Published online Oct 28, 2019. doi: 10.3748/wjg.v25.i40.6158
Peer-review started: June 25, 2019
First decision: July 21, 2019
Revised: September 4, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: October 28, 2019
Processing time: 126 Days and 1.2 Hours
The optimal way to home-monitor patients with inflammatory bowel disease (IBD) for disease progression or relapse remains to be found.
To determine whether an electronic health (eHealth) screening procedure for disease activity in IBD should be implemented in clinical practice, scheduled every third month (3M) or according to patient own decision, on demand (OD).
Adult IBD patients were consecutively randomized to 1-year open-label eHealth interventions (3M vs OD). Both intervention arms were screening for disease activity, quality of life and fatigue and were measuring medical compliance with the constant care web-application according to the screening interventions OD or 3M. Disease activity was assessed using home measured fecal calprotectin (FC) and a disease activity score.
In total, 102 patients were randomized (n = 52/50 3M/OD) at baseline, and 88 patients completed the 1-year study (n = 43 3M; n = 45 OD). No difference in the two screening procedures could be found regarding medical compliance (P = 0.58), fatigue (P = 0.86), quality of life (P = 0.17), mean time spent in remission (P > 0.32), overall FC relapse rates (P = 0.49), FC disease courses (P = 0.61), FC time to a severe relapse (P = 0.69) and remission (P = 0.88) during 1 year. Median (interquartile range) numbers of FC home-monitoring test-kits used per patient were significantly different, 3M: 6.0 (5.0-8.0) and OD: 4.0 (2.0-9.0), P = 0.04.
The two eHealth screening procedures are equally good in capturing a relapse and bringing about remission. However, the OD group used fewer FC home test-kits per patient. Individualized screening procedures can be recommended for adult IBD patients in clinical web-practice.
Core tip: Involving patients with inflammatory bowel disease (IBD) in their disease by home-monitoring of disease activity has been shown to bring patients with IBD significantly faster in remission relative to standard care. However, the optimal way to home-monitor patients with IBD for disease progression or relapse remains to be found. We randomized 102 patients with IBD to screen for disease activity either every third month or whenever the patients felt a need for screening on the ibd.constant-care.com web-application for 1 year. We found that the two screening procedures were equally good in capturing a relapse and bringing about remission.
- Citation: Ankersen DV, Weimers P, Marker D, Bennedsen M, Saboori S, Paridaens K, Burisch J, Munkholm P. Individualized home-monitoring of disease activity in adult patients with inflammatory bowel disease can be recommended in clinical practice: A randomized-clinical trial. World J Gastroenterol 2019; 25(40): 6158-6171
- URL: https://www.wjgnet.com/1007-9327/full/v25/i40/6158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i40.6158
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory disease of the gastrointestinal tract that results from interactions of the intestinal immune system, the gut microbiome, epigenetics and environmental factors[1]. Relapse rates of patients with IBD range from 30%-50% on a yearly basis[2-4]. Relapses in IBD are categorized according to severity[2-4], which has been linked to increased health care costs and reduced quality of life relative to patients in remission[5].
IBD is associated with significant direct and indirect costs for patients and society, and as the number of patients is expected to grow worldwide during the coming years, IBD constitutes a significant health care burden[5-7]. Therefore, new initiatives capable of reducing the burden of IBD are warranted[7]. Screening of disease activity using electronic health (eHealth) or mobile health applications has previously been shown to reduce time to remission[8] and hospital admissions and outpatients visits[9], to increase compliance with medical therapy and quality of life and to empower IBD patients[8]. eHealth offers patients a quick and easily understandable tool for disease monitoring, with faster recognition of disease relapse, greater engagement with their treatment and easier access to health care. All of these benefits support patients in optimizing their individualized treatment[10,11]. Close monitoring of disease activity is vital for optimizing individualized treatments and to improve potentially the long-term disease course. However, the type and frequency of optimal disease monitoring remain unknown.
The primary aim of this study was to determine if an eHealth home monitoring screening procedure for disease activity in adult IBD patients should be implemented in clinical practice every third month (3M) or according to IBD patients own belief, on demand (OD).
Adult UC and CD patients (18 years or older) who fulfilled the Copenhagen diagnostic criteria for IBD[2,3], being on any medical IBD therapy and in remission [Simple Clinical Colitis Activity Index (SCCAI) ≤ 2 or Harvey-Bradshaw index (HBI) < 5] or with mild-to-moderate disease activity (SCCAI 3-4 and HBI 5-16) were consecutively enrolled from the outpatient clinic at the Department of Gas-troenterology, North Zealand University Hospital, Denmark, between July 8 2015 and July 25, 2016. It was mandatory for patients to speak Danish and have a smartphone. Patients were excluded if they had severe disease activity (SCCAI ≥ 5 or HBI > 16), a severe mental disorder or were abusing drugs at the time of inclusion.
This study was a 1-year open-label randomized trial (1:1) of adult IBD patients using the constant care platform[10] for self-monitoring of disease activity. Patients were randomized to either be screened for disease activity whenever they felt necessary (OD group) or scheduled to be screened every 3M.
Following their inclusion, patients allocated to either the OD or 3M group received training (approximately 1 hour) by IBD eHealth nurses in how to use the Constant Care platform, how to score themselves, and how to measure their levels of fecal calprotectin (FC) at home. Both groups of patients were instructed in how to screen for disease activity at baseline and at their 1-year follow-up. Patients randomized to the OD group were instructed in how to screen for disease activity when they felt a need to, whereas patients allocated to the 3M group were instructed in how to screen themselves every 3M. If patients in the 3M group felt a recurrence in disease activity between the scheduled evaluations, they were required to take new disease activity home measures.
If patients experienced a recurrence of disease visualized on constant care web-application (web-app), they were instructed to contact the electronic care (eCare) personnel by phone or via the patient’s personal web-wall, for an early consultation to assess the need of individualized treatment adjustment or diagnostic investigation. Daily web ward rounds were performed by the eCare nurses in close collaboration with a medical doctor.
The Ethical Committee in Denmark (H-15005603) and the Danish Data Protection Agency (Suite no.: 03806, NOH-2015-021) approved the study. The study was also registered at clinicaltrials.gov, No. NCT02492555. All patients in the study gave written informed consent prior to their inclusion.
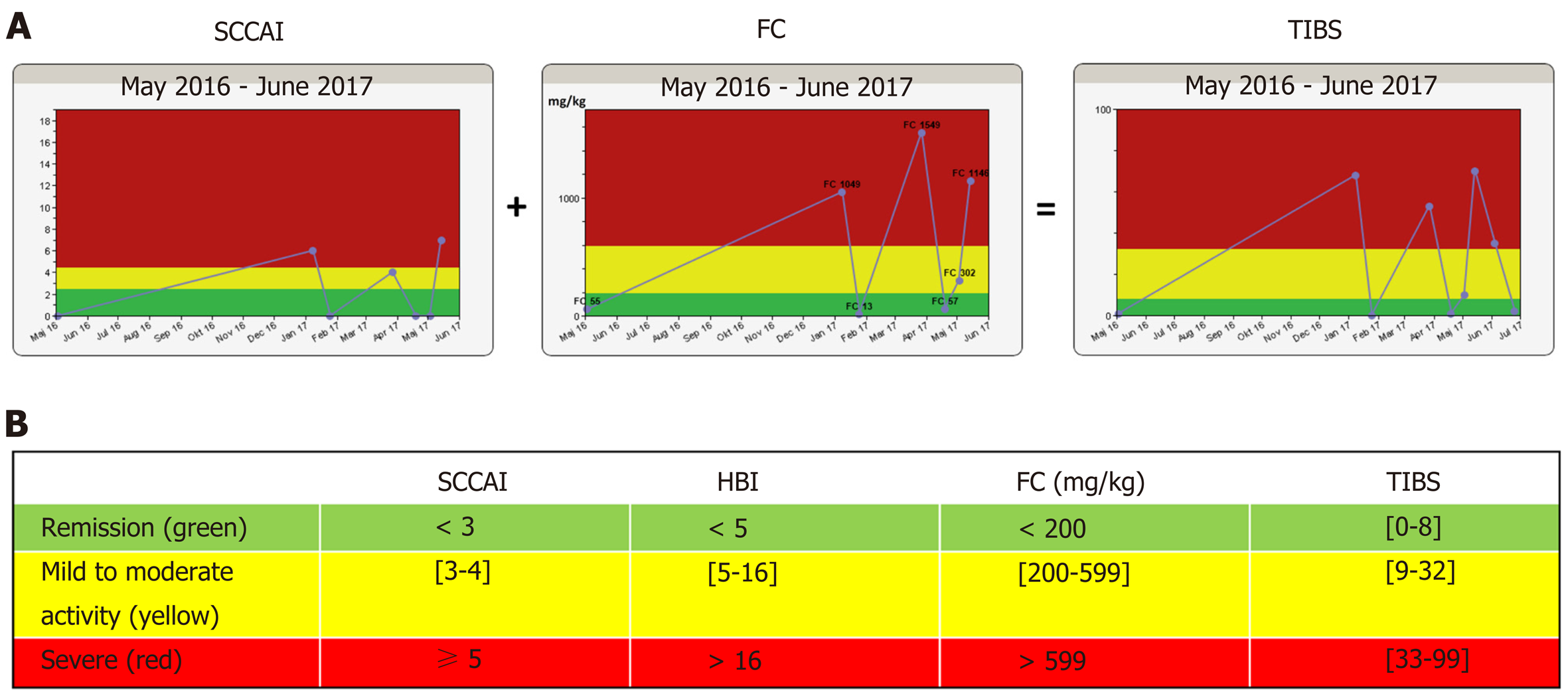
The disease algorithm in the constant care application[10] for UC and CD consists of a subjective score, either SCCAI[12] for UC or HBI[13] for CD, plus a validated FC home testing kit[14]. These two measures were added together in a weighted manner, providing the total inflammation burden score (TIBS). Disease activity measures (SCCAI, HBI, FC and TIBS) are shown in a “traffic light” form to the patient and eCare providers (Figure 1). The TIBS score can only be generated if subjective disease activity scores (HBI/SCCAI) and FC were registered on the same day. In a few cases where FC and the subjective disease scores were up to 14 d apart, they were manually calculated. The cut-off values used for disease activity measures, defining remission (green zone), mild-to-moderate activity (yellow zone) and severe activity (red zone), are shown in Figure 1. A flare-up (FC/SCCAI) was defined as a green disease activity score for at least 30 d followed by either a yellow or a red score.
Based on disease activity measures, an electronic list (green, yellow and red) was automatically generated for surveillance purposes (web rounds). Beside the disease activity algorithm, the constant care web-app also consists of electronic questionnaires {concerning medical adherence [medical adherence report scale (MARS)], fatigue and quality of life} and additional features (e.g., an e-learning quiz and personal web wall where patients can communicate with eCare personnel at the hospital).
FC measurements were performed by the patients at home using the CalproSmartTM application (Calpro AS, Lysaker, Norway). This home test can be performed in 18 min and is integrated into the constant care web-app, giving the patients the opportunity to see FC results longitudinally and in a traffic light form on the constant care web-app (Figure 1). The FC home test[14] and the disease activity algorithm in constant care (Figure 1) have been described in greater detail elsewhere[10,14].
Montreal classifications[15,16] (extent, location and behavior) were derived from electronic patient files at inclusion and stating the highest progression step. One-year disease courses were generated assuming linearity between measuring points of HBI, SCCAI, FC and TIBS. Two internal assessors (Munkholm P and Ankersen DV) characterized the individual disease courses according to the definitions listed below: (1) Chronic continuous course, red throughout 1 year; (2) Chronic continuous course, yellow throughout 1 year; (3) Chronic continuous course, red and yellow throughout 1 year; (4) Continuous remission course, green throughout 1 year; (5) Intermittent course; green, yellow and red throughout 1 year; and (6) Intermittent course; green with a single relapse (yellow or red) throughout 1 year. Assessors had a consensus meeting on disease courses in any instances of disagreement.
Medicine registrations were carried out retrospectively and based upon electronic patient files and the electronic prescription management system. IBD medications were registered at baseline and after 1 year of follow-up. Medical treatments were categorized as in Burisch et al 2018[4]: None: No IBD medicine; 5-aminosalysalic acid (5ASA): Oral and/or topical 5ASA treatment ± topical steroids; Corticosteroids: Oral steroids ± 5ASA or topical steroids; Immunomodulators: Azathioprine (and derivatives) and methotrexate ± steroids; and Biologicals: Any anti-tumor necrosis factor treatment (including certolizumab) or vedolizumab (anti-α4β7-integrin) in combination with any of the above. Adherence to medication was measured by MARS, a self-assessment questionnaire, consisting of five questions that has been used previously in IBD web trials[17,18].
Disease-related quality of life was assessed using the Short IBD Questionnaire, which consists of 10 questions (score 10-70)[19]. In the constant care application, a score of 10-50 is presented as red and indicates a poor quality of life, while a score above 50 is represented as green and indicates a good quality of life[20,21]. The functional assessment of chronic illness therapy-fatigue questionnaire (13 questions, score 0-52) was used to assess fatigue in IBD patients during the preceding 7 d[22,23]. Patient satisfaction with the study was assessed by a questionnaire prepared by the authors, consisting of seven yes/no questions.
In order to compare the effects of the two interventions across 1 year of home monitoring, two ways of modeling longitudinal disease activity measures were performed: (1) FC, HBI, SCCAI and TIBS modeling was based on the assumption of linearity between repeated measures and normalization of 1 year of data to 100% for all patients that completed the study. Based on normalized data for each patient, the mean time spent in the green, yellow and red zones (Figure 1) throughout the year were calculated as percentages. In addition, FC SCCAI, HBI and TIBS sample intensity (defined as the number of registrations in green, yellow and red divided by the mean time spent in remission, moderate and severe activity) was also calculated; and (2) Based on longitudinal FC measures, area under the curve was calculated using the inclusion date and the first observation made 357 d after inclusion.
For dichotomous and categorical variables, Fisher’s exact test was used. For quality of life and fatigue data, repeated measures analysis of covariance was performed, with a correction for baseline values. Kaplan-Meier survival analysis was used to compare the interventions regarding time to a relapse and time to remission on disease activity indices (FC/SCCAI). The possible effect of intervention group on time-to-relapse and time-to-remission was tested by the log rank test. To compare 1-year disease courses of symptoms and FC to TIBS, which were blinded to two assessors, a simple concordance analysis was made by deriving the relative number of agreements. Data were analyzed using SAS (9.4). Results were considered significant when two-sided P values were less than 0.05.
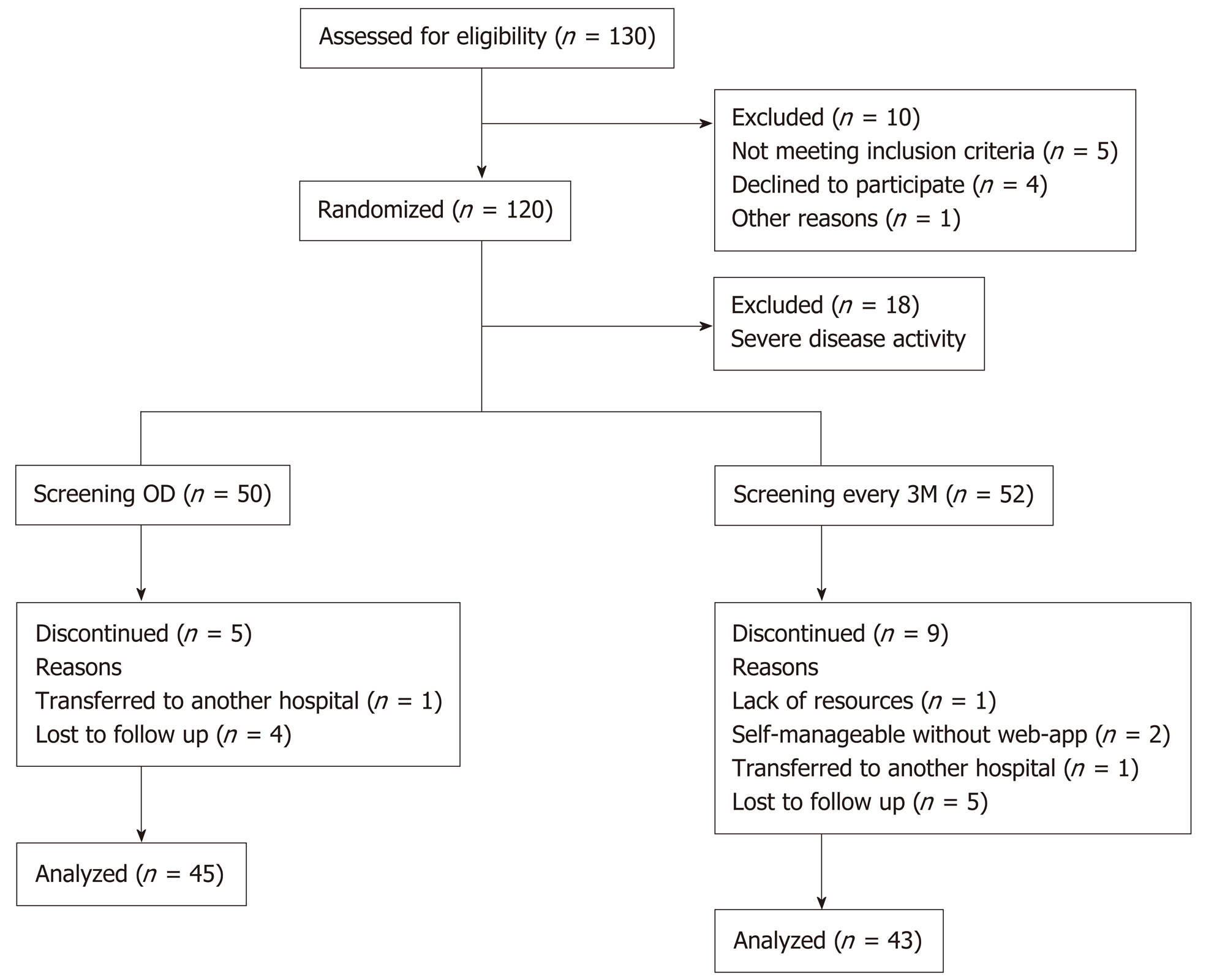
Overall, 102 IBD patients, fulfilling the inclusion criteria, were randomized in the study, with 50 in the OD group and 52 in the 3M group. Table 1 shows the clinical and patient-reported characteristics of each group. Twenty-one patients (21%) had technical problems getting FC home test to work at inclusion. In these cases (n = 9), the patients’ first FC measurement after inclusion was used as baseline if it was within 14 d of the inclusion date; median days from inclusion was 5 [interquartile range (IQR): 4-8]. Baseline data (Table 1) across the two groups showed similar cha-racteristics.
| Characteristics | Screening every 3M, n = 52 | Screening OD, n = 50 |
| Sex | ||
| Male | 26 (50%) | 24 (48%) |
| Female | 26 (50%) | 26 (52%) |
| Mean body mass index, SD; kg/m2 | 24.7 (3.7) | 25.8 (5.1) |
| Smoking | ||
| Current | 5 (9.6%) | 6 (12.0%) |
| Former | 30 (57.7%) | 25 (50.0%) |
| Never | 17 (32.7%) | 19 (38.0%) |
| Mean age at inclusion, SD; yr | 44.9 (15.2) | 48.4 (16.0) |
| Mean age at diagnosis, SD; yr | 32.0 (13.1) | 37.3 (14.9) |
| IBD diagnosis | ||
| UC | 39 (75%) | 35 (70%) |
| CD | 10 (19.2%) | 13 (26.0%) |
| IBD-U | 3 (5.8%) | 2 (4.0%) |
| UC extent, n = 39; 35 | ||
| E1, proctitis | 13 (33.3%) | 7 (20.0%) |
| E2, left side | 17 (43.6%) | 18 (51.4%) |
| E3, extensive | 9 (23.1%) | 10 (28.6%) |
| CD location, n = 10; 13 | ||
| L1, small bowel | 0 (0%) | 1 (7.7%) |
| L2, colonic | 3 (30.0%) | 3 (23.1%) |
| L3, ilea-colonic | 7 (70.0%) | 9 (69.2%) |
| CD behavior, n = 10; 13 | ||
| B1, inflammatory | 9 (90.0%) | 8 (61.5%) |
| B2, stricturing | 0 (0%) | 5 (38.5%) |
| B3, penetrating | 1 (10.0%) | 0 (0%) |
| Previous surgery CD, n = 10; 13 | 7 (70%) | 5 (38%) |
| Median IBD duration, IQR; yr | 10.5 (4.5-19.5) | 8.0 (4.0-16.0) |
| Median short-IBD-quality of life (IQR), n = 50; 50 | 60.5 (54.0-65.0) | 58.0 (51.0--63.0) |
| Median fatigue, FACIT-F (IQR), n = 48; 50 | 11.0 (9.0-16.0) | 11 (9.0-21.0) |
| Median compliance, MARS (IQR), n = 41; 84 | 24.0 (23.0-25.0) | 24.0 (24.0-25.0) |
| Medical treatment | ||
| None | 10 (19.2%) | 9 (18.0%) |
| 5ASA | 24 (46.2%) | 27 (54.0%) |
| Corticosteroids | 4 (7.7%) | 4 (8.0%) |
| Immunomodulators | 9 (17.3%) | 3 (6.0%) |
| Biological therapy | 5 (9.6%) | 7 (14.0%) |
| Median UC disease activity, SCCAI (IQR), | 0.5 (0-2.0) | 1.0 (0-2.0) |
| n = 42; 36, Incl. 4 with IBD-U | ||
| Green < 3 | 36 (85.7%) | 33 (91.7%) |
| Yellow [3-4] | 6 (14.3%) | 3 (8.3%) |
| Median CD disease activity, HBI (IQR), | 1.5 (0.0–2.0) | 3.0 (1.0–6.0) |
| n = 10; 14, Incl. 1 with IBD-U | ||
| Green < 5 | 9 (90.0%) | 10 (71.4%) |
| Yellow [5-16] | 1 (10.0%) | 4 (28.6%) |
| Median fecal calprotectin (IQR; mg/kg), n = 47; 43 | 64.0 (20.0-382.0) | 70.0 (20.0-547.0) |
| Green < 200 | 32 (61.5%) | 30 (60.0%) |
| Yellow [200-599] | 9 (17.3%) | 4 (8.0%) |
| Red > 599 | 6 (11.5%) | 9 (18.0%) |
| Missing | 5 (9.6%) | 7 (14.0%) |
| Median TIBS (IQR), n = 47; 43 | 4.0 (0-20.0) | 4.0 (2.0-14.0) |
| Green [0-8] | 28 (53.8%) | 26 (52.0%) |
| Yellow [9-32] | 13 (25.0%) | 8 (16.0%) |
| Red [33-99] | 6 (11.5%) | 9 (18.0%) |
| Missing | 5 (9.6%) | 7 (14.0%) |
| Patient-reported co-morbidities | ||
| None | 23 (42.2%) | 18 (36.0%) |
| 1 | 23 (44.2%) | 24 (48.0%) |
| > 1 | 6 (11.5%) | 8 (16.0%) |
| Education1 | ||
| Short | 4 (7.7%) | 2 (4.0%) |
| Student | 4 (7.7%) | 2 (4.0%) |
| Medium | 31 (59.6%) | 40 (80.0%) |
| Higher/Academic | 13 (25%) | 6 (12.0%) |
| Occupation | ||
| Yes | 42 (80.8%) | 38 (76.0%) |
| No | 10 (19.2%) | 12 (24.0%) |
Five patients from the OD group and nine from the 3M group withdrew from the study (Figure 2). These 14 patients registered symptoms on the constant care web-app within a median 146 d (IQR: 18-202). In total, 88 (86%) patients completed the study and made registrations on the web-app, for a mean of 12 [standard deviation (SD) = 1.3] mo. Of the 88 patients that completed the study, only one measured FC throughout the year without measuring symptoms. Four out of the 88 patients only measured FC at baseline (but completed 1-year symptom registrations on constant care). Patients were only excluded from data analysis if data were missing.
The number of FC home tests per patient, and sample intensities according to FC activity zones (green, yellow and red), were calculated in relation to the screening procedures (3M and OD). The median (IQR) number of FC home-monitoring test kits per patient used in the 3M and OD groups were 6.0 (5.0-8.0) and 4.0 (2.0-9.0), respectively (P = 0.04). Sample intensities of FC in the red zone were in median (IQR) 8.8 (5.9-15.8) vs 12.0 (8.8-20.7) in the 3M (n = 22) and OD (n = 18) groups, respectively (P = 0.22). Median (IQR) sample intensity in the yellow zone were 6.1 (2.9-10.6) vs 8.6 (3.0-18.0) in the 3M (n = 29) and OD (n = 23) groups, respectively (P = 0.28), and median (IQR) sample intensity in the green zone were 6.0 (5.0-9.6) vs 3.7 (2.0-11.9) in the 3M (n = 41) and OD (n = 39) groups, respectively (P = 0.009). Similar results were observed for SCCAI and TIBS, data not shown.
There was no statistical difference between the 3M and OD groups in the proportion of time spent in the different disease activity zones (Table 2). The close similarity regarding disease activities as measured using FC only between the two intervention groups are also shown in Figure 3. In addition, the area under the curve for FC (n = 80), adjusted for age at inclusion (P = 0.11) and IBD duration (P = 0.68), showed no significant difference (mean difference: 6250, P = 0.77) between the two screening procedures.
| FC | SCCAI | HBI | TIBS | P value | |||||
| | 3M, n = 43 | OD, n = 41 | 3M, n = 37 | OD, n = 35 | 3M, n = 6 | OD, n = 9 | 3M, n = 43 | OD, n = 39 | |
| Green, mean of % | 66 | 65 | 82 | 87 | 72 | 66 | 60 | 61 | NS |
| Yellow, mean of % | 18 | 19 | 15 | 10 | 28 | 34 | 26 | 22 | |
| Red, mean of % | 16 | 16 | 3 | 3 | 0 | 0 | 14 | 16 | |
| Total of % | 101 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
We analyzed the number of relapses (FC and SCCAI) in each intervention group based on 83 (99%) and 70 (97%) patients respectively, as one and two (1% and 3 %) patients were not at risk for having a FC and SCCAI relapse respectively (not having consecutive 30 d in remission during the study period). The number of patients experiencing a severe FC and SCCAI relapse (i.e., changing from a green score to a red score) did not differ significantly between the two groups FC: [3M: 15 (36.6%) and OD: 12 (28.6%), P = 0.49] and SCCAI: [3M: 9 (25.0%) and OD: 7 (20.6%), P = 0.78]. Similarly, the number of moderate and severe relapses combined (i.e., changing from a green score to a yellow or red score) did not differ significantly between groups FC: [3M: 22 (53.7%) and OD: 17 (40.5%), P = 0.27] and SCCAI: [3M: 14 (38.9%) and OD: 9 (26.5%), P = 0.31].
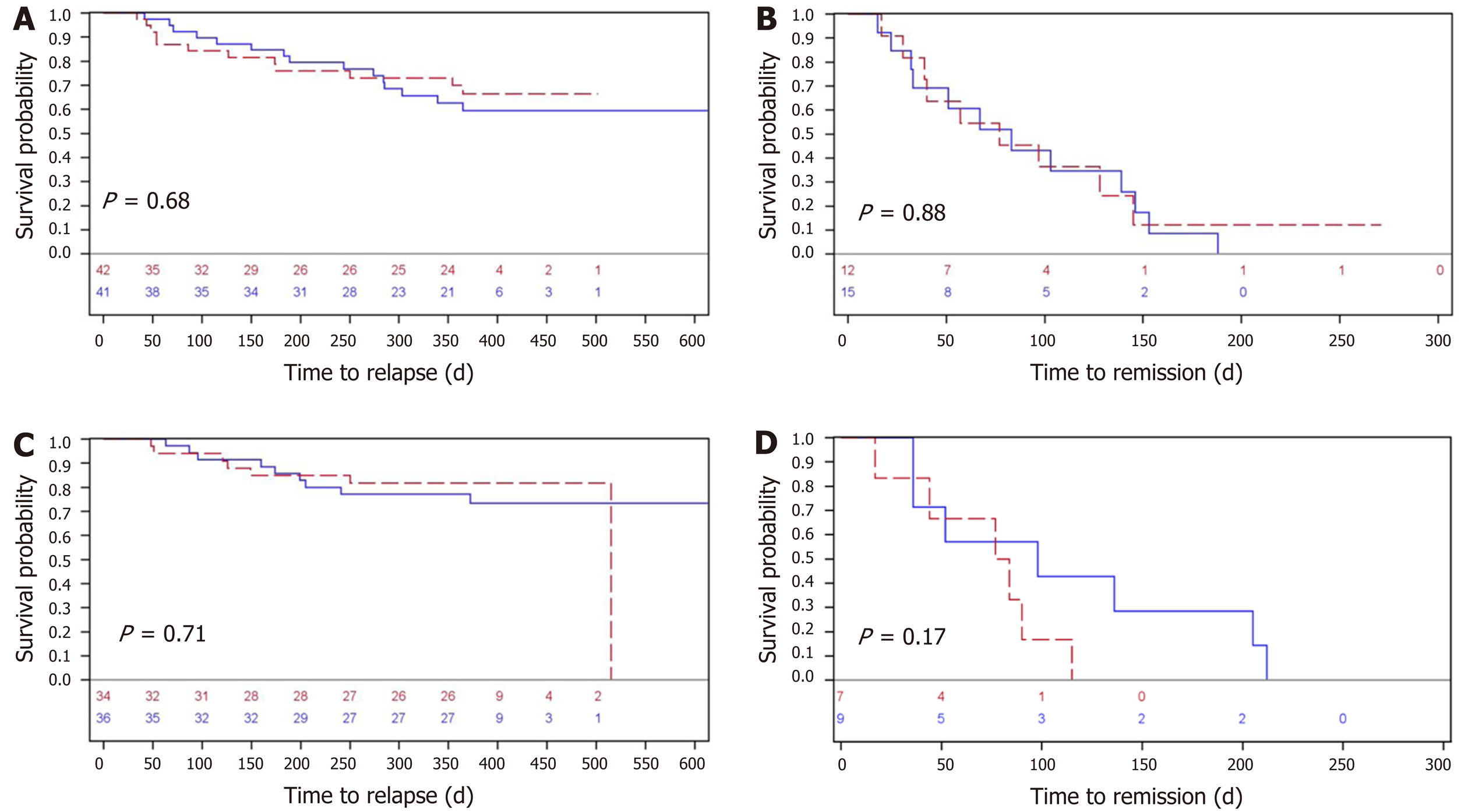
The performance of the two screening procedures in relation to experiencing a severe relapse and the corresponding time to remission are illustrated for all patients (FC) and separately for UC/IBD-U patients (SCCAI) at risk in Figure 4. There was no statistical difference for all patients as measured by FC or UC/IBD-U patients alone using SCCAI, regarding time to relapse (regardless of a red flare or a yellow/red flare). Median times from a FC severe relapse to remission were approximately 75 d (P = 0.88) for both groups, whereas median time to remission for UC patients (SCCAI) was approximately 75-100 d (P = 0.17) (Figure 4). The corresponding Kaplan-Meier curves of time to a moderate-severe relapse and to remission are shown in Supplementary Figure 1.
Regarding the disease courses (A-F), there were no statistically significant differences between the 3M and OD groups (Supplementary Table 1). Agreement between FC disease courses (A-F) and TIBS disease courses (A-F) were 78%. Likewise, agreement between disease courses for SCCAI and TIBS were 62%, while between HBI and TIBS they were 31%.
Medication at baseline and after 1 year of follow-up is shown in Supplementary Table 2. Overall, 27 medical changes were registered throughout the year (14 3M and 13 OD). Independent of intervention, patients (n = 80) were compliant with medical therapy (i.e., they had a MARS score higher than 21) for a median of 3M: 23.57 (IQR 21.50-24.25) and OD: 24.17 (IQR 23.50-24.80), P = 0.58. Eight patients were not measured for compliance, as they did not receive any IBD treatment.
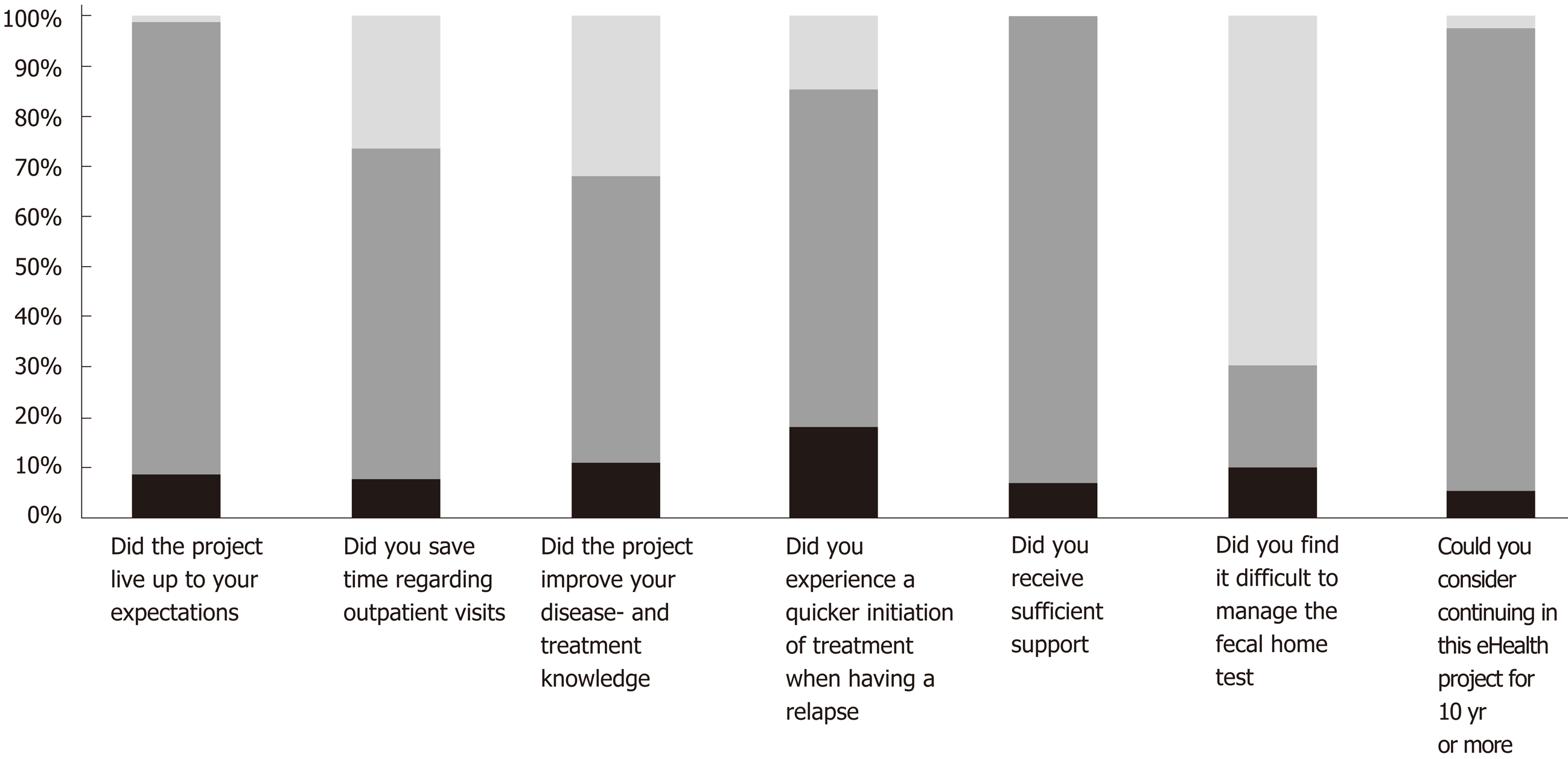
Mean (SD) changes in quality of life throughout the year in the 3M group was 0.56 (6.78) and 4.04 (9.24) in the OD group. The difference between the two interventions in quality of life, and when taking quality of life at baseline into account, showed no statistical difference between the two screening procedures, where the mean difference was 2.3, P = 0.17. Similarly, the mean difference in fatigue between the two interventions, and adjusting for fatigue baseline measures, was 0.19, P = 0.86. The 88 patients that completed the study were asked seven questions at follow-up (Figure 5). There was no statistical difference (all P values > 0.2) between the two intervention groups on any of the seven yes/no questions assessing patient satisfaction (Figure 5).
In this study we have demonstrated that monitoring IBD patients according to patients’ own requests (OD) or every 3M resulted in similar disease-related outcomes, including time to relapse and time to remission, disease course, medical compliance, fatigue, quality of life and patient satisfaction. Therefore, we recommend using an “on demand” approach to monitoring IBD, where fewer CalproSmart tests kits are needed, thus lowering costs, while maintaining outcomes comparable to a more expensive, scheduled monitoring approach.
The optimal way to monitor IBD patients for disease progression or relapse remains to be found. Recently, the STRIDE[24] (Selecting Therapeutic Targets in IBD) Steering Committee recommended that screening in IBD should be performed every 3 mo during a relapse and once every 6 or 12 mo during remission, and it should include assessing both clinical symptoms and inflammation via endoscopy. However, the CALM[25] study has shown that close monitoring of CD patients according to clinical symptom scores and objective markers (including FC) results in better endoscopic and clinical outcomes at 1 year follow-up, relative to standard clinical management. FC measures have also been able to identify UC patients at risk of a relapse 3[26], 6 and 12 mo before one occurs[27] and to correlate to histological mucosal healing[27], indicating that FC is a clinically valuable marker for disease monitoring. In addition, Pedersen et al[18] showed that FC was the first marker (relative to SCCAI and TIBS scores) to decrease in response to treatment. Furthermore, IBD patients enrolled in the web-outpatient clinic at our institution will undergo the same practice as standard care, which includes colonoscopy when needed and at least every 3 to 5 years for surveillance of colorectal cancer.
In this eHealth study, the overall purpose was to detect a relapse as soon as possible and to involve patients in their own treatment, including starting and changing medications. Therefore, a FC home test that can be performed by the patients in just 18 min seems to be a good, quick and cheap alternative to endoscopy targeting inflammation[27,28]. The present study showed that screening for disease activity OD is equally good on all parameters as 3M; however, the 3M group used significantly more FC test kits per patient relative to the OD group, most likely due to the significant greater sample intensity/registrations in the green FC zone. In addition to being more cost-effective, an OD approach might also be preferable from the patients’ perspective, some of whom (especially those in remission) find it inconvenient to monitor themselves every 3 mo. However, even if an OD approach became common, in some cases (adaptive ones), it might be advisable to begin patients with a pre-defined screening interval. Later on, when the patients have better knowledge of their disease, they could then be transitioned to an OD arrangement. In a previous study, Pedersen et al[18] concluded that the constant care application for mild-to-moderate UC patients helped them to recognize symptoms and thereby improved their understanding of the disease – something fundamental to self-management. Fifty-seven percent of patients in this study stated that they had benefited from an improved understanding of their disease and its treatment.
Previous research has shown that an advantage of screening for inflammation at home and using eHealth monitoring is that it detects a relapse sooner, and therefore helps bring about remission quicker, than with standard care[10]. This prompts the question of whether one of the screening procedures (OD or 3M) can detect a relapse sooner than the other one. Our results showed no statistical differences in time to a flare up as measured by FC and SCCAI between the two groups. However, due to the small sample, these findings need further confirmation.
The disease activity algorithm has shown that UC patients with mild-to-moderate disease activity have a median of approximately 30 d to SCCAI time to remission (and median 7 d for FC)[18]. In the present study, UC patients with either a severe or moderate-to-severe relapse had a median of approximately 75 -100 d to remission, figures similar to the overall IBD population in the study examined by FC. Plausible explanations for the difference in FC/SCCAI median time to remission (7-30 vs 75-100 d) are that this study is more representative of clinical practice (including both UC and CD on different medical therapies), as well as the fact that in the study by Pedersen et al[18], UC patients measured themselves every week.
eHealth treatments have previously been shown to be feasible and safe for UC patients[8,18], CD patients on infliximab[29] and children and adolescents with IBD[17]. Therefore, we have not assessed feasibility and adverse events in relation to the two eHealth screening procedures. For children and adolescents, a fixed eHealth screening interval of once every month have been shown to be feasible[17]. One could speculate if the OD screening approach could apply to pediatric IBD patients as well. In general, all patients in this study were satisfied: Treatment lived up to the patients’ expectations (90%), they felt they received enough support (93%) and they were saved time spent on outpatient visits by home-monitoring instead (66%). Nonetheless, 20% of participants experienced difficulties with the CalproSmart app due to early problems in the study when the app was not CE marked and not available to download from app store and google play. According to Bossuyt et al[30], one of the major concerns regarding implementation of eHealth in IBD is a less robust digital infrastructure that is not sufficiently workable for patients and healthcare providers. Although there might be room for improving the digital infrastructure, the constant care platform has in this study shown to be satisfactory from a patient perspective.
The strength of this study is its use of a novel eHealth application in a representative IBD population in clinical practice, including both CD and UC patients with different disease phenotypes.
A limitation of the study is that the TIBS disease activity algorithm has not yet been validated by endoscopy. Furthermore, monitoring FC for small bowel inflammation in cases of CD has been questioned based on whether FC is a valid marker for disease activity and nor do symptoms necessarily worsen in these patients. Another limitation of the study is its design, which might have been improved by using a non-inferior design for the statistical analysis and power calculation.
In conclusion, the two eHealth screening procedures (OD and 3M) proved equally good in detecting a relapse and bringing about remission in adult IBD patients. There were no statistically significant differences between the OD and 3M groups on any of the disease measures, compliance or quality of life, although the OD protocol resulted in fewer FC home test kits used per patient. Therefore, individualized, i.e., OD, eHealth screening for disease activity can be recommend in clinical web-practice. Based on our results, OD screening was implemented in clinical practice in June 2018 at our institution and all IBD patients in North Zealand, Denmark are now offered the opportunity of home monitoring using the constant care application. The latter is available for free in Denmark to any IBD patients and in collaboration with their IBD clinic.
Home-monitoring of disease activity in inflammatory bowel disease (IBD) has previously been shown to reduce time to remission, hospital admissions and outpatient visits, to increase compliance with medical therapy and quality of life and to empower patients. However, no study has investigated how often adult patients with IBD should home-monitor for disease activity. This study showed that the electronic health (eHealth), on demand (OD) screening procedure was cheaper and equally good on all disease measures as screening every third month (3M).
The optimal way to home-monitor adult patients with IBD for disease progression or relapse remains to be found.
To determine whether an eHealth screening procedure for disease activity in adult patients with IBD should be implemented in clinical practice, scheduled every third month, 3M or according to patient own decision, OD.
A randomized 1-year open-label eHealth trial where adult patients were randomized to screen for disease activity, quality of life, fatigue and medical compliance every 3M or OD on the web-application ibd.constant-care.com.
There was no statistical difference between the two screening procedures regarding medical compliance, fatigue, quality of life, mean time spent in remission, overall fecal calprotectin (FC) relapse rates, FC disease courses and FC time to a severe relapse and remission. The on-demand screening approach used fewer FC home-monitoring test-kits than screening every third month.
The two eHealth screening procedures were equally good in capturing a relapse and bringing about remission. The on-demand screening protocol used fewer FC home test-kits per patient. Individualized screening procedures can be recommended for adult patients with IBD in clinical web-practice.
A validation of the eHealth disease algorithm [total inflammation burden score (TIBS)] by endoscopy should be performed and time to a moderate and severe relapse of the TIBS between the two screening protocols should also be further examined.
We thank Thomas Janum (J-Consult) for help and support in developing the constant care application, integration of CalproSmart, and the export function from the constant care database.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gangl A, Serban ED, Sergi CM S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull. 1999;46:400-415. [PubMed] |
| 3. | Munkholm P. Crohn's disease--occurrence, course and prognosis. An epidemiologic cohort-study. Dan Med Bull. 1997;44:287-302. [PubMed] |
| 4. | Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, Salupere R, Pedersen N, Kjeldsen J, D'Incà R, Valpiani D, Schwartz D, Odes S, Olsen J, Nielsen KR, Vegh Z, Lakatos PL, Toca A, Turcan S, Katsanos KH, Christodoulou DK, Fumery M, Gower-Rousseau C, Chetcuti Zammit S, Ellul P, Eriksson C, Halfvarson J, Magro FJ, Duricova D, Bortlik M, Fernandez A, Hernández V, Myers S, Sebastian S, Oksanen P, Collin P, Goldis A, Misra R, Arebi N, Kaimakliotis IP, Nikuina I, Belousova E, Brinar M, Cukovic-Cavka S, Langholz E, Munkholm P; Epi-IBD group. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2018;0:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Burisch J, Jess T, Martinato M, Lakatos PL; ECCO -EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 738] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 6. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4102] [Article Influence: 512.8] [Reference Citation Analysis (110)] |
| 7. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1869] [Article Influence: 186.9] [Reference Citation Analysis (1)] |
| 8. | Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, Avnstrøm S, Langholz E, O'Morain C, Lynge E, Munkholm P. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided 'Constant-care' approach. Gut. 2010;59:1652-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, Becx MC, Maljaars JP, Cilissen M, van Bodegraven AA, Mahmmod N, Markus T, Hameeteman WM, Dijkstra G, Masclee AA, Boonen A, Winkens B, van Tubergen A, Jonkers DM, Pierik MJ. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Burisch J, Munkholm P. Telemonitoring and Self-Care in Patients with IBD. In: Cross RK, Watson AR, eds. Telemanagement of Inflammatory Bowel Disease. Springer US;. 2015;85-100. [DOI] [Full Text] |
| 11. | Ankersen DV, Weimers P, Burisch J. Whats 'App-ening': the help of new technologies in nutrition in digestive diseases. Curr Opin Clin Nutr Metab Care. 2017;20:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Best WR. Predicting the Crohn's disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M, Marker D, Carlsen K, Burisch J, Munkholm P. Fecal Calprotectin Measured By Patients at Home Using Smartphones--A New Clinical Tool in Monitoring Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2367] [Article Influence: 215.2] [Reference Citation Analysis (0)] |
| 16. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2349] [Article Influence: 123.6] [Reference Citation Analysis (2)] |
| 17. | Carlsen K, Jakobsen C, Houen G, Kallemose T, Paerregaard A, Riis LB, Munkholm P, Wewer V. Self-managed eHealth Disease Monitoring in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm Bowel Dis. 2017;23:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Pedersen N, Thielsen P, Martinsen L, Bennedsen M, Haaber A, Langholz E, Végh Z, Duricova D, Jess T, Bell S, Burisch J, Munkholm P. eHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014;20:2276-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571-1578. [PubMed] |
| 20. | Burisch J, Weimers P, Pedersen N, Cukovic-Cavka S, Vucelic B, Kaimakliotis I, Duricova D, Bortlik M, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, Krabbe S, Andersen V, Dahlerup JF, Kjeldsen J, Salupere R, Olsen J, Nielsen KR, Manninen P, Collin P, Katsanos KH, Tsianos EV, Ladefoged K, Lakatos L, Ragnarsson G, Björnsson E, Bailey Y, O'Morain C, Schwartz D, Odes S, Valpiani D, Boni MC, Jonaitis L, Kupcinskas L, Turcan S, Barros L, Magro F, Lazar D, Goldis A, Nikulina I, Belousova E, Fernandez A, Sanroman L, Almer S, Zhulina Y, Halfvarson J, Arebi N, Diggory T, Sebastian S, Lakatos PL, Langholz E, Munkholm P; EpiCom-group. Health-related quality of life improves during one year of medical and surgical treatment in a European population-based inception cohort of patients with inflammatory bowel disease--an ECCO-EpiCom study. J Crohns Colitis. 2014;8:1030-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Jowett SL, Seal CJ, Barton JR, Welfare MR. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96:2921-2928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Tinsley A, Macklin EA, Korzenik JR, Sands BE. Validation of the functional assessment of chronic illness therapy-fatigue (FACIT-F) in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Cohen BL, Zoëga H, Shah SA, Leleiko N, Lidofsky S, Bright R, Flowers N, Law M, Moniz H, Merrick M, Sands BE. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39:811-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1408] [Article Influence: 140.8] [Reference Citation Analysis (115)] |
| 25. | Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, Danalioglu A, Novacek G, Armuzzi A, Hébuterne X, Travis S, Danese S, Reinisch W, Sandborn WJ, Rutgeerts P, Hommes D, Schreiber S, Neimark E, Huang B, Zhou Q, Mendez P, Petersson J, Wallace K, Robinson AM, Thakkar RB, D'Haens G. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 671] [Article Influence: 83.9] [Reference Citation Analysis (1)] |
| 26. | De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, Dʼhaens GR, Franchimont D, Baert FJ, Torp RA, Henriksen M, Potvin PM, Van Hootegem PP, Hindryckx PM, Moreels TG, Collard A, Karlsen LN, Kittang E, Lambrecht G, Grimstad T, Koch J, Lygren I, Coche JC, Mana F, Van Gossum A, Belaiche J, Cool MR, Fontaine F, Maisin JM, Muls V, Neuville B, Staessen DA, Van Assche GA, de Lange T, Solberg IC, Vander Cruyssen BJ, Vermeire SA. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Zittan E, Kelly OB, Kirsch R, Milgrom R, Burns J, Nguyen GC, Croitoru K, Van Assche G, Silverberg MS, Steinhart AH. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohn's Disease. Inflamm Bowel Dis. 2016;22:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Pedersen N, Elkjaer M, Duricova D, Burisch J, Dobrzanski C, Andersen NN, Jess T, Bendtsen F, Langholz E, Leotta S, Knudsen T, Thorsgaard N, Munkholm P. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Bossuyt P, Pouillon L, Bonnaud G, Danese S, Peyrin-Biroulet L. E-health in inflammatory bowel diseases: More challenges than opportunities? Dig Liver Dis. 2017;49:1320-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |