Published online Dec 28, 2019. doi: 10.3748/wjg.v25.i48.6928
Peer-review started: October 21, 2019
First decision: November 9, 2019
Revised: December 13, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: December 28, 2019
Processing time: 68 Days and 1.2 Hours
Alterations in health-related quality of life (HRQoL) and neuropsychological disorders were described in the hepatitis C virus (HCV) patients. Although several studies investigated the modifications of HRQoL after HCV eradication, no data exists on the modifications of neuropsychological symptoms.
To investigate the effect of directly acting antivirals (DAAs) treatment on HRQoL and neuropsychological symptoms.
Thirty nine patients with HCV infection underwent a neuropsychological assessment, including Zung-Self Depression-Rating-Scale, Spielberg State-Trait Anxiety Inventory Y1-Y2 and the Toronto-Alexithymia Scale-20 items before and after DAAs treatment. HRQoL was detected by Short-Form-36 (SF-36).
All HRQoL domains, but role limitation physical and bodily pain, significantly improved after treatment. Interestingly, after DAAs treatment, all domains of HRQoL returned similar to those of controls. Each neuropsychological test significantly improved after HCV eradication. A significant correlation was observed among each psychological test and the summary components of SF-36. At multiple linear regression analysis including each psychological test as possible covariates, Zung-Self Depression Rating Scale (Zung-SDS) score was independently and significantly related to summary components of the SF-36 in the basal state and the difference between Zung-SDS score before and after treatment was the only variable significantly and independently related to the modification of HRQoL induced by the treatment.
Neuropsychological symptoms strongly influenced HRQoL in HCV patients and there was a significant improvement of neuropsychological tests and HRQoL after DAAs treatment.
Core tip: In patients with the hepatitis C virus (HCV) infection, alterations in health-related quality of life (HRQoL) and neuropsychological disturbances were described also in the absence of liver cirrhosis. During the last years, HCV therapy has evolved from interferon-based to directly acting antiviral (DAA)-based therapy, with excellent tolerability and efficacy. Until now, few data exist on the modifications of neuropsychological symptoms before and after DAAs treatment and on the relationship of these symptoms on HRQoL. With this study we demonstrated that HCV eradication with DDAs treatment significantly improves health-related quality of life and neuropsychological symptoms.
- Citation: Nardelli S, Riggio O, Rosati D, Gioia S, Farcomeni A, Ridola L. Hepatitis C virus eradication with directly acting antivirals improves health-related quality of life and psychological symptoms. World J Gastroenterol 2019; 25(48): 6928-6938
- URL: https://www.wjgnet.com/1007-9327/full/v25/i48/6928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i48.6928
The hepatitis C virus (HCV) infection is one of the most frequent causes of liver cirrhosis in the Western Word with consequent significant social and health burden[1]. It is known that patients with chronic medical illness, such as liver cirrhosis, may be afflicted by symptoms of depression[2] anxiety[3] and alexithymia[4,5], especially in the end stage of their liver disease, which is characterized by frequent medical complications, hospitalization, functional limitation and change of body image. In patients with HCV infection, alterations in health-related quality of life (HRQoL) and neuropsychological disturbances were described also in the absence of liver cirrhosis[6,7]. In fact, even in the absence of debilitating symptoms, HCV may adversely affects the HRQoL by negatively impacting on the physical and mental well-being of the patients[8]. Nonspecific symptoms such as asthenia, irritability, general malaise, muscle and joint aches and headaches may occur in HCV patients[9]. In most cases these symptoms do not require medical intervention but affect the sense of physical well-being and cause emotional problems. It has been suggested that the virus may impact on cognitive functions thereby evoking abnormal changes in sleep patterns and attention deficit regardless of the stage of the disease[10,11]. Finally, apprehension for themself and for the patients’ family may contribute to the development of anxiety and depressive symptoms.
Many authors have postulated a direct action of the HCV virus at the CNS level and viral replicates have been detected in the cerebral tissue but it is difficult to determine whether the neuropsychiatric symptoms are due to a direct neurotoxicity of the virus per se or to emotional stress related to functional deficit, social stigma and to apprehension for long-term prognosis[10,11].
In the past, patients with a diagnosed mental health disease (MHD), defined as either a Diagnostic and Statistical Manual of Mental Disorders (DSM) (DSM IV) diagnosis of major depression, bipolar disorder, schizophrenia, generalized anxiety, and post-traumatic stress disorder or requiring anti-depressants, antipsychotics, mood stabilizers or psychotropic prescribed by a psychiatrist, were marginalized with respect to HCV therapy and MHD was one of the most frequently cited reason for exclusion from HCV interferon-based therapy[12]. During the last years, HCV therapy has evolved from interferon-based to directly acting antiviral (DAA)-based therapy, with excellent tolerability and efficacy[13]. Although several studies investigated the modifications of HRQoL after HCV eradication[14-18], until now, to our knowledge, no data exists on the modifications of neuropsychological symptoms (depression, anxiety and alexithymia symptoms) before and after directly acting antivirals (DAAs) treatment and on the relationship of these symptoms on HRQoL. Thus, aim of the present study was to investigate the effect of HCV infection on HRQoL and neuropsychological symptoms and to analyse the modifications of these parameters after DAAs treatment.
From January 2018 to January 2019, thirty-nine patients with HCV infection submitted to Ledipasvir/Sofosbuvir treatment, were consecutively enrolled at “Santa Maria Goretti” Hospital of Latina, Sapienza University of Rome. The diagnosis of HCV infection was based on history, clinical examination, biochemical, elastography and ultrasound findings. Liver fibrosis was quantified according to Metavir staging system[19], based on Fibroscan® score. Exclusion criteria were: HIV co-infection; presence of a previously diagnosed mental health disease, defined as either a DSM IV diagnosis of major depression, bipolar disorder, schizophrenia, generalized anxiety, and post-traumatic stress disorder or requiring anti-depressants, antipsychotics, mood stabilizers or psychotropics prescribed by a psychiatrist; alcohol abuse in the last 3 mo; other causes of liver disease different from HCV infection.
An informed written consent to participate to the study was obtained by all the patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethical and the Research Committees of the “Sapienza” University of Rome.
All neuro-psychological assessment was carried on by an expert (D.R.) who administered the questionnaires. Depressive symptoms were assessed by the Zung-Self Depression Rating Scale (Zung-SDS)[20], which includes 20 items with negative and positive contents. Patients have to mark the answer that best suits their present state of mind. Each answer is given 1 to 4 points. A higher total score corresponds to the presence of depressive symptoms; the cut-off is 50 out of 80.
Anxiety symptoms were detected by the Spielberg State-Trait Anxiety Inventory (STAI)Y1-Y2[21], a widely used anxiety rating scale. It consists of 40 items, each graded from 1 to 4. The scale differentiates anxiety into (1) anxiety caused by a specific condition (state anxiety); (2) anxiety as a more permanent patient disorder (trait anxiety). The main variables that the questionnaire measures are anxiety, apprehension, nervousness and tension. A high score is associated with higher anxiety level; the cut-off is 60 points out of 80.
Alexithymic symptoms were detected by 20-item Toronto alexithymia scale (TAS-20)[22]. This test comprises three scales: Difficulty identifying feelings (DIF: Seven items), difficulty describing feelings (DDF: Five items), and Externally-Oriented Thinking (EOT: Eight items). Items are rated using a 5-point Likert scale whereby 1 = strongly disagree and 5 = strongly agree. The total alexithymia score is the sum of responses to all 20 items, the cut-off is a score > 60.
HRQoL was evaluated with Short Form-36[23], a paper-pencil questionnaire that consists of 36 multple-choice questions. It measures eight domains, four in the area of “physical health” (physical functioning, role limitation-physical, bodily pain, general health) and four in the area of “mental health” (role limitation-emotional, vitality, mental health and social functioning). Each domain earns between 0 and 100 points, with higher scores indicating a better HRQoL. Two comprehensive indices of HRQoL may also be computed: The physical component summary (PCS) and the mental component summary (MCS). The Short-Form-36 (SF-36) was evaluated according to the Apolone et al[24]’s instructions, using a free, downloadable software which allows each patient to be compared with one healthy Italian man or woman. With this software we obtain control values of healthy Italian population matched for age, gender, degree of education, job and marital status. All questionnaires were administered before DAAs treatment and after the evidence of SVR, defined as undetectable viral load 12 weeks after the end of the therapy.
Data are presented as means ± SD. Comparisons among groups and before and after DAA treatment were performed by unpaired or paired Student-t-test or paired χ2 for quantitative and qualitative data, respectively. For continuous variables that showed abnormal distribution Wilcoxon Signed-Rank Test was used, likewise McNemar Test was used to compare nonparametric dependent groups. The relationship between the scores of each psychological test and the PCS or MCS of SF-36 was evaluated by Pearson’s correlation coefficients. P < 0.05 were considered statistically significant. Two multiple linear regression analyses were used to identify the variables related to the component summary of SF-36 at basal state and to its variations before and after treatment (i.e. the difference between the final and initial value of each test). Zung-SDS, TAS-20, STAI Y1 and STAI Y2 scores were included in the multivariate analyses. All the analyses will be performed using the SPSS software Version 16.0.
The sample included 39 patients with HCV infections, 23 men and 16 women; among them 9 patients were classified as affected by well compensated cirrhosis (7 Child-Pugh class A and 2 Child-Pugh class B) on clinical and radiological findings and on the degree of the fibrosis according to Metavir score = F4. Comparing patients with and without cirrhosis there was no statistical difference in albumin levels (3.75 ± 0.59 vs 4.10 ± 0.38 g/dL; P = 0.15), bilirubin levels (1.04 ± 0.36 vs 0.67 ± 0.29 mg/dL; P = 0.09), INR (1.17 ± 0.54 vs 0.98 ± 0.16). Given the similarity of the two groups and the small number of the patients, we decided to consider all patients as a single group.
The mean age of the patients was 59.8 ± 14.2 years; twenty-five of these had genotype 1, five patients genotype 2, seven patients genotype 3 and two patients genotype 4. Only 14 patients were naive, the remaining twenty five patients were previously submitted to IFN-based regimens. The main clinical and demographic features of the patients are reported in Table 1. After DAAs treatment, all patients achieved sustained virological response and, as expected, there was a significant amelioration of serum transaminases: ALT (U/L) 75.1 ± 77.8 vs 26 ± 7.1, P < 0.001; AST (U/L) 101.9 ± 86.5 vs 27.3 ± 5.5, P < 0.001.
| Characteristic | Data |
| Men/Women | 23/16 |
| Age (yr) | 59.8 ± 14.2 |
| Hepatitis C virus-RNA (UI/mL) | 1.624 × 103 ± 1.522 × 103 |
| Hepatitis C virus genotype (n) | |
| 1a/1b | 4/21 |
| 2 | 5 |
| 3 | 7 |
| 4 | 2 |
| Fibrosis according to Metavir (n) | |
| F0-F2 | 21 |
| F3-F4 | 18 |
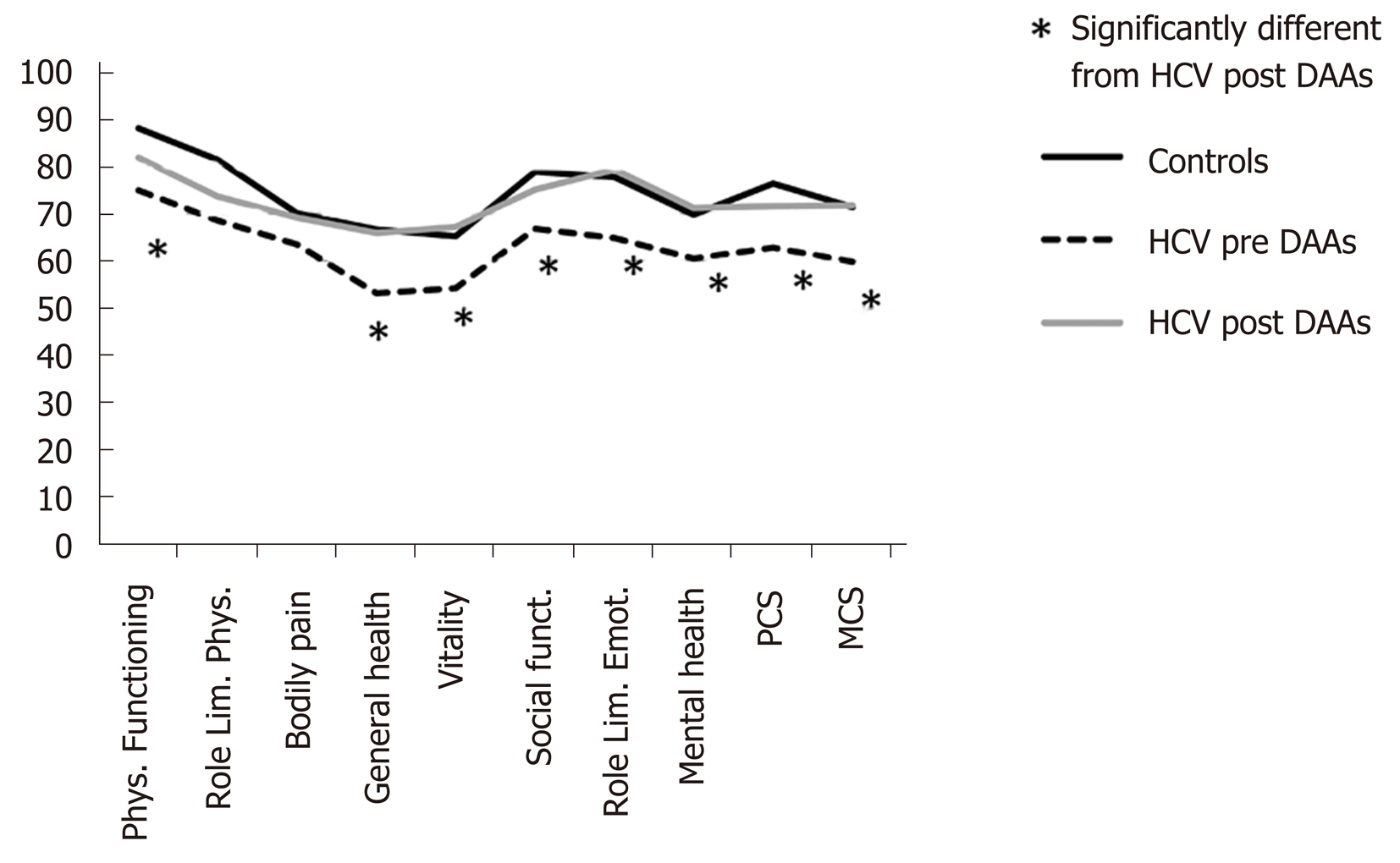
The comparison between HRQoL domains of controls and patients before and after treatment with DAAs is shown in Figure 1. As widely described in literature, also in our sample the quality of life was significantly worse in HCV patients compared to controls values. The results of SF-36 before and after HCV eradication are shown in Figure 1 and Table 2. All HRQoL domains, but role limitation physical, bodily pain and social functioning, significantly improved after DAAs treatment. Interestingly, after DAAs treatment, all domains of HRQoL returned similar to those of healthy controls (Figure 1).
| Pre DAAs (n = 39) | Post DAAs (n = 39) | P value | |
| Physical functioning | 75 ± 27.3 | 81.9 ± 22.2 | 0.005 |
| Role limitation physical | 68.6 ± 40.4 | 73.7 ± 36.3 | 0.35 |
| Bodily pain | 63.5 ± 32.2 | 69.2 ± 27.1 | 0.19 |
| General health | 53.1 ± 21.2 | 65.9 ± 17.5 | < 0.001 |
| Vitality | 54.2 ± 20.4 | 67.2 ± 18,1 | < 0.001 |
| Social functioning | 66.8 ± 25.4 | 75.1 ± 20.6 | 0.03 |
| Role limitation emotional | 64.8 ± 39.7 | 79.4 ± 34.7 | 0.02 |
| Mental health | 60.5 ± 22.2 | 71.2 ± 18.9 | 0.002 |
| Physical component summary | 62.8 ± 22.7 | 71.6 ± 21.2 | 0.002 |
| Mental component summary | 59.8 ± 21.9 | 71.8 ± 19.1 | < 0.001 |
The results of Zung-SDS, STAI Y1-Y2 and TAS-20 before and after DAAs treatment are reported in Table 3. A significant improvement after DAAs treatment was observed for each test. According to the cut of value of each test, 8/39 (20%) had depressive symptoms (Zung ≥ 50), 2/39 (5%) had symptoms related to state anxiety (STAI-Y2 ≥ 60), 4/39 (10%) symptoms related to trait anxiety (STAI-Y1 ≥ 60), and 11/39 (28%) had alexithymia (TAS-20 ≥ 60) at basal evaluation. The prevalence of neuropsychological disorders decreased after HCV eradication without reaching the statistical significance (Table 4).
| Pre DAAs (n = 39) | Post DAAs (n = 39) | P value | |
| TAS-20 | 49.2 ± 14.2 | 42.5 ± 12.6 | < 0.001 |
| Zung-SDS | 41.7 ± 9.3 | 37.1 ± 8.6 | < 0.001 |
| STAI-Y1 | 39.8 ± 11.9 | 32.8 ± 7.8 | < 0.001 |
| STAI-Y2 | 41.4 ± 12.2 | 35.7 ± 9.2 | < 0.001 |
| Pre DAAs (n = 39) | Post DAAs (n = 39) | P value | |
| Zung SDS ≥ 50 (no/yes) | 8/31 (20) | 4/35 (10) | Ns |
| STAI Y1 ≥ 60 (no/yes) | 37/2 (5) | 39/0 (0) | Ns |
| STAI Y2 ≥ 60 (no/yes) | 35/4 (10) | 39/0 (0) | Ns |
| TAS-20 ≥ 60 (no/yes) | 28/11 (28) | 34/5 (13) | Ns |
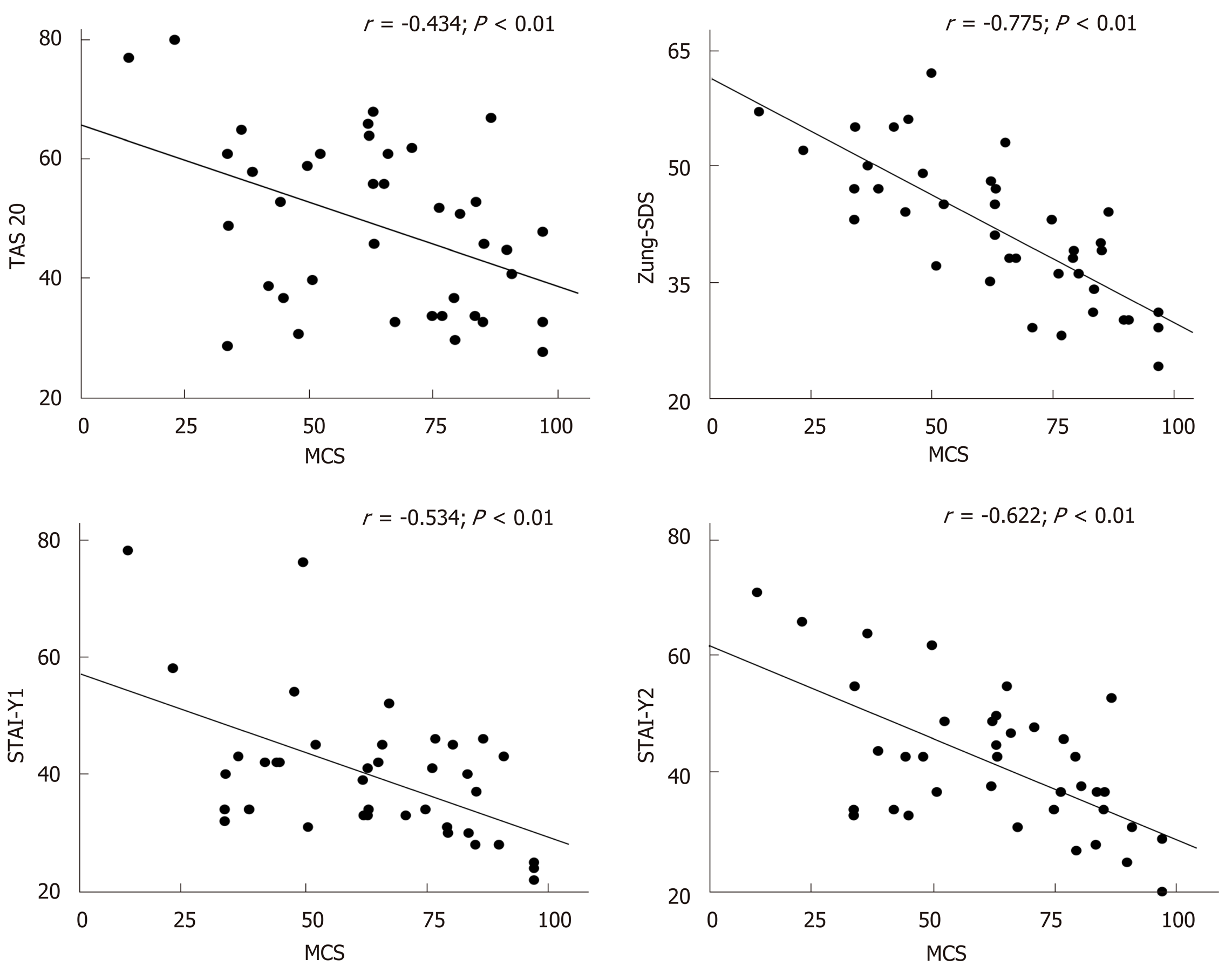
A significant correlation between each neuropsychological test and the two components of HRQoL (PCS and MCS) at basal evaluation was found (Figure 2 and Table 5). Significant correlations were also observed between the variations of Zung-SDS, TAS-20, STAI-Y1 and Y2 and HRQoL components (i.e., the difference between the final and initial value of each test) before and after DAAs treatment, as shown in Figure 3 and Table 6.
Multiple linear regression analysis was used to identify the possible covariates related to the physical and mental component of the SF-36. Scores tests evaluating depression, anxiety and alexithymia symptoms were included in the analysis as possible covariates. Zung score was independently and significantly related to both the mental and physical component of the SF-36 in the basal state and the difference between Zung score before and after treatment was the only variable significantly and independently related to the modification of HRQoL induced by the treatment (Table 7).
| Beta | SE (Beta) | T value | P value | |
| Physical component summary | ||||
| Zung-SDS | -1.99 | 0.37 | -5.38 | < 0.001 |
| Mental component summary | ||||
| Zung-SDS | -1.53 | 0.33 | -4.62 | < 0.001 |
| Δ Physical component summary | ||||
| Δ Zung-SDS | -1.58 | 0.32 | -4.86 | < 0.001 |
| Δ Mental component summary | ||||
| Δ Zung-SDS | -1.49 | 0.29 | -4.98 | < 0.001 |
It is estimated that approximately 71 million individuals are infected with HCV worldwide[1]. Diagnosis of HCV infection can significantly affect the patients’ social life. In the decompensated state of the disease, due to the inability to hold down a job, many HCV patients experience financial difficulties along with a sensation of awkwardness, social denial and all of this may lead to a worsening of their socioeconomic status and quality of life[25].
As previously demonstrated in a cohort of cirrhotic patients[5], among the factors that could influence HRQoL there are psychological symptoms such as anxiety, depression and alexithymia. Even in the absence of decompensated cirrhosis, patients with chronic HCV infection, can develop an altered emotional status as well as anxiety and depressive symptoms. Many unfounded reasons could be involved: The awareness of having a chronic disease potentially with a poor long-term prognosis, the shame for having been infected, the fear of infecting loved ones, the guilt of having an infection which still today may represent a social stigma as sexually transmitted or substances abuse diseases. Moreover, until a few years ago, the patients were also worried about the limited eradication capacity of interferon based regimens and by their innumerable side effects[25]. The present study confirms that the HRQoL is impaired in HCV population as already observed[8,12-16,25] even in patients with compensated disease. Fortunately, the emergence of DAAs, with high efficacy rates and tolerability profile, have completely revolutionized the treatment of chronic HCV treatment[13].
Health-related quality of life is a key component in the evaluation of any therapeutic intervention. It may be more relevant than length of life, because patients are frequently more concerned about quality and disability than about longevity[26]. Several studies have already investigated the modifications of HRQoL after HCV eradication with the new IFN-free regimen[14]. In particular the trial conducted by Ng et al[17] demonstrated that HCV treatment with Elbasvir/Grazoprevir resulted in a significant improvement of patients reported outcomes compared to Sofosbuvir/Peg-IFN/Ribavirin regimen. Antoher Brasilian study[18] evaluated the modifications of HRQoL measured with SF36 and CLDQ in a cohort of 56 HCV patients treated with different DAAs regimens and they found a significant amelioration of both tests after HCV eradication. These results were confirmed in the present study. In fact, all domains of HRQoL, but role limitation physical and bodily pain, evaluated after the end of therapy significantly improved after DAAs treatment. Interestingly, differently from that observed in the study of Juanbeltz et al[18] in which HRQoL remains lower than that of general population despite viral clearance, in our population HRQoL after HCV eradication returned similar to that of healthy controls. These different results may be related to the greater number of cirrhotics enrolled in the Juanbeltz et al[18] study.
Especially in patients with HCV related compensated disease the improvement of HRQoL observed after HCV eradication could be likely due to an amelioration of the emotional and psychological well being. Therefore, the hypothesis of the present study was to test the role of emotional status evaluated by standardized tests able to detect symptoms of anxiety, depression and alexithymia on HRQoL in HCV patients. A second aim was to relate the modification of HRQoL to those of neuropsychological test after DAA treatment. At basal evaluation, significant correlations were found between all neuropsychological test and component summary of HRQoL. When the questionnaires used to evaluate anxiety, depression and alexithymia were included in a multivariate analysis, Zung score, a measure of depressive tract, was the only variable independently correlated to both physical and mental component of HRQoL, confirming the hypothesis that chronic HCV infection could induce the occurrence of depressive symptoms. Moreover and more interestingly, we found a significant improvement of neuropsychological tests after HCV eradication and a significant correlation between the modification of these tests and of HRQoL, after therapy. As for the basal state the modification of the Zung score was the major determinant of the variation of the HRQoL. To our knowledge, no study investigated the modifications of depression, anxiety and alexithymia symptoms before and after DAAs treatment.
The study has some limitation: The sample size is limited and the majority of patients were affected by chronic hepatitis or very well compensated cirrhosis. Consequently the conclusion on the relationship between the modification of neuropsychological disorders and HRQoL cannot be extended to more advanced stage of HCV disease. Moreover, all patients were treated with a single regimen (Ledipasvir/Sofosbuvir). Thus, the observed effects cannot be formally extended to other treatments. On the other hand, the homogeneity of the study is higher than that obtainable using different drugs.
In conclusion, depression, state and trait anxiety and alexithymia symptoms are among the major determinants of the altered HRQoL, even in patients with chronic HCV infection, without liver cirrhosis. Therefore, the detection of these symptoms appears to be important when the patients’ health status perception has to be considered.
In patients with hepatitis C virus (HCV) infection, alterations in health-related quality of life (HRQoL) and neuropsychological disturbances were described also in the absence of liver cirrhosis.
During the last years, HCV therapy has evolved from interferon-based to directly acting antiviral (DAA)-based therapy, with excellent tolerability and efficacy.
No data exists on the modifications of neuropsychological symptoms before and after DAAs treatment and on the relationship of these symptoms on HRQoL.
All patients included in the study underwent a neuropsychological assessment, including Zung-Self Depression-Rating-Scale, Spielberg State-Trait Anxiety Inventory Y1-Y2 and the Toronto-Alexithymia Scale-20 items before and after DAAs treatment. HRQoL was detected by Short-Form-36.
In this study we demonstrated for the first time that HCV eradication strongly improves depression, anxiety and alexithymia symptoms and HRQoL.
HCV eradication is important non only in patients with liver cirrhosis, but also in patients with chornic hepatitis because significantly improves health related quality of life and neuropsychological symptoms.
Further studies are needed to confirm improvements in HRQoL and neuropsychological symptoms even after years of DAAs treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Montasser I, Xu XY S-Editor: Wang J L-Editor: A E-Editor: Zhang YL
| 1. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 514] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (7)] |
| 2. | Singh N, Gayowski T, Wagener MM, Marino IR. Depression in patients with cirrhosis. Impact on outcome. Dig Dis Sci. 1997;42:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | De Bona M, Ponton P, Ermani M, Iemmolo RM, Feltrin A, Boccagni P, Gerunda G, Naccarato R, Rupolo G, Burra P. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Marwaha S, He Z, Broome M, Singh SP, Scott J, Eyden J, Wolke D. How is affective instability defined and measured? A systematic review. Psychol Med. 2014;44:1793-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Nardelli S, Pentassuglio I, Pasquale C, Ridola L, Moscucci F, Merli M, Mina C, Marianetti M, Fratino M, Izzo C, Merkel C, Riggio O. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metab Brain Dis. 2013;28:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Prell T, Dirks M, Arvanitis D, Braun D, Peschel T, Worthmann H, Schuppner R, Raab P, Grosskreutz J, Weissenborn K. Cerebral patterns of neuropsychological disturbances in hepatitis C patients. J Neurovirol. 2019;25:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | García-Guerrero MA, Sánchez Gómez P, Peña Lasa J, Portu Zapirain J, Elizagárate Zabala E, Gorria Bernal V, Ojeda Del Pozo N. Effect of psychiatric symptoms and quality of life on cognitive performance in HCV patients. Rev Psiquiatr Salud Ment. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Foster GR. Quality of life considerations for patients with chronic hepatitis C. J Viral Hepat. 2009;16:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31 Suppl 1:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Forton DM, Thomas HC, Taylor-Robinson SD. Central nervous system involvement in hepatitis C virus infection. Metab Brain Dis. 2004;19:383-391. [DOI] [Full Text] |
| 11. | Forton DM, Hamilton G, Allsop JM, Grover VP, Wesnes K, O'Sullivan C, Thomas HC, Taylor-Robinson SD. Cerebral immune activation in chronic hepatitis C infection: a magnetic resonance spectroscopy study. J Hepatol. 2008;49:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Tang LS, Masur J, Sims Z, Nelson A, Osinusi A, Kohli A, Kattakuzhy S, Polis M, Kottilil S. Safe and effective sofosbuvir-based therapy in patients with mental health disease on hepatitis C virus treatment. World J Hepatol. 2016;8:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ioannou GN, Feld JJ. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology. 2019;156:446-460.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 14. | Goñi Esarte S, Juanbeltz R, Martínez-Baz I, Castilla J, San Miguel R, Herrero JI, Zozaya JM. Long-term changes on health-related quality of life in patients with chronic hepatitis C after viral clearance with direct-acting antiviral agents. Rev Esp Enferm Dig. 2019;111:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Juanbeltz R, Castilla J, Martínez-Baz I, O'Leary A, Sarobe M, San Miguel R. Health-related quality of life in hepatitis C patients who achieve sustained virological response to direct-acting antivirals: a comparison with the general population. Qual Life Res. 2019;28:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Juanbeltz R, Martínez-Baz I, San Miguel R, Goñi-Esarte S, Cabasés JM, Castilla J. Impact of successful treatment with direct-acting antiviral agents on health-related quality of life in chronic hepatitis C patients. PLoS One. 2018;13:e0205277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Ng X, Nwankwo C, Arduino JM, Corman S, Lasch KE, Lustrino JM, Patel S, Platt HL, Qiu J, Sperl J. Patient-reported outcomes in individuals with hepatitis C virus infection treated with elbasvir/grazoprevir. Patient Prefer Adherence. 2018;12:2631-2638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Siqueira FM, Ferreira VL, Borba HHL, Pontarolo R. Quality of life of Brazilian chronic hepatitis C patients treated with interferon-free therapies. Rev Inst Med Trop Sao Paulo. 2018;60:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 20. | Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 761] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467-S472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1092] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 22. | Leisinga D, Grandeb T, Faberb R. The Toronto Alexithymia Scale (TAS-20): A measure of general psychological distress. J Research Personality. 2009;43:707-710. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 24. | Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 870] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 25. | Cillo U, Amodio P, Ronco C, Soni SS, Zanus G, Minazzato L, Salari A, Neri D, Bombonato G, Schiff S, Bianco T. Hepatitis C virus adversely affects quality of life. Blood Purif. 2011;32:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | McNeil BJ, Weichselbaum R, Pauker SG. Speech and survival: tradeoffs between quality and quantity of life in laryngeal cancer. N Engl J Med. 1981;305:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 448] [Article Influence: 10.2] [Reference Citation Analysis (0)] |