Published online Jan 7, 2020. doi: 10.3748/wjg.v26.i1.55
Peer-review started: November 11, 2019
First decision: November 22, 2019
Revised: November 29, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: January 7, 2020
Processing time: 56 Days and 22 Hours
Ulcerative colitis (UC) is a main form of inflammatory bowel disease. Due to complicated etiology and a high rate of recurrence, it is quite essential to elucidate the underlying mechanism of and search for effective therapeutic methods for UC.
To investigate the effects of astragalus polysaccharides (APS) combined with matrine on UC and associated lung injury.
UC was induced in rats by colon mucosal tissue sensitization combined with trinitro-benzene-sulfonic acid-ethanol. Then, the effects of the treatments of salazopyrine, APS, matrine, and APS combined with matrine on histopathological changes of lung and colon tissues, disease activity index (DAI), colon mucosal damage index (CMDI), serum endotoxin (ET) level, serum diamine oxidase (DAO) activity, the contents of tumor necrosis factor-α and interleukin-1β, and the activities of myeloperoxidase, superoxide dismutase, and malondialdehyde in lung tissues, as well as the protein expression of zonula occludens (ZO)-1, Occludin, and trefoil factor 3 (TFF3) were detected in UC rats.
The treatments of salazopyrine, APS, matrine, and APS combined with matrine reduced DAI scores and improved histopathological changes of colon and lung tissues, as well as decreased CMDI scores, ET levels, and DAO activities in UC rats. Moreover, in lung tissues, inflammatory response and oxidative stress injury were relieved after the treatments of salazopyrine, APS, matrine, and APS combined with matrine in UC rats. Furthermore, the expression of ZO-1, Occludin, and TFF3 in lung and colon tissues was increased after different treatments in UC rats. Notably, APS combined with matrine exerted a better protective effect against UC and lung injury compared with other treatments.
APS combined with matrine exert a synergistic protective effect against UC and lung injury, which might be associated with regulating TFF3 expression.
Core tip: The results of the present study show that astragalus polysaccharides combined with matrine exert a synergistic protective effect against ulcerative colitis and lung injury, which might be associated with regulating trefoil factor 3 expression.
- Citation: Yan X, Lu QG, Zeng L, Li XH, Liu Y, Du XF, Bai GM. Synergistic protection of astragalus polysaccharides and matrine against ulcerative colitis and associated lung injury in rats. World J Gastroenterol 2020; 26(1): 55-69
- URL: https://www.wjgnet.com/1007-9327/full/v26/i1/55.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i1.55
As a main form of inflammatory bowel disease (IBD), ulcerative colitis (UC) is characterized by chronic inflammation and ulcerative lesions in the intestinal mucosa[1,2]. Clinical manifestations of UC mainly include persistent or recurrent diarrhea, stools with mucus, blood, and pus, abdominal pain, and various systemic symptoms[1,2]. Recently, due to the changes in diet structure, living habits, and environments, the incidence and diagnosis rates of UC in China have increased year by year[3]. Despite the substantial progress in the diagnosis and treatment of UC, unsatisfactory therapeutic effects remain an issue due to complicated etiology and a high rate of recurrence[4]. Therefore, elucidating the underlying mechanism of and searching for effective therapeutic methods for UC are quite essential.
Previous studies have demonstrated that lung injury is closely involved in IBD, including UC, thus, the treatment for UC should be focused on large intestine and lung injury simultaneously[5-7]. In recent years, several traditional Chinese medicines (TCMs) have been evidenced to exert protective effects against UC[8] and lung diseases[9]. Astragalus membranaceus is a popular TCM that has been widely used for its anti-fatigue, anti-sepsis, anti-inflammation, anti-hypertension, and anti-tumor properties[10]. Astragalus polysaccharides (APS) are one of the primary bioactive ingredients extracted from Astragalus membranaceus, and play important roles in treating oxidative stress, immunological diseases, and cancers based on their pharmacological and biological effects[11,12]. It has been shown that APS can attenuate experimental colitis by regulating the immune response[13,14]. Also, APS are reported to protect against several lung diseases, such as pulmonary arterial hypertension[15] and chronic obstructive pulmonary disease[16]. Matrine, the extracts from another common TCM Sophora flavescens Ait., is also proved to be effective in treating colitis[17] and lung injury[18] due to its diverse pharmacological properties, including anti-virus, anti-inflammation, antioxidant, and anti-tumor activities[19]. However, few studies have investigated the effect of APS combined with matrine on UC and associated lung injury.
In the current study, we established a UC rat model by colon mucosal tissue sensitization combined with trinitro-benzene-sulfonic acid (TNBS)-ethanol, and then explored the effects and mechanisms of the treatments of salazopyrine, APS, matrine, and APS combined with matrine on histopathological changes of colon and lung tissues, intestinal mucosa injury, and lung injury in UC rats.
Approval from the Animal Ethics Committee of North China University of Science and Technology was obtained prior to experiments.
Totally, ten healthy male New Zealand white rabbits weighing 2.5 kg were obtained from Jinmuyang Laboratory Animal Breeding Co., Ltd (Beijing, China). The rabbits were sacrificed by air embolization, and then colon tissues were dissected and collected. The colonic mucosa tissue was scraped using a disinfecting blade, and added into the same amount of pre-cooled physiological saline to make the tissue homogenate. After centrifugation at 3000 rpm for 30 min at 4 °C, the supernatant was obtained and mixed with the same volume of complete Freund's adjuvant to prepare an antigen emulsion.
UC was induced in rats by colon mucosal tissue sensitization combined with TNBS-ethanol. Briefly, the antigen emulsion (8 mg/each rat) was injected into the toes and groin of the rats on the 1st, 15th, and 22th days, respectively. On the 29th day, the rats were fasted with free access to water for 24 h, and then anesthetized with 10% chloral hydrate (0.35 mL/100g) by intraperitoneal injection. TNBS-50% ethanol was prepared by mixing TNBS solution (120 mg/mL) with 50% ethanol at a ratio of 1:1 (v/v). The rats were inverted, and then TNBS-50% ethanol was slowly injected into the intestine at 100 mg/kg body weight using a silicone tube inserted 8 cm proximal to the anus. After pinching the anus and keeping the anus elevated for 1 min, the rats were placed in the cage, with the buttock raised to prevent the drug outflow. The rats naturally awakened after the anesthetic failed.
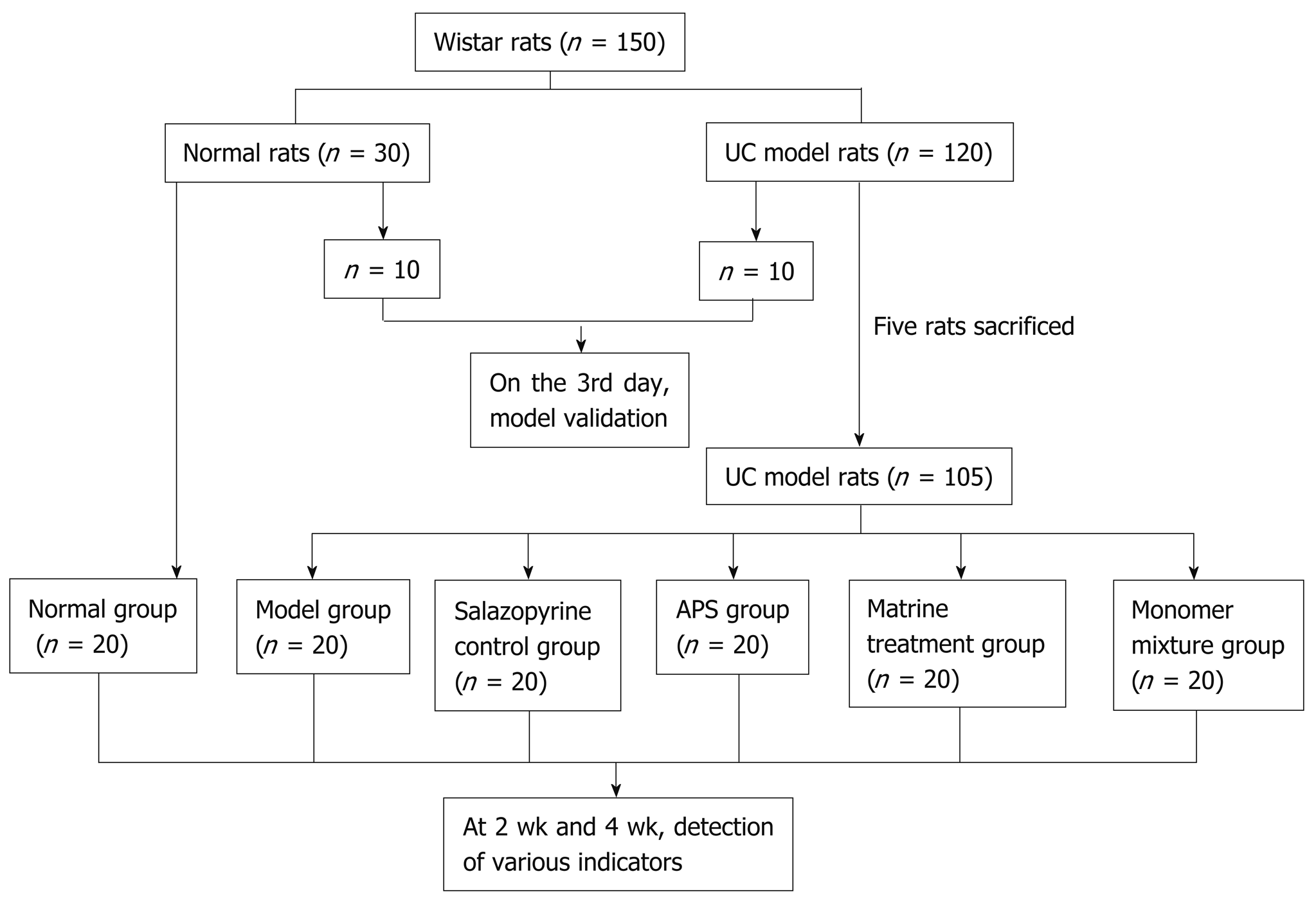
A total of 150 healthy male Wistar rats (weighing 200 ± 10 g, purchased from Charles River, Beijing, China) were used for the following experiments after one week of acclimation. The grouping is shown as Figure 1. Briefly, 30 Wistar rats were randomly selected as a normal group, and the remaining 120 Wistar rats were induced as UC models. On the 3rd day after modeling, 10 rats in the normal group and 10 UC model rats were randomly selected and used for model validation by histopathological observation (0 wk). The remaining 105 UC model rats (five rats sacrificed after modeling) were randomly assigned to five groups: Model group (n = 25), salazopyrine control group (n = 25), APS treatment group (n = 15), matrine treatment group (n = 15), and monomer mixture group (n = 25). The rats in the salazopyrine group received 0.125 g/mL of salazopyrine (SASP, Sunve, Shanghai, China); rats in the APS treatment group were given 0.6 g/mL of APS (Fuzhou Rimian Technology Development Co. LTD, China); rats in the matrine treatment group were given 12 mg/mL of matrine (Xi'an Linhe Biotechnology Co. LTD, China); and rats in the monomer mixture group were administered with the mixture of APS and matrine at a ratio of 1:1. All drugs in these groups were intragastrically administered at eight times of the dose for an adult human (60 kg body weight); and rats in the normal group and model group were intragastrically administered with equal volume of drinking water once a day. Among all the experimental animals, dynamic observation at 2 wk and 4 wk after administration was performed in the normal group, model group, salazopyrine control group, and monomer mixture group. Various indicators at 4 wk before and after the treatment were observed in the APS treatment group and matrine treatment group. Rats were anesthetized with 10% chloral hydrate (3.5 μL/g) by intraperitoneal injection, and lung and colon tissues were removed aseptically and stored in liquid nitrogen for the following experiments. Meanwhile, blood samples were collected from the abdominal aorta, and then serum was separated by centrifugation at 4 °C for 15 min and stored at ﹣80 °C.
The general conditions, including coating gloss, mental state, activity, diet, respiration, and feces, of rats at 0 wk, 2 wk, and 4 wk after administration in each group were observed. Meanwhile, body weight was measured, and disease activity index (DAI)[20] and colon mucosal damage index (CMDI)[21] were evaluated.
Lung and colon tissues were fixed at 4 °C for 24 h with 4% paraformaldehyde. Following paraffin embedding, the tissues were sliced into sections. After dehydration with gradient ethanol, the sections underwent hematoxylin-eosin (HE) staining and mounting with neutral resin. Lastly, the sections were observed by light microscopy (Olympus, Japan).
Serum samples were thawed on ice, and then determined with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Boster, Wuhan, China) for the content of endotoxin (ET). In addition, lung tissues in each group were cut into pieces, and then lung tissue homogenate was obtained with an ultrasonic cell disruptor (VCX130PB, Sonics, United States). After centrifugation at 4 °C for 15 min, the supernatant was determined with commercial ELISA kits (Boster) for the contents of tumor necrosis factor (TNF)-α, and interleukins (IL)-1β according to the manufacturer’s instructions.
Diamine oxidase (DAO) activity in serum samples was detected by spec-trophotometry. Briefly, the standard curve of DAO was made. Then, 100 μL of serum samples were incubated with 1 mL of phosphate buffer, 50 μL of HPRO (200 μg), 50 μL of o-dianisidine (50 μg), and 50 μL of pentanediamine (1750 μg) for 1.5 h in a water bath at 37 °C. Lastly, 200 μL of mixture was added into 96-well plates, and the absorbance at 436 nm was read with a microplate reader (Molecular Devices, United States).
Lung tissues were cut into pieces, and then lung tissue homogenate was obtained with an ultrasonic cell disruptor (VCX130PB, Sonics, United States). The activities of myeloperoxidase (MPO), superoxide dismutase (SOD), and malondialdehyde (MDA) were determined with MPO, SOD, and MDA detection kits (Nanjing Jiancheng Bioengineering Institute, China), respectively, according to the manufacturer’s instructions.
Lung and colon tissues were lysed with RIPA lysis buffer (Applygen, Beijing, United States), and protein was extracted by centrifugation and detected with the BCA kit (CW Biotech Co., Beijing, China). Then, protein samples were separated on an SDS-PAGE gel, and transferred to polyvinylidene fluoride membranes, followed by blockage with 5% nonfat milk for 1 h. Next, the membrane was incubated with anti-rat zonula occludens (ZO)-1 antibody (1:200, Santa Cruz, Santa Cruz, United States) or GAPDH antibody (Zhongshan Biotech, Beijing, China) overnight at 4 °C, and then washed with phosphate buffer saline, followed by the incubation with a secondary antibody (1:1000, Applygen, Beijing, United States) for 2 h at room temperature, respectively. Lastly, enhanced chemiluminescence (ECL, Millipore, United States) was used to detect the protein levels.
Lung and colon tissues were fixed at 4 °C for 24 h with 4% paraformaldehyde. Following paraffin embedding, the tissues were sliced into sections. After dehydration with gradient ethanol, the sections were immunostained with Occludin (Abcam, Cambridge, MA, United States) or trefoil factor 3 (TFF3, ProSci, Poway, CA, United States) antibody, followed by staining with a secondary antibody (Zhongshan Biotech, Beijing, China), and incubation with diaminobenzidine (Zhongshan Biotech, Beijing, China). Ultimately, the sections were observed by light microscopy (Olympus, Japan).
SPSS Statistics 20.0 software (IBM, Armonk, NY, United States) was used for data statistical analyses. Data are expressed as the mean ± SD. The differences between groups were analyzed by one-way ANOVA followed by multiple comparisons by the LSD test. P values < 0.05 were considered statistically significant.
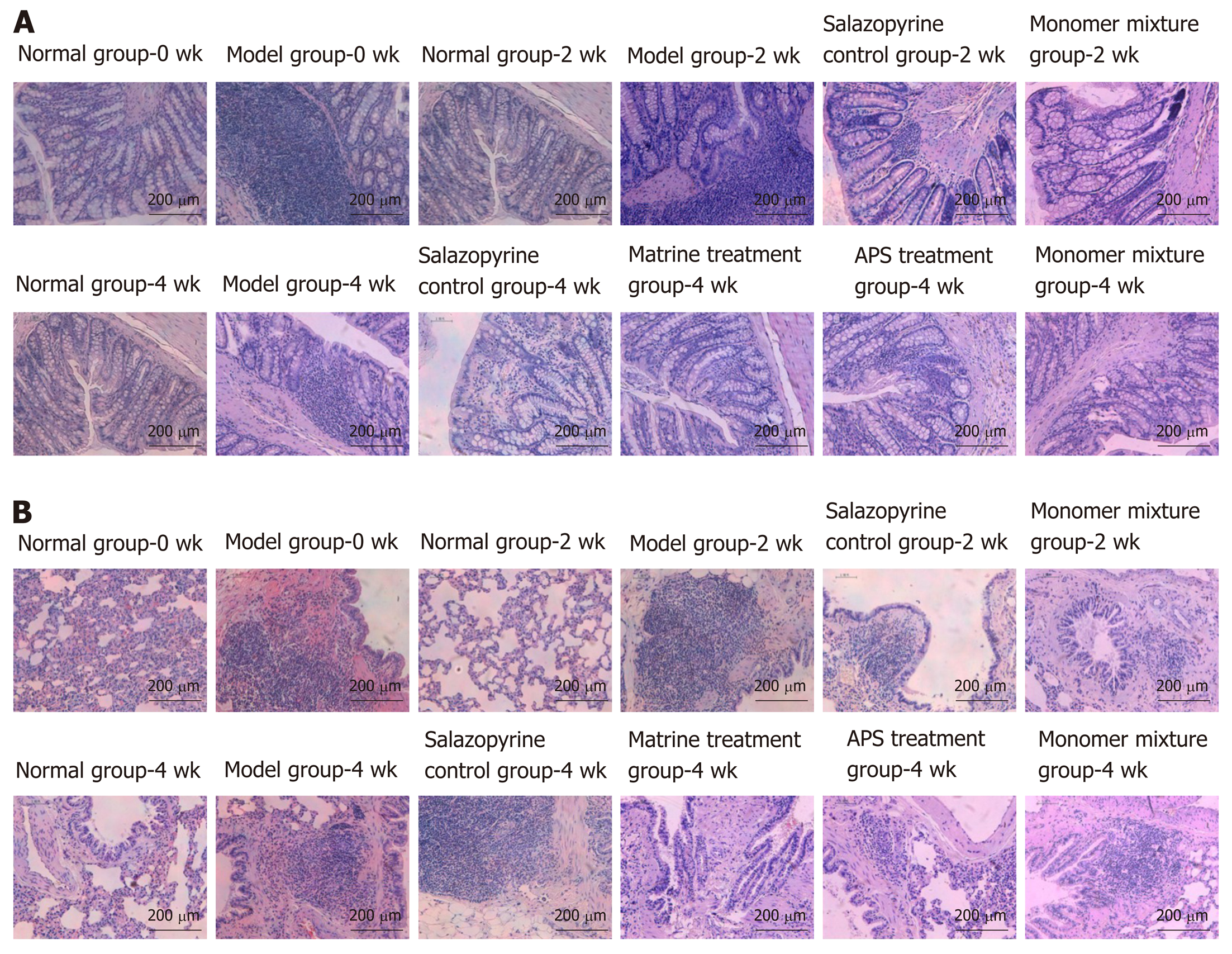
The rats in the normal group showed smooth hair, sensitive response, high activity, normal diet, normal feces, and stable breathing, while the rats in the model group presented pale yellow color hair, lack of energy, sleepiness, decreased appetite, softened feces, and dyspnea. At 2 wk after administration, compared with the model group, rats in the salazopyrine control group had increased coating glossiness, less autonomic activity, and improved feces. Meanwhile, the mental state, activity, food intake, defecation, and respiratory symptoms of rats in the matrine treatment group, APS treatment group, and the monomer mixture group were also improved compared with the model group. In addition, these general conditions of rats were obviously improved at 4 wk than those at 2 wk. Moreover, the weight of rats in the model group was significantly lower than that in the normal group (P < 0.01, Table 1) at 0 wk. At 2 wk and 4 wk, compared with the control group, the weight of rats was also lower in the other groups, and the weight of rats was increased in comparison with rats at 0 wk, especially in the APS treatment group and the monomer mixture group (Table 1). Compared with the normal group, DAI score was significantly increased in the model group (P < 0.01, Table 2) at 0 wk. Similarly, at 2 wk and 4 wk, DAI scores were also increased in the other groups compared with the normal group (Table 2). However, rats in the other groups had lower DAI scores than the model group, especially in the APS treatment group and the monomer mixture group (Table 2). Furthermore, HE staining revealed that the colonic mucosa of rats in the model group showed congestion, edema, ulceration, and a large amount of lymphocyte and neutrophil infiltration, and lung tissue of rats in the model group showed obvious congestion, edema, and a large amount of inflammatory cell infiltration (Figure 2). Nonetheless, compared with the model group, histopathological observation was improved in the other treatment groups at 2 wk and 4 wk after administration (Figure 2).
| Group | n | 0 wk | n | 2 wk | n | 4 wk |
| Normal | 10 | 414.00 ± 29.28 | 10 | 467.50 ± 21.44 | 10 | 507.60 ± 49.21 |
| Model | 10 | 355.90 ± 25.60b | 10 | 413.10 ± 27.91b | 10 | 461.30 ± 37.54 |
| Salazopyrine control | 10 | 420.70 ± 35.29b | 11 | 451.91 ± 38.31b | ||
| Matrine treatment | 13 | 429.23 ± 38.04a | 12 | 458.83 ± 45.36a | ||
| APS treatment | 13 | 443.62 ± 45.06 | 13 | 486.92 ± 55.67 | ||
| Monomer mixture | 13 | 426.92 ± 30.74b | 12 | 489.75 ± 32.06 |
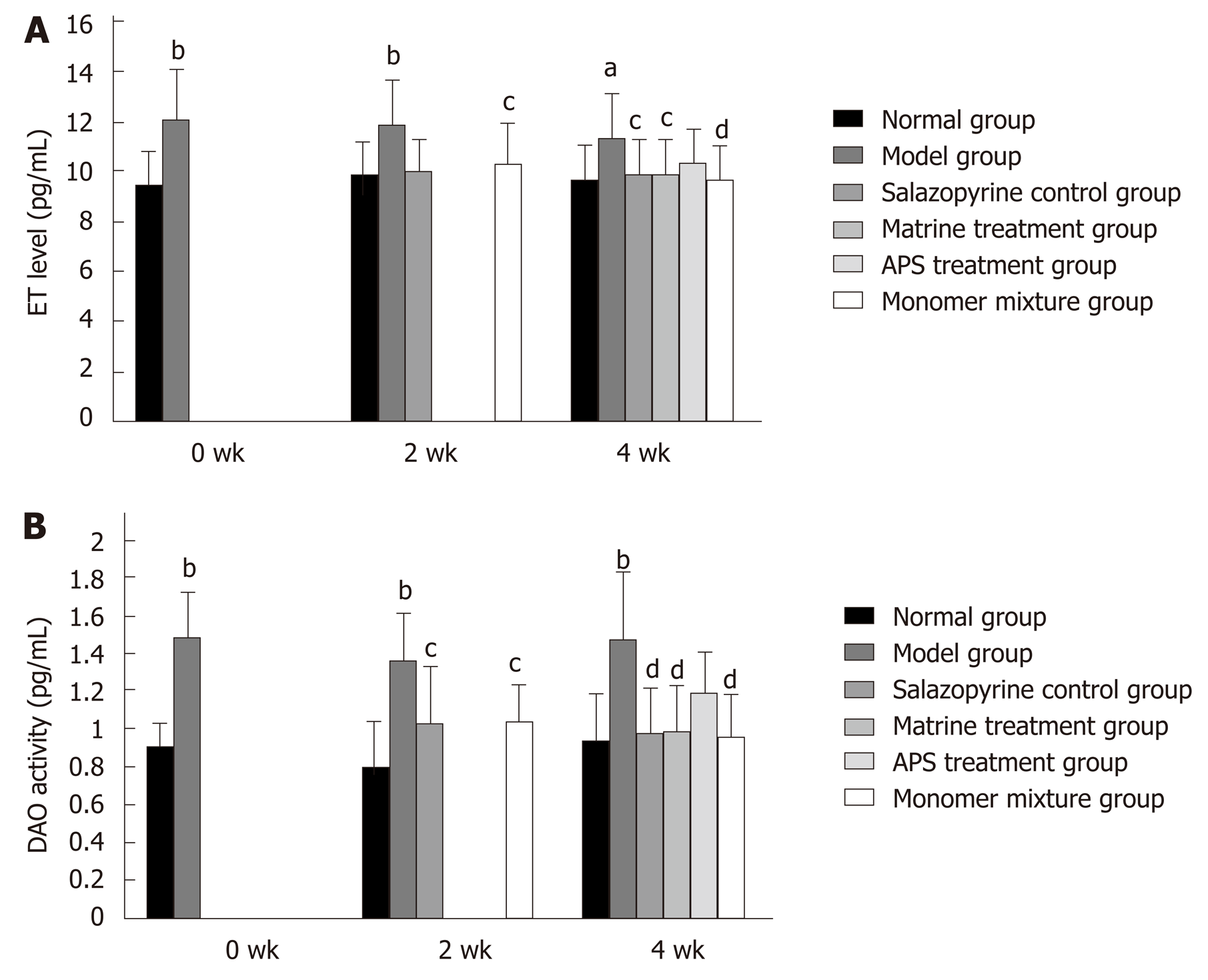
CMDI score was used to evaluate intestinal mucosa injury. Compared with the normal group, CMDI score was significantly elevated in the model group (P < 0.01, Table 3) at 0 wk. Consistently, CMDI scores were also increased in other groups compared with those in the normal group at 2 wk and 4 wk (Table 3). However, rats in other treatment groups had lower CMDI scores than that in the model group, especially in the matrine treatment group and the monomer mixture group (Table 3). In addition, compared with the normal group, serum ET levels were obviously increased in the model group at 0 wk, 2 wk, and 4 wk (P < 0.05, Figure 3A), while lower serum ET levels were found in the other treatment groups than in the model group (Figure 3A). Similarly, the trend of DAO activity was consistent with ET level (Figure 3B).
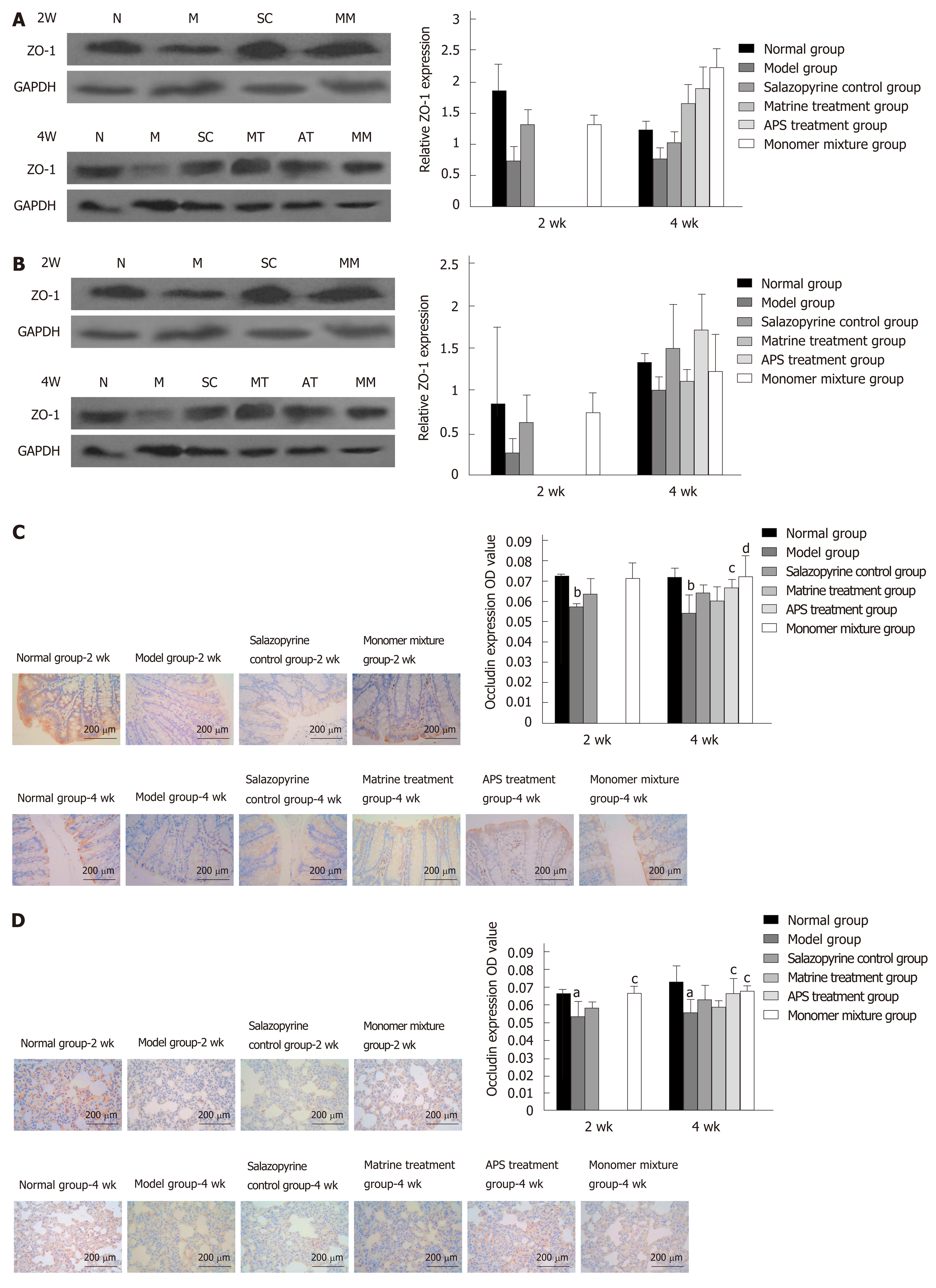
Western blot analysis showed that ZO-1 protein expression in colon tissues was significantly decreased in the model group at 2 wk and 4 wk compared with the normal group (P < 0.05, Figure 4A), while ZO-1 protein expression in colon tissues was increased in the other treatment groups than in the model group, especially in the monomer mixture group at 4 wk (P < 0.05, Figure 4A). Similarly, in lung tissues, ZO-1 protein expression was obviously decreased in the model group at 2 wk and 4 wk compared with the normal group (P < 0.01, Figure 4B); however, the other treatment groups showed increased protein expression of ZO-1 compared with the model group, especially in the monomer mixture group at 2 wk (P < 0.05, Figure 4B). Moreover, immunohistochemistry analysis revealed that Occludin was usually expressed in intestinal mucosal epithelial cells and glandular cells in colon tissues in normal rats. Occludin protein expression in colon tissues was remarkably decreased in the model group at 2 wk and 4 wk compared with the normal group (P < 0.05, Figure 4C), while Occludin protein expression in colon tissues was increased in the other treatment groups than in the model group, especially in the APS treatment group and monomer mixture group at 4 wk (P < 0.05, Figure 4C). In addition, Occludin was usually expressed in alveolar epithelial cell membrane in lung tissues of normal rats. Consistently, in lung tissues, Occludin protein expression at 2 wk and 4 wk was prominently decreased in the model group compared with the normal group (P < 0.05, Figure 4D); however, the other treatment groups showed increased protein expression of Occludin compared with the model group, especially in the APS treatment group and monomer mixture group at 4 wk (P < 0.05, Figure 4D).
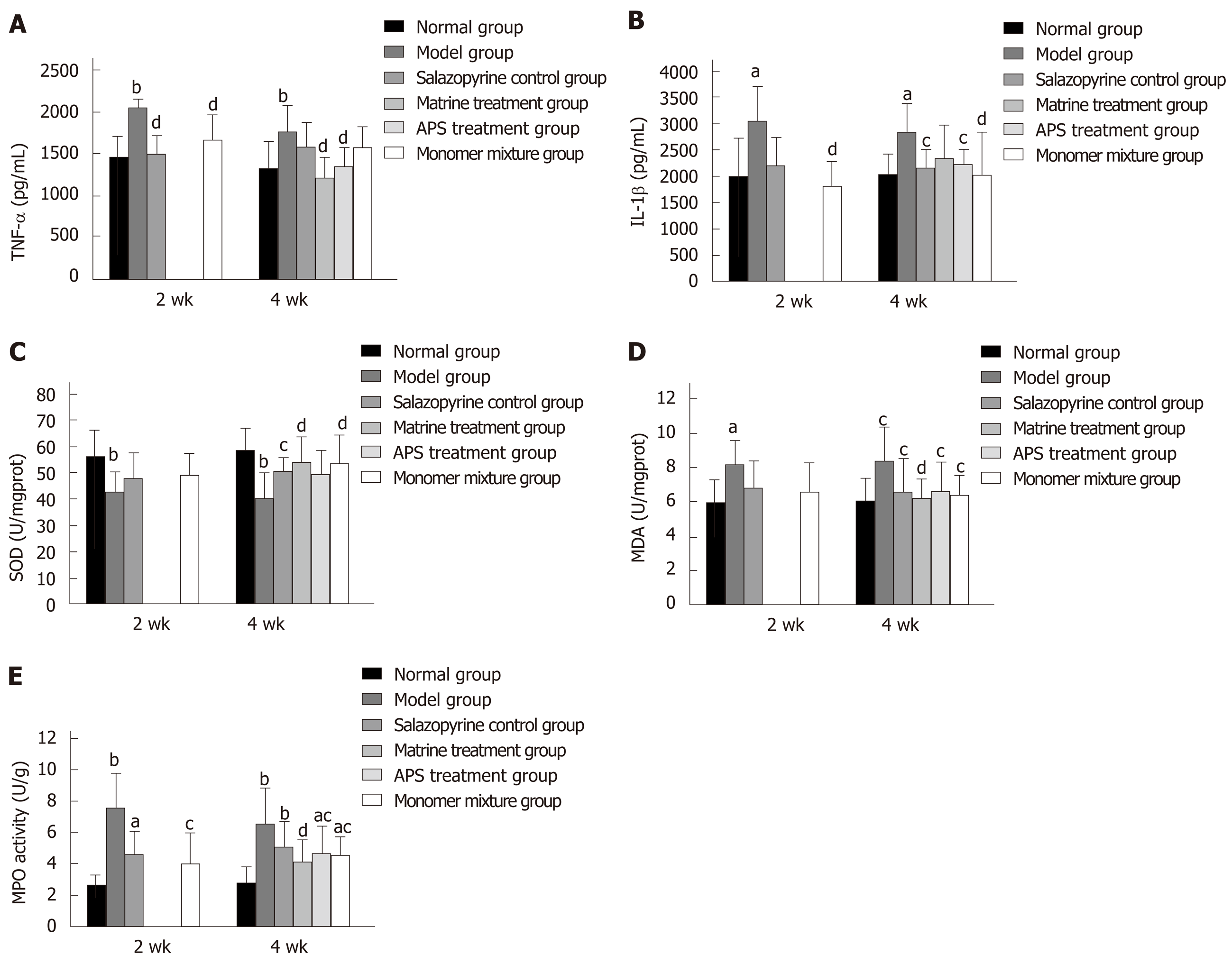
ELISA showed that the expression of inflammatory factors such as TNF-α and IL-1β in lung tissues was significantly increased in the model group at 2 wk and 4 wk compared with the normal group (P < 0.05, Figure 5A and B), while their expression was decreased in the other treatment groups compared with the model group (P < 0.05, Figure 4A and B). In addition, in lung tissues, SOD activity was obviously decreased in the model group at 2 wk and 4 wk compared with the normal group (P < 0.01, Figure 5C); however, the other treatment groups showed an increased activity of SOD compared with the model group, especially at 4 wk (P < 0.05, Figure 5C). Furthermore, the activities of MDA and MPO were conspicuously enhanced in the model group at 2 wk and 4 wk compared with the normal group (P < 0.05, Figure 5D and E); however, the activities of MDA and MPO were weakened in the other treatment groups compared with the model group (Figure 5D and E).
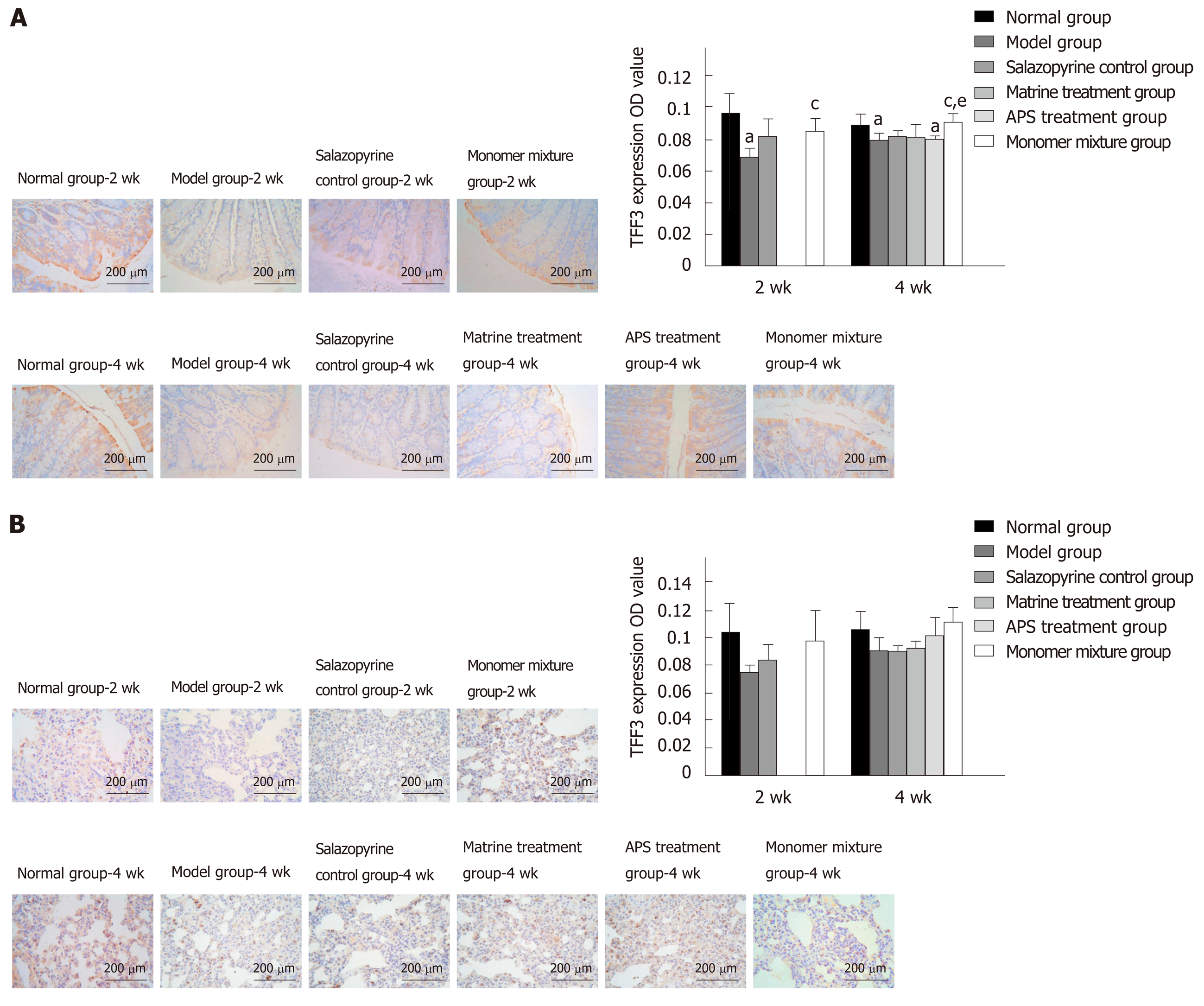
Immunohistochemistry analysis revealed that TFF3 was usually expressed in intestinal mucosa goblet cells and intestinal gland acini in colon tissues of normal rats. TFF3 protein expression in colon tissues was remarkably decreased in the model group at 2 wk and 4 wk compared with the normal group (P < 0.05, Figure 6A), while TFF3 protein expression in colon tissues was increased in the other treatment groups compared with the model group, especially in the monomer mixture group at 2 wk and 4 wk (P < 0.05, Figure 6A). In addition, compared with the salazopyrine control group, TFF3 protein expression in colon tissues was higher in the monomer mixture group at 4 wk (P < 0.05, Figure 6A). Similarly, in lung tissues, TFF3 protein expression was decreased in the model group at 2 wk and 4 wk compared with the normal group, but without a significant difference (Figure 6B). Meanwhile, increased protein expression of TFF3 was found in the APS treatment group and monomer mixture group at 4 wk compared with the model group without a significant difference (Figure 6B).
In the present study, a UC rat model was successfully established by colon mucosal tissue sensitization combined with TNBS-ethanol. The treatments of salazopyrine, APS, matrine, or APS combined with matrine inhibited DAI scores, increased body weight, and improved histopathological changes of colon and lung tissues in UC rats. In addition, the treatments of salazopyrine, APS, matrine, or APS combined with matrine alleviated intestinal mucosa injury and inhibited ET levels and DAO activity in UC rats. Moreover, in lung tissues, the inflammatory response and oxidative stress injury were relieved after the treatments of salazopyrine, APS, matrine, or APS combined with matrine in UC rats. Furthermore, the results revealed that the expression of ZO-1, Occludin, and TFF3 in lung and colon tissues was increased after different treatments in UC rats. Notably, APS combined with matrine exerted a better protective effect against UC and lung injury compared with other treatments.
The roles of APS and matrine have been widely investigated in various diseases. Previous studies have demonstrated that APS can significantly improve histological and DAI scores, and increase body weight by regulating the inflammatory response in dextran sulfate sodium-induced colitis[22,23]. It is also reported that matrine can ameliorate colitis by regulating the inflammatory response[17,24]. These results are consistent with our findings. Meanwhile, CMDI, ET levels, and DAO activity were detected in this study. CMDI is a common indicator to evaluate intestinal mucosa injury in colitis, and a high CMDI score represents a serious degree of intestinal mucosa injury[25,26]. In addition, ET is a lipopolysaccharide that is present in the cell wall of Gram-negative bacteria, and is particularly accumulated in the intestine[27]. The healthy human intestinal barrier can protect ET from entering the blood circulation, while the dysfunctional intestinal barrier causes ET to pass through the intestinal mucosa and enter the blood circulation, thereby resulting in endotoxemia[28,29]. DAO is present in the mucosa or villi of mammals, and is normally abundant in intestinal mucosa, kidney, and placental tissues, but rarely in serum[30]. After intestinal mucosal epithelial damage, the cytoplasm DAO can be released into the blood circulation[30,31]. Therefore, serum ET level and DAO activity are ideal indicators reflecting the structure and function of the intestinal mucosa. This study revealed that the treatments of APS or/and matrine inhibited CMDI scores, ET levels, and DAO activity in UC rats, indicating that they can alleviative intestinal mucosa injury. Moreover, the dysfunctional intestinal barrier is closely related to the tight junctions between cells[32]; thus, the expression of tight junction-associated proteins ZO-1 and Occludin in colon tissues was determined in this study. The tight junction mainly consists of the ZO protein family, Occludin protein, Claudin protein, and connective adhesion molecules, which is an important structure for maintaining mucosal permeability[33]. Occludin can interact with the intracellular protein ZO-1, and then bind to the backbone protein[33]; thus, ZO-1 and Occludin play important roles in performing tight junction barrier functions. Several studies have suggested that both ZO-1 and occludin were lowly expressed during colitis[34,35], which is consistent with our study. Taken together, these results suggest that APS combined with matrine might exert a synergistic protective effect against UC.
It is well known that the inflammatory response and oxidative stress are involved in the pathophysiology of lung injury[36]. This study detected the expression of inflammatory factors such as TNF-α and IL-1β in lung tissues. Accumulating evidence has indicated that TNF-α and IL-1β levels were significantly elevated during lung injury[37,38], which is consistent with our results. In addition, the activities of SOD, MDA, and MPO in lung tissues were measured in this study, which are most representative and important indicators during the oxidation-antioxidant balance systems[39]. A previous study has confirmed the widespread abnormality of oxygen free radical metabolism in UC[40]. UC contributes to increased permeability of the intestinal mucosa and elevated oxygen consumption, then a large number of O2-, OH, and lipid peroxides are produced, which damage the intestinal mucosa[40]. In addition, the oxygen free radical produced in UC can destroy lung tissue, thereby leading to inflammatory injury of the lungs[40]. This study revealed that SOD activity in lung tissues was obviously decreased, and the activities of MDA and MPO in lung tissues were conspicuously enhanced during UC, which indicated that UC was accompanied with lung injury. The anti-inflammatory role of APS and matrine has also been reported. Consistently, this study showed that the treatments of APS or/and matrine inhibited the levels of TNF-α and IL-1β, reduced the activities of MDA and MPO, and increased SOD activity. All these results indicated that APS combined with matrine might exert a synergistic protective effect against lung injury by regulating the inflammatory response and oxidative stress.
Furthermore, this study detected TFF3 expression in lung and colon tissues after different treatments in UC rats. The TFF family are a kind of small molecule polypeptides, and play roles in mucosal protection, inhibition of inflammatory mediators, regulation of cellular immunity, and apoptosis[41]. TFF3 is specifically distributed in the surface of the intestinal mucosa, and is confirmed to be closely related with the onset of UC[42]. It is generally believed that the expression of TFF3 is down-regulated during the acute onset of IBD, while the up-regulated expression of TFF3 is found during the recovery phase[43,44]. Thus, TFF3 not only has a protective effect on the intestinal mucosal barrier, but its reduction is also related to the progression of UC. This study found decreased expression of TFF3 in UC rats at 2 wk and 4 wk, indicating that the down-regulation of TFF3 reduced the protective and repairing effects on the mucosa, promoted the formation of ulcers, and slowed the repair of damaged mucosa. After 2 wk and 4 wk of treatment with APS and matrine, the expression of TFF3 increased, suggesting that APS combined with matrine might increase the expression of TFF3, and then promote intestinal mucosal injury repair and protect intestinal mucosal barrier function. Therefore, we speculated that the synergistic protective effect of APS and matrine against UC and lung injury might be associated with regulating TFF3 expression.
In conclusion, APS combined with matrine exert a synergistic protective effect against UC and lung injury, which might be associated with regulating TFF3 expression.
Astragalus polysaccharides (APS) are bioactive components extracted from the radix of Astragalus membranaceus, a commonly used herbal compound in traditional Chinese medicine.
APS was reported to have anti-inflammatory, anti-oxidative, anti-tumor, and anti-diabetic properties.
To evaluate the therapeutic effect of APS and its potential mechanisms in a ulcerative colitis (UC) rat model induced by colon mucosal tissue sensitization combined with trinitro-benzene-sulfonic acid-ethanol.
First, we used two groups of Wistar rats: UC models and controls. Then, 105 UC model rats were randomly divided to five groups: Model group (n = 25), salazopyrine control group (n = 25), APS treatment group (n = 15), matrine treatment group (n = 15), and monomer mixture group (n = 25).
The inflammatory response and oxidative stress injury was relieved in colitis observed in APS combined with matrine-treated mice.
APS combined with matrine may represent a potential therapeutic approach for treating inflammatory bowel disease.
Drug research can provide a valuable resource to help clinicians make strategic treatment choices that will ultimately benefit patients at many levels.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goral V, Kochhar R S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin Gastroenterol Hepatol. 2018;16:343-356.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 323] [Article Influence: 46.1] [Reference Citation Analysis (1)] |
| 3. | Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis. 2010;11:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Sharp RM, Peterson DR, Kerr DE, Ward AK, Ross AB, Coulson RJ, Nau WH. Devices, systems, and methods for treating ulcerative colitis and other inflammatory bowel diseases. United States patent US 20170143400A1. 2016;Nov 1. |
| 5. | Yilmaz A, Yilmaz Demirci N, Hoşgün D, Uner E, Erdoğan Y, Gökçek A, Cağlar A. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol. 2010;16:4952-4957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Tang ZP, Wu JW, Dai YC, Zhang YL, Bi RR. Relationship between Ulcerative Colitis and Lung Injuries. Chin Med Sci J. 2015;30:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 470] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 8. | Zheng K, Shen H, Jia J, Lu Y, Zhu L, Zhang L, Shen Z. Traditional Chinese medicine combination therapy for patients with steroid-dependent ulcerative colitis: study protocol for a randomized controlled trial. Trials. 2017;18:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Zhou MX, Wei X, Li AL, Wang AM, Lu LZ, Yang Y, Ren DM, Wang XN, Wen XS, Lou HX, Shen T. Screening of traditional Chinese medicines with therapeutic potential on chronic obstructive pulmonary disease through inhibiting oxidative stress and inflammatory response. BMC Complement Altern Med. 2016;16:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 10. | Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Guo L, Liu J, Hu Y, Wang D, Li Z, Zhang J, Qin T, Liu X, Liu C, Zhao X, Fan YP, Han G, Nguyen TL. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression. Carbohydr Polym. 2012;90:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Li R, Chen W-c, Wang W-p, Tian W-y, Zhang X-g. Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr Polym. 2010;82:240-244. [RCA] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Zhao HM, Wang Y, Huang XY, Huang MF, Xu R, Yue HY, Zhou BG, Huang HY, Sun QM, Liu DY. Astragalus polysaccharide attenuates rat experimental colitis by inducing regulatory T cells in intestinal Peyer's patches. World J Gastroenterol. 2016;22:3175-3185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Gao YJ, Zhu F, Qian JM, Dai JY. Therapeutic and immunoregulatory effect of GATA-binding protein-3/T-box expressed in T-cells ratio of astragalus polysaccharides on 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Chin J Integr Med. 2016;22:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 15. | Yuan LB, Hua CY, Gao S, Yin YL, Dai M, Meng HY, Li PP, Yang ZX, Hu QH. Astragalus Polysaccharides Attenuate Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats. Am J Chin Med. 2017;45:773-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Chu X, Liu X, Qiu J, Zeng X, Bao H, Shu J. Protection of Astragalus Polysaccharides and Codonopsis Pilosula Polysaccharides on Alveolar Macrophage Phagocytosis of Chronic Obstructive Pulmonary Disease Mice With PM2. 5 Inhalation. Chest. 2016;149:382A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Li P, Lei J, Hu G, Chen X, Liu Z, Yang J. Matrine Mediates Inflammatory Response via Gut Microbiota in TNBS-Induced Murine Colitis. Front Physiol. 2019;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Li WW, Wang TY, Cao B, Liu B, Rong YM, Wang JJ, Wei F, Wei LQ, Chen H, Liu YX. Synergistic protection of matrine and lycopene against lipopolysaccharideinduced acute lung injury in mice. Mol Med Rep. 2019;20:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Huang J, Xu H. Matrine: Bioactivities and Structural Modifications. Curr Top Med Chem. 2016;16:3365-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 21. | Vilaseca J, Salas A, Guarner F, Rodríguez R, Martínez M, Malagelada JR. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990;31:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Lv J, Zhang Y, Tian Z, Liu F, Shi Y, Liu Y, Xia P. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κВ activation. Int J Biol Macromol. 2017;98:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 23. | Tian Z, Liu Y, Yang B, Zhang J, He H, Ge H, Wu Y, Shen Z. Astagalus Polysaccharide Attenuates Murine Colitis through Inhibiton of the NLRP3 Inflammasome. Planta Med. 2017;83:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Wu C, Xu Z, Gai R, Huang K. Matrine ameliorates spontaneously developed colitis in interleukin-10-deficient mice. Int Immunopharmacol. 2016;36:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Yang Y, Yan H, Jing M, Zhang Z, Zhang G, Sun Y, Shan L, Yu P, Wang Y, Xu L. Andrographolide derivative AL-1 ameliorates TNBS-induced colitis in mice: involvement of NF-кB and PPAR-γ signaling pathways. Sci Rep. 2016;6:29716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Shi L, Dai Y, Jia B, Han Y, Guo Y, Xie T, Liu J, Tan X, Ding P, Li J. The inhibitory effects of Qingchang Wenzhong granule on the interactive network of inflammation, oxidative stress, and apoptosis in rats with dextran sulfate sodium-induced colitis. J Cell Biochem. 2019;120:9979-9991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Morris MC, Gilliam EA, Li L. Innate immune programing by endotoxin and its pathological consequences. Front Immunol. 2014;5:680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | van Deventer SJ, ten Cate JW, Tytgat GN. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988;94:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Gardiner KR, Anderson NH, Rowlands BJ, Barbul A. Colitis and colonic mucosal barrier dysfunction. Gut. 1995;37:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Honzawa Y, Nakase H, Matsuura M, Chiba T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm Bowel Dis. 2011;17:E23-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Fogel WA, Lewinski A. The effects of diamine oxidase administration on experimental ulcerative colitis in rats. Inflamm Res. 2006;55 Suppl 1:S63-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009;1165:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 33. | Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 514] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 34. | Yin J, Wu M, Duan J, Liu G, Cui Z, Zheng J, Chen S, Ren W, Deng J, Tan X, Al-Dhabi NA, Duraipandiyan V, Liao P, Li T, Yulong Y. Pyrrolidine Dithiocarbamate Inhibits NF-KappaB Activation and Upregulates the Expression of Gpx1, Gpx4, Occludin, and ZO-1 in DSS-Induced Colitis. Appl Biochem Biotechnol. 2015;177:1716-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Castro-Ochoa KF, Vargas-Robles H, Chánez-Paredes S, Felipe-López A, Cabrera-Silva RI, Shibayama M, Betanzos A, Nava P, Galinski EA, Schnoor M. Homoectoine Protects Against Colitis by Preventing a Claudin Switch in Epithelial Tight Junctions. Dig Dis Sci. 2019;64:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 37. | Lei J, Wei Y, Song P, Li Y, Zhang T, Feng Q, Xu G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur J Pharmacol. 2018;818:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 38. | Butt Y, Kurdowska A, Allen TC. Acute Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med. 2016;140:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 660] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 39. | Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2006] [Cited by in RCA: 1937] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 40. | Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 41. | Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 42. | Nakov R, Velikova T, Nakov V, Ianiro G, Gerova V, Tankova L. Serum trefoil factor 3 predicts disease activity in patients with ulcerative colitis. Eur Rev Med Pharmacol Sci. 2019;23:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 43. | Vestergaard EM, Brynskov J, Ejskjaer K, Clausen JT, Thim L, Nexø E, Poulsen SS. Immunoassays of human trefoil factors 1 and 2: measured on serum from patients with inflammatory bowel disease. Scand J Clin Lab Invest. 2004;64:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Dossinger V, Kayademir T, Blin N, Gött P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell Physiol Biochem. 2002;12:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |