mL; DNA titer was 105 copies/mL; genotype 1a) obtained from TTV-infected people were inoculated into two chimpanzees, respectively. TTV DNA was detected in the blood of one chimpanzee (the fecal supernatant) at 7 wk after inoculation and in that of the other (the infected serum) at 5 wk. The chimpanzee inoculated with the fecal supernatant was observed to have TTV infection persisting for longer than 30 wk. There were moderate biochemical and morphological changes, as evidenced by puncture liver biopsy[62].
Parenteral route: Studying the virus at early stages suggested its high hepatotropism; therefore, the parenteral route of transmission seemed most likely[63-66]. It includes infection through transfusion of contaminated blood and its products, during hemodialysis procedures and injections. Therefore, the people who are at increased risk for TTV infection are: (1) Blood donors[23,67-69]; (2) Hemophilic patients[70-72]; (3) Patients having multiple blood transfusions[73-76]; (4) Patients treated with hemodialysis[22,76-81]; (5) Patients who have undergone organ transplantation[69,82-87]; and (6) Intravenous drug addicts[20,79].
The spread of infection is due to TTV contamination of blood and its products[23,67,68,73,78,88,89]. So the contamination of blood products and coagulation factors VIII and IX accounts for 44%-56%, as reported by Simmonds et al[88]. AbuOdeh et al[90] consider that the rate of TTV infection in healthy blood donors and in HBV- or HCV-infected patients is 81.4%, 90.8%, and 84.9%, respectively.
Charlton et al[91] revealed a relationship between the intravenous administration of blood products and the detection of TTV DNA in the serum of recipients. Konishi et al[92] identified serum TTV DNA in some of 447 patients examined at 6-10 wk after blood transfusions.
Matsumoto et al[63] described the correlation of the detection rate of TTV with the volumes of transfused blood. On the other hand, Wolff et al[69] detected no correlation between the frequency of blood transfusions and the detection of TTV when examining 600 blood donors, 100 healthy individuals, and 495 patients after heart transplantation. In this case, the authors identified only two recipient-donor pairs with the same virus isolates.
There are a number of works devoted to the TTV detection in hemodialysis patients[22,76,78,81]. Summarizing the data presented in these works, it can be concluded that the detection rate of TTV DNA in the blood of patients on hemodialysis is statistically higher than that in blood donors.
A number of works confirm that the detection rate of TTV DNA in patients after organ transplantation is statistically higher than that before surgery, or than that in blood donors[81-83,87]. Kanda et al[93] reported that 60% of 25 bone marrow transplant recipients became infected with TTV at 6-12 wk post-transplantation.
In their paper, Burra et al[82] estimated the frequency and levels of viral DNA (copies/mL) in 25 patients before and after liver transplantation and in 80 donors. TTV DNA was detected in 75% of blood donors and in all the 25 patients before and after surgery (P < 0.01). There was a significant rise in posttransplantation TTV DNA levels: 4.2 log copies/mL ± 0.6 log copies/mL prior to surgery vs 6.8 log copies/mL ± 0.4 log copies/mL at 3 postoperative mo (P < 0.001). The authors suggest that this may be due to the use of immunosuppressive therapy[82].
According to other authors, either age, or gender, or time after transplantation, or the number of blood transfusions, or the cause of transplantation are not associated with TTV load in recipients after liver transplantation[86,94].
Sexual transmission route: Detection of TTV DNA in physiological media (semen, vaginal secretions, cervical mucus, and saliva), the contact with which is possible during sexual intercourse, and in both permanent sexual partners, confirms that TTV can be sexually transmitted[95]. In the group at risk for sexual transmission of TTV, the prevalence of the latter is 86% or more, and does not differ from that in the general population[19,96,97]. In this connection, the genital tract is believed to play an insignificant role in the spread of TTV infection[9,92,98,99].
The vertical transmission route: Involves the possible transfer of infection from mother to fetus during pregnancy[99,100] and breast-feeding[101]. The data obtained by Gerner et al[102] suggest that there may be TTV infection during pregnancy. The viral genome was detected in serum in 57 (41.3%) of the 138 examined pregnant women and in the umbilical cord venous blood in 19 (13.8%) of the 138 infants. Schröter et al[101] hold that 99% of babies born to infected mothers are seropositive for TTV.
There are data on the dynamics of TTV persistence within the first year of a child’s life, which assumes that viral load increases during the first year of life, reaching a plateau after 6 mo[33]. The mean fecal contamination rate in infants of the first year of life who did not receive blood transfusions was 22.4%, as reported by Lin et al[103]. The data obtained by Uchaykin et al[104] suggest that the detection rate of anelloviruses is high in children aged 8 mo to 16 years. It has been shown that the identification rate of TTV amounts to as much as 94% in healthy children, 82.5% in patients with hepatitis of unknown etiology, and 100% in those with hepatitis A or chronic hepatitis B and C[104]. All the three TT viruses TTV, TTMDV, and TTMV circulate in the blood of almost all apparently healthy children. There is no direct relationship between the detection of TT viruses (TTV, TTMDV, and TTMV) and liver damage. It is noteworthy that 77% of cases are infants aged 8 mo to 1.5 years.
That there may be also a postpartum route of infection transmission because of the detection of virus DNA in the milk of breast-feeding mothers is not ruled out[101]. The rate of TTV DNA detection in breast-fed infants increased significantly with prolonged lactation[105,106].
Acquisition of anelloviruses just at an early age is indirect evidence that there are likely to be different routes of contamination with these agents[104]. Manzin et al[107] have summarized the data on viral replication in wild and domestic animals, which may suggest that there is interspecies transmission of the virus.
So far, the issue of viral pathogenicity has been controversial. Since the discovery of TTV, numerous studies have been underway to determine its target organs. Initial studies of TTV indicate its hepatotropism and most likely replication in liver cells. Since its discovery, hopes have thus arisen to put TTV on a par with previously known hepatitis viruses[59,64,108-110].
On the one hand, it is the problem of searching for viral tropism, which is most addressed to date. On the other hand, the available data are quite contradictory, which cannot fail to pose difficulties of gaining a deeper understanding of TTV persistence in the human body.
The target organs for primary TTV replication have been unknown so far. Moreover, there is no doubt that the virus replicates in a number of organs and tissues, such as the liver, bone marrow, lung, lymphoid tissue, as well as in blood mononuclear cells and granulocytes[30,57,111-115].
A number of authors have described cases of TTV DNA detection in liver tissue by hybridization and attempted to establish the contribution of the virus to the identified changes in the liver[35,66,112,116]. Rodríguez-Iñigo et al[112] followed up 30 TTV seropositive patients with liver diseases. In these patients, TTV DNA titers in liver were 10 times higher than that in serum[112]. TTV DNA was found in both serum and liver tissue of some patients with the normal histological pattern of the latter. The data obtained by Kazemi et al[117] also indicate the higher rate of TTV DNA detection in liver tissue than that in plasma. TTV DNA was found in the plasma and liver tissue of 11.1% and 25.9% of patients with HBV and HCV cirrhosis, respectively, as well as in 23.5% and 26.9% of those with cryptogenic cirrhosis[117].
It has been suggested that some TTV genotypes, 3 and 4 in particular, take refuge in peripheral blood mononuclear cells that become a reservoir of the virus and contribute to its persistence[118]. Using cultivation and hybridization, Mariscal et al[114] have confirmed that TTV DNA is present in the cytoplasm of peripheral blood mononuclear cells. Moreover, Focosi et al[115] have shown that the most likely site of TTV replication is T lymphocytes: By inducing the immunosuppression specific for T lymphocytes (antithymocyte globulin, basiliximab), the investigators found a statistically significant decrease in viral load (P < 0.001). This is confirmed by the data obtained by Tyschik et al[50] who have reported that because of the presence of lymphocytes, TTV viral load is 100 times higher in whole blood than that in plasma.
Tanaka et al[113] found the viral genome in the bone marrow cells, lymph nodes, and serum of patients with acute leukemia, malignant lymphoma, and aplastic anemia at the same frequencies. Kikuchi et al[111] revealed a high DNA titer only in bone marrow in a quantitative study of viral DNA in liver and bone marrow autopsy and biopsy specimens from patients with subacute hepatitis and aplastic anemia. This may suggest that the virus replicates in bone marrow, thus leading to aplastic anemia. In this case, only viral DNA is found in hepatocytes, but mRNA is absent.
Deng et al[119] have demonstrated the high rate of viral DNA detection in saliva (in 38% of cases). TTV DNA titers were higher in saliva than those in serum of the examinees (in 21% of cases). The authors presume that this fact may point to virus replication in the salivary gland too.
The data presented confirm viral polytropism and rule out the possibility of attributing TTV exclusively to hepatitis viruses.
Viral persistence in the body provokes an immune response to a foreign agent. TTV actively synthesizes the proteins that are required for long-term viral replication in various tissues and organs and that possess immunogenicity[13,29,38,40]. The TTV replication cycle in humans has been shown to be accompanied by the synthesis of microRNAs (miRNAs)[120,121]. The latter inhibits interferon signaling, promotes the evasion of TTV from an immune response, and facilitates its persistence in the host[12,120-124]. Despite the known effects of miRNAs, it is impossible to say unequivocally what contribution is made by TTV miRNAs synthesis to viral persistence in the body and whether this effect is clinically significant. The complex influence of pathological factors helps reduce the effectiveness of the immune system in eliminating the penetrated infectious agent.
An update on the immunobiology of TTV confirms that the immune system cells recognize the antigenic determinants of the virus and respond to its penetration by producing the corresponding proinflammatory proteins and cytokines: Interferon-gamma, tumor necrosis factor-alpha and by elevating the concentrations of IL, such as IL-6, IL-12, IL-28, and IL-29, the chemokine CCL7, and a number of antiviral proteins[12,29,125,126]. To date, the macroorganism’s immunocompetent cells are known to interact with the virus through Toll-like receptors 9[17].
Triggering the whole spectrum of antiviral defense mechanisms can keep the level of TTV DNA at a controllable one; i.e., viral persistence proceeds under persistent immune surveillance. In this case, TTV cannot be completely eliminated, as confirmed by its high prevalence rate in the human population, even among the people belonging to a healthy population.
The high prevalence of TTV among healthy people assumes that there is a high risk for its coinfection with other viral agents. The features of TTV persistence during coinfection with human immunodeficiency virus[32,127], cytomegalovirus[128], and BK polyomavirus[129] have been studied.
The discovery of TTV seemed important due to the lack of detection of known viral agents in 10%-20% of cases of acute hepatitis, in 5%-10% of those of chronic hepatitis and in up to 50% of those of fulminant hepatitis[9,104,130]. Therefore, after the discovery of TTV, studies by many scientists have been aimed at assessing the potential hepatotropism of the virus, at investigating its coinfection with known hepatitis viruses, and at detecting TTV in patients with cryptogenic liver diseases. Studies that have been underway in this area are very ambiguous.
TTV infection has been shown to be diagnosed in 15%-28% of patients with acute viral hepatitis (AVH) A, in 22%-24% of those with AVH B, in 40%-60% of those with AVH C, and in 20% of those with HIV[63,131,132]. There is a slight increase in the detection rate of TTV DNA in patients with hepatitides of known etiology compared to healthy donors: 94.3% vs 81.7%, respectively; P < 0.05)[133]; 11.0% vs 7.5% (P = 0.13)[63]; 65% and 69% vs 21%, (P < 0.0005)[134]. TTV DNA was most frequently detected in a group of patients with AVH (HBV, HCV, HGV, and cryptogenic hepatitis)[135,136]. The researchers have shown that the detection of TTV in patients with AVH and fulminant liver failure is much higher than that in blood donors (80.6% and 76% vs 52%, respectively; P < 0.05)[135]. Moreover, the detection rate of TTV in patients with AVH was higher than in those with fulminant liver failure, chronic hepatitis, or cirrhosis (P < 0.001)[136]. The authors note that the presence of TTV does not increase mortality rates in patients with fulminant liver failure.
There are debates about the role of TTV in the development of CLD. Gromova et al[137] state that the prevalence of TTV among patients with chronic viral hepatitis B and C is 61.0% and 45.1%, respectively. The detection rate of TTV ranges from 17% to 57% in chronic non-B non-G hepatitis cases[138,139], from 9% to 66% in non-B non-G liver cirrhosis patients[140], and from 9% to 51% in hepatocellular carcinoma cases[141]. A number of works dedicated to this topic have revealed no signs of the effect of TTV on the course of CLD[108,138,140,142,143].
TTV DNA was detected in patients with enhanced hepatic aminotransferase activities in the absence of viral hepatitis markers[15,57,64,144,145]. According to de Oliveira et al[15], the detection rate of TTV DNA in peripheral blood cells was 31.48% in patients with increased aminotransferase activities and 5.26% in the control group. Piaggio et al[145] have published data confirming the relationship between the spontaneous postoperative increase in the level of hepatic aminotransferases and the detection of TTV in the serum of an examined patient. Mikhailov et al[57] have obtained data suggesting that TTV DNA is detected at the same frequencies in donors with enhanced or normal ALT activities (17.6% and 16.7%, respectively), which is indirect evidence that there is no effect of TTV on the development of hyperenzymemia.
Sharafanova et al[59] reports that TTV monoinfection occurs in 9.8% of patients with liver diseases[64]. A biochemical study of TTV-monoinfected patients revealed a significant increase in bilirubin levels and AST and gammaglutamyltranspeptidase activities compared to healthy individuals[59,64]. When comparing patients seronegative for TTV and those monoinfected with TTV, a number of authors note that the latter have enhanced ALT, lactate dehydrogenase, gammaglutamyltranspeptidase, and alkaline phosphatase activities[138,146,147]. Moreover, ALT activity does not depend on serum TTV DNA persistence length and titers[63].
The data presented in the first decade since the discovery of TTV show a wide scatter and the low detection rate of the virus in liver diseases, which is most likely to be due to the use of incomplete primers for its PCR diagnosis. The higher sensitivity and specificity of PCR for the diagnosis of TTV in the last decade could revealed the virus in 77.4% of patients with hepatitis A, in 87.6% of HBV-positive patients, in 77% of HCV-positive one, and in 92.8% of patients with non-A-E hepatitis[67]. Moreover, the authors have shown that there are no statistically significant differences in biochemical parameters between the groups of TTV-positive and TTV-negative patients with hepatitis A-E viral infection[67].
Thus, a great body of data on the coinfection of TTV with hepatitis viruses of known and unknown etiology goes back to the first decade after its dis-covery[1,63,67,112,142,148-152]. The coexistence of several infectious agents is always the most difficult link for understanding the role of each of them in the pathogenesis of the disease. In recent years, there has been a decline in the number of works devoted to the clinical significance of TTV infection. This is likely to be due to the fact that TTV is a common virus that is found in patients with different viral hepatitides, in cases of hepatitis with no obvious viral agent, as well as in a healthy population[67]. Moreover, many authors note that the virus has no aggravating effect on the progression and course of different liver diseases. Perhaps future investigations in this area will clarify conflicting data and unclear aspects of the effect of TTV on the development and course of liver diseases.
Of the most interest are liver tissue changes in case of TTV monoinfection. The morphological pattern of liver tissue biopsy samples from TTV-monoinfected patients corresponds predominantly to moderate focal portal and lobular hepatitis[59]. According to histological findings in liver biopsy samples from TTV-infected individuals, most authors note lymphocytic infiltration of portal tracts, focal necroses, portal fibrosis, hydropic and granular hepatocyte dystrophy varying in severity, single necroses, and desquamated epithelial cells in the bile ducts[59,153-156]. Similar morphological changes in a liver tissue biopsy specimen from a TTMDV-infected individual who had no markers of hepatitis A-E virus infection were described by Morozov et al[47,157]. Sharafanova et al[59] hold that the changes in the bile duct epithelium can be attributed to the peculiarities of morphological alterations during TTV monoinfection.
Koltunov depicted the histological degree of hepatic inflammatory activity in patients with TTV monoinfection according to the Metavir scoring system: Low (A1), moderate (A2), and high (A3) activities were detected in 70%, 20%, and 10% of patients, respectively. According to the Metavir scoring system, the stages of fibrosis (F) in the same patients corresponded as follows: F1 (30%), F2 (50%), F3 (10%), and F4 (10%)[156].
Tokita et al[158] investigated liver tissue from TTV-infected patients with nonalcoholic steatohepatitis who had no markers of other viral hepatitides. The most pronounced differences were found in patients infected with TTV genotype 1. Focal necrosis and pericellular, perivenular fibrosis occurred more frequently in the TTV-positive group than in the TTV-negative group. Arakawa et al[159] noted the appearance of irregular regeneration of hepatocytes in patients with chronic hepatitis in the presence of TTV.
Based on these data of the study of liver biopsy samples, it can be concluded that TTV has histological signs sufficient for a nonpathogenic virus with extensive polytropism, which confirm the possibility of undergoing its replicative cycle just in the liver cells.
Along with this, cytological hybridization in the TTV-infected cells revealed no substantial cytopathic (cell-damaging) effects that were characteristic of pathogenic hepatotropic viruses, such as hepatitis B and C viruses[22,32]. Many authors found no statistically significant clinical, biochemical, or histological differences between TTV-positive and TTV-negative patients[63,65,112,142,149-151]. Authors conclude that the presence of TTV does not complicate the course or worsen liver functional parameters in patients with known hepatitis A-E viruses, and it does not affect the efficiency of interferon therapy[59,150-152]. Long-term detection of TTV DNA in the serum of patients with the liver morphofunctional integrity indicates the existence of asymptomatic carriage of the virus[150,155,160]. Most likely, these data suggest that there may be an independent persistence of TTV and viral hepatitis of established etiology.
Some authors call the virus nonpathogenic and, moreover, identify it as part of the human virome[12,161,162]. This judgment is substantiated mainly by that the virus infection cannot be linked with the development of a specific disease. However, this statement cannot be called absolutely justified: It is not so long ago that there was a study linking the persistence of TTV DNA within the body with possibly increased mortality in elderly patients[163].
Rezahosseini et al[12] consider that TTV rarely causes disease in healthy individuals. Moreover, the viral load of TTV in immunosuppressed patients is higher than that in a healthy population[164]. In this connection, a number of authors propose to use TTV viral load as an endogenous marker of the human immune status[12,34,85,162,165,166]. In this case, TTV DNA levels in the serum of patients after organ transplantation are proposed to be used to control the development of a transplant rejection response. Thus, Görzer et al[85] found that TTV DNA concentrations below 7 log10 copies/mL were statistically associated with postoperative lung transplant dysfunction. Solis et al[162] established a similar correlation in patients undergoing kidney transplantation. They concluded that patients with TTV DNA levels lower than 4.2 log10 copies/mL one mo after surgery had a higher risk for a transplant rejection response over the next two years. The development of a method to determine the immune status on the basis of an assessment of TTV viremia can assist in predicting severe postoperative complications in patients undergoing organ transplantation and can implement necessary preventive measures. This certainly makes it possible to set entirely new goals of studying TTV.
TT virus is at the interface of many clinical and fundamental disciplines, thereby being of interest to many specialists. Therefore, the main task of current investigations is to generalize and systematize the available data for further prospective studies.
Since the discovery of TTV and the Aneloviridae family as a whole, the virus has been the subject of a detailed study by many investigators around the world. The molecular genetic structure of the virus has been well studied; methods for its qualitative and quantitative determination have been developed[167]; the virus has shown an upward spread in different regions of the world; routes of its transmission have been established; and the feasibility of independent elimination of the virus has been indicated. Moreover, TTV DNA was found in many tissues and biological fluids of not only patients, but also in those of healthy individuals.
The identification of TTV DNA considerably depends on virus detection methods, used primers, and biological material. In this connection, the TTV data available in the literature are largely contradictory and cannot be cross-compared, which greatly complicates the process of studying the characteristics of the virus. Despite the fact that the virus does not replicate predominantly in hepatocytes, it is detectable in both acute and CLD. There are reports of liver disease cases with the detection of serum TTV as the only viral marker. The variants of TTV monoinfection with moderate deviations of biochemical liver tests, which are accompanied by morphological changes in liver tissue, by predominantly involving the bile canaliculi, suggest that the virus may have a damaging effect primarily on the bile duct epithelium.
Due to the significant prevalence of the virus in the population, it is suggested that TTV is part of the human virome. At the same time, the human immune system via immunosurveillance maintains a safe balance, by protecting the body from the pathogenic effects of the virus.
After contamination with TTV, it seems that there may be two variants of its presence in the body: (1) The non-pathogenic persistence of TTV in humans as its virome; and (2) In the event of adverse situations in the body, the participation of TTV in the infectious process, by causing damage to susceptible cells, including hepatocytes and the epithelium of biliary canaliculi. The first variant is supported by the observations made by Shibayama et al[138]. The investigators found that the virus persisted in all seventeen TTV-infected patients for 1-7 years. Matsumoto et al[63] identified TTV DNA in 33% of the examinees (using the serum bank) 22 years later. Lefrere et al[168] traced the persistence of TTV after blood transfusions in 27.7% of 173 cases over an average of 3.1 years. The second variant is supported by data on clinical, laboratory and morphological changes in the liver during TTV monoinfection. It can perhaps be said that the pathogenicity of TTV is conditional.
Finally, a number of recent studies have shown that TTV can serve as an endogenous marker of the human immune status[169-171]. Further investigations in this area can be extremely useful for practical public health.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang K, Neri V S-Editor: Wang YQ L-Editor: A E-Editor: Zhang YL
| 1. | Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 852] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Okamoto H. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol. 2009;331:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Wei Y, Chen M, Yang X, Zhang L, Rao L, Yuan F, Wang Y, Gong J, Li L. Molecular characterization of human Torque Teno virus. Biomed Rep. 2015;3:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Mankotia DS, Irshad M. Cloning and expression of N22 region of Torque Teno virus (TTV) genome and use of peptide in developing immunoassay for TTV antibodies. Virol J. 2014;11:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Focosi D, Antonelli G, Pistello M, Maggi F. Torquetenovirus: the human virome from bench to bedside. Clin Microbiol Infect. 2016;22:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 6. | Biagini P. Classification of TTV and related viruses (anelloviruses). Curr Top Microbiol Immunol. 2009;331:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Spandole S, Cimponeriu D, Berca LM, Mihăescu G. Human anelloviruses: an update of molecular, epidemiological and clinical aspects. Arch Virol. 2015;160:893-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 193] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 8. | Bostan N, Amen NE, Bokhari H. Current and Future Prospects of Torque Teno Virus. J Vaccines Vaccin. 2013;S1:004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 9. | Cherednichenko TV, Filipova EA. TT – viral infection. J Children Infections. 2008;3:43-48 . Available from: https://cyberleninka.ru/article/n/tt-virusnaya-infektsiya/viewer. |
| 10. | Mushahwar IK, Erker JC, Muerhoff AS, Leary TP, Simons JN, Birkenmeyer LG, Chalmers ML, Pilot-Matias TJ, Dexai SM. Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc Natl Acad Sci USA. 1999;96:3177-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 281] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Jarkasi NS, Sekawi Z, Yoke Kqueen C, Othman Z. A Review on the Global Widespread of TTV Infection Among Humans Population. PJSRR. 2018;4:10-24 . Available from: https://www.researchgate.net/publication/337240870_PJSRR_2018_41_10-24. |
| 12. | Rezahosseini O, Drabe CH, Sørensen SS, Rasmussen A, Perch M, Ostrowski SR, Nielsen SD. Torque-Teno virus viral load as a potential endogenous marker of immune function in solid organ transplantation. Transplant Rev (Orlando). 2019;33:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Kakkola L, Bondén H, Hedman L, Kivi N, Moisala S, Julin J, Ylä-Liedenpohja J, Miettinen S, Kantola K, Hedman K, Söderlund-Venermo M. Expression of all six human Torque teno virus (TTV) proteins in bacteria and in insect cells, and analysis of their IgG responses. Virology. 2008;382:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Chernobrovkina TY, Litvinova OS, Yankovskaya YD. TTV-Infection: clinical, epidemiological and diagnostic aspects. Archive of Internal Medicine. 2016;6:28-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | de Oliveira JC, Nasser TF, Oda JM, Aoki MN, Carneiro JL, Barbosa DS, Reiche EM, Watanabe MA. Detection of TTV in peripheral blood cells from patients with altered ALT and AST levels. New Microbiol. 2008;31:195-201. [PubMed] |
| 16. | Bazykina EA, Trotsenko OE, Turkutukov VB, Balakhontseva LA, Kotova VO. Molecular-epidemiologic characteristics of viral hepatitis G and TTV in the world and on the territory of the Russian Federation (review). Dalnevostochnyi Zhurnal Infektsionnoy Patologii. 2017;32:80-86. |
| 17. | Rocchi J, Ricci V, Albani M, Lanini L, Andreoli E, Macera L, Pistello M, Ceccherini-Nelli L, Bendinelli M, Maggi F. Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology. 2009;394:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Gallian P, Berland Y, Olmer M, Raccah D, de Micco P, Biagini P, Simon S, Bouchouareb D, Mourey C, Roubicek C, Touinssi M, Cantaloube JF, Dussol B, de Lamballerie X. TT virus infection in French hemodialysis patients: study of prevalence and risk factors. J Clin Microbiol. 1999;37:2538-2542. [PubMed] |
| 19. | Gallian P, Biagini P, Zhong S, Touinssi M, Yeo W, Cantaloube JF, Attoui H, de Micco P, Johnson PJ, de Lamballerie X. TT virus: a study of molecular epidemiology and transmission of genotypes 1, 2 and 3. J Clin Virol. 2000;17:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Alzahrani AJ, Dela Cruz DM, Obeid OE, Bukhari HA, Al-Qahtani AA, Al-Ahdal MN. Molecular detection of hepatitis B, hepatitis C, and torque teno viruses in drug users in Saudi Arabia. J Med Virol. 2009;81:1343-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Dencs A, Hettmann A, Szomor KN, Kis Z, Takács M. Prevalence and genotyping of group 3 torque teno viruses detected in health care workers in Hungary. Virus Genes. 2009;39:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | El-Taher SM, Fouad NA, Fouad MA, Mahedy AW, Elnazi AK. Transfusion-transmitted virus infection in hemodialysis patients in Arar, Saudi Arabia: Prevalence, predictors and genotyping. Saudi J Kidney Dis Transpl. 2015;26:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Al-Qahtani AA, Alabsi ES, AbuOdeh R, Thalib L, El Zowalaty ME, Nasrallah GK. Prevalence of anelloviruses (TTV, TTMDV, and TTMV) in healthy blood donors and in patients infected with HBV or HCV in Qatar. Virol J. 2016;13:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Najafimemar Z, Tabarraei A, Talei G, Moradi A. Prevalence and Genotyping of Torque Teno Virus in HBV/HIV and Chronic HBV Patients in Iran. Iran Biomed J. 2018;22:338-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Spandole-Dinu S, Cimponeriu DG, Crăciun AM, Radu I, Nica S, Toma M, Alexiu OA, Iorga CS, Berca LM, Nica R. Prevalence of human anelloviruses in Romanian healthy subjects and patients with common pathologies. BMC Infect Dis. 2018;18:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Cancela F, Ramos N, Mirazo S, Mainardi V, Gerona S, Arbiza J. Detection and molecular characterization of Torque Teno Virus (TTV) in Uruguay. Infect Genet Evol. 2016;44:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kim HS, Kim JS, Park MJ, Song W, Kang HJ, Lee KM. [Distribution of TT virus genotypes and genogroups in 69 healthy and 59 hepatitis B virus infected Korean individuals]. Korean J Lab Med. 2007;27:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Qiu J, Kakkola L, Cheng F, Ye C, Söderlund-Venermo M, Hedman K, Pintel DJ. Human circovirus TT virus genotype 6 expresses six proteins following transfection of a full-length clone. J Virol. 2005;79:6505-6510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Maggi F, Focosi D, Albani M, Lanini L, Vatteroni ML, Petrini M, Ceccherini-Nelli L, Pistello M, Bendinelli M. Role of hematopoietic cells in the maintenance of chronic human torquetenovirus plasma viremia. J Virol. 2010;84:6891-6893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Kosulin K, Kernbichler S, Pichler H, Lawitschka A, Geyeregger R, Witt V, Lion T. Post-transplant Replication of Torque Teno Virus in Granulocytes. Front Microbiol. 2018;9:2956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Osiowy C, Sauder C. Detection of TT virus in human hair and skin. Hepatol Res. 2000;16:155–162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Pirouzi A, Bahmani M, Feizabadi MM, Afkari R. Molecular characterization of Torque teno virus and SEN virus co-infection with HIV in patients from Southern Iran. Rev Soc Bras Med Trop. 2014;47:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Tyschik EA, Rasskazova AS, Degtyareva AV, Rebrikov DV, Sukhikh GT. Torque teno virus dynamics during the first year of life. Virol J. 2018;15:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Wohlfarth P, Leiner M, Schoergenhofer C, Hopfinger G, Goerzer I, Puchhammer-Stoeckl E, Rabitsch W. Torquetenovirus Dynamics and Immune Marker Properties in Patients Following Allogeneic Hematopoietic Stem Cell Transplantation: A Prospective Longitudinal Study. Biol Blood Marrow Transplant. 2018;24:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Comar M, Ansaldi F, Morandi L, Dal Molin G, Foschini PM, Crocè SL, Bonin S, Stanta G, Tiribelli C, Campello C. In situ polymerase chain reaction detection of transfusion-transmitted virus in liver biopsy. J Viral Hepat. 2002;9:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Macera L, Spezia PG, Medici C, Rofi E, Del Re M, Focosi D, Mazzetti P, Navarro D, Antonelli G, Danesi R, Pistello M, Maggi F. Comparative evaluation of molecular methods for the quantitative measure of torquetenovirus viremia, the new surrogate marker of immune competence. J Med Virol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Kenar Koohi A, Ravanshad M, Rasouli M, Falahi S, Baghban A. Phylogenetic analysis of torque teno virus in hepatitis C virus infected patients in shiraz. Hepat Mon. 2012;12:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Ott C, Duret L, Chemin I, Trépo C, Mandrand B, Komurian-Pradel F. Use of a TT virus ORF1 recombinant protein to detect anti-TT virus antibodies in human sera. J Gen Virol. 2000;81:2949-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Tsuda F, Takahashi M, Nishizawa T, Akahane Y, Konishi K, Yoshizawa H, Okamoto H. IgM-class antibodies to TT virus (TTV) in patients with acute TTV infection. Hepatol Res. 2001;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Maggi F, Bendinelli M. Immunobiology of the Torque teno viruses and other anelloviruses. Curr Top Microbiol Immunol. 2009;331:65-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Mankotia DS, Irshad M. Development of an Immunoassay for Detection of Torque Teno Virus (TTV) Antibodies Using the N22 Expression Product from TTV Genotype 2. Intervirology. 2017;60:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Tsuda F, Okamoto H, Ukita M, Tanaka T, Akahane Y, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. Determination of antibodies to TT virus (TTV) and application to blood donors and patients with post-transfusion non-A to G hepatitis in Japan. J Virol Methods. 1999;77:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Chen T, Väisänen E, Mattila PS, Hedman K, Söderlund-Venermo M. Antigenic diversity and seroprevalences of Torque teno viruses in children and adults by ORF2-based immunoassays. J Gen Virol. 2013;94:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Prescott LE, MacDonald DM, Davidson F, Mokili J, Pritchard DI, Arnot DE, Riley EM, Greenwood BM, Hamid S, Saeed AA, McClure MO, Smith DB, Simmonds P. Sequence diversity of TT virus in geographically dispersed human populations. J Gen Virol. 1999;80:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Vasilyev EV, Trofimov DY, Tonevitsky AG, Ilinsky VV, Korostin DO, Rebrikov DV. Torque Teno Virus (TTV) distribution in healthy Russian population. Virol J. 2009;6:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Tri Rinonce H, Yano Y, Utsumi T, Heriyanto DS, Anggorowati N, Widasari DI, Ghozali A, Utoro T, Lusida MI, Soetjipto, Prasanto H, Hayashi Y. Prevalence and genotypic distribution of GB virus C and torque Teno virus among patients undergoing hemodialysis. Mol Med Rep. 2017;15:2843–2852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Morozov IA, Zwerkova EA, Kyuregyan KK, Karlsen AA, Isaeva OV, Ilchenko LY, Fedorov IG, Kozhanova TV, Gordeychuk IV, Petrenko NV, Mikhailov MI. Genus Anelloviridae Viruses in Chronic Liver Disease. Eksp Klin Gastroenterol. 2015;4-11. [PubMed] |
| 48. | Osipkina OV, Voropayev EV, Mitsura VM, Zyatkov AA, Tereshkov DV, Perevolotskaya TV, Perevolotskiy AN. Torque Teno Virus: prevalence and features of PCR analysis. Problems of Health and Ecol. 2018;3:85-90. |
| 49. | Abraham P, John GT, Raghuraman S, Radhakrishnan S, Thomas PP, Jacob CK, Sridharan G. GB virus C/hepatitis G virus and TT virus infections among high risk renal transplant recipients in India. J Clin Virol. 2003;28:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Tyschik EA, Shcherbakova SM, Ibragimov RR, Rebrikov DV. Transplacental transmission of torque teno virus. Virol J. 2017;14:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Naganuma M, Tominaga N, Miyamura T, Soda A, Moriuchi M, Moriuchi H. TT virus prevalence, viral loads and genotypic variability in saliva from healthy Japanese children. Acta Paediatr. 2008;97:1686-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | McElvania TeKippe E, Wylie KM, Deych E, Sodergren E, Weinstock G, Storch GA. Increased prevalence of anellovirus in pediatric patients with fever. PLoS One. 2012;7:e50937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Vecchia AD, Kluge M, dos Santos da Silva JV, Comerlato J, Rodrigues MT, Fleck JD, da Luz RB, Teixeira TF, Roehe PM, Capalonga R, Oliveira AB, Spilki FR. Presence of Torque teno virus (TTV) in tap water in public schools from Southern Brazil. Food Environ Virol. 2013;5:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Charest AJ, Plummer JD, Long SC, Carducci A, Verani M, Sidhu JP. Global occurrence of Torque teno virus in water systems. J Water Health. 2015;13:777-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Chikasue K, Kimura M, Ikeda K, Ohnishi T, Kawanishi S, Iio T, Kataoka M, Arao Y. Detection of Torque teno virus DNA in exhaled breath by polymerase chain reaction. Acta Med Okayama. 2012;66:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Haloschan M, Bettesch R, Görzer I, Weseslindtner L, Kundi M, Puchhammer-Stöckl E. TTV DNA plasma load and its association with age, gender, and HCMV IgG serostatus in healthy adults. Age (Dordr). 2014;36:9716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Mikhailov MI, Polyakov AN, Isaeva OV, Kyuregyan KK, Popova OE. TTV is a novel virus associated with posttransfusion hepatitis. Russian J Gastroenterol, Hepatol, Coloproctol. 1999;3:36-40. |
| 58. | Ukita M, Okamoto H, Kato N, Miyakawa Y, Mayumi M. Excretion into bile of a novel unenveloped DNA virus (TT virus) associated with acute and chronic non-A-G hepatitis. J Infect Dis. 1999;179:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Sharafanova TI, Reshetnyak VI, Ilchenko LYu, Serova TI, Shepeleva SD, Tkachev VD. Morphological changes in liver tissue in patients infected with hepatitis TT virus. Ross Gastroenterol Zh. 2001;2:158-162. |
| 60. | Ross RS, Viazov S, Runde V, Schaefer UW, Roggendorf M. Detection of TT virus DNA in specimens other than blood. J Clin Virol. 1999;13:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Luo K, Liang W, He H, Yang S, Wang Y, Xiao H, Liu D, Zhang L. Experimental infection of nonenveloped DNA virus (TTV) in rhesus monkey. J Med Virol. 2000;61:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Tawara A, Akahane Y, Takahashi M, Nishizawa T, Ishikawa T, Okamoto H. Transmission of human TT virus of genotype 1a to chimpanzees with fecal supernatant or serum from patients with acute TTV infection. Biochem Biophys Res Commun. 2000;278:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Matsumoto A, Yeo AE, Shih JW, Tanaka E, Kiyosawa K, Alter HJ. Transfusion-associated TT virus infection and its relationship to liver disease. Hepatology. 1999;30:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Loginov AS, Sharafanova TI, Reshetniak VI, Il'chenko LIu, Shepeleva SD, Serova TI, Tkachev VD. [HGV and TTV - new hepatitis viruses]. Ter Arkh. 2000;72:9-13. [PubMed] |
| 65. | Moriyama M, Matsumura H, Shimizu T, Shioda A, Kaneko M, Miyazawa K, Miyata H, Tanaka N, Uchida T, Arakawa Y. Histopathologic impact of TT virus infection on the liver of type C chronic hepatitis and liver cirrhosis in Japan. J Med Virol. 2001;64:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Okamoto H, Ukita M, Nishizawa T, Kishimoto J, Hoshi Y, Mizuo H, Tanaka T, Miyakawa Y, Mayumi M. Circular double-stranded forms of TT virus DNA in the liver. J Virol. 2000;74:5161-5167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Magu SK, Kalghatgi AT, Bhagat MR. Incidence and clinical implication of TT virus in patients with hepatitis and its frequency in blood donors in India. Med J Armed Forces India. 2015;71:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Mazzola JC, Saito PK, Yamakawa RH, Watanabe MA, da Silva Junior WV, Matta AC, Borelli SD. Prevalence of Torque teno virus in healthy donors of Paraná State, southern Brazil. Rev Bras Hematol Hemoter. 2015;37:336-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Wolff C, Diekmann A, Boomgaarden M, Körner MM, Kleesiek K. Viremia and excretion of TT virus in immunosuppressed heart transplant recipients and in immunocompetent individuals. Transplantation. 2000;69:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Takayama S, Miura T, Matsuo S, Taki M, Sugii S. Prevalence and persistence of a novel DNA TT virus (TTV) infection in Japanese haemophiliacs. Br J Haematol. 1999;104:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Yokozaki S, Toyoda H, Nakano I, Katano Y, Ebata M, Fukuda Y, Takamatsu J, Saito H, Hayakawa T. Infection with TT virus, a novel transfusion-transmissible DNA virus, in haemophiliacs and in blood products. Br J Haematol. 1999;105:1114-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Grabarczyk P, Brojer E, Windyga J, łopaciuk S, Klukowska A, Mikulska M. [GBV-C/HGV and TTV infection markers in Polish blood donors and haemophilia patients]. Przegl Epidemiol. 2006;60:581-588. [PubMed] |
| 73. | Ali S, van Pelt JF, Verslype C, Nevens F, Fevery J, Yap SH. TT virus infection in acute and chronic liver diseases and in patients regularly receiving blood products in Belgium. Acta Gastroenterol Belg. 2004;67:161-165. [PubMed] |
| 74. | de Castro Amarante MF, Kashima S, Covas DT. TT virus (TTV) genotyping in blood donors and multiple transfused patients in Brazil. Virus Genes. 2007;35:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Hu YW, Al-Moslih MI, Al Ali MT, Uzicanin S, Perkins H, Yi QL, Rahimi Khameneh S, Wu J, Brown EG. Clinical outcome of frequent exposure to Torque Teno virus (TTV) through blood transfusion in thalassemia patients with or without hepatitis C virus (HCV) infection. J Med Virol. 2008;80:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Jalali H, Mahdavi MR, Zaeromali N. Torque Teno Virus (TTV) Among β-Thalassemia and Haemodialysis Patients in Mazandaran Province (North of Iran). Int J Mol Cell Med. 2017;6:56-60. [PubMed] |
| 77. | Rivanera D, Lozzi MA, Idili C, Lilli D. Prevalence of TT virus infection in Italian dialysis patients. Pathol Biol (Paris). 2009;57:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Irshad M, Mandal K, Singh S, Agarwal SK. Torque teno virus infection in hemodialysis patients in North India. Int Urol Nephrol. 2010;42:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Ataei B, Emami Naeini A, Khorvash F, Yazdani MR, Javadi AA. Prevalence of transfusion transmitted virus infection in hemodialysis patients and injection drug users compared to healthy blood donors in isfahan, iran. Gastroenterol Res Pract. 2012;2012:671927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Massaú A, Martins C, Nachtigal GC, Araújo AB, Rossetti ML, Niel C, da Silva CM. The high prevalence of Torque teno virus DNA in blood donors and haemodialysis patients in southern Brazil. Mem Inst Oswaldo Cruz. 2012;107:684-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Takemoto AY, Okubo P, Saito PK, Yamakawa RH, Watanabe MA, Veríssimo da Silva Junior W, Borelli SD, Bedendo J. Torque teno virus among dialysis and renal-transplant patients. Braz J Microbiol. 2015;46:307-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Burra P, Masier A, Boldrin C, Calistri A, Andreoli E, Senzolo M, Zorzi M, Sgarabotto D, Guido M, Cillo U, Canova D, Bendinelli M, Pistello M, Maggi F, Palù G. Torque Teno Virus: any pathological role in liver transplanted patients? Transpl Int. 2008;21:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Young JC, Chehoud C, Bittinger K, Bailey A, Diamond JM, Cantu E, Haas AR, Abbas A, Frye L, Christie JD, Bushman FD, Collman RG. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant. 2015;15:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 84. | Görzer I, Haloschan M, Jaksch P, Klepetko W, Puchhammer-Stöckl E. Plasma DNA levels of Torque teno virus and immunosuppression after lung transplantation. J Heart Lung Transplant. 2014;33:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 85. | Görzer I, Jaksch P, Strassl R, Klepetko W, Puchhammer-Stöckl E. Association between plasma Torque teno virus level and chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2017;36:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Béland K, Dore-Nguyen M, Gagné MJ, Patey N, Brassard J, Alvarez F, Halac U. Torque Teno virus in children who underwent orthotopic liver transplantation: new insights about a common pathogen. J Infect Dis. 2014;209:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Akbari H, Piroozmand A, Dadgostar E, Nikoueinejad H, Chitsazian Z, Einollahi B, Amini Mahabadi J. Prevalence of Transfusion-transmitted Virus (TTV) Infection and its Association with Renal Post-transplantation Complications in Iran. Int J Organ Transplant Med. 2018;9:126-131. [PubMed] |
| 88. | Simmonds P, Davidson F, Lycett C, Prescott LE, MacDonald DM, Ellender J, Yap PL, Ludlam CA, Haydon GH, Gillon J, Jarvis LM. Detection of a novel DNA virus (TTV) in blood donors and blood products. Lancet. 1998;352:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 282] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 89. | Pisani G, Cristiano K, Wirz M, Bisso G, Beneduce F, Morace G, Rapicetta M, Gentili G. Prevalence of TT virus in plasma pools and blood products. Br J Haematol. 1999;106:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | AbuOdeh R, Al-Mawlawi N, Al-Qahtani AA, Bohol MF, Al-Ahdal MN, Hasan HA, AbuOdeh L, Nasrallah GK. Detection and genotyping of torque teno virus (TTV) in healthy blood donors and patients infected with HBV or HCV in Qatar. J Med Virol. 2015;87:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology. 1998;28:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 92. | Konishi K, Ueyama T. [Involvement of TTV, a new infectious factor in post-transfusion hepatitis, non A-non G]. Nihon Rinsho. 1999;57:1279-1284. [PubMed] |
| 93. | Kanda Y, Tanaka Y, Kami M, Saito T, Asai T, Izutsu K, Yuji K, Ogawa S, Honda H, Mitani K, Chiba S, Yazaki Y, Hirai H. TT virus in bone marrow transplant recipients. Blood. 1999;93:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Simonetta F, Pradier A, Masouridi-Levrat S, van Delden C, Giostra E, Morard I, Mueller N, Muellhaupt B, Valli PV, Semmo N, Seebach J, Chalandon Y, Kaiser L, Roosnek E; Swiss Transplant Cohort Study (STCS). Torque Teno Virus Load and Acute Rejection After Orthotopic Liver Transplantation. Transplantation. 2017;101:e219-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 95. | 10th International Symposium on Viral Hepatitis and Liver Disease. Antivir Ther. 2000;5 Suppl 1:1-82, A1-17, B1-89, C1-133, passim. [PubMed] |
| 96. | Huang YH, Wu JC, Lin CC, Sheng WY, Lee PC, Wang YJ, Chang FY, Lee SD. Prevalence and risk factor analysis of TTV infection in prostitutes. J Med Virol. 2000;60:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 97. | Yazici M, Cömert MR, Mas R, Guney C, Cinar E, Kocar IH. Transfusion-transmitted virus prevalence in subjects at high risk of sexually transmitted infection in Turkey. Clin Microbiol Infect. 2002;8:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Inami T, Konomi N, Arakawa Y, Abe K. High prevalence of TT virus DNA in human saliva and semen. J Clin Microbiol. 2000;38:2407-2408. [PubMed] |
| 99. | Matsubara H, Michitaka K, Horiike N, Kihana T, Yano M, Mori T, Onji M. Existence of TT virus DNA and TTV-like mini virus DNA in infant cord blood: mother-to-neonatal transmission. Hepatol Res. 2001;21:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Xin X, Xiaoguang Z, Ninghu Z, Youtong L, Liumei X, Boping Z. Mother-to-infant vertical transmission of transfusion transmitted virus in South China. J Perinat Med. 2004;32:404-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Schröter M, Polywka S, Zöllner B, Schäfer P, Laufs R, Feucht HH. Detection of TT virus DNA and GB virus type C/Hepatitis G virus RNA in serum and breast milk: determination of mother-to-child transmission. J Clin Microbiol. 2000;38:745-747. [PubMed] |
| 102. | Gerner P, Oettinger R, Gerner W, Falbrede J, Wirth S. Mother-to-infant transmission of TT virus: prevalence, extent and mechanism of vertical transmission. Pediatr Infect Dis J. 2000;19:1074-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Lin CL, Kyono W, Tongson J, Chua PK, Easa D, Yanagihara R, Nerurkar VR. Fecal excretion of a novel human circovirus, TT virus, in healthy children. Clin Diagn Lab Immunol. 2000;7:960-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Uchaykin VF, Cherednichenko TV, Samokhvalov EI, Filipova EV, Chaplygina UV, Kovalev OB, Konev VA. Present-day Understanding of TT-virus Infection in Children. J Child Infect. 2010;4:15-18. |
| 105. | Inaba N, Oshima K, Okajima Y, Nagase T. [TTV materno-infantile infection--a study on the TTV frequency in Japanese pregnant women and the natural history of TTV mother-to-infant infection]. Nihon Rinsho. 1999;57:1406-1409. [PubMed] |
| 106. | Goto K, Sugiyama K, Ando T, Mizutani F, Terabe K, Tanaka K, Nishiyama M, Wada Y. Detection rates of TT virus DNA in serum of umbilical cord blood, breast milk and saliva. Tohoku J Exp Med. 2000;191:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Manzin A, Mallus F, Macera L, Maggi F, Blois S. Global impact of Torque teno virus infection in wild and domesticated animals. J Infect Dev Ctries. 2015;9:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Naoumov NV, Petrova EP, Thomas MG, Williams R. Presence of a newly described human DNA virus (TTV) in patients with liver disease. Lancet. 1998;352:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 220] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 109. | Kanda T, Yokosuka O, Ikeuchi T, Seta T, Kawai S, Imazeki F, Saisho H. The role of TT virus infection in acute viral hepatitis. Hepatology. 1999;29:1905-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Fabris P, Biasin MR, Infantolino D, Tositti G, Venza E, Floreani A, Zanetti A, de Lalla F. TTV infection in patients with acute hepatitis of defined aetiology and in acute non-A-E hepatitis. J Hepatol. 2000;32:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Kikuchi K, Miyakawa H, Abe K, Kako M, Katayama K, Fukushi S, Mishiro S. Indirect evidence of TTV replication in bone marrow cells, but not in hepatocytes, of a subacute hepatitis/aplastic anemia patient. J Med Virol. 2000;61:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 112. | Rodríguez-Iñigo E, Casqueiro M, Bartolomé J, Ortiz-Movilla N, López-Alcorocho JM, Herrero M, Manzarbeitia F, Oliva H, Carreño V. Detection of TT virus DNA in liver biopsies by in situ hybridization. Am J Pathol. 2000;156:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Tanaka Y, Mizokami M, Orito E, Ohno T, Nakano T, Kato T, Iida S, Ueda R. Lack of integrated TT virus (TTV) genomes in cellular DNA in infected human hematopoietic cells. Leuk Lymphoma. 2000;38:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Mariscal LF, López-Alcorocho JM, Rodríguez-Iñigo E, Ortiz-Movilla N, de Lucas S, Bartolomé J, Carreño V. TT virus replicates in stimulated but not in nonstimulated peripheral blood mononuclear cells. Virology. 2002;301:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Focosi D, Macera L, Boggi U, Nelli LC, Maggi F. Short-term kinetics of torque teno virus viraemia after induction immunosuppression confirm T lymphocytes as the main replication-competent cells. J Gen Virol. 2015;96:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 116. | Ohbayashi H, Tanaka Y, Ohoka S, Chinzei R, Kakinuma S, Goto M, Watanabe M, Marumo F, Sato C. TT virus is shown in the liver by in situ hybridization with a PCR-generated probe from the serum TTV-DNA. J Gastroenterol Hepatol. 2001;16:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Kazemi MJ, Yaghobi R, Iravani Saadi M, Geramizadeh B, Moayedi J. Association Between TT Virus Infection and Cirrhosis in Liver Transplant Patients. Hepat Mon. 2015;15:e28370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Albert E, Solano C, Pascual T, Torres I, Macera L, Focosi D, Maggi F, Giménez E, Amat P, Navarro D. Dynamics of Torque Teno virus plasma DNAemia in allogeneic stem cell transplant recipients. J Clin Virol. 2017;94:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 119. | Deng X, Terunuma H, Handema R, Sakamoto M, Kitamura T, Ito M, Akahane Y. Higher prevalence and viral load of TT virus in saliva than in the corresponding serum: another possible transmission route and replication site of TT virus. J Med Virol. 2000;62:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Kincaid RP, Sullivan CS. Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog. 2012;8:e1003018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 121. | Boss IW, Renne R. Viral miRNAs: tools for immune evasion. Curr Opin Microbiol. 2010;13:540-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 122. | Cullen BR. MicroRNAs as mediators of viral evasion of the immune system. Nat Immunol. 2013;14:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 123. | Kincaid RP, Burke JM, Cox JC, de Villiers EM, Sullivan CS. A human torque teno virus encodes a microRNA that inhibits interferon signaling. PLoS Pathog. 2013;9:e1003818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 124. | Vignolini T, Macera L, Antonelli G, Pistello M, Maggi F, Giannecchini S. Investigation on torquetenovirus (TTV) microRNA transcriptome in vivo. Virus Res. 2016;217:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Garbuglia AR, Grasso F, Donà MG, Mochi S, Conti P, De Lutiis MA, Giorgi C, Iezzi T. TT virus infection: role of interferons, interleukin-28 and 29, cytokines and antiviral proteins. Int J Immunopathol Pharmacol. 2007;20:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Zanotta N, Maximova N, Campisciano G, Del Savio R, Pizzol A, Casalicchio G, Berton E, Comar M. Up-regulation of the monocyte chemotactic protein-3 in sera from bone marrow transplanted children with torquetenovirus infection. J Clin Virol. 2015;63:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | García-Álvarez M, Berenguer J, Alvarez E, Guzmán-Fulgencio M, Cosín J, Miralles P, Catalán P, López JC, Rodríguez JM, Micheloud D, Muñoz-Fernández MA, Resino S. Association of torque teno virus (TTV) and torque teno mini virus (TTMV) with liver disease among patients coinfected with human immunodeficiency virus and hepatitis C virus. Eur J Clin Microbiol Infect Dis. 2013;32:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Maggi F, Focosi D, Statzu M, Bianco G, Costa C, Macera L, Spezia PG, Medici C, Albert E, Navarro D, Scagnolari C, Pistello M, Cavallo R, Antonelli G. Early Post-Transplant Torquetenovirus Viremia Predicts Cytomegalovirus Reactivations In Solid Organ Transplant Recipients. Sci Rep. 2018;8:15490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Handala L, Descamps V, Morel V, Castelain S, François C, Duverlie G, Helle F, Brochot E. No correlation between Torque Teno virus viral load and BK virus replication after kidney transplantation. J Clin Virol. 2019;116:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 130. | Osipova LA, Makarova TE, Medvedeva EA. The experience of the viral hepatitis caused by TT-virus type, treatment. Zdravookhranenie Dalnego Vostoka (Health Care Far East). 2018;1:41-43. |
| 131. | Hayashi K, Fukuda Y, Hayakawa T, Kumada T, Nakano S. [TT virus(TTV) infection in patients with acute hepatitis]. Nihon Rinsho. 1999;57:1322-1325. [PubMed] |
| 132. | Tangkijvanich P, Theamboonlers A, Hirsch P, Kullavanijaya P, Suwangool P, Poovorawan Y. TT virus infection in chronic liver disease. Hepatogastroenterology. 1999;46:1053-1058. [PubMed] |
| 133. | Kato T, Mizokami M, Orito E, Nakano T, Tanaka Y, Ueda R, Hirashima N, Iijima Y, Kato T, Sugauchi F, Mukaide M, Shimamatsu K, Kage M, Kojiro M. High prevalance of TT virus infection in Japanese patients with liver diseases and in blood donors. J Hepatol. 1999;31:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Gerner P. TT virus infection in healthy children and in children with chronic hepatitis B or C. J Pediatr. 2000;136:606-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 135. | Das K, Kar P, Gupta RK, Das BC. Role of transfusion-transmitted virus in acute viral hepatitis and fulminant hepatic failure of unknown etiology. J Gastroenterol Hepatol. 2004;19:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Asim M, Singla R, Gupta RK, Kar P. Clinical & molecular characterization of human TT virus in different liver diseases. Indian J Med Res. 2010;131:545-554. [PubMed] |
| 137. | Gromova NI, Grankova TM, Gordeychuk IV, Kyuregyan KK, Ilchenko LYu, Mikhaylov MI. Effect of TT virus infection on the course and efficacy of treatment of chronic viral hepatitis. Infect Dis. 2011;9:14-18. |
| 138. | Shibayama T, Igari T, Tsuda F. [The prevalence of TTV infection in non-A to G chronic liver disease, especially non-A to G hepatocellular carcinoma, and the clinical significance of TTV infection]. Nihon Rinsho. 1999;57:1356-1361. [PubMed] |
| 139. | Cao K, Mizokami M, Orito E, Ding X, Ge XM, Huang GY, Ueda R. TT virus infection among IVDUs in south western China. Scand J Infect Dis. 1999;31:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 140. | Giménez-Barcons M, Forns X, Ampurdanés S, Guilera M, Soler M, Soguero C, Sánchez-Fueyo A, Mas A, Bruix J, Sánchez-Tapias JM, Rodés J, Saiz JC. Infection with a novel human DNA virus (TTV) has no pathogenic significance in patients with liver diseases. J Hepatol. 1999;30:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 141. | Rosa AS, Araujo OC, Savassi-Ribas F, Fernandes CA, Coelho HS, Niel C, Villela-Nogueira CA, Araujo NM. Prevalence of occult hepatitis B virus infection and Torque teno virus infection and their association with hepatocellular carcinoma in chronic hepatitis C patients. Virus Res. 2017;242:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Hagiwara H, Hayashi N, Mita E, Oshita M, Kobayashi I, Iio S, Hiramatsu N, Sasaki Y, Kasahara A, Kakinuma K, Yamauchi T, Fusamoto H. Influence of transfusion-transmitted virus infection on the clinical features and response to interferon therapy in Japanese patients with chronic hepatitis C. J Viral Hepat. 1999;6:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 143. | Nishizawa Y, Tanaka E, Orii K, Rokuhara A, Ichijo T, Yoshizawa K, Kiyosawa K. Clinical impact of genotype 1 TT virus infection in patients with chronic hepatitis C and response of TT virus to alpha-interferon. J Gastroenterol Hepatol. 2000;15:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 144. | Cleavinger PJ, Persing DH, Li H, Moore SB, Charlton MR, Sievers C, Therneau TM, Zein NN. Prevalence of TT virus infection in blood donors with elevated ALT in the absence of known hepatitis markers. Am J Gastroenterol. 2000;95:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 145. | Piaggio F, Dodi F, Bottino G, Andorno E, Gentile R, Ferrari C, Barabino G, Giannone A, Immordino G, Miggino M, Magoni Rossi A, Moraglia E, Gasloli G, Gelli M, Ferrante R, Morelli N, Casaccia M, Valente U. Torque Teno Virus--cause of viral liver disease following liver transplantation: a case report. Transplant Proc. 2009;41:1378-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 146. | Abe K, Okano T, Miyasaka A, Sato S, Suzuki K, Sato S, Ishikawa K. [Clinical characteristics of acute non A to G hepatitis caring TTV-DNA]. Nihon Rinsho. 1999;57:1285-1289. [PubMed] |
| 147. | Sakamoto M, Akahane Y. [TTV superinfection on acute hepatitis B]. Nihon Rinsho. 1999;57:1326-1329. [PubMed] |
| 148. | Ikeda H, Takasu M, Inoue K, Okamoto H, Miyakawa Y, Mayumi M. Infection with an unenveloped DNA virus (TTV) in patients with acute or chronic liver disease of unknown etiology and in those positive for hepatitis C virus RNA. J Hepatol. 1999;30:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Watanabe H, Saito T, Kawamata O, Shao L, Aoki M, Terui Y, Mitsuhashi H, Matsuo T, Takeda Y, Saito K, Togashi H, Shinzawa H, Takahashi T. Clinical implications of TT virus superinfection in patients with chronic hepatitis C. Am J Gastroenterol. 2000;95:1776-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 150. | Kao JH, Chen W, Chen PJ, Lai MY, Chen DS. TT virus infection in patients with chronic hepatitis B or C: influence on clinical, histological and virological features. J Med Virol. 2000;60:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 151. | Kristian P, Schreter I, Siegfried L, Jarcuska P, Jarcuska P, Paralicova Z, Porubcin S. Impact of TTV and SENV infection in chronic hepatitis B or C on liver histology and therapy outcome. Bratisl Lek Listy. 2010;111:629-634. [PubMed] |
| 152. | Alavi S, Sharifi Z, Valeshabad AK, Nourbakhsh K, Shamsian BS, Arzanian MT, Safarisharari A, Navidinia M. Clinical outcomes of Torque teno virus-infected thalassemic patients with and without hepatitis C virus infection. Korean J Hematol. 2011;46:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 153. | Hasebe C, Kawashima T, Kohgo Y. [Histological findings of TTV-positive non-B non-C chronic hepatitis patients]. Nihon Rinsho. 1999;57:1350-1355. [PubMed] |
| 154. | Hu ZJ, Lang ZW, Zhou YS, Yan HP, Huang DZ, Chen WR, Luo ZX. Clinicopathological study on TTV infection in hepatitis of unknown etiology. World J Gastroenterol. 2002;8:288-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | Khomeriki SG, Ilchenko LYu, Morozov IA, Karlovich TI. Clinicomorphological features of liver damage in patients infected with hepatitis TT. World Viral Hepat. 2006;2:2-8. |
| 156. | Koltunov AS, Alekseenko SA, Evseev AN, Koltunov SS. Clinical and morphological characteristic of chronic hepatitis associated with TTV-infection. Far East Med J. 2013;3:12-14. |
| 157. | Morozov IA, Ilchenko LYu, Kyuregyan KK, Karlsen AA, Fediukina ES, Fedorov IG, Petrenko NV. The first two cases of chronic hepatitis associated with Torque Teno midi virus (TTMDV), genus gammatorquevirus. Archive of Internal Medicine. 2017;7:71-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 158. | Tokita H, Murai S, Kamitsukasa H, Yagura M, Harada H, Hebisawa A, Takahashi M, Okamoto H. Influence of TT virus on the histopathological features of nonalcoholic fatty liver disease. Hepatol Res. 2001;19:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Arakawa Y, Shioda A, Moriyama M. [Incidence of TT virus infection in patients with non-A to G liver disease]. Nihon Rinsho. 1999;57:1290-1294. [PubMed] |
| 160. | Wang JT, Lee CZ, Kao JH, Sheu JC, Wang TH, Chen DS. Incidence and clinical presentation of posttransfusion TT virus infection in prospectively followed transfusion recipients: emphasis on its relevance to hepatitis. Transfusion. 2000;40:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 161. | De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, Okamoto J, Snyder TM, Cornfield DN, Nicolls MR, Weill D, Bernstein D, Valantine HA, Quake SR. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 368] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 162. | Solis M, Velay A, Gantner P, Bausson J, Filipputtu A, Freitag R, Moulin B, Caillard S, Fafi-Kremer S. Torquetenovirus viremia for early prediction of graft rejection after kidney transplantation. J Infect. 2019;79:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 163. | Giacconi R, Maggi F, Macera L, Pistello M, Provinciali M, Giannecchini S, Martelli F, Spezia PG, Mariani E, Galeazzi R, Costarelli L, Iovino L, Galimberti S, Nisi L, Piacenza F, Malavolta M. Torquetenovirus (TTV) load is associated with mortality in Italian elderly subjects. Exp Gerontol. 2018;112:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 164. | Focosi D, Macera L, Pistello M, Maggi F. Torque Teno virus viremia correlates with intensity of maintenance immunosuppression in adult orthotopic liver transplant. J Infect Dis. 2014;210:667-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 165. | Schiemann M, Puchhammer-Stöckl E, Eskandary F, Kohlbeck P, Rasoul-Rockenschaub S, Heilos A, Kozakowski N, Görzer I, Kikić Ž, Herkner H, Böhmig GA, Bond G. Torque Teno Virus Load-Inverse Association With Antibody-Mediated Rejection After Kidney Transplantation. Transplantation. 2017;101:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 166. | Ruiz P, Martínez-Picola M, Santana M, Muñoz J, Pérez-Del-Pulgar S, Koutsoudakis G, Sastre L, Colmenero J, Crespo G, Navasa M. Torque Teno Virus Is Associated With the State of Immune Suppression Early After Liver Transplantation. Liver Transpl. 2019;25:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 167. | Kulifaj D, Durgueil-Lariviere B, Meynier F, Munteanu E, Pichon N, Dubé M, Joannes M, Essig M, Hantz S, Barranger C, Alain S. Development of a standardized real time PCR for Torque teno viruses (TTV) viral load detection and quantification: A new tool for immune monitoring. J Clin Virol. 2018;105:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 168. | Lefrère JJ, Roudot-Thoraval F, Lefrère F, Kanfer A, Mariotti M, Lerable J, Thauvin M, Lefèvre G, Rouger P, Girot R. Natural history of the TT virus infection through follow-up of TTV DNA-positive multiple-transfused patients. Blood. 2000;95:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 169. | Strassl R, Schiemann M, Doberer K, Görzer I, Puchhammer-Stöckl E, Eskandary F, Kikic Ž, Gualdoni GA, Vossen MG, Rasoul-Rockenschaub S, Herkner H, Böhmig GA, Bond G. Quantification of Torque Teno Virus Viremia as a Prospective Biomarker for Infectious Disease in Kidney Allograft Recipients. J Infect Dis. 2018;218:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 170. | Schmitz J, Kobbe G, Kondakci M, Schuler E, Magorsch M, Adams O. The Value of Torque Teno Virus (TTV) as a Marker for the Degree of Immunosuppression in Adult Patients after Hematopoietic Stem Cell Transplantation (HSCT). Biol Blood Marrow Transplant. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 171. | Frye BC, Bierbaum S, Falcone V, Köhler TC, Gasplmayr M, Hettich I, Dürk T, Idzko M, Zissel G, Hengel H, Müller-Quernheim J. Kinetics of Torque Teno Virus-DNA Plasma Load Predict Rejection in Lung Transplant Recipients. Transplantation. 2019;103:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |