Published online Feb 21, 2019. doi: 10.3748/wjg.v25.i7.859
Peer-review started: October 22, 2018
First decision: December 12, 2018
Revised: January 11, 2019
Accepted: January 18, 2019
Article in press: January 18, 2019
Published online: February 21, 2019
Processing time: 123 Days and 19.8 Hours
Disorders of primary bile acid synthesis may be life-threatening if undiagnosed, or not treated with primary bile acid replacement therapy. To date, there are few reports on the management and follow-up of patients with Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency. We hypothesized that a retrospective analysis of the responses to oral bile acid replacement therapy with chenodeoxycholic acid (CDCA) in patients with this bile acid synthesis disorder will increase our understanding of the disease progression and permit evaluation of this treatment regimen as an alternative to the Food and Drug Administration (FDA) approved drug cholic acid, which is currently unavailable in China.
To evaluate the therapeutic responses of patients with AKR1D1 deficiency to oral bile acid therapy, specifically CDCA.
Twelve patients with AKR1D1 deficiency, confirmed by fast atom bombardment ionization-mass spectrometry analysis of urine and by gene sequencing for mutations in AKR1D1, were treated with differing doses of CDCA or ursodeoxycholic acid (UDCA). The clinical and biochemical responses to therapy were monitored over a period ranging 0.5-6.4 years. Dose adjustment, to optimize the therapeutic dose, was based on changes in serum biochemistry parameters, notably liver function tests, and suppression of the urinary levels of atypical hepatotoxic 3-oxo-Δ4-bile acids measured by mass spectrometry.
Physical examination, serum biochemistry parameters, and sonographic findings improved in all 12 patients during bile acid therapy, except one who underwent liver transplantation. Urine bile acid analysis confirmed a significant reduction in atypical hepatotoxic 3-oxo-Δ4 bile acids concomitant with clinical and biochemical improvements in those patients treated with CDCA. UDCA was ineffective in down-regulating endogenous bile acid synthesis as evidenced from the inability to suppress the urinary excretion of atypical 3-oxo-Δ4-bile acids. The dose of CDCA required for optimal clinical and biochemical responses varied from 5.5-10 mg/kg per day among patients based on maximum suppression of the atypical bile acids and improvement in serum biochemistry parameters, and careful titration of the dose was necessary to avoid side effects from CDCA.
The primary bile acid CDCA is effective in treating AKR1D1 deficiency but the therapeutic dose requires individualized optimization. UDCA is not recommended for long-term management.
Core tip: Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency presents as particularly severe and rapidly progressive cholestasis. Treatment with oral primary bile acid has been shown to be effective in normalizing liver function, circumventing the only alternative treatment of liver transplantation. The primary bile acid cholic acid is an approved drug for treating this genetic defect but is not available in China and many other countries. Here we report on the use of the alternative primary bile acid, chenodeoxycholic acid, in the largest cohort of patients with AKR1D1 deficiency studied to date, showing beneficial effects in a personalized regimen approach.
- Citation: Zhang MH, Setchell KD, Zhao J, Gong JY, Lu Y, Wang JS. Δ4-3-oxosteroid-5β-reductase deficiency: Responses to oral bile acid therapy and long-term outcomes. World J Gastroenterol 2019; 25(7): 859-869
- URL: https://www.wjgnet.com/1007-9327/full/v25/i7/859.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i7.859
Inborn errors in primary bile acid synthesis from cholesterol now represent a specific category of metabolic liver disease[1,2]. The Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency[3] [also referred to as 5β-reductase deficiency, congenital bile acid synthesis defect type 2 (CBAS 2), MIM604741] is the second most common of these bile acid synthesis disorders. Unlike the more commonly diagnosed 3β-hydroxy-Δ5-C27-steroid oxidoreductase (HSD3B7) deficiency which frequently accounts for late-onset chronic cholestasis[4,5], the AKR1D1 deficiency tends to be diagnosed in early infancy and presents as particularly severe and rapidly progressive cholestasis[6]. Early diagnosis of these defects is consequently crucial, because the related liver disease may be life-threatening, but is treatable by oral primary bile acid replacement[2,4,7-11].
Due to the failure to synthesize primary bile acids in the AKR1D1 deficiency and the production instead of a spectrum of highly cholestatic and hepatotoxic 3-oxo-Δ4 bile acids[3], bile flow is impaired, leading to cholestasis. Since bile acids are essential for micellar solubilization of lipids, fat and fat-soluble vitamin deficiency occurs quite often.
The AKR1D1 deficiency was first discovered by the application of fast atom bombardment ionization mass spectrometry (FAB-MS) and based on the detection of increased concentrations of 3-oxo-Δ4 bile acids in urine concomitant with a lack of normal primary bile acid conjugates[11]. Following the cloning of the AKR1D1 gene[7], genetic analysis is now a clinically useful tool for confirming the diagnosis based on identified mutations in the AKR1D1 gene[7]. Oral primary bile acid therapy is now the therapy of choice for treating bile acid synthesis disorders, circumventing the need for liver transplantation, which is the only alternative in these progressive and frequently fatal forms of cholestasis. Although cholic acid (CA) has been approved by the United States Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) for the treatment of bile acid synthesis disorders, the data of its effects on patients with AKR1D1 deficiency is very limited[7,8]. At the same time, this drug is presently unavailable to many patients due to the limit of accessibility and affordability. We now describe the clinical and biochemical responses to another primary bile acid - chenodeoxycholic acid (CDCA) therapy in 12 patients, the largest cohort to date, diagnosed with AKR1D1 deficiency based on genetic and mass spectrometric identifications. We report on the effectiveness of CDCA therapy in this bile acid synthesis disorder, and the lack of effect of ursodeoxycholic acid (UDCA) in patients treated with the latter.
From December 2012 to April 2017, 12 patients with AKR1D1 deficiency (Table 1) were identified by urinary FAB-MS analysis at Cincinnati Children’s Hospital Medical Center from the screening of 193 patients with unexplained cholestasis. Gene sequencing performed at the Department of Pediatrics of Jinshan Hospital of Fudan University and the Center for Pediatric Liver Disease of the Children’s Hospital of Fudan University confirmed mutations in AKR1D1, in all 12 patients (Table 1).
| Patient No. | Sex | Age at first symptom | Age at first visit (mo) | TB (μmol/L) | DB (μmol/L) | ALT (U/L) | AST (U/L) | GGT (U/L) | TBA (μmol/L) | Organomegaly | INR2 | Molecular defect3 |
| 1 | F | 14 d | 2.5 | 292 | 214 | 169 | 309 | 46 | 4.6 | Hepatomegaly | 1.23 | c.933delG/c.1023+5A>C |
| 2 | M | 1 mo | 11 | 146 | 108 | 101 | 405 | 40 | 1.6 | Hepatosplenomegaly | 1.17 | c.158A>G/ c.158A>G |
| 3 | M | 3 d | 2 | 96 | 64 | 176 | 183 | 33 | 3 | Hepatomegaly | 1.03 | c.396C>A/c.722A>T |
| 4 | M | 3 d | 1 | 310 | 218 | 76 | 213 | 51 | 5.5 | Hepatomegaly | 1.52 | c.919G>T/c.919G>T |
| 5 | M | 10 d | 10 | 146 | 113 | 210 | 207 | 65 | 6.1 | Hepatosplenomegaly | 1.14 | c.797G>A/- |
| 6 | F | 1 mo | 8 | 324 | 252 | 267 | 584 | 108 | 1.4 | Hepatomegaly | 2.17 | c.148C>T/- |
| 7 | M | 1 mo | 8 | 211 | 126 | 234 | 734 | 66 | 7.9 | Hepatomegaly | 1.1 | c.797G>A/C.797G>A |
| 8 | M | 3 d | 1 | 320 | 158 | 523 | 703 | 51 | 2.5 | Hepatomegaly | 1.36 | c.797G>A/C.797G>A |
| 9 | F | 10 d | 1 | 378 | 232 | 1317 | 1416 | 62 | 8.5 | Hepatomegaly | 1.9 | c.614delT/C.797G>A |
| 10 | M | 3 d | 2 | 160 | 126 | 531 | 896 | 64 | 5.9 | Hepatosplenomegaly | 1.28 | c.593C>T/c.797G>A |
| 11 | M | 3 d | 3 | 125 | 76 | 79 | 161 | 38 | 1.4 | Hepatomegaly | 1.02 | c.278delA/- |
| 12 | M | 1 mo | 2.5 | 204 | 112 | 339 | 619 | 50 | 1.8 | Hepatomegaly | 2.11 | c.919C>T/C.919C>T |
| Normal range | 5-21 | 0-3.4 | 0-40 | 0-40 | 7-50 | 0-10 | 0.8-1.2 | |||||
With the exception of patients 2 and 8, the other ten patients were from non-consanguineous healthy families. Blood samples were collected after parenteral administration of vitamin K when they were transferred to our hospitals for evaluation. Investigations of all patients for congenital infection, including toxoplasmosis, rubella, cytomegalovirus, herpes simplex and hepatotropic virus, and metabolic disease, including tyrosinaemia, galactosaemia and α1-antitrypsin deficiency, were negative. All patients had jaundice and dark urine. The clinical, biochemical, and physical information at referral is summarized in Table 1. All presented with varying degrees of conjugated hyperbilirubinemia, elevated serum transaminases, normal or slightly elevated γ-glutamyltranspeptidase (GGT), and low or normal serum total bile acids. All patients had hepatomegaly, and three had splenomegaly (Table 1). Evaluations at the beginning and follow-up included physical examination, serum biochemistry tests, and urinary bile acid analysis by FAB-MS. After initiating bile acid therapy, the patients were seen on a weekly basis in order to make dose adjustments based on the serum biochemistry parameters to minimize possible hepatotoxicity of the medications. When their clinical status stabilized, the patients were seen every 2-4 wk and finally after normalization of serum biochemistries, every 6 mo thereafter.
Urine samples were collected before treatment with primary bile acid. In those patients that had been treated with UDCA prior to the initial presentation, the therapy was stopped for 5-7 d before urine was collected. The initial negative ion FAB-MS spectrum of the urine was used to confirm the diagnosis of an AKR1D1 deficiency before treatment was started with CDCA. Confirmation of the AKR1D1 deficiency was based on the finding of a lack or absence of normal primary bile acids and the presence of elevated levels of taurine and/or glycine conjugated Δ4-3-oxo bile acids[2,3]. Urine was collected at frequent intervals during the course of therapy with CDCA in order to monitor the extent of suppression of atypical Δ4-3-oxo bile acids and to confirm compliance to therapy. Dose adjustments were optimized to achieve maximum suppression of atypical bile acids.
With approval by the Ethics Committees on Human Research of the Jinshan Hospital of Fudan University, and of the Children’s Hospital of Fudan University, and after obtaining informed consent from the parents, a 1.5-mL peripheral blood sample was obtained. To confirm the diagnosis and establish the molecular basis of the disorder, genomic DNA was isolated from white blood cells. Prior to December 2015, all of the coding exons and adjacent introns of the AKR1D1 gene were Sanger sequenced. After January 2016, panel sequencing[12] and Sanger confirmation were performed. Molecular data are listed in Table 1 with NM_005989 as reference.
With the exception of patients 2, 10, and 12, all other patients had received UDCA therapy before being referred for evaluation (Table 2). Fat-soluble vitamins were routinely supplemented for all patients after admission. With the informed consent from the parents, UDCA therapy was terminated, urine collections were obtained, and patients were then switched to the primary bile acid CDCA at an initial dose of 5-12 mg/kg bw/d (usually lower initial dose in more severe cases), taken in two divided doses. Baseline urine samples were not available for mass spectrometric analysis from patients 3 and 5. Patient 5 declined treatment with CDCA and continued with a high dose of UDCA (40 mg/kg per day). Patient 3 also continued to take UDCA (30 mg/kg per day) but after 4 mo was switched to CDCA because of abnormal bile acid profiles, even though the biochemistries improved. The initial dose of CDCA was adjusted based on the findings from the urine bile acid analyses and serum biochemistry parameters, including transaminases and GGT. Generally, the dose was reduced if serum alanine aminotransferase (ALT) and aspartic transaminase (AST) became elevated with concomitant elevated serum total bile acids level, and conversely was increased if there was inadequate suppression of atypical bile acids as demonstrated by FAB-MS, even after the resolution of jaundice. The primary bile acid administration dosage and duration of treatment for each patient are summarized in Tables 2 and 3.
| Patient No. | Starting age | Dosage (d.kg)/duration of therapy | Before UDCA administration | At the end of UDCA therapy | ||||||
| TB / DB (μmol/L) | ALT/AST (U/L) | ALB (g/L) | INR | TB/DB (μmol/L) | ALT/AST (U/L) | ALB (g/L) | INR | |||
| 1 | 2.5 mo | 20 mg/3 mo | 241/177 | 73/172 | 38 | 1.23 | 292/214 | 169/309 | 33 | 1.46 |
| 3 | 2 mo | 30 mg/4 mo | 96/64 | 176/183 | 43 | 1.03 | 8.7/5.9 | 51/29 | 44 | n.a. |
| 4 | 1 mo | 20 mg/1.5 mo; 40 mg/1 wk | 150/58 | 76/159 | 35 | 1.43 | 310/218 | 76/273 | 36 | 1.52 |
| 5 | 10 mo | 18 mg/1 mo; 40 mg/4 mo | 126/66 | 340/262 | 49 | n.a. | 6.7/2.9 | 8/16 | 42 | n.a. |
| 6 | 8 mo | 20 mg/6 mo | 221/142 | 235/204 | 32 | 1.24 | 324/252 | 267/584 | 33 | 2.17 |
| 7 | 8 mo | 10 mg/2 mo | 268/121 | 532/897 | 38 | 1.16 | 19/15 | 100/124 | 52 | 0.9 |
| 8 | 1 mo | 16 mg/6 mo | 308/131 | 150/187 | 41 | 1.13 | 273/148 | 448/410 | 28 | 1.36 |
| 9 | 1 mo | 18 mg/3 mo | 316/243 | 1240/1565 | 37 | 1.71 | 358/258 | 195/283 | 32 | n.a. |
| 11 | 3 mo | 18 mg/2 mo | 122/87 | 106/155 | n.a. | 1.02 | 85/68 | 83/167 | 39 | n.a. |
| Normal range | 5-21/0-3.4 | 0-40 | 35-52 | 0.8-1.2 | 5-21/0-3.4 | 0-40 | 35-52 | 0.8-1.2 | ||
| Patient No. | Starting age (mo) | Starting dosage (mg/kg per day) | Dosage adjustment (mg/kg per day × duration) | Age (mo) at LFTs normalization | Dosage maintaining normal LFTs and suppressing atypical bile acids (mg/kg per day) | Status/age at last follow-up |
| 1 | 5.5 | 8 | 8 mo × 7 mo; 10 × - | 9 | 10 | Normal/4 yr 11 mo |
| 2 | 11 | 12 | 12 mo × 1 mo; 8 mo × 0.75 mo; 4.5 mo × 5 mo; 5.5 × - | 31 | 5.5 | Normal/4 yr |
| 3 | 6 | 10 | 10 mo × 24 mo; 9 mo × 12 mo, 8 × - | 13 | 8 | Normal/4 yr 9 mo |
| 4 | 3 | 5 | 5 mo × 1mo; 3 mo × 2 mo; 7 mo × 4.5 mo; 8 mo × 2.5 mo; 11 mo × 13 mo; 8 × - | 26 | 8 | Normal/6 yr 7 mo |
| 6 | 14 | 8 | 8 wk × 3 wk; 6 wk × 1 wk; 5 mo × 2.5 mo; 6 mo × 3 mo; 7 × - | 18 | 7 | Normal/2 yr 11 mo |
| 7 | 10 | 10 | 10 wk × 1 wk; 8 mo × 1 mo; 0 wk × 1 wk; 5 × - | 14 | 5 | Normal/2 yr 4 mo |
| 8 | 7 | 10 | 10 wk × 1 wk; 4 mo × 2 mo; 5 mo × 6 mo; 6 mo × 2 mo | N.A. | N.A. | Transplanted/1 yr 6 mo |
| 9 | 4 | 8 | 8 mo × 2 mo; 9 mo × 2 mo; 10 × - | 10 | 10 | Normal/1 yr 5 mo |
| 10 | 3.5 | 10 | 10 × - | 6.5 | 10 | Normal/11 mo |
| 11 | 5 | 10 | 10 mo × 13 mo; 7 × - | 8 | 7 | Normal/2 yr |
| 12 | 2.5 | 10 | 10 × - | 5 | 10 | Normal/8 mo |
Nine patients (Table 2) had received UDCA therapy prior to diagnosis of AKR1D1 deficiency, of whom three (patients 3, 4, and 5) were administered with a period of high dose UDCA (30-40 mg/kg bw/d). Complete normalization of liver function tests (LFTs) was seen only in patient 5, and in patients 3 and 7 serum bilirubin levels almost normalized but serum aminotransferases levels remained elevated. Biochemistry parameters did not improve or worsen in the remaining patients. Overall, UDCA was largely ineffective in treating AKR1D1 deficiency.
Eleven patients received CDCA therapy at an initial dose of 5-12 mg/kg bw/d (Table 3). Five patients (patients 2, 4, 6, 7, and 8) had to temporarily stop or have the dose reduced because of a marked elevation of serum ALT, AST, and total bile acid levels after beginning the therapy. This was believed due to the intrinsic hepatotoxicity of CDCA. However, with the exception of patient 8, the clinical status of all the other patients gradually improved during CDCA administration after dose adjustment. Jaundice disappeared after several months and hepatomegaly improved gradually. After the resolution of jaundice, the dose of CDCA was increased in some cases due to the insufficient suppression of atypical bile acids as determined from the follow-up urine analysis. The duration of therapy ranged from 5.5 mo to 6.4 years (median = 1.5 years) in the 11 patients undergoing CDCA therapy. At the last follow-up of these patients (median age, 2.3 years; range: 0.7-6.6 years; n = 11), the liver function tests had normalized and all were clinically well (Table 3). When the dose of CDCA was optimized, no obvious side effects were subsequently reported or observed with continued treatment. Patient 8 showed hepatotoxicity to a dose of 10 mg/kg bw/d CDCA, and still no improvement on a low dose of CDCA (4-6 mg/kg bw/d), and 9 mo later, after further deterioration in liver function tests, the patient underwent liver transplantation at the age of 17 mo. At the time of writing, all the remaining patients are thriving with continued CDCA therapy.
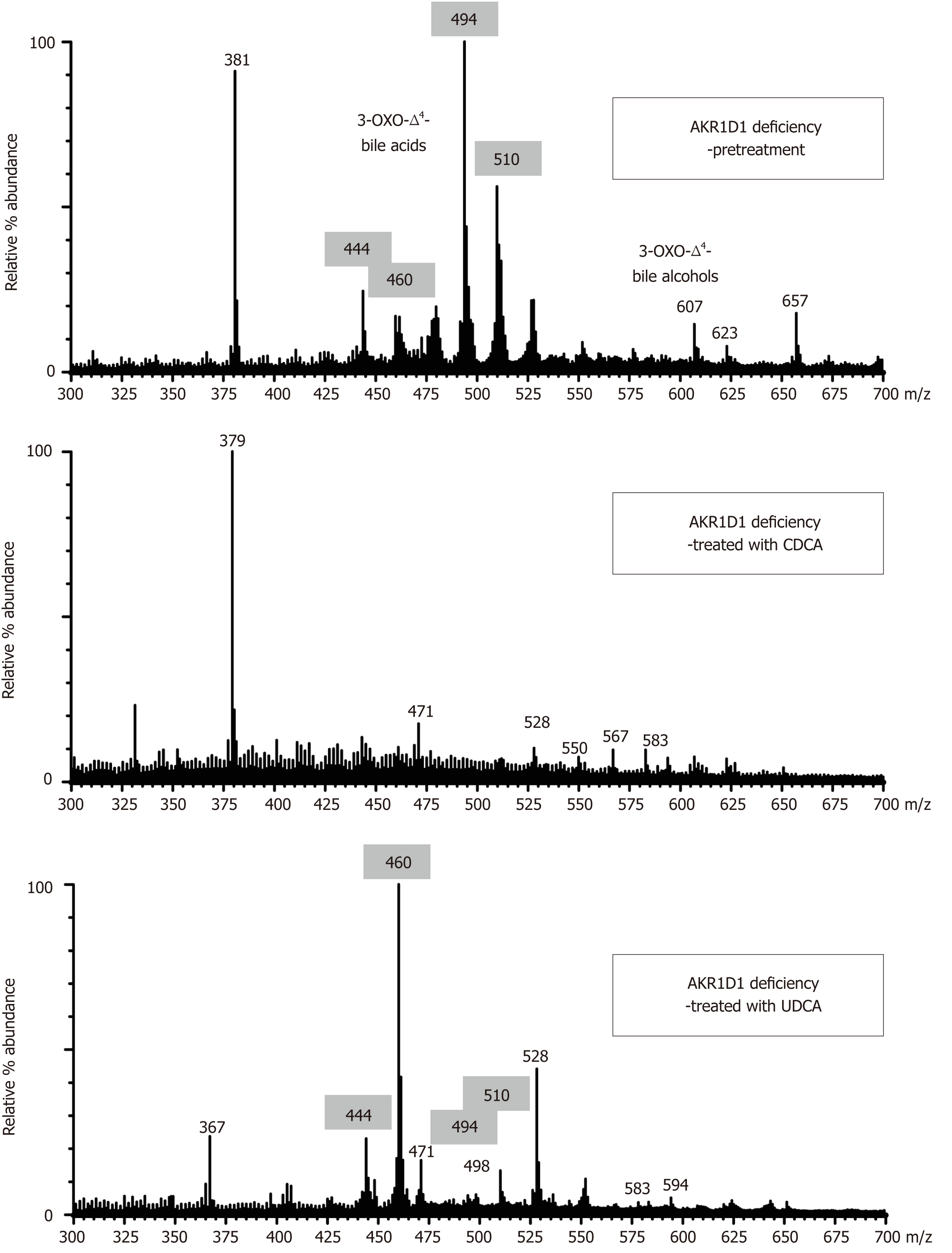
Urine bile acid analysis by FAB-MS conclusively established a deficiency in the activity of AKR1D1 in all patients prior to initiation of bile acid therapy. The negative ion mass spectra of the urines were characterized by an absence of ions representing normal primary bile acid conjugates of CDCA and CA (m/z 448, 464, 498, and 514), and the presence of intense ions at m/z 444, 460, 494 and 510. These represent molecular ions for glycine and taurine conjugated forms of 3-oxo-Δ4 bile acids that are the biomarkers for the AKR1D1 deficiency[2,3,13]. When diagnosed in the first few months of life, it is the taurine conjugated 3-oxo-Δ4 bile acids (m/z 494 and 510) that generally dominate the mass spectra because in early life bile acids are predominantly conjugated with taurine[3,14]. After weaning and in older infants, the glycine conjugated forms of these atypical bile acids (m/z 444 and 460) become the predominant species present.
The biochemical response to CDCA therapy was confirmed by the successful reduction in the intensity of the ions for these 3-oxo-Δ4 bile acid conjugates (Figure 1), and on this basis, the dose of CDCA was adjusted to optimize maximum suppression. With optimized CDCA therapy, most patients showed a marked suppression, or complete disappearance of the atypical bile acids (Figure 1), although it should be noted that it can be difficult to attain complete disappearance of these metabolites. The presence of ions in the mass spectrum that reflect metabolites of CDCA was a common observation and served to confirm compliance to therapy. Ions at m/z 448 (glyco-CDCA), m/z 498 (tauro-CDCA), m/z 471 (CDCA-sulfate), and m/z 528 (glyco-CDCA-sulfate) represent major metabolites of CDCA (Figure 1). Those patients showing the best clinical response to CDCA therapy had the greatest suppression in the urinary excretion of atypical 3-oxo-Δ4 bile acids. Patient 2 did not show adequate suppression of 3-oxo-Δ4 bile acids initially, presumed to be due to poor compliance. Likewise, the urinary FAB-MS analysis for patient 8, who was on a relatively low dose of CDCA (4-6 mg/kg bw/d), failed to show adequate suppression of the 3-oxo-Δ4 bile acids, and this patient’s serum transaminases progressively increased and liver function deteriorated to the point of requiring a liver transplant. In patient 5, who refused CDCA therapy and was maintained on high dose UDCA, the FAB-MS showed no reduction in the intensity of the 3-oxo-Δ4 bile acids even in the face of improvements in serum biochemistries. Figure 1 shows the mass spectra of the urine after UDCA treatment and these findings are consistent with the inability of UDCA to suppress endogenous bile acid synthesis. Ions consistent with the atypical 3-oxo-Δ4 bile acids remained in the mass spectra but were inter-dispersed with ions reflecting metabolites of exogenous UDCA.
The AKR1D1 gene encodes the enzyme AKR1D1, which is essential for the synthesis of the primary bile acids, cholic acid and CDCA. This NADPH-dependent enzyme, first isolated from rat liver by Berséus and Björkhem in 1967[14,15], catalyzes the reduction of the Δ4-bond of the sterol intermediate 7α-hydroxy-4-cholesten-3-one (often now referred to as C4 or sterol-C4, and used as a surrogate marker for bile acid synthesis) to give rise to a 7α-hydroxy-5-cholestan-3-one intermediate in the pathway to cholic acid and CDCA synthesis. When the activity of AKR1D1 enzyme is deficient or reduced, the liver synthesizes a spectrum of atypical bile acids that retain this 3-oxo-Δ4 structure. Since the description of a primary enzyme defect in twins by Setchell et al[3], there have been a number of reports of patients with liver disease exhibiting elevated levels of Δ4-3-oxo bile acids[8,16-20], but discerning whether these were due to a ‘primary’ genetic defect or ‘secondary’ to reduced enzyme activity because of loss of hepatic synthetic function as occurs in end-stage liver disease is challenging. Furthermore, Δ4-3-oxo bile acids are typically found in urine of all healthy neonates[21-23] due to developmental immaturity in bile acid synthesis and transport in a period of physiological cholestasis that is a natural phenomenon at this time of life[24]. These atypical bile acids usually disappear with time so that in the absence of genetic testing, repeat testing of urine is important to enable differential diagnosis of a primary vs secondary AKR1D1 deficiency. Elevated levels of Δ4-3-oxo bile acids have been reported in patients with neonatal hemochromatosis and tyrosinemia[17-20]. However, when arising from a ‘secondary’ defect, these are usually accompanied by the presence of primary bile acids. In the original description of the AKR1D1 deficiency[3], primary bile acids were absent but the 5β-reduced metabolites, allo-cholic acid and allo-CDCAs, were elevated and used at the time to differentiate a ‘primary’ vs ‘secondary’ enzyme defect. The original description of the AKR1D1 deficiency as a cause of cholestasis predated by 6 years the cloning and expression of a cDNA for AKR1D1 by Kondo et al[25], so genetic confirmation was not possible. Now, complementing MS analysis with genetic analysis of AKR1D1 (and vice versa) permits an accurate diagnosis by identifying specific gene mutations for this autosomal recessive disorder.
Over a period of 4-5 years, urine samples from 193 cholestatic patients presenting at Jinshan Hospital and Children’s Hospital of Fudan University were screened for CBAS and 12 were positive for AKR1D1 deficiency. In the 12 patients described here, an AKR1D1 deficiency was conclusively established from MS by the finding of predominantly Δ4-3-oxo-bile acids in urine (Figure 1), and confirmed by gene sequencing analysis (Table 1).
Molecular studies identified homozygous or compound heterozygous mutations in nine patients and simple heterozygous mutations in three patients. A total of 11 different mutations were identified, including three frame-shifting (c.278delA, c.614delT, and c.933delG), two stop code (c.148C>T, c.396C>A), and six missense mutations (c.158A>G, c.472A>G, c.593C>T, c.722A>T, c.797G>A, and c.919G>A) (Table 1, Supplementary Table 1). Among them, c.148C>T[26] and c.593 C>T[7] were reported previously as disease-causing mutations, and c.797G>A is recorded as an SNP (rs182820353) but predicted as disease-causing by mutation taster. The other eight were novel and predicted as disease-causing. All five new missense mutations were in highly conserved residues. All mutations were seen in one allele each, except for two recurrent mutations (c.797G>A and c.919C>T). Mutation c.797G>A was seen in seven alleles (two homozygous and three heterozygous). Its frequency in patients (7/24) was much higher than that in 1000 Genomes (1/5008, P = 5.77308222349e-17) and ExAC (8/121368, P = 2.88738419819e-23). c.919C>T was seen in four alleles (two homozygous). For the three patients (patients 5, 6, and 11) in whom only one single heterozygous mutation was found, we speculate that there might be a large fragment deletion or duplication or mutation in the intron region leading to splice site changes.
All 12 patients presented with neonatal onset cholestasis with markedly elevated serum transaminases, normal or slightly elevated GGT, and low levels of serum total bile acids (sTBA) when measured with routine enzyme/immunoassay kits. PT was prolonged and not corrected by parenteral vitamin K in seven patients. A routine sTBA was not included in the initial battery of tests in some patients, which may have a delayed diagnosis because a low or normal sTBA when combined with a low serum GGT can be a helpful indicator of a possible bile acid synthesis disorder[27], particularly in the case of the HSD3B7 and AKR1D1 deficiencies. Most of our patients had been placed on UDCA therapy for their cholestasis prior to referral for evaluation, and it is worth cautioning that routine sTBA measurements in UDCA treated patients are of little guidance, or may even mislead, as these will be elevated due to cross-reactivity in the assay from UDCA and its metabolites. In contrast to the HSD3B7 deficiency, which is frequently diagnosed as a cause of late-onset chronic cholestasis in older children[28,29] and even some adults[30], the AKR1D1 deficiency is usually diagnosed early in life. In the 12 patients described, the first symptoms of cholestasis were evident from 3 d-1 mo of age. The average age at diagnosis was 4.3 mo (range, 1-11 mo) and the rate of progression of liver disease was rapid, even to dysfunction.
Given the rapid onset of liver failure in the AKR1D1 defect, early diagnosis is crucial. Limited reports showed that treatment with primary bile acids was extremely effective in patients with AKR1D1 deficiency[3,7,31,32]. Primary bile acids inhibit cholesterol 7α-hydroxylase expression to down-regulate bile acid synthesis through feedback inhibition, leading to a decrease in production of atypical Δ4-3-oxo-bile acids, while additionally stimulating bile acid-dependent bile flow. In a number of patients with AKR1D1 deficiency, CA and CDCA[2,7,9,33] were combined. However, in some young infants, CDCA may be cathartic and lead to diarrhea. Furthermore, an increase in serum transaminases was observed in the National Cooperative Gallstone Trial when CDCA was evaluated for the dissolution of cholesterol gallstones[34]. For these reasons, CA monotherapy was favored and the FDA in 2015 approved CA for the treatment of bile acid synthesis disorders, including the AKR1D1 deficiency with evidence from a very limited number of cases due to the rarity of the disease[8,9]. In China, CA has to date been unavailable for patients with bile acid synthesis disorders and is currently unapproved by the Chinese FDA. All of the patients apart from one were therefore treated with CDCA, despite several reports indicating that CDCA should not be recommended for patients with AKR1D1 deficiency[4,14]. In our patients, although significant elevations in serum ALT and AST occurred in some patients, after temporarily stopping therapy and/or making dose adjustments, we finally achieved normalization of LFTs. These patients became symptom free from CDCA treatment over periods ranging 0.5-7.0 years and there were no observed serious adverse effects reported, even in those with an initial abnormal international normalized ratio (INR), save one patient that underwent liver transplantation. The optimal dose of CDCA administered varied from 4-10 mg/kg per day among the patients and was assessed by monitoring the disappearance of the atypical Δ4-3-oxo-bile acids in urine and evaluating the improvement in liver function. During CDCA therapy, LFTs remained within the normal range and FAB-MS analysis confirmed concomitant reductions, although variably, in the levels of urinary Δ4-3-oxo-bile acids. Those patients showing good compliance to therapy had almost normal development and the lack of reported side effects supports the safety of CDCA provided that doses are individually optimized.
UDCA, a potent choleretic widely used in the treatment of cholestatic liver diseases, has been used to treat some patients with the HSD3B7 deficiency[8,11,35]. It stimulates bile flow, lowers serum transaminases, and may improve liver histology[11,30]. However, its inability to suppress bile acid synthesis (it is slightly stimulatory) makes it unsuitable for long-term use in patients with bile acid synthesis disorders because the common therapeutic goal in these patients is to stop the production and accumulation of the hepatotoxic and cholestatic atypical bile acids being synthesized. Nine of our patients had been placed on UDCA prior to diagnosis of AKR1D1 deficiency, and this was stopped in all but one. In that patient, even though LFTs normalized with UDCA, the urinary levels of Δ4-3-oxo-bile acids increased over time (Figure 1). Thus, UDCA may be helpful in the short-term but it is not recommended for long-term therapy.
In conclusion, early diagnosis of the AKR1D1 deficiency by biochemical and genetic testing is critical because this bile acid synthesis disorder manifests as a particularly severe and rapidly progressive cholestatic disease with fatal outcome. CDCA, despite earlier reports cautioning against its use[29,31], was effective in the treatment of our patients (up to now the largest cohort) with AKR1D1 deficiency provided that the dose was optimized in individual patients. UDCA is not recommended as the long-term treatment as it is unable to suppress the production of the atypical hepatotoxic Δ4-3-oxo-bile acids and did not improve the clinical outcome of these patients.
The Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency is a rare bile acid synthesis disorder. There have been few reports describing the effectiveness of treatment regimens for this rare genetic disease and the long-term outcomes of oral primary bile acid therapy are unclear. This study provides evidence on how these patients should ideally be managed.
Oral cholic acid (CA), one of the two primary bile acids synthesized by the liver, is an approved therapy for the treatment of bile acid synthesis disorders but is not available to many patients. Chenodeoxycholic acid (CDCA), the other primary bile acid, offers an alternative potential therapy but has been contraindicated in a few reported cases of AKR1D1 deficiency due to its more hydrophobic and potentially hepatotoxic effects. However, in the absence of access to CA in China, we evaluated the effectiveness of CDCA in patients with AKR1D1 deficiency and showed that provided that the therapeutic doses is individually optimized, it is an effective treatment for this disorder.
Through retrospective analysis of the clinical and biochemical responses to bile acid therapy with CDCA in patients with AKR1D1 deficiency, our objective was to better understand the disease progression and long-term outcomes of treatment in order to make recommendations regarding its effectiveness and safety.
Twelve patients with confirmed AKR1D1 deficiency, diagnosed by fast atom bombardment ionization-mass spectrometry analysis of the urinary bile acid profile and by gene sequencing of AKR1D1, were treated with oral bile acids. The clinical and biochemical responses to CDCA therapy were retrospectively evaluated and analyzed, including the results on urinary bile acid profiles and serum liver function indices.
Physical examination, biochemistry parameters, and sonographic findings improved in the patients during CDCA therapy, except for one who underwent liver transplantation. The urinary levels of atypical hepatotoxic 3-oxo-Δ4 bile acids were suppressed concomitantly with clinical improvements in those patients treated with CDCA, but not with ursodeoxycholic acid (UDCA). The dose of CDCA varied from 5.5-10 mg/kg per day among patients based on maximum suppression of the atypical bile acids.
CDCA is an effective alternative therapy to CA, provided that the dose is carefully optimized on an individual patient basis to minimize side effects. UDCA does not achieve the therapeutic goal of suppressing the production of atypical hepatotoxic bile acids and is not recommended for long-term treatment.
CDCA is effective in the treatment of the patients with AKR1D1 deficiency. However, CDCA is intrinsically hepatotoxic, and therefore its dose must be optimized to individual patients. Future studies should focus on continued long-term monitoring of these patients to provide more detailed information of the natural history of this rare metabolic liver disease and to determine long-term outcomes of therapy with primary bile acids.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Brecelj J, Colak Y S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Setchell KDR, Suchy FJSR, Balistreri WF. Disorders of bile acid synthesis. Suchy FJSR, Balistreri WF. Liver Disease in Children. Cambridge: Cambridge University Press 2014; . [DOI] [Full Text] |
| 2. | Setchell KD, Heubi JE. Defects in bile acid biosynthesis--diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 1:S17-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Setchell KD, Suchy FJ, Welsh MB, Zimmer-Nechemias L, Heubi J, Balistreri WF. Delta 4-3-oxosteroid 5 beta-reductase deficiency described in identical twins with neonatal hepatitis. A new inborn error in bile acid synthesis. J Clin Invest. 1988;82:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 173] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Setchell KDR, Flick R, Watkins JB, Piccoli DA. Chronic hepatitis in a 10 yr old due to an inborn error in bile acid synthesis—diagnosis and treatment with oral bile acid. Gastroenterology. 1990;98:A578. |
| 5. | Fischler B, Bodin K, Stjernman H, Olin M, Hansson M, Sjövall J, Björkhem I. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med. 2007;262:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Heubi JE, Setchell KD, Bove KE. Inborn errors of bile acid metabolism. Semin Liver Dis. 2007;27:282-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Lemonde HA, Custard EJ, Bouquet J, Duran M, Overmars H, Scambler PJ, Clayton PT. Mutations in SRD5B1 (AKR1D1), the gene encoding delta(4)-3-oxosteroid 5beta-reductase, in hepatitis and liver failure in infancy. Gut. 2003;52:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Gonzales E, Gerhardt MF, Fabre M, Setchell KD, Davit-Spraul A, Vincent I, Heubi JE, Bernard O, Jacquemin E. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology. 2009;137:1310-1320.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Daugherty CC, Setchell KD, Heubi JE, Balistreri WF. Resolution of liver biopsy alterations in three siblings with bile acid treatment of an inborn error of bile acid metabolism (delta 4-3-oxosteroid 5 beta-reductase deficiency). Hepatology. 1993;18:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Gonzales E, Cresteil D, Baussan C, Dabadie A, Gerhardt MF, Jacquemin E. SRD5B1 (AKR1D1) gene analysis in delta(4)-3-oxosteroid 5beta-reductase deficiency: evidence for primary genetic defect. J Hepatol. 2004;40:716-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Setchell KD, Bragetti P, Zimmer-Nechemias L, Daugherty C, Pelli MA, Vaccaro R, Gentili G, Distrutti E, Dozzini G, Morelli A. Oral bile acid treatment and the patient with Zellweger syndrome. Hepatology. 1992;15:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Wang NL, Lu YL, Zhang P, Zhang MH, Gong JY, Lu Y, Xie XB, Qiu YL, Yan YY, Wu BB, Wang JS. A Specially Designed Multi-Gene Panel Facilitates Genetic Diagnosis in Children with Intrahepatic Cholestasis: Simultaneous Test of Known Large Insertions/Deletions. PLoS One. 2016;11:e0164058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Setchell KD, Dumaswala R, Colombo C, Ronchi M. Hepatic bile acid metabolism during early development revealed from the analysis of human fetal gallbladder bile. J Biol Chem. 1988;263:16637-16644. [PubMed] |
| 14. | Berséus O, Björkhem L. Enzymatic conversion of a delta-4-3-ketosteroid into a 3-alpha-hydroxy-5-beta steroid: mechanism and stereochemistry of hydrogen transfer from NADPH. Bile acids and steroids 190. Eur J Biochem. 1967;2:503-507. [PubMed] |
| 15. | Berséus O. Conversion of cholesterol to bile acids in rat: purification and properties of a delta-4-3-ketosteroid 5-beta-reductase and a 3-alpha-hydroxysteroid dehydrogenase. Eur J Biochem. 1967;2:493-502. [PubMed] |
| 16. | Zhao J, Fang LJ, Setchell KD, Chen R, Li LT, Wang JS. Primary ∆4-3-oxosteroid 5β-reductase deficiency: two cases in China. World J Gastroenterol. 2012;18:7113-7117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Kimura A, Endo F, Kagimoto S, Inoue T, Suzuki M, Kurosawa T, Tohma M, Fujisawa T, Kato H. Tyrosinemia type I-like disease: a possible manifestation of 3-oxo-delta 4-steroid 5 beta-reductase deficiency. Acta Paediatr Jpn. 1998;40:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Siafakas CG, Jonas MM, Perez-Atayde AR. Abnormal bile acid metabolism and neonatal hemochromatosis: a subset with poor prognosis. J Pediatr Gastroenterol Nutr. 1997;25:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Shneider BL, Setchell KD, Whitington PF, Neilson KA, Suchy FJ. Delta 4-3-oxosteroid 5 beta-reductase deficiency causing neonatal liver failure and hemochromatosis. J Pediatr. 1994;124:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Clayton PT, Patel E, Lawson AM, Carruthers RA, Tanner MS, Strandvik B, Egestad B, Sjövall J. 3-Oxo-delta 4 bile acids in liver disease. Lancet. 1988;1:1283-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Strandvik B, Wahlén E, Wikström SA. The urinary bile acid excretion in healthy premature and full-term infants during the neonatal period. Scand J Clin Lab Invest. 1994;54:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kimura A, Suzuki M, Murai T, Kurosawa T, Tohma M, Sata M, Inoue T, Hoshiyama A, Nakashima E, Yamashita Y, Fujisawa T, Kato H. Urinary 7alpha-hydroxy-3-oxochol-4-en-24-oic and 3-oxochola-4,6-dien-24-oic acids in infants with cholestasis. J Hepatol. 1998;28:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Inoue T, Kimura A, Aoki K, Tohma M, Kato H. Developmental pattern of 3-oxo-delta 4 bile acids in neonatal bile acid metabolism. Arch Dis Child Fetal Neonatal Ed. 1997;77:F52-F56. [PubMed] |
| 24. | Heubi JE, Balistreri WF, Suchy FJ. Bile salt metabolism in the first year of life. J Lab Clin Med. 1982;100:127-136. [PubMed] |
| 25. | Kondo KH, Kai MH, Setoguchi Y, Eggertsen G, Sjöblom P, Setoguchi T, Okuda KI, Björkhem I. Cloning and expression of cDNA of human delta 4-3-oxosteroid 5 beta-reductase and substrate specificity of the expressed enzyme. Eur J Biochem. 1994;219:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Ueki I, Kimura A, Chen HL, Yorifuji T, Mori J, Itoh S, Maruyama K, Ishige T, Takei H, Nittono H, Kurosawa T, Kage M, Matsuishi T. SRD5B1 gene analysis needed for the accurate diagnosis of primary 3-oxo-Delta4-steroid 5beta-reductase deficiency. J Gastroenterol Hepatol. 2009;24:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Al-Hussaini AA, Setchell KDR, AlSaleem B, Heubi JE, Lone K, Davit-Spraul A, Jacquemin E. Bile Acid Synthesis Disorders in Arabs: A 10-year Screening Study. J Pediatr Gastroenterol Nutr. 2017;65:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Jacquemin E, Setchell KD, O'Connell NC, Estrada A, Maggiore G, Schmitz J, Hadchouel M, Bernard O. A new cause of progressive intrahepatic cholestasis: 3 beta-hydroxy-C27-steroid dehydrogenase/isomerase deficiency. J Pediatr. 1994;125:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Setchell KDR, Balistreri WF, Piccoli DA, Clerici C, Paumgartner G, Stiehl A, Gerok W. Oral bile acid therapy in the treatment of inborn errors in bile acid synthesis associated with liver disease. Paumgartner G, Stiehl A, Gerok W. Bile Acids as Therapeutic Agents: From Basic Science to Clinical Practice. London: Kluwer Academic Publishers 1990; 367-373. |
| 30. | Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology. 2012;55:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Horslen SP, Lawson AM, Malone M, Clayton PT. 3 beta-hydroxy-delta 5-C27-steroid dehydrogenase deficiency; effect of chenodeoxycholic acid therapy on liver histology. J Inherit Metab Dis. 1992;15:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Drury JE, Mindnich R, Penning TM. Characterization of disease-related 5beta-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency. J Biol Chem. 2010;285:24529-24537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Clayton PT, Mills KA, Johnson AW, Barabino A, Marazzi MG. Delta 4-3-oxosteroid 5 beta-reductase deficiency: failure of ursodeoxycholic acid treatment and response to chenodeoxycholic acid plus cholic acid. Gut. 1996;38:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Schoenfield LJ, Lachin JM. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann Intern Med. 1981;95:257-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 319] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Riello L, D'Antiga L, Guido M, Alaggio R, Giordano G, Zancan L. Titration of bile acid supplements in 3beta-hydroxy-Delta 5-C27-steroid dehydrogenase/isomerase deficiency. J Pediatr Gastroenterol Nutr. 2010;50:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |