Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1674
Peer-review started: November 11, 2019
First decision: January 17, 2020
Revised: March 10, 2020
Accepted: March 14, 2020
Article in press: March 14, 2020
Published online: April 14, 2020
Processing time: 115 Days and 10.1 Hours
Gastrointestinal hemangiomas are rare benign tumors. According to the size of the affected vessels, hemangiomas are histologically classified into cavernous, capillary, or mixed-type tumors, with the cavernous type being the most common and racemose hemangiomas being very rare in the clinic. Melena of uncertain origin and anemia are the main clinical manifestations, and other presentations are rare. Due to the rarity of gastrointestinal hemangiomas and lack of specific manifestations and diagnostic methods, preoperative diagnoses are often delayed or incorrect.
We report a 5-year-old girl who presented with abdominal pain, nausea, and vomiting for a duration of 10 h. The laboratory studies showed prominent anemia. Computed tomography and contrast-enhanced computed tomography of the abdomen revealed a small bowel obstruction caused by a giant abdominal mass. Segmental resection of the ileal lesions was performed through surgery, and the final pathology results revealed a diagnosis of racemose hemangioma complicated by a small bowel obstruction and simultaneous chronic anemia.
The current report will increase the understanding of the diagnosis and treatment of gastrointestinal hemangiomas and provide a review of the related literature.
Core tip: Gastrointestinal hemangiomas are rare benign tumors, and small bowel racemose hemangiomas complicated by obstructions and chronic anemia are even more rare clinically. Here, we report a 5-year-old girl who presented with abdominal pain, nausea, and vomiting for a duration of 10 h. The preoperative examination revealed an acute obstruction and anemia. A segmental resection of the ileum was performed, and the final pathology revealed a small bowel racemose hemangioma complicated by an obstruction and simultaneous chronic anemia. To improve the diagnosis and treatment of gastrointestinal hemangiomas, we present this unusual report and review some of the related literature.
- Citation: Fu JX, Zou YN, Han ZH, Yu H, Wang XJ. Small bowel racemose hemangioma complicated with obstruction and chronic anemia: A case report and review of literature. World J Gastroenterol 2020; 26(14): 1674-1682
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1674
Gastrointestinal hemangiomas are rare benign tumors, representing 0.05% of all gastrointestinal tumors[1]. These tumors usually present in young people with no sex predilection. Their main clinical manifestation is gastrointestinal bleeding of uncertain origin, which is defined as chronic or recurrent gastrointestinal bleeding of an unknown cause. Other forms of presentation include obstruction, intussusception, intramural hematoma, perforation, and platelet sequestration[2]. According to the size of the affected vessels, hemangiomas are histologically classified into cavernous, capillary, or mixed-type tumors, with the cavernous type being the most common and racemose hemangioma being very rare in the clinic[3]. In the gastrointestinal tract, these tumors are more frequently found in the jejunum. Computed tomography (CT) and contrast-enhanced computed tomography (CECT) are the main methods for diagnosing such lesions preoperatively, and capsule endoscopy is significantly helpful for diagnosing small bowel lesions[4]. Surgical resection is the ideal treatment. This study presents the unusual case of a 5-year-old girl who underwent segmental resection, and the final pathology results revealed a small bowel racemose hemangioma complicated by an obstruction and simultaneous chronic anemia. A review of the current literature was also provided to contextualize the findings of the present study.
A 5-year-old female child was admitted to the Emergency Department of our hospital complaining of abdominal pain, nausea, and vomiting for a duration of 10 h.
The patient suddenly developed abdominal pain 10 h ago, which was total abdominal pain accompanied by nausea and vomiting. There was no pulsatile vomiting. The vomitus was the previously ingested food and yellow-green bile-like substance, and she vomited three times. There was no hematemesis, no fever, no chest tightness or suffocation, and no diarrhea. The abdominal symptoms gradually became aggravated.
The patient was born after a full-term pregnancy by spontaneous vaginal delivery and had a history of iron deficiency anemia for 1 year. Prior to this admission, the patient had been treated with supplemental iron as recommended by her pediatrician for her symptoms but had shown no improvement. Her parents were healthy, and there were no close relatives. Her mother had a healthy pregnancy.
On the physical examination, her heart rate was 99 beats per minute, and her blood pressure was 12/8 KPa. There were no lesions in the oropharynx, and her neck was supple. The lungs were clear, and her heart rate was regular, without a murmur. Her abdomen was soft, and an abdominal mass could be felt on the left lower abdomen, which was tender. The neurologic examination was unremarkable.
The white cell count was 5.41 × 109/L, with 77.8% of neutrophils; hemoglobin was 78 g/L, with a hematocrit level of 27.7%, and the platelet count was 356 × 109/L. The serum ferritin level was less than 1.0 µg/L (normal range: 15-200). The ele-ctrocardiogram and chest X-ray were normal.
An initial imaging evaluation by ultrasound revealed an enormous tumor mass in the middle of the abdomen and pelvis with an inhomogeneous echo pattern that was 10.3 cm × 4.0 cm in size, and several strong echoes and grid-like structures could be seen in the mass with a low blood flow signal on color Doppler flow imaging.
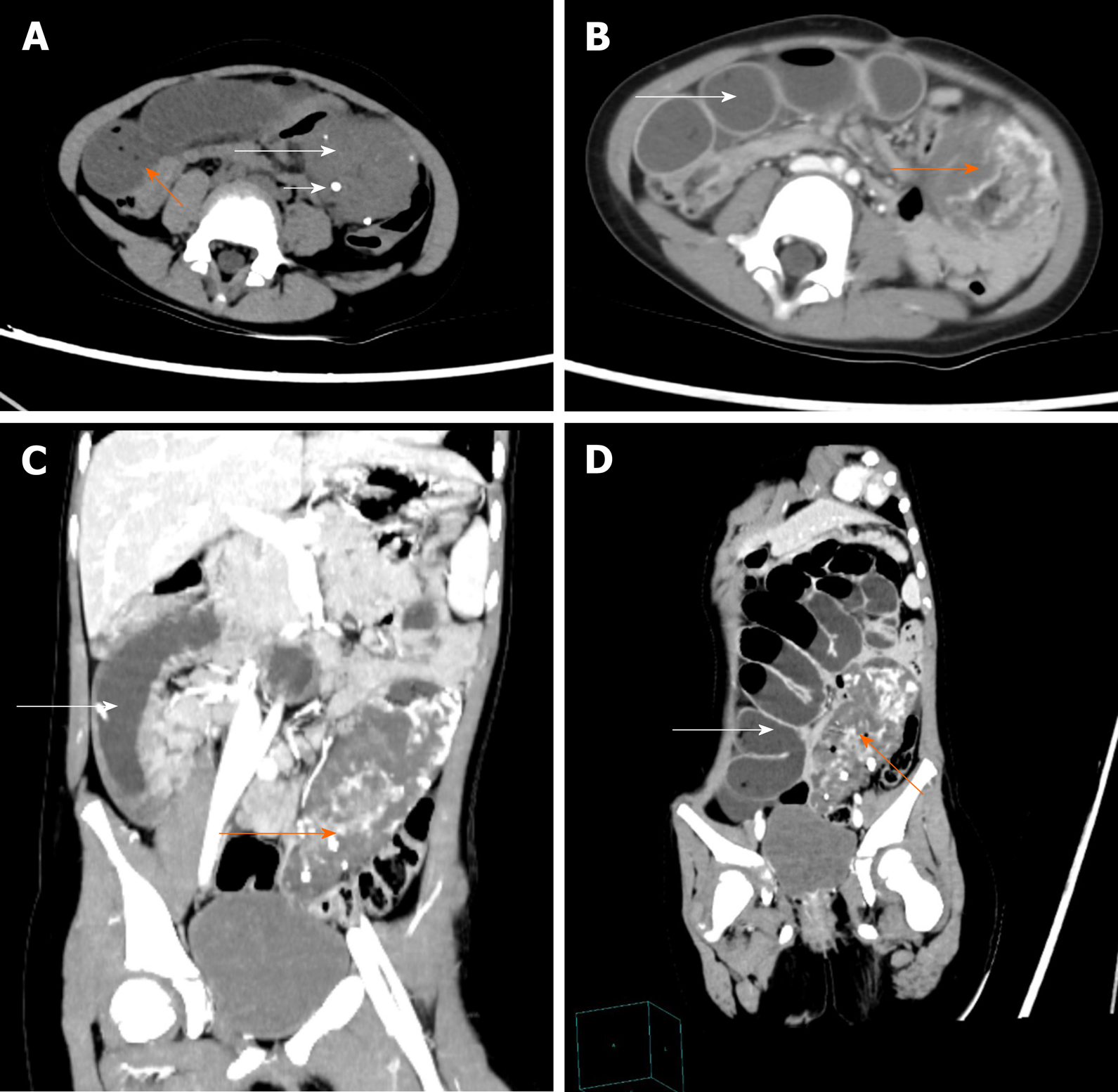
The abdominal lesions were further evaluated by an abdominal CT scan and CECT. The former revealed an ill-circumscribed mass of mixed density in the left lower abdomen that extended to the pelvis. There were multiple high-density nodes in the mass (Figure 1A). The latter revealed that the mass exhibited heterogeneous enhancement following contrast administration. In the venous phase, there were thick and tortuous blood vessels in the mass, which were connected to each other by a honeycomb or racemose appearance (Figure 1B-1D).
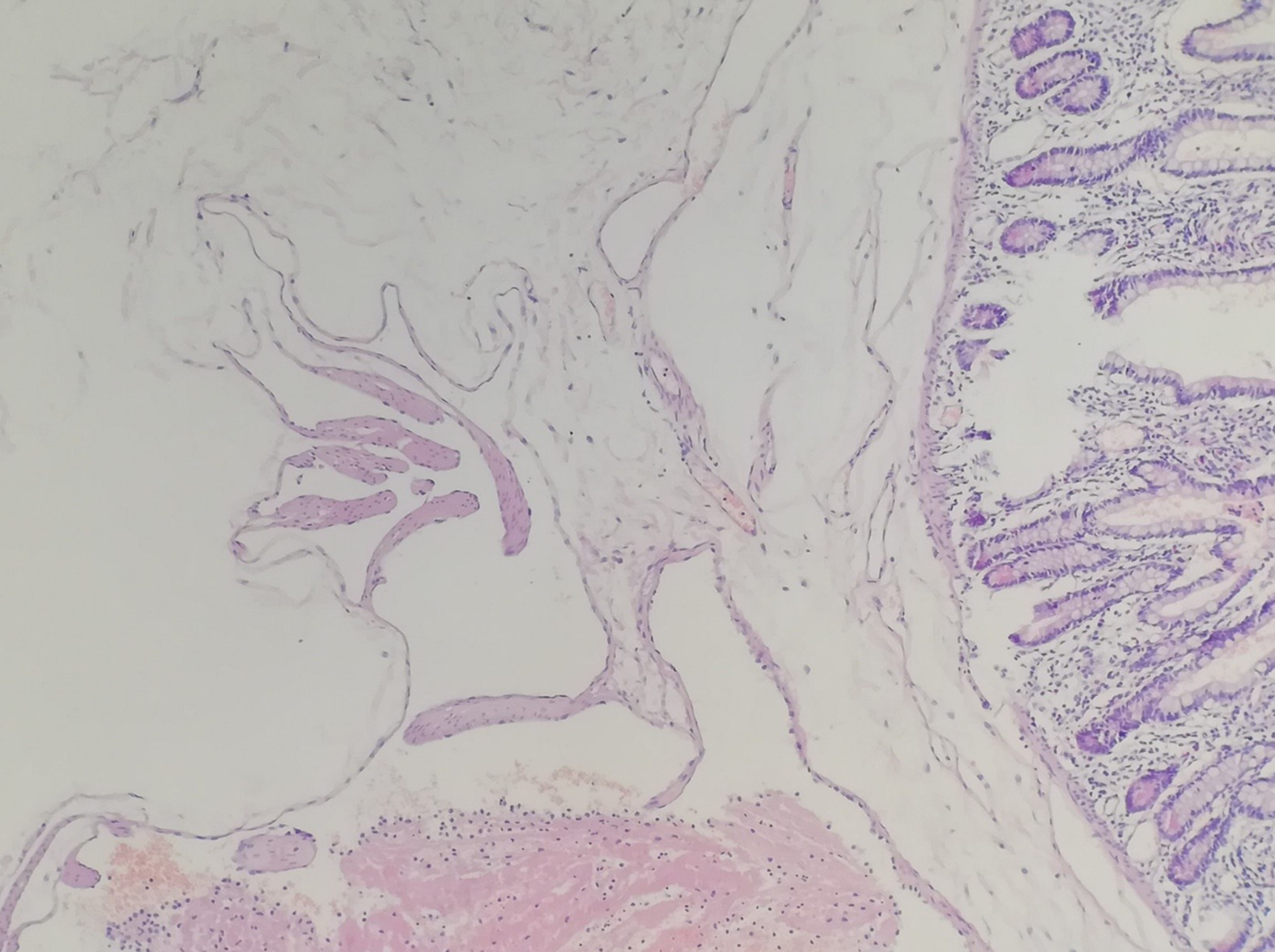
Considering the large abdominal mass in a young woman with multiple calcifications, the most likely preoperative diagnosis was a teratoma complicated by a small bowel obstruction. However, the final diagnosis by histopathology was small bowel racemose hemangioma complicated by an obstruction and anemia (Figure 2).
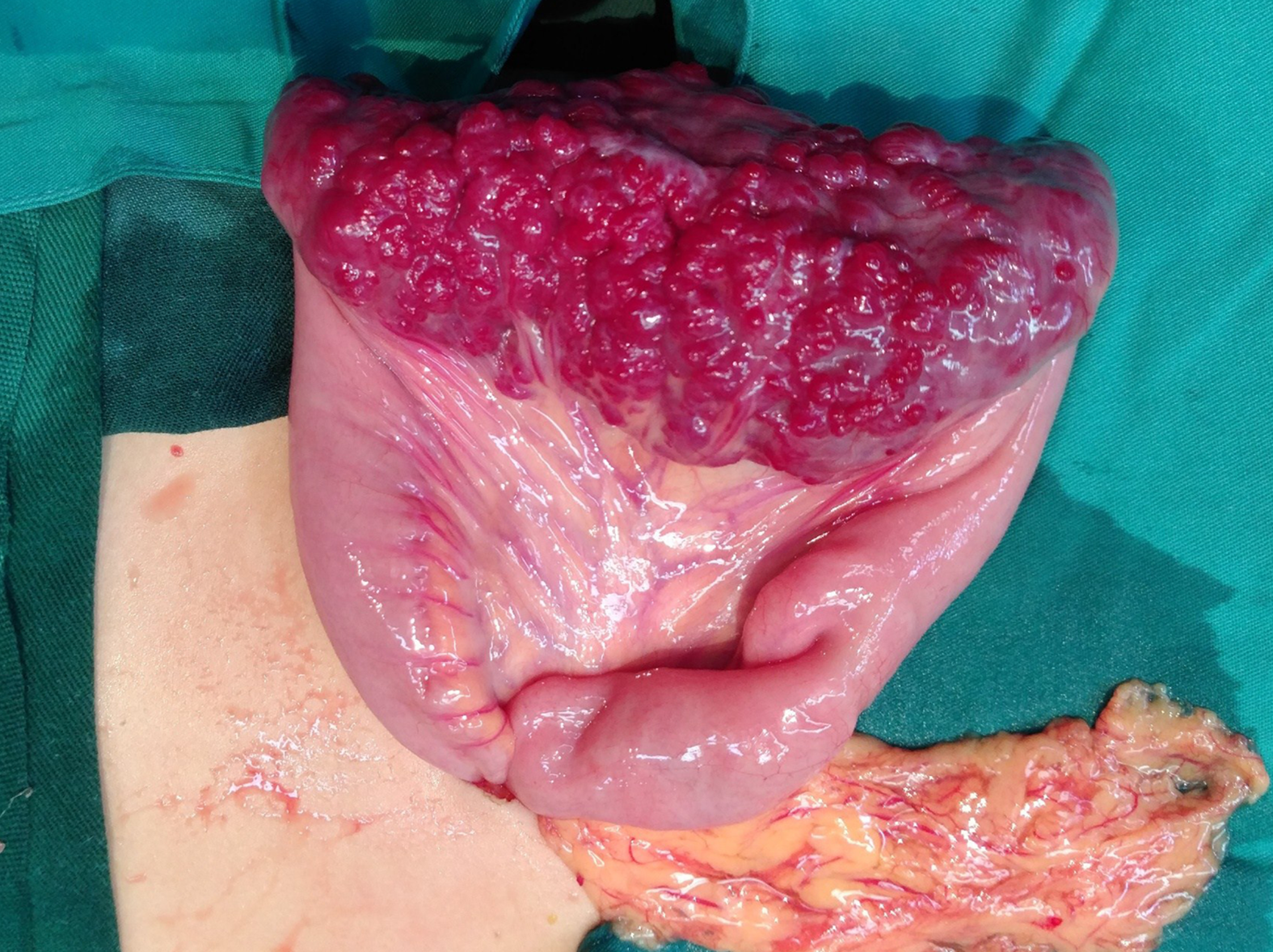
Laparoscopy was performed, and the result revealed a 10 cm × 4 cm lesion on the ileum; a vascular nature was suspected due to the bluish purple coloration, compressibility, and presence of varices on the surface (Figure 3). The mass invaded the intestinal canal and required a dilated proximal intestinal and segmental small bowel resection.
The patient was discharged without immediate complications on the 8th day, and the hemoglobin increased to 123 g/L at the second month after the operation.
Hemangiomas are defined as congenital benign vascular lesions that are venous malformations, not true tumors. Hemangiomas are classified into cavernous, capillary, or mixed tumors; the cavernous type is the most common, and racemose hemangioma is very rare[3]. According to the biological characteristics of hemangioma, Fishman et al[5] divided them into two categories: Hemangioma and vascular malformations. According to the angiographic findings, vascular malformations can be divided into high-flow and low-flow types, and racemose hemangioma is a complex high-flow type of arteriovenous malformation, which accounts for approximately 1.5% of all hemangiomas and mostly occurs in the head, neck, and limbs[6,7]. Hemangiomas of the gastrointestinal tract are rare, accounting for only 0.05% of all intestinal neoplasms and 7%-10% of all benign tumors of the small bowel[8]. According to the literature, small bowel racemose hemangiomas with obstructions and chronic anemia were rarely reported, which makes our case even more unusual.
The PubMed (https://www.ncbi.nlm.nih.gov/pubmed), WanFang Data (http://www.wanfangdata.com.cn/index.html), and China National Knowledge Infrastructure (CNKI; http://kns.cnki.net/kns/brief/default_result.aspx) databases were investigated between 2009 and 2019 to analyze the clinicopathological features and outcomes of patients with gastrointestinal hemangiomas by searching for MeSH terms and keywords such as “hemangioma”, “capsule endoscopy”, “double balloon enteroscopy”, “anemia”, and “gastrointestinal bleeding”. The reference lists were screened to identify additional relevant studies, and a standardized form was used for data extraction. Finally, there were approximately 25 cases of gastrointestinal hemangiomas[9-31]. The patient information is summarized in Table 1 to analyze the clinicopathological features (Table 2). The mean age of the patients with gastrointestinal hemangioma was 42.9 years (range: 0-75 years). The sex distribution included 14 males and 11 females (Male:Female = 1.27:1), which is consistent with the results of Durer C et al[14]. Gastrointestinal hemangiomas were mainly located in the jejunum and ileum, accounting for 36% and 24% of all gastrointestinal hemangiomas, respectively. The sizes of the gastrointestinal hemangiomas ranged widely from 0.3 cm to 32.5 cm, and the average size was approximately 7.44 cm. In our case, the patient was a 5-year-old girl, and the lesion was confirmed to be located in the ileum with a size of 9 cm x 6 cm.
| Ref. | Age (yr) | Sex | Presentation | Preoperative diagnosis study | Hemangioma size (cm) | Location | Histology | Treatment |
| Attash et al[9] | 3 | M | Hematemesis, anemia | EGD, CECT | 17.6 × 13.2 | Stomach | Cavernous | Resembling sleeve gastrectomy |
| Peng et al[10] | 47 | M | Fatigue, dizziness, melena | VCE, CECT | 50 × 15 | Ileum | Cavernous | Partial small bowel resection |
| Ocampo et al[11] | 29 | M | Anemia | Abdominal X-ray, CECT | 10 | Ileum | ND | Segmental small bowel resection |
| Kaya et al[12] | 2 d | F | Melena | EGD | ND | Stomach | ND | Propranolol |
| Fernandes et al[13] | 56 | F | Hematochezia, dizziness | VCE, VECT | 14 | Ileum | Cavernous | Laparotomy and vascular tumor resection |
| Durer et al[14] | 66 | M | Anemia | VCE, DBE | 2.5 | Jejunum | Cavernous | Surgery |
| Amati et al[15] | 20 | F | Abdominal distention, pain | CT | 28 × 26 × 12 | Sigmoid colon | Cavernous | Resection of the sigmoid |
| Wang et al[16] | 73 | M | Melena, weakness, dizziness | VCE, DBE | 2 × 1 | Ileum | Capillary | Laparotomy |
| Andrade et al[17] | 44 | F | Melena | Colonoscopy, MRI | 7.5 × 3.5 | Rectal | ND | Managed conservatively |
| Li et al[18] | 68 | M | Epigastric discomfort | EUS, CECT | 4 × 2 | Stomach | Hemolymphangioma | Endoscopic ultrasonogra-phy treatment |
| Iwaya et al[19] | 70 | M | Anemia, melena | VCE, DBE | 2 × 1.7 × 1.2 | Jejunum | Hemangiolymphangioma | Laparoscopic small bowel resection |
| Vitor et al[20] | 18 | M | Melena | Colonoscopy, MRI | ND | Rectum | Cavernous | Iron supplementat-ion |
| Parker et al[21] | 32 | M | Abdominal pain, anorexia, constipation | CT biopsy, MRI | 14 × 7 × 7 | Ileum | Cavernous | Laparotomy |
| Ganesananthan et al[22] | 65 | M | Rectal bleeding | Colonoscopy, CT | ND | Left colon and rectum | Cavernous | Managed Conservatively |
| Takase et al[23] | 62 | M | Anemia, melena | VCE, DBE | 1.5 | Jejunum | Cavernous | Laparoscopic enterectomy |
| 52 | M | Anemia | CECT, VCE, DBE | 1 | Ileum | Cavernous | Laparoscopic enterectomy | |
| Kuo et al[24] | 20 | F | Lower abdominal pain, postprandial bloating | CECT | 5.6 × 4.6 × 1.5 | Jejunum | Cavernous | Segmental resection |
| Zhang et al[25] | 44 | M | Melena | VCE | ND | Jejunum | Cavernous | Laparotomy with segmental resection |
| Moein Jahromi et al[26] | 75 | F | Anemia, melena | VCE, DBE | 2.7 × 1.7 | Jejunum | Capillary | Laparoscopic partial small bowel resection |
| Fu et al[27] | 54 | F | Painless rectal bleeding | Colonoscopy, CT, MRI | ND | Rectum | Cavernous | 3-D laparoscopica-lly assisted surgery |
| 22 | F | Recurrent intermittent rectal bleeding | Colonoscopy, CT | ND | Rectum | Cavernous | 3-D laparoscopica-lly assisted surgery | |
| Liao et al[28] | 11 | F | Hematochezia, palpitation, cold sweat | Angiography | 1.2 × 1.0 | Jejunum | Capillary | Laparoscopic segmental resection |
| Hu et al[29] | 31 | F | Melena, dizziness | Angiography | 0.3 × 0.3 | Jejunum | Racemose hemangioma | Laparoscopic segmental resection |
| Lian et al[30] | 57 | M | Abdominal pain with anus exhausting and defecating | CT | 16 × 12 × 8 | Jejunum | Cavernous | Reduction of the volvulus and segmental resection |
| Li et al[31] | 54 | F | Hematemesis, melena, dizziness | EGD, CECT | 2 × 2 × 2 | Stomach | Racemose hemangioma | Local excision of lesion |
| No. of cases | 25 | ||
| Age | 42.92 ± 22.75 (0-75) | Preoperative diagnosis study | |
| Sex | CT or CECT | 14 (56%) | |
| Male | 14 (54%) | Colonoscopy, EGD, or EUS | 9 (36%) |
| Female | 11 (44%) | VCE | 9 (36%) |
| Location | DBE | 6 (24%) | |
| Jejunum | 9 (36%) | MRI | 4 (16%) |
| Ileum | 6 (24%) | Angiography | 2 (8%) |
| Colorectum | 6 (24%) | Abdominal X-ray | 1 (4%) |
| Stomach | 4 (16%) | Histology | |
| Size | 7.44 ± 8.601 (0.3-32.5) | Cavernous hemangioma | 15 (68.2%) |
| Main symptom | Capillary hemangioma | 3 (13.7%) | |
| Melena | 11 (44%) | Racemose hemangioma | 2 (9.1%) |
| Anemia | 7 (28%) | Hemolymphangioma | 1 (4.5%) |
| Dizziness | 5 (20%) | Hemangiolymphangioma | 1 (4.5%) |
| Abdominal distention or pain | 5 (20%) | Treatment | |
| Rectal bleeding or Hematochezia | 5 (20%) | Operation | 20 (80%) |
| Hematemesis | 2 (8%) | Endoscopy | 1 (4%) |
| Fatigue or weakness | 2 (8%) | Medication (propranolol) | 1 (4%) |
| Anorexia or postprandial bloating | 2 (8%) | Iron supplementation | 1 (4%) |
| Palpitations or cold sweat | 1 (4%) | None | 2 (8%) |
| Anus exhausting and defecating | 1 (4%) | ||
Clinically, gastrointestinal hemangiomas are symptomatic in 90% of cases, unlike other benign tumors of the gastrointestinal tract that tend to present as an incidental finding[32]. The most frequent sign is chronic gastrointestinal bleeding, which causes anemia of an unknown origin and rarely leads to massive bleeding. Occasionally, these tumors may cause intestinal obstructions, intussusception, intramural hematoma, perforation, and platelet sequestration[2]. Among the 25 patients analyzed in our literature, melena, which was observed in 11 (44%) patients, was the main clinical symptom, followed by anemia in 7 (28%), and dizziness in 5 (20%). However, shock and intestinal obstructions caused by gastrointestinal hemangioma were only observed in 1 (4%) patient. Based on the histological examinations, there have been 15 reported cases of cavernous hemangioma, 3 cases of capillary hemangioma[16,26,28], 2 cases of racemose hemangioma[29,31], 1 case of hemolymphangioma[18], and 1 case of hemangiolymphangioma[19]. Overall, acute intestinal obstruction and chronic anemia caused by a small intestinal racemose hemangioma as in our case are extremely rare.
Gastrointestinal hemangioma is difficult to diagnose preoperatively, especially for small intestinal hemangiomas. Since the most frequent clinical presentation in these patients is gastrointestinal bleeding, the patients frequently undergo gastroscopy and colonoscopy studies with normal results, as in the reported case. However, when the lesions are located in the stomach or colorectal region, gastroscopy and colonoscopy can still have great value in the diagnosis and treatment of this disease. In the literature, among the 25 patients, 10 hemangiomas were located in the stomach or colorectum, 9 of which were diagnosed by endoscopy before the operation. A simple abdominal X-ray may be useful if phleboliths (50% of cases), obstructions, or perforations are present[1,33]. In our literature review, phleboliths were recognized overlying the right sacrum by a preoperative abdominal X-ray in one case[11]. CT and CECT are fundamental tools in the preoperative diagnosis of gastrointestinal hemangiomas, especially in emergency situations, because of their speed, availability, and ability to diagnose extraintestinal lesions. Due to the large degree of vascularity, gastrointestinal hemangiomas are homogenously and significantly enhanced on CECT. Magnetic resonance imaging (MRI), unlike CT, can demonstrate blood flow in the lesion without the administration of contrast medium, and phleboliths are usually void of signal on T1- and T2-weighted images[1]. For colorectal hemangioma, preoperative MRI can define the size of the lesion, which has great significance for treatment. In our retrospective analysis of 25 patients, half of all positive results before surgery were acquired by CT and/or CECT, and 16% were from MRI. Small bowel video capsule endoscopy (VCE) is a noninvasive imaging test and can be recommended when the source of the bleeding remains unidentified after upper and lower endoscopy. On the other hand, double-balloon enteroscopy (DBE) is an invasive and highly sensitive diagnostic tool that provides both therapeutic and diagnostic interventions[2]. There were 15 cases of small intestinal hemangioma in our literature, 9 of which were preoperatively diagnosed by small bowel VCE and 6 by DBE. Undoubtedly, small bowel VCE and DBE are very important for the diagnosis of small intestinal hemangiomas. However, small bowel VCE and DBE are not suitable for critical patients with gastrointestinal hemangiomas, such as those with massive hemorrhage, intestinal obstructions, or intussusception. In addition, 30% of the results were false positives, and 20% of the examinations were incomplete[11].
Based on the literature we reviewed and the CT images of our patient, we summarized the following features of gastrointestinal hemangiomas: (1) CT scan: Tumors tend to appear with mixed density, and there is a blurred boundary between the tumor and the surrounding intestinal tissue; additionally, multiple calcifications representing phleboliths can be recognized inside the tumor on approximately half of all CT studies; (2) CECT: The masses exhibit heterogeneous enhancement following contrast administration; in the venous phase, thick and tortuous blood vessels are present inside the tumor on CT images, and phleboliths can be found in some cases; and (3) For racemose hemangiomas, the characteristic CT manifestations include a dilated feeding artery, malformed vessels, and thick and tortuous draining veins[11,32,34]. Phleboliths are secondary to thrombosis of the intralesional vessels and subsequent partial or total calcifications of the thrombus and are an important diagnostic criterion that can be observed in 26%-50% of adult patients, especially in young patients, and phleboliths are virtually pathognomonic of hemangiomas if they are grouped[33-35]. Phleboliths and malformed vessels were evident in our case.
The main treatment for hemangiomas is surgical resection of the affected segment[1,10,36]. Since hemangiomas never metastasize to the lymph nodes or distant organs, local resection is sufficient. However, in some cases of polypoid lesions accessible by endoscopy, especially those located in the stomach or colorectal region, it may be possible to perform polypectomy and cauterization. However, these are still controversial options because of the risk for uncontrollable bleeding and intestinal perforation. In the literature, surgical resection was still the main treatment for gastrointestinal hemangiomas and was applied in 80% of all cases. However, there was an endoscopic resection performed for a stomach hemangioma with a size of 4 cm × 2 cm, resulting in a good clinical course[18]. In terms of drug therapy, Kaya et al[12] reported a case of neonatal gastric hemangioma successfully cured by propranolol. However, in symptomatic hemangiomas, which may be associated with potentially life-threatening massive bleeding, perforations, intestinal obstructions, and intussusception, surgical resection is the preferred treatment option. Gastrointestinal hemangiomas usually have a satisfying prognosis, and there is no evidence in the literature on the recurrence of hemangiomas[10,13]. Our patient underwent partial small bowel resection, and two months after the operation, her hemoglobin increased to 123 g/L, with a hematocrit level of 40.6%.
In conclusion, hemangiomas of the small intestine are a rare but significant source of gastrointestinal tract bleeding. Since the main symptoms of hemangiomas are not specific, the clinical diagnosis is often delayed or incorrect, and the preoperative diagnosis was mistaken for a teratoma in our case. However, rare pathologies do occur and most importantly, they can present in an unspecific presentation. Therefore, we can say that although gastrointestinal hemangiomas are rare tumors, they should be considered in the differential diagnoses of patients, especially children, who present with gastrointestinal bleeding of an obscure origin or other abdominal symptoms.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chowdhury F, Velnar T S-Editor: Wang J L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Corsi A, Ingegnoli A, Abelli P, De Chiara F, Mancini C, Cavestro GM, Fanigliulo L, Di Mario F, Franzi A, Zompatori M. Imaging of a small bowel cavernous hemangioma: report of a case with emphasis on the use of computed tomography and enteroclysis. Acta Biomed. 2007;78:139-143. [PubMed] |
| 2. | Pera M, Márquez L, Dedeu JM, Sánchez J, Garcia M, Ramón JM, Puigvehí M. Solitary cavernous hemangioma of the small intestine as the cause of long-standing iron deficiency anemia. J Gastrointest Surg. 2012;16:2288-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Regula J, Wronska E, Pachlewski J. Vascular lesions of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:313-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | de Mascarenhas-Saraiva MN, da Silva Araújo Lopes LM. Small-bowel tumors diagnosed by wireless capsule endoscopy: report of five cases. Endoscopy. 2003;35:865-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Fishman SJ, Mulliken JB. Hemangiomas and vascular malformations of infancy and childhood. Pediatr Clin North Am. 1993;40:1177-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 185] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Seccia A, Salgarello M, Farallo E, Falappa PG. Combined radiological and surgical treatment of arteriovenous malformations of the head and neck. Ann Plast Surg. 1999;43:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Dimakakos PB, Kotsis TE. Arteriovenous Malformations. In: Liapis CD, Balzer K, Benedetti-Valentini F, Fernandes e Fernandes J. Vascular Surgery. Berlin: Springer Berlin Heidelberg, 2007: 573-583. Available from: https://link.springer.com/chapter/10.1007%2F978-3-540-30956-7_50. |
| 8. | Boyle L, Lack EE. Solitary cavernous hemangioma of small intestine. Case report and literature review. Arch Pathol Lab Med. 1993;939-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Attash SM, Ali MS, Al-Nuaimy HA. Isolated cavernous haemangioma of the stomach in a 3-year-old child: an unusual cause of upper GI bleeding. BMJ Case Rep. 2012;6:2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Peng C, Chen H, Li W, Xu R, Zhuang W. A Rare Cause of Recurrent Gastrointestinal Bleeding: Giant Diffuse and Cavernous Intestinal Mesentery Hemangioma in an Adult. Dig Dis Sci. 2016;61:3363-3365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ocampo Toro WA, Corral Ramos B, Concejo Iglesias P, Cubero Carralero J, Blanco García DF, Barón Ródiz P. Haemangiomas of the Small Intestine: Poorly Known Cause of Gastrointestinal Bleeding of Uncertain Origin. Cureus. 2018;10:e3155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Kaya H, Gokce IK, Gungor S, Turgut H, Ozdemir R. A Newborn with Gastric Hemangioma Treated Using Propranolol. Pediatr Gastroenterol Hepatol Nutr. 2018;21:341-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Fernandes D, Dionísio I, Neves S, Duarte P. Cavernous hemangioma of small bowel: a rare cause of digestive hemorrhage. Rev Esp Enferm Dig. 2014;106:214-215. [PubMed] |
| 14. | Durer C, Durer S, Sharbatji M, Comba IY, Aharoni I, Majeed U. Cavernous Hemangioma of the Small Bowel: A Case Report and Literature Review. Cureus. 2018;10:e3113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Amati AL, Hecker A, Schwandner T, Ghanem H, Holler J, Reichert M, Padberg W. A hemangioma of the sigmoid colon mesentery presenting as a retroperitonealtumor: a case report and review. World J Surg Oncol. 2014;12:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Wang B, Lou Z, Zheng W, Zhang J, Liu J. Capillary hemangioma in the ileum: Obscure small-bowel bleeding in an elderly person. Turk J Gastroenterol. 2018;29:520-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Andrade P, Lopes S, Macedo G. Diffuse cavernous hemangioma of the rectum: case report and literature review. Int J Colorectal Dis. 2015;30:1289-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Li QY, Xu Q, Fan SF, Zhang Y. Gastric haemolymphangioma: a literature review and report of one case. Br J Radiol. 2012;85:e31-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Iwaya Y, Streutker CJ, Coneys JG, Marcon N. Hemangiolymphangioma of the small bowel: A rare cause of chronic anemia. Dig Liver Dis. 2018;50:1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Vitor S, Oliveira Ferreira A, Lopes J, Velosa J. Hemangioma of the rectum - How misleading can hematochezia be? Rev Esp Enferm Dig. 2016;108:500-501. [PubMed] |
| 21. | Parker WT, Harper JG, Rivera DE, Holsten SB, Bowden T. Mesenteric cavernous hemangioma involving small bowel and appendix: a rare presentation of a vascular tumor. Am Surg. 2009;75:811-816. [PubMed] |
| 22. | Ganesananthan S, Barlow J, Durai D, Hawthorne AB. Multiple venous malformations in the left colon and rectum: a long-standing case managed conservatively and an update of current literature. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Takase N, Fukui K, Tani T, Nishimura T, Tanaka T, Harada N, Ueno K, Takamatsu M, Nishizawa A, Okamura A, Kaneda K. Preoperative detection and localization of small bowel hemangioma: Two case reports. World J Gastroenterol. 2017;23:3752-3757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Kuo LW, Chuang HW, Chen YC. Small bowel cavernous hemangioma complicated with intussusception: report of an extremely rare case and review of literature. Indian J Surg. 2015;77:123-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Zhang GY, Luo CJ, Zhao B, Zhan H, Long B, Guo LY, Zhou HN, Jiao ZY. [Small intestinal cavernous hemangioma causing chronic hemorrhage: a case report]. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:866-868. [PubMed] |
| 26. | Moein Jahromi B, Tsai F. Small-bowel hemangioma: rare and hard to find. Gastrointest Endosc. 2019;89:436-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Fu ZW, Wang LX, Zhang ZY, Luo QF, Ge HY. Three-dimensional laparoscopy-assisted bowel resection for cavernous hemangioma of the rectum: Report of two cases. Asian J Endosc Surg. 2019;12:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Liao Ch, Tang HR, Tang HR, Zhang JY, Wang H. Gastrointestinal hemorrhage caused by small intestinal hemangioma in children: a case report. Zhonghua Waikeputong Zazhi. 2017;32:682. [DOI] [Full Text] |
| 29. | Hu XD, Liu M, Zhang HL, Zhang YN, Feng Y. Lower gastrointestinal hematorrhea induced by hemangioma of jejunum: a case report. Weichangbingxue He Ganbingxue Zazhi. 2016;25:839-840 Available from: http://www.en.cnki.com.cn/Article_en/CJFDTotal-WCBX201607034.htm. |
| 30. | Lian YJ. Cavernous hemangioma of small intestine with volvulus: a case report. Zhonghua Waikeputong Zazhi. 2018;33:33. [DOI] [Full Text] |
| 31. | Li SH, Zou WY, Shi SL. Stomach hemangioma: report of one case. Yixue Yingxiangxue Zazhi. 2011;22:829-830. [DOI] [Full Text] |
| 32. | Huprich JE, Barlow JM, Hansel SL, Alexander JA, Fidler JL. Multiphase CT enterography evaluation of small-bowel vascular lesions. AJR Am J Roentgenol. 2013;201:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Chen HH, Tu CH, Lee PC, Chiu HH, Wu MS, Wang HP. Endoscopically diagnosed cavernous hemangioma in the deep small intestine: a case report. Advances in Digestive Medicine. 2015;2:74-78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Lee NK, Kim S, Kim GH, Jeon TY, Kim DH, Jang HJ, Park DY. Hypervascular subepithelial gastrointestinal masses: CT-pathologic correlation. Radiographics. 2010;30:1915-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Levy AD, Abbott RM, Rohrmann CA, Frazier AA, Kende A. Gastrointestinal hemangiomas: imaging findings with pathologic correlation in pediatric and adult patients. AJR Am J Roentgenol. 2001;177:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Yoo S. GI-Associated Hemangiomas and Vascular Malformations. Clin Colon Rectal Surg. 2011;24:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |