Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1463
Peer-review started: December 16, 2019
First decision: February 18, 2020
Revised: March 6, 2020
Accepted: March 19, 2020
Article in press: March 19, 2020
Published online: April 7, 2020
Processing time: 109 Days and 12.1 Hours
The prognosis of hepatocellular carcinoma (HCC) patients remains poor despite advances in treatment modalities and diagnosis. It is important to identify useful markers for the early detection of HCC in patients. Preneoplastic antigen (PNA), originally reported in a rat carcinogenesis model, is increased in the tissues and serum of HCC patients.
To determine the diagnostic value of PNA for discriminating HCC and to characterize PNA-positive HCC.
Patients with hepatitis C virus (HCV)-related hepatic disorders were prospectively enrolled in this study, which included patients with hepatitis, with cirrhosis, and with HCC. A novel enzyme-linked immunosorbent assay was developed to measure serum PNA concentrations in patients.
Serum PNA concentrations were measured in 89 controls and 141 patients with HCV infections (50 hepatitis, 44 cirrhosis, and 47 HCC). Compared with control and non-HCC patients, PNA was increased in HCC. On receiver operating characteristic curve analysis, the sensitivity of PNA was similar to the HCC markers des-γ-carboxy-prothrombin (DCP) and α-fetoprotein (AFP), but the specificity of PNA was lower. There was no correlation between PNA and AFP and a significant but weak correlation between PNA and DCP in HCC patients. Importantly, the correlations with biochemical markers were completely different for PNA, AFP, and DCP; glutamyl transpeptidase was highly correlated with PNA, but not with AFP or DCP, and was significantly higher in PNA-high patients than in PNA-low patients with HCV-related HCC.
PNA may have the potential to diagnose a novel type of HCC in which glutamyl transpeptidase is positively expressed but AFP or DCP is weakly or negatively expressed.
Core tip: Despite advances in treatment modalities and diagnosis, the prognosis of hepatocellular carcinoma (HCC) patients remains poor. It is important to identify useful markers for the early detection of HCC. Preneoplastic antigen (PNA) is increased in the tissues and serum of HCC patients. Therefore, we investigated the diagnostic value of PNA to discriminate HCC. We found that PNA had a comparable diagnostic value to α-fetoprotein and des-γ-carboxy-prothrombin. PNA may have the potential to diagnose a novel type of HCC in which glutamyl transpeptidase is positively expressed, but α-fetoprotein or des-γ-carboxy-prothrombin is weakly or negatively expressed.
- Citation: Yamashita S, Kato A, Akatsuka T, Sawada T, Asai T, Koyama N, Okita K. Clinical relevance of increased serum preneoplastic antigen in hepatitis C-related hepatocellular carcinoma. World J Gastroenterol 2020; 26(13): 1463-1473
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1463.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1463
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and the primary risk factors for HCC include chronic infection by hepatitis B virus (HBV) or hepatitis C virus (HCV) and nonalcoholic fatty liver disease[1,2]. The overall survival rate of HCC patients has improved with advances in treatment modalities and diagnosis[3,4]. The surveillance rate for HCC patients has increased in Japan; thus, patients are more likely to be diagnosed in the earlier stage of the disease, and the survival rate has increased[5]. The HCC markers α-fetoprotein (AFP) and des-γ-carboxy-prothrombin (DCP), also known as PIVKA-II, have been used for the early-stage screening and diagnosis of HCC[6].
Although HCC treatment modalities and diagnosis have developed and improved, the prognosis of HCC patients at advanced stages remains poor[7,8]. Therefore, it is important to develop useful markers for the early detection of HCC.
The expression of preneoplastic antigen (PNA) was originally reported in a study of hyperplastic nodules in a rat experimental carcinogenesis model[9]. Immunostaining approaches showed that PNA was present in the cytoplasm of hepatocytes in hyperplastic nodules and in primary hepatomas[9,10]. Purification and biochemical characterization indicated that PNA was composed of microsomal epoxide hydrolase (mEH) and other binding proteins[11,12]. The mEH gene was hypomethylated in nodules and hepatomas induced by chemical carcinogens[13].
Evidence that PNA expression in human liver tissues is increased in pathological states has been accumulating. Immunohistochemical analysis of human tissues showed that mEH was positive in normal hepatocytes and HCC tissues, but less or negatively expressed in other tumors, even if they metastasized to liver tissues[14,15]. Localization of mEH in the membrane changed during liver pathogenesis, such as neoplasia[16] or hepatitis infection[17]. An autoantibody response to mEH was detected in the serum of patients infected with HCV[18]. PNA was also detected in the culture medium of human HCC cell lines[19]. These data suggest that immunological detection of PNA may be a promising diagnostic tool for HCC.
To gain insight into PNA expression in liver diseases, we developed a highly sensitive enzyme-linked immunosorbent assay (ELISA) to measure PNA and determined serum PNA concentrations in patients with HCV-related hepatitis, cirrhosis, or HCC. The characteristics of PNA-positive HCC are also discussed in relation to biochemical markers.
In Japan Community Health Care Organization (JCHO) Shimonoseki Medical Center, patients with HCV-related hepatic disorders were prospectively enrolled in this study, which included patients with hepatitis, with cirrhosis, and with HCC. All subjects fulfilled the criteria for a diagnosis of hepatitis, cirrhosis, or HCC with HCV infection, regardless of treatment history. The study protocol was approved by the Human Ethics Committee of JCHO Shimonoseki Medical Center (Approval date: May 29, 2015). Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Blood samples were collected from patients after written, informed consent was confirmed. Biochemical markers were assessed routinely in JCHO Shimonoseki Medical Center including albumin, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), and total bilirubin levels, as well as HCC markers including DCP and AFP. A fibrosis marker of type IV collagen was measured with a latex immunoassay by Sekisui Medical Co., Ltd. (Tokyo, Japan).
Human mEH, a component of PNA, was produced in a recombinant baculovirus system[20], and the solubilized form of mEH was purified with sequential steps by column chromatography[16]. The development of anti-mEH monoclonal antibody 2G2 has been previously described[19]. For preparation of the PNA-specific antibody, PNA fractions were purified from the culture medium of Huh.1 (human HCC) and LN-71 (human glioblastoma) cells by ammonium sulfate precipitation followed by the same methods for mEH purification. Female BALB/c mice aged 6 wk, purchased from Tokyo Laboratory Animal Science Co., Ltd. (Tokyo, Japan), were injected s.c. with 2 μg of PNA four times, along with Freund's complete adjuvant for the first injection, with incomplete adjuvant for the second injection, and no adjuvant for the third and fourth injections. Their spleen cells were harvested and hybridized with NS-1 myeloma cells, as described previously[21]. Hybridoma clone 6G2 producing PNA-specific antibody was selected by screening each culture supernatant by ELISA against mEH and PNA. The mEH-specific 2G2 antibody (IgG1) was purified using an IgG Purification Kit-A (Chemical Dojin Co., Ltd., Tokyo, Japan), and PNA-specific antibody 6G2 (IgM) was purified using a HiTrap IgY Purification HP column (GE Healthcare Japan, Tokyo, Japan).
The ELISA for PNA was developed using a combination of anti-PNA antibody fixed on the plate and horseradish peroxidase-conjugated anti-mEH antibody for detection. Sera from control individuals were collected from healthy volunteers in Japan or purchased from BioreclamationIVT (Westbury, NY, United States).
The receiver operating characteristic (ROC) curve was obtained by calculating the sensitivity and specificity of the assay at every possible cut-off point and plotting sensitivity against [1-specificity] in SPSS for Windows (SPSS Japan, Tokyo, Japan). The area under the ROC curve (AUROC) was calculated to determine the diagnostic accuracy of the assay. Appropriate cut-off points were examined for balancing the sensitivity and specificity of the ROC curve, and the optimal cut-off point was identified as that yielding the minimal value for [(1 - sensitivity)2 + (1 - specificity)2] or the maximal value for [sensitivity + specificity - 1][22].
Statistical analysis was performed using SPSS for Windows (SPSS Japan, Tokyo, Japan). Differences in mean values between groups were assessed by the Mann-Whitney U test. χ2 tests were used to compare univariate associations of categorical variables. Correlations between two parameters were analyzed using Spearman’s rank correlation coefficient. A statistical review of this study was performed by a biomedical statistician.
Of the 141 patients diagnosed as HCV-positive, 50 had hepatitis, 44 had cirrhosis, and 47 had HCC (Table 1). Compared with patients with hepatitis and cirrhosis, HCC patients had higher percentages of male and older patients. The percentage of male patients was 40% (20/50) in the hepatitis group, 41% (18/44) in the cirrhosis group, and 53% (25/47) in the HCC group. The median age was 70.2 years, 71.7 years, and 75.3 years in the three groups, respectively. The results of biochemical markers differed among the groups (Table 1). With the progression of hepatic disorders from hepatitis to cirrhosis, serum albumin decreased, whereas ALP, total bilirubin, and type IV collagen increased significantly. With the progression to HCC, AST and GTP increased significantly. It is important to note that no biological markers differed between cirrhosis and HCC, suggesting no significant deterioration of liver functions in HCV-related HCC.
| 1: Hepatitis (n = 50) | 2: Cirrhosis (n = 44) | 3: HCC (n = 47) | 1 vs 2 | 1 vs 3 | 2 vs 3 | ||||
| Mean | SD | Mean | SD | Mean | SD | P value | P value | P value | |
| Sex (male/female) | 20/30 | 18/26 | 25/22 | 0.360 | 0.048 | 0.101 | |||
| Age (yr) | 70.2 | (11.7) | 71.8 | (10.4) | 75.3 | (9.0) | 0.558 | 0.018 | 0.102 |
| Albumin (g/L) | 4.2 | (0.3) | 3.7 | (0.6) | 3.7 | (0.9) | < 0.001 | < 0.001 | 0.962 |
| AST (U/L) | 44 | (35) | 46 | (20) | 60 | (51) | 0.056 | 0.033 | 0.861 |
| ALT (U/L) | 41 | (42) | 33 | (20) | 45 | (48) | 0.967 | 0.667 | 0.674 |
| ALP (U/L) | 259 | (60) | 345 | (141) | 362 | (312) | 0.001 | 0.084 | 0.212 |
| Bilirubin total (mg/dL) | 0.8 | (0.4) | 1.2 | (0.7) | 1.2 | (1.1) | 0.001 | 0.003 | 0.477 |
| GTP (U/L) | 46 | (61) | 47 | (44) | 105 | (212) | 0.186 | 0.001 | 0.078 |
| Type IV collagen (ng/mL) | 159 | (69) | 237 | (88) | 207 | (94) | < 0.001 | 0.003 | 0.075 |
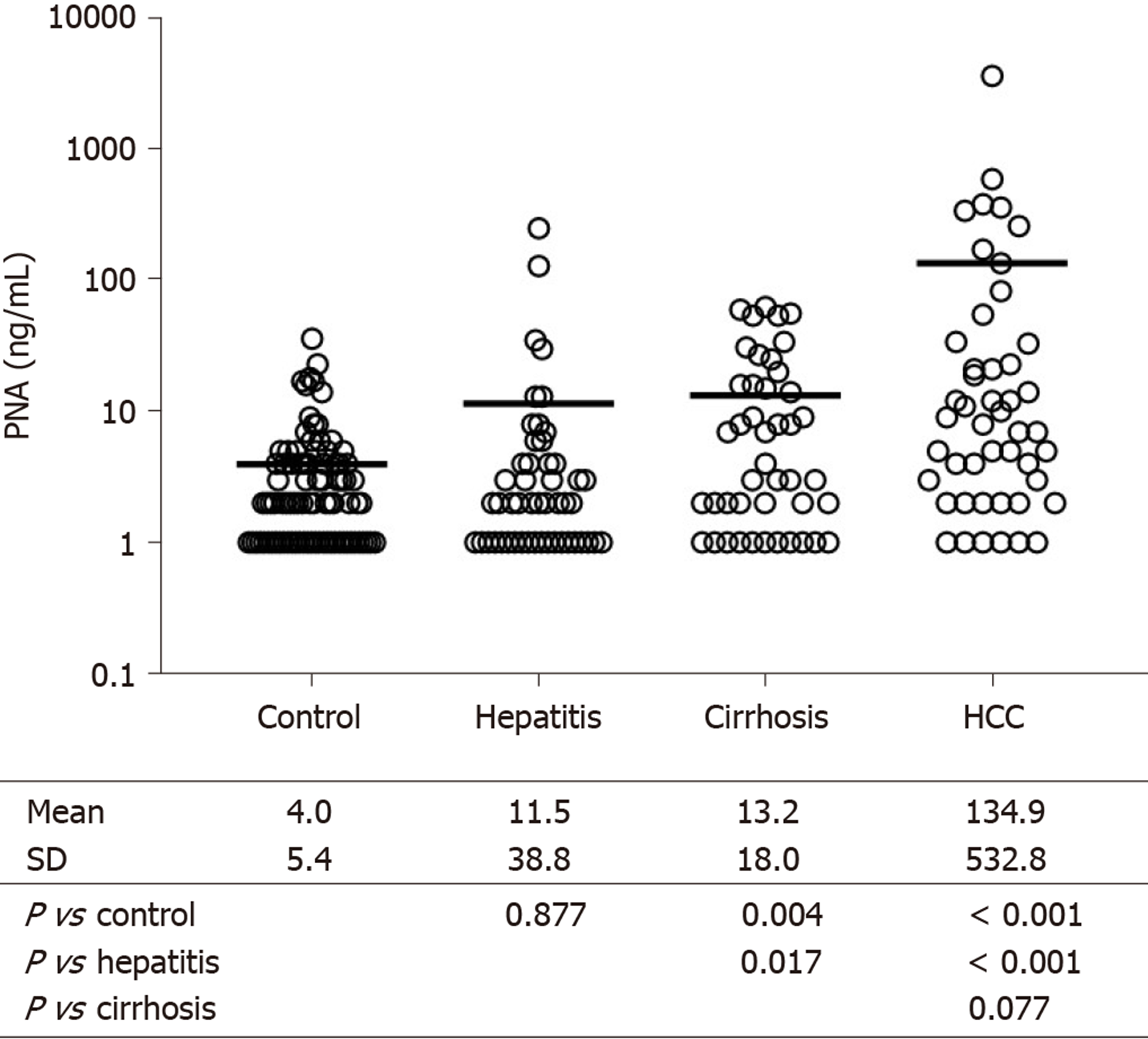
Next, serum PNA was measured in patients with hepatitis, cirrhosis, and HCC, and the results were compared with those of 89 control subjects (Figure 1). Serum PNA did not differ between control and hepatitis patients, but it was slightly higher in cirrhosis than in control (P = 0.004) and hepatitis patients (P = 0.017). In contrast, PNA increased significantly in HCC compared with control (P < 0.001) and hepatitis patients (P < 0.001). PNA concentrations were over 10 times higher in HCC patients than in cirrhosis patients, although the difference between the two groups was not significant (P = 0.077) due to the wide variations in data, especially in HCC patients.
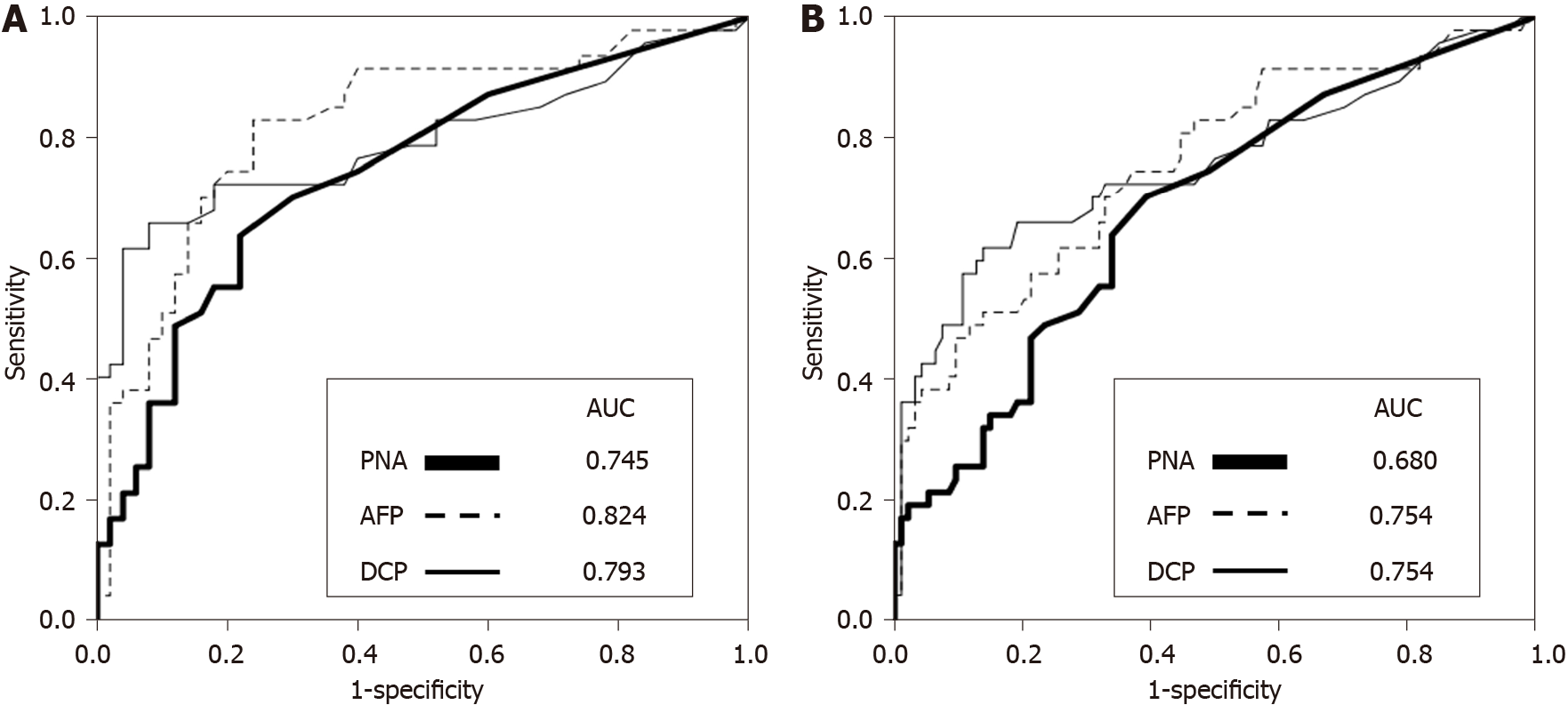
The diagnostic value of serum PNA in HCC was determined and compared with the two HCC markers AFP and DCP (Figure 2). The ROC curves were obtained by calculating the sensitivity and specificity of these markers to differentiate HCC from hepatitis. The AUROC was 0.745 for PNA, 0.824 for AFP, and 0.793 for DCP. The ROC curve was also obtained to differentiate HCC from hepatitis and cirrhosis, and the AUROC was 0.680 for PNA and 0.754 for AFP and DCP. These data indicate that the diagnostic value of PNA was comparable to AFP and DCP to differentiate HCC from hepatitis or from hepatitis and cirrhosis.
Balancing sensitivity and specificity of the ROC curve indicated that the optimal cut-off point of serum PNA for predicting HCC was 5 ng/mL. Using this cut-off point, the sensitivity and specificity of PNA were determined and compared with those of AFP and DCP (Table 2). In differentiating HCC from hepatitis, the sensitivity and specificity were 63.8% and 78.0% for PNA, 61.7% and 88.0% for AFP, and 61.7% and 94.0% for DCP, respectively. In differentiating HCC from hepatitis and cirrhosis, the sensitivity and specificity were 63.8% and 66.0% for PNA, 61.7% and 75.5% for AFP, and 61.7% and 83.0% for DCP, respectively. These data suggest that the sensitivity of PNA was similar to AFP and DCP, but the specificity of PNA was lower than of AFP and DCP.
| (A) HCC vs hepatitis | (B) HCC vs hepatitis/cirrhosis | |||
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| PNA | 63.8 | 78.0 | 63.8 | 66.0 |
| AFP | 61.7 | 88.0 | 61.7 | 75.5 |
| DCP | 61.7 | 94.0 | 61.7 | 83.0 |
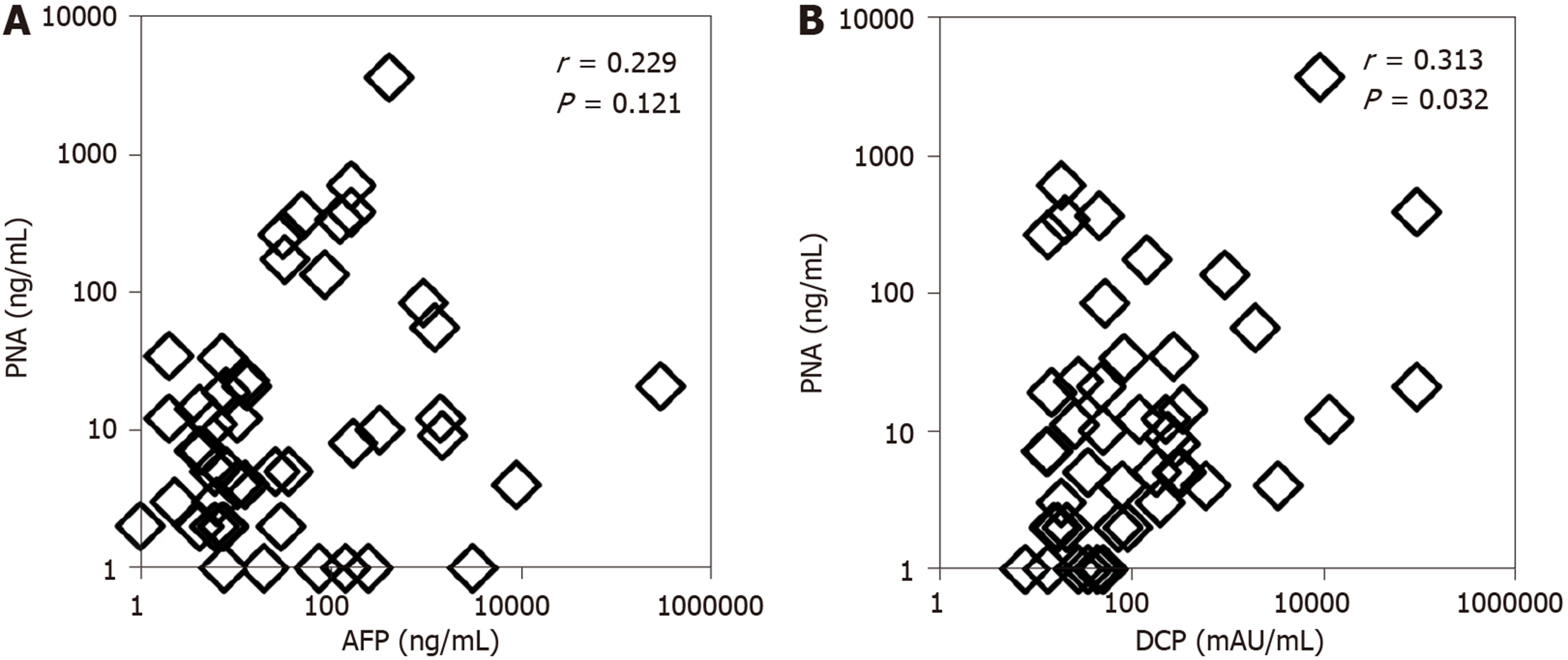
The correlations of PNA with AFP and DCP in the serum of HCC patients were evaluated (Figure 3). Spearman’s correlation analysis clearly indicated no correlation between PNA and AFP, with a correlation index of 0.229 (P = 0.121). A significant but weak correlation was seen between PNA and DCP, with a correlation index of 0.313 (P = 0.032). These data indicated that PNA was positive in many patients who were negative for AFP or DCP.
To determine the differences in disease characteristics, the correlations of PNA, AFP, and DCP with biochemical markers in the sera of HCC patients were compared (Table 3). Spearman’s correlation analysis indicated significant correlations of PNA with AST, ALP, and GTP. The highest correlation was seen between PNA and GTP, with a correlation index of 0.666 (P < 0.001). In contrast, AFP was significantly correlated with all biochemical markers with the exception of GTP. DCP was significantly correlated only with albumin and ALP. These data indicate that the correlations with biochemical markers were completely different for PNA, AFP, and DCP in HCC patients, and that GTP was highly correlated with PNA, but not with AFP or DCP. HCC patients were then divided into PNA-high and PNA-low patients by the cut-off of the median PNA value (8 ng/mL), and biochemical markers were compared between PNA-high and PNA-low patients (Table 4). Statistical analyses indicated that AST, ALP, and GTP were significantly higher in PNA-high patients than in PNA-low patients with HCV-related HCC. The mean GTP value was 183.2 U/L in PNA-high patients, which was almost 6 times higher than the mean GTP value (31.0 U/L) in PNA-low patients (P < 0.0001).
| PNA | AFP | DCP | ||||
| r | P value | r | P value | r | P value | |
| Albumin | -0.173 | 0.245 | -0.416 | 0.004 | -0.339 | 0.020 |
| AST | 0.325 | 0.026 | 0.626 | < 0.001 | 0.232 | 0.117 |
| ALT | 0.227 | 0.125 | 0.438 | 0.002 | -0.038 | 0.799 |
| ALP | 0.582 | < 0.001 | 0.459 | 0.001 | 0.302 | 0.039 |
| Bilirubin | 0.226 | 0.130 | 0.366 | 0.012 | 0.030 | 0.841 |
| GTP | 0.666 | < 0.001 | 0.274 | 0.062 | 0.082 | 0.585 |
| Type IV collagen | 0.107 | 0.476 | 0.498 | < 0.001 | 0.246 | 0.095 |
| PNA-high patients (n = 23) | PNA-low patients (n = 24) | ||||
| Mean | SD | Mean | SD | P value | |
| Albumin (g/L) | 3.5 | 0.7 | 3.9 | 1.1 | 0.1348 |
| AST (U/L) | 73.2 | 58.6 | 47.0 | 36.1 | 0.0247 |
| ALT (U/L) | 58.3 | 61.8 | 31.7 | 18.9 | 0.3025 |
| ALP (U/L) | 463.4 | 393.2 | 264.2 | 135.9 | 0.0004 |
| Bilirubin total (mg/dL) | 1.4 | 1.4 | 1.0 | 0.7 | 0.6345 |
| GTP (U/L) | 183.2 | 278.7 | 31.0 | 12.5 | < 0.0001 |
| Type IV collagen (ng/mL) | 225.2 | 104.3 | 189.4 | 76.3 | 0.2133 |
HCC patients consist of heterogeneous populations, and the subtypes of HCC are characterized by clinical phenotypes such as cell differentiation and tumor size, and molecular phenotypes associated with gene mutations and transcriptional modification[23,24]. In addition, the immune microenvironment, which is affected by both clinical and molecular phenotypes, may have a large impact on patient prognosis[25] and be activated in a certain population of HCC patients[26]. Therefore, the development of a novel biomarker that can diagnose HCC and further characterize its phenotype in the clinical setting is warranted.
In this study, PNA was aberrantly increased in the serum of HCV-related HCC patients. The mechanism and function of the PNA increase in the serum of HCC patients are unclear. A component of PNA, mEH, is a drug metabolizing enzyme on the endoplasmic reticulum membrane that catalyzes the hydration of reactive epoxide. In addition, mEH plays a role in bile acid transport on the plasma membrane of hepatocytes[27], and bile acid regulates hepatic tumor development[28]. Studies with a number of monoclonal antibodies recognizing different portions of mEH showed that mEH was located predominantly inside hepatocytes, while located abundantly on the surface of HCC cells[19,29]. Recent studies have shown that mEH plays a key role in the hydrolysis of fatty acids such as epoxyeicosatrienoic acid (EET)[30,31]. As EET regulates tumor cell growth, metastasis, angiogenesis, and inflammation in cancer[30,32,33], the induction of mEH in HCC may reflect the dynamic change of localization and functions of mEH during carcinogenesis. The present data showing that serum PNA was increased in a limited number of non-HCC cirrhotic patients may suggest the change of mEH in preneoplastic stages.
The early detection of HCC allows patients to receive curative treatment and achieve long-term survival. The routine practice of screening high-risk patients for HCC contributes to the detection of HCC nodules in the early stages in more than 60% of patients in Japan[34]. The HCC markers AFP and DCP have been used to screen and diagnose HCC at an earlier stage. An immunohistochemical study of small HCC tissues showed that AFP-positive HCCs were more malignant than AFP-negative and DCP-positive HCCs[35]. DCP was more efficient than AFP for the diagnosis of early HCC and for the prediction of microvascular invasion[36]. The combined use of AFP and DCP was useful for predicting the aggressiveness of early-stage HCC in patients given local treatment[37,38]. However, their sensitivity and specificity are lower for early-stage HCC than for advanced stage HCC[6]. It appears that PNA has the potential to diagnose a novel population of HCC patients in which AFP or DCP is weakly or negatively expressed. Further analysis of PNA may clarify its potential for the detection of HCC nodules in the early stage.
In the present study, serum GTP was highly correlated with PNA, but not with AFP or DCP, and it was significantly higher in PNA-high patients than in PNA-low patients with HCV-related HCC. Therefore, PNA could potentially be used to diagnose a novel type of HCC in which GTP is positively expressed, but AFP or DCP is weakly or negatively expressed.
HCC patients with elevated serum GTP had a lower survival rate after resection[39] and transarterial chemoembolization[40]. The increase of GTP in early-stage HCC and the aggressive phenotype of HCC highly expressing GTP have been examined. Immunohistochemical and enzyme histochemical analysis showed the localization of GTP in preneoplastic foci at the early stage in a rat carcinogenesis model[41] and in hepatocellular foci of HCC patients with HCV infection[42]. The meta-analysis indicated that pretreatment serum GTP was a predictor of poor overall survival, recurrence-free survival, and disease-free survival in HCC patients[43,44]. The ectopic expression of GTP accelerated tumor cell growth, metastasis, and resistance to chemotherapy[45,46]. Therefore, PNA-positive HCC may have an aggressive phenotype with a higher risk for disease progression and a poor prognosis. The prognostic value of PNA for survival will be determined in the follow-up evaluation of the patients enrolled in this study.
A limitation of this study is the etiologies of the hepatic disorders. The risk factors for hepatitis, cirrhosis, and HCC include chronic infection by HBV or HCV and hepatic steatosis and inflammation in nonalcoholic fatty liver disease. In the present study, however, only patients with HCV-related hepatic disorders were enrolled. Further studies are warranted to identify the changes of PNA in hepatitis, cirrhosis, and HCC with other etiologies.
In conclusion, the diagnostic value of PNA to discriminate HCC was comparable to AFP and DCP. PNA may have the potential to diagnose a novel type of HCC in which GTP is positively expressed, but AFP or DCP is weakly or negatively expressed. Further studies are needed to determine the diagnostic value of PNA for HCC patients in comparison with other HCC markers.
The prognosis of hepatocellular carcinoma (HCC) patients remains poor despite advances in treatment modalities and diagnosis. The early detection of HCC allows patients to receive curative treatment and achieve long-term survival. It is important to identify useful markers for the early detection of HCC in patients.
Preneoplastic antigen (PNA), originally reported in a rat carcinogenesis model, is increased in the tissues and serum of HCC patients. However, the diagnostic value of PNA for discriminating HCC remains to be determined.
The objectives of this study are to determine the diagnostic value of PNA for discriminating HCC and to characterize PNA-positive HCC.
Patients with hepatitis C virus-related hepatic disorders were prospectively enrolled in this study, which included patients with hepatitis, with cirrhosis, and with HCC. A novel enzyme-linked immunosorbent assay was developed to measure serum PNA concentrations in patients.
Compared with control and non-HCC patients, PNA was increased in HCC. The sensitivity of PNA was similar to the HCC markers des-γ-carboxy-prothrombin (DCP) and αα-fetoprotein (AFP), but the specificity of PNA was lower. There was no correlation between PNA and AFP and a significant but weak correlation between PNA and DCP in HCC patients. Importantly, the correlations with biochemical markers indicated that GTP was highly correlated with PNA, but not with AFP or DCP, and that it was significantly higher in PNA-high patients than in PNA-low patients with hepatitis C virus-related HCC.
PNA may have the potential to diagnose a novel type of HCC in which GTP is positively expressed but AFP or DCP is weakly or negatively expressed.
Further studies are needed to determine the diagnostic value of PNA for HCC patients in comparison with other HCC markers and to determine its potential for the detection of HCC nodules in the early stage. PNA-positive HCC may have an aggressive phenotype with a higher risk for disease progression and a poor prognosis. The prognostic value of PNA for survival will be determined in a follow-up evaluation of the patients enrolled in this study.
The authors would like to thank all of the patients and their families who participated in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A S-Editor: Zhang L L-Editor: Webster JR E-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (2)] |
| 2. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (4)] |
| 3. | Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: An evidence-based approach. World J Gastroenterol. 2019;25:1550-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (4)] |
| 4. | Kudo M, Izumi N, Sakamoto M, Matsuyama Y, Ichida T, Nakashima O, Matsui O, Ku Y, Kokudo N, Makuuchi M; Liver Cancer Study Group of Japan. Survival Analysis over 28 Years of 173,378 Patients with Hepatocellular Carcinoma in Japan. Liver Cancer. 2016;5:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Johnson P, Berhane S, Kagebayashi C, Satomura S, Teng M, Fox R, Yeo W, Mo F, Lai P, Chan SL, Tada T, Toyoda H, Kumada T. Impact of disease stage and aetiology on survival in hepatocellular carcinoma: implications for surveillance. Br J Cancer. 2017;116:441-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573-10583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (7)] |
| 7. | Désert R, Nieto N, Musso O. Dimensions of hepatocellular carcinoma phenotypic diversity. World J Gastroenterol. 2018;24:4536-4547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 8. | Akada K, Koyama N, Taniguchi S, Miura Y, Aoshima K. Database analysis of patients with hepatocellular carcinoma and treatment flow in early and advanced stages. Pharmacol Res Perspect. 2019;7:e00486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Okita K, Kligman LH, Farber E. A new common marker for premalignant and malignant hepatocytes induced in the rat by chemical carcinogens. J Natl Cancer Inst. 1975;54:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Okita K, Esaki T, Kurokawa F, Takemoto T, Fujikura Y, Fukumoto T. An antigen specific to hyperplastic liver nodules defined with monoclonal antibody: a new marker for preneoplastic cells in rat chemical hepatocarcinogenesis. Tumour Biol. 1988;9:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Levin W, Lu AY, Thomas PE, Ryan D, Kizer DE, Griffin MJ. Identification of epoxide hydrase as the preneoplastic antigen in rat liver hyperplastic nodules. Proc Natl Acad Sci USA. 1978;75:3240-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Ogino M, Okita K, Tsubota W, Numa Y, Kodama T, Takemoto T. Some biochemical properties of the preneoplastic antigen in rat liver hyperplastic nodules. Gan. 1982;73:349-353. [PubMed] |
| 13. | Ding VD, Cameron R, Pickett CB. Regulation of microsomal, xenobiotic epoxide hydrolase messenger RNA in persistent hepatocyte nodules and hepatomas induced by chemical carcinogens. Cancer Res. 1990;50:256-260. [PubMed] |
| 14. | Fritz P, Behrle E, Zanger UM, Mürdter T, Schwarzmann P, Kroemer HK. Immunohistochemical assessment of human microsomal epoxide hydrolase in primary and secondary liver neoplasm: a quantitative approach. Xenobiotica. 1996;26:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Coller JK, Fritz P, Zanger UM, Siegle I, Eichelbaum M, Kroemer HK, Mürdter TE. Distribution of microsomal epoxide hydrolase in humans: an immunohistochemical study in normal tissues, and benign and malignant tumours. Histochem J. 2001;33:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gill SS, Ota K, Ruebner B, Hammock BD. Microsomal and cytosolic epoxide hydrolases in rhesus monkey liver, and in normal and neoplastic human liver. Life Sci. 1983;32:2693-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Akatsuka T, Tohmatsu J, Abe K, Shikata T, Ishikawa T, Nakajima K, Yoshihara N, Odaka T. Non-A, non-B hepatitis related AN6520 Ag is a normal cellular protein mainly expressed in liver. II. J Med Virol. 1986;20:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Akatsuka T, Kobayashi N, Ishikawa T, Saito T, Shindo M, Yamauchi M, Yoshihara N, Odaka T. Autoantibody response to microsomal epoxide hydrolase in hepatitis C and A. J Autoimmun. 2007;28:7-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Duan H, Yoshimura K, Kobayashi N, Sugiyama K, Sawada J, Saito Y, Morisseau C, Hammock BD, Akatsuka T. Development of monoclonal antibodies to human microsomal epoxide hydrolase and analysis of "preneoplastic antigen"-like molecules. Toxicol Appl Pharmacol. 2012;260:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Morisseau C, Newman JW, Dowdy DL, Goodrow MH, Hammock BD. Inhibition of microsomal epoxide hydrolases by ureas, amides, and amines. Chem Res Toxicol. 2001;14:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Akatsuka T, Tohmatsu J, Yoshihara N, Katsuhara N, Okamoto T, Shikata T, Odaka T. Detection of an antigen (AN6520), possibly related to non-A, non-B hepatitis, by monoclonal antibodies. I. J Med Virol. 1986;20:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1357] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 23. | Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385-7392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 945] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 24. | Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage P, Nault JC, Zucman-Rossi J. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 538] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 25. | Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 333] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 26. | Sadeghi M, Lahdou I, Oweira H, Daniel V, Terness P, Schmidt J, Weiss KH, Longerich T, Schemmer P, Opelz G, Mehrabi A. Serum levels of chemokines CCL4 and CCL5 in cirrhotic patients indicate the presence of hepatocellular carcinoma. Br J Cancer. 2015;113:756-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Peng H, Zhu QS, Zhong S, Levy D. Transcription of the Human Microsomal Epoxide Hydrolase Gene (EPHX1) Is Regulated by PARP-1 and Histone H1.2. Association with Sodium-Dependent Bile Acid Transport. PLoS One. 2015;10:e0125318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Takahashi S, Tanaka N, Fukami T, Xie C, Yagai T, Kim D, Velenosi TJ, Yan T, Krausz KW, Levi M, Gonzalez FJ. Role of Farnesoid X Receptor and Bile Acids in Hepatic Tumor Development. Hepatol Commun. 2018;2:1567-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Duan H, Takagi A, Kayano H, Koyama I, Morisseau C, Hammock BD, Akatsuka T. Monoclonal antibodies reveal multiple forms of expression of human microsomal epoxide hydrolase. Toxicol Appl Pharmacol. 2012;260:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Rand AA, Barnych B, Morisseau C, Cajka T, Lee KSS, Panigrahy D, Hammock BD. Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids. Proc Natl Acad Sci USA. 2017;114:4370-4375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Marowsky A, Meyer I, Erismann-Ebner K, Pellegrini G, Mule N, Arand M. Beyond detoxification: a role for mouse mEH in the hepatic metabolism of endogenous lipids. Arch Toxicol. 2017;91:3571-3585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30:525-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnés CM, Mammoto A, Mammoto T, Luria A, Benny O, Chaponis DM, Dudley AC, Greene ER, Vergilio JA, Pietramaggiori G, Scherer-Pietramaggiori SS, Short SM, Seth M, Lih FB, Tomer KB, Yang J, Schwendener RA, Hammock BD, Falck JR, Manthati VL, Ingber DE, Kaipainen A, D'Amore PA, Kieran MW, Zeldin DC. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122:178-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 34. | Song P, Gao J, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and china. Liver Cancer. 2013;2:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of α-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (4)] |
| 37. | Suh SW, Lee KW, Lee JM, You T, Choi Y, Kim H, Lee HW, Lee JM, Yi NJ, Suh KS. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J Hepatol. 2014;60:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Park H, Kim SU, Park JY, Kim DY, Ahn SH, Chon CY, Han KH, Seong J. Clinical usefulness of double biomarkers AFP and PIVKA-II for subdividing prognostic groups in locally advanced hepatocellular carcinoma. Liver Int. 2014;34:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Xu XS, Wan Y, Song SD, Chen W, Miao RC, Zhou YY, Zhang LQ, Qu K, Liu SN, Zhang YL, Dong YF, Liu C. Model based on γ-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol. 2014;20:10944-10952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Ventura Y, Carr BI, Kori I, Guerra V, Shibolet O. Analysis of aggressiveness factors in hepatocellular carcinoma patients undergoing transarterial chemoembolization. World J Gastroenterol. 2018;24:1641-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Satoh K, Takahashi G, Miura T, Hayakari M, Hatayama I. Enzymatic detection of precursor cell populations of preneoplastic foci positive for γ-glutamyltranspeptidase in rat liver. Int J Cancer. 2005;115:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Paolicchi A, Marchi S, Petruccelli S, Ciancia E, Malvaldi G, Pompella A. Gamma-glutamyltransferase in fine-needle liver biopsies of subjects with chronic hepatitis C. J Viral Hepat. 2005;12:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Ou Y, Huang J, Yang L. The prognostic significance of pretreatment serum γ-glutamyltranspeptidase in primary liver cancer: a meta-analysis and systematic review. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Sun P, Li Y, Chang L, Tian X. Prognostic and clinicopathological significance of Gamma-Glutamyltransferase in patients with hepatocellular carcinoma: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2019;98:e15603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Hanigan MH, Gallagher BC, Townsend DM, Gabarra V. γ-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis. 1999;20:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Obrador E, Carretero J, Ortega A, Medina I, Rodilla V, Pellicer JA, Estrela JM. γ-Glutamyl transpeptidase overexpression increases metastatic growth of B16 melanoma cells in the mouse liver. Hepatology. 2002;35:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |