Published online Oct 21, 2019. doi: 10.3748/wjg.v25.i39.5918
Peer-review started: August 1, 2019
First decision: August 27, 2019
Revised: September 6, 2019
Accepted: September 27, 2019
Article in press: September 27, 2019
Published online: October 21, 2019
Processing time: 80 Days and 20.4 Hours
Intestinal inflammation and epithelial injury are the leading actors of inflammatory bowel disease (IBD), causing an excessive pro-inflammatory cytokines expression. Tristetraprolin (TTP), an mRNA binding protein, plays a role in regulating the inflammatory factors, recognizing specific sequences on the 3’ untranslated region of cytokine mRNAs. TTP activity depends on its phosphorylation state: the unphosphorylated TTP degrades pro-inflammatory cytokine mRNAs; on the contrary, the phosphorylated TTP fails to destabilize mRNAs furthering their expression. The phospho-TTP forms a complex with the chaperone protein 14-3-3. This binding could be one of the factors that promote intestinal inflammation as a cause of disease progression.
To assess if TTP phosphorylation has a role in paediatric IBD.
The study was carried out on a cohort of paediatric IBD patients. For each patient enrolled, a specimen of inflamed and non-inflamed colonic mucosa was collected. Furthermore, the experiments were conducted on macrophages differentiated from blood samples of the same patients. Macrophages from healthy donors’ blood were used as controls. Co-immunoprecipitation assay and immunoblotting analyses were performed to observe the formation of the phospho-TTP/14-3-3 complex. In the same samples TNF-α expression was also evaluated as major factor of the pro-inflammatory activity.
In this work we studied indirectly the phosphorylation of TTP through the binding with the chaperone protein 14-3-3. In inflamed and non-inflamed colon mucosa of IBD paediatric patients immunoblot assay demonstrated a higher expression of the TTP in inflamed samples respect to the non-inflamed; the co-immunoprecipitated 14-3-3 protein showed the same trend of expression. In the TNF-α gene expression analysis higher levels of the cytokine in inflamed tissues compared to controls were evident. The same experiments were conducted on macrophages from IBD paediatric patients and healthy controls. The immunoblot results demonstrated a high expression of both TTP and co-immunoprecipitated 14-4-3 protein in IBD-derived macrophages in comparison to healthy donors. TNF-α protein levels from macrophages lysates showed the same trend of expression in favour of IBD paediatric patients compared to healthy controls.
In this work, for the first time, we describe a relation between phospho-TTP/14-3-3 complex and IBD. Indeed, a higher expression of TTP/14-3-3 was recorded in IBD samples in comparison to controls.
Core tip: We report a preliminary study describing the role of tristetraprolin (TTP)/14-3-3 protein complex in a small cohort of paediatric patients affected by inflammatory bowel disease (IBD). The experiments were conducted on IBD inflamed and non-inflamed colonic tissues and in IBD-derived macrophages in comparison to healthy donors. This study demonstrates for the first time the relation between the phosphorylation of TTP and IBD in paediatric patients. Indeed, a higher expression of the TTP/14-3-3 protein complex was recorded both in inflamed compared to non-inflamed colon specimens and in IBD-derived in comparison to healthy donors’ macrophages.
- Citation: Di Silvestre A, Lucafò M, Pugnetti L, Bramuzzo M, Stocco G, Barbi E, Decorti G. Role of tristetraprolin phosphorylation in paediatric patients with inflammatory bowel disease. World J Gastroenterol 2019; 25(39): 5918-5925
- URL: https://www.wjgnet.com/1007-9327/full/v25/i39/5918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i39.5918
Inflammatory bowel disease (IBD) is a chronic immune-mediated condition of the gastro-intestinal tract that includes ulcerative colitis (UC) and Crohn’s disease (CD). The incidence and prevalence of IBD is increasing and the disease is frequently diagnosed in adolescents and young adults[1], where the negative consequences on growth, development and psychosocial function are more evident[2]. Genetic and environmental factors have a crucial role in the pathogenesis of IBD, a combination of both seems to initiate an alteration in the epithelial barrier that causes an aberrant cytokine response, promoting chronic inflammation of the gastrointestinal tract[3]. Indeed, cytokines drive intestinal inflammation and associated symptoms in progressive and destructive forms with intestinal stenosis, rectal bleeding, abscesses and fistula formation[4].
Cytokine mRNA sequences contain adenosine/uridine-rich elements (ARE) on the 3’ untranslated region (3’UTR) that contribute to the regulation of the transcripts[5]. Degradation or stability are guided by a group of proteins, called mRNA-binding proteins, that recognize and bind the sequences on the 3’UTR. The most characterized protein of this family is tristetraprolin (TTP), a member of the Cys-Cys-Cys-His class, encoded by the ZFP36 gene[6], that, through the two-zinc finger domains, binds the ARE sequence of pro-inflammatory cytokine mRNAs, like tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2 and IL-6. Once bound, TTP promotes their degradation by recruiting several proteins that participate in mRNA regulation. These include the carbon catabolite repression 4/chromatin assembly factor-1/negative on TATA 1 (Ccr4/Caf1/Not1) deadenylase complex, that shortens the 3’-poly(A) tail. When the poly(A) tail is shortened, the decappining enzymes remove the 7-methylguanylate cap from the 5’-end of mRNA, and exonucleases promote a rapid degradation of mRNA from either the 5’ or 3’ end[7,8]. TTP expression has been observed in different tissues with a peculiar distribution in macrophages: Very low levels of the protein were detected in resting macrophages but, after an appropriate pro-inflammatory stimulus, TTP expression was rapidly induced[9,10]. Following stimulation, TTP becomes phosphorylated in almost 30 sites but only for two the function is known, the serines (S) 52 and 178 in mouse (corresponding to human S60 and S186).
The process of TTP phosphorylation is not completely clear, but different authors proposed the involvement of the mitogen-activated protein kinase (MAPK) p38 signalling pathway, that, via the downstream kinase MAPK-activated protein kinase 2 (MAPKAP kinase 2 or MK2), induces the phosphorylation of the two serines[11,12]. Furthermore, in primary cultures of macrophages and in a murine macrophages cell line (RAW264.7) the addition of MAPK p38 inhibitor leads to a rapid degradation of TTP through the proteasome complex, demonstrating that phosphorylation is also necessary to protect TTP from destruction[13].
The phosphorylation process promotes the binding of TTP protein with a low molecular-weight adaptor protein called 14-3-3[14,15]. This interaction changes the structure of TTP in a more stable one, since the unphosphorylated form is rapidly degraded by the proteasome, inhibits the recruitment of the Ccr4/Caf1/Not1 complex and consequently the target mRNAs are stabilized[16,17]. Ross and collaborators generated a knock-in mouse strain, in which S52 and 178 were replaced with alanines that cannot be phosphorylated. In primary macrophages of knock-in mice, low concentrations of unphosphorylated, rapidly degraded TTP, were observed and a reduced expression of different pro-inflammatory cytokines was recorded[18]. Furthermore, experiments in TTP-deficient mice revealed that the protein is necessary in orchestrating the response to pro-inflammatory stimuli; a severe syndrome of growth retardation, cachexia, arthritis, inflammation, autoimmunity, together with an over-expression of TNF-α in macrophages, was observed in these animals, underlining the importance of TTP[19]. Recently, TTP expression was studied in rheumatoid arthritis patients; confocal images revealed a high expression of the phosphorylated TTP in synovial tissues, corroborating the hypothesis that the protein can be involved in prolonged inflammation[11]. In this work, we investigated whether the TTP protein and 14-3-3 complex are differently expressed in inflamed and non-inflamed colonic tissues of paediatric patients with IBD.
Thirty-four IBD paediatric patients (mean age at enrolment 12.6 years, 16 UC and 18 CD, 18 males and 16 females) were enrolled at diagnosis at the Gastroenterology department of Paediatric Clinic of IRCCS Burlo Garofolo in Trieste. Local ethical committee approval for the study was obtained and the parents of all the participating children gave written informed consent. For each patient, during a colonoscopy, inflamed and non-inflamed tissues were collected in TRIzol® for RNA isolation.
Inflamed and non-inflamed colonic biopsies obtained from four additional patients (mean age at enrolment 13.8 years, 2 UC and 2 CD, 3 males and 1 females) were immediately frozen on dry ice and used for protein analysis. Blood samples were also collected and peripheral blood mononuclear cells (PBMCs) were isolated. PBMCs were also collected from four healthy donors (HD) from the Transfusion Center, Azienda Ospedaliera Universitaria, Trieste. Blood was obtained by venipuncture between 08.00 a.m. and 10.00 a.m. to minimize the variability due to circadian rhythm, and immediately processed.
PBMCs of patients and HD were obtained by density gradient centrifugation on Ficoll PaqueTM Plus (Healthcare, Milan, Italy). Human macrophages were differentiated from PBMCs by stimulation with GM-CSF (50 ng/ml, Millipore, Milan, Italy) for 7 days in RPMI 1640 containing 100 g/L of foetal calf serum, 10 g/L L-glutamine 200 mM, 10 g/L penicillin 10000 UI/mL and streptomycin 10 g/L (Sigma-Aldrich, St. Louis, MO, United States).
Total RNA was extracted from patients’ colonic mucosa using TRIzol® reagent (Thermo Scientific, Carlsbad, CA, United States) according to manufacturer’s instructions. The RNA concentration and purity were calculated by NanoDrop instrument (NanoDrop 2000, EuroClone®, Milan, Italy).
Expression levels of ZFP36 and TNF were evaluated by real-time RT-PCR TaqMan® analysis using the CFX96 real-time system-C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, United States). The reverse transcription reaction was carried out with the High Capacity RNA-to-cDNA Kit (Applied Biosystem, Carlsbad, CA, United States), and the real-time PCR was performed in triplicate using the TaqMan® Gene Expression Assay to assess ZFP36 and TNF mRNA expression, according to the manufacturer’s instructions. ZFP36 and TNF expression values were normalized using the ribosomal protein lateral stalk subunit P0 (RPLP0) as reference gene. The results were expressed as Log2 of the ΔCt relative expression (RE).
The ab206996 immunoprecipitation kit (Abcam, Cambridge, United Kingdom) was used on proteins derived from patients’ colon biopsies and macrophages differentiated from PBMCs obtained from patients and HD according to the manufacturer’s instructions. Briefly, samples were homogenized in non-denaturating lysis buffer, complete of protease inhibitor cocktail and inhibitor tablets for phosphatase (PhosSTOPTM, Sigma-Aldrich, St. Louis, MO, United States). The cell extracts were mixed at 4 °C and then centrifuged at 10000 x g for 10 min at 4 °C. The supernatants were incubated with TTP antibody (1:1000, Millipore, Milan, Italy) for 4 h at 4 °C. This antibody recognizes both the unphosphorylated and phosphorylated forms. After antibody binding to protein lysate, 40 μL of protein A/G Sepharose® beads were added and incubated for an hour at 4 °C. After three steps of washes with the appropriate buffer at 4 °C, the lysates were eluted with the 2X SDS-PAGE loading buffer and subsequently analysed for TTP (immunoprecipitated) and 14-3-3 (Co-immunoprecipitated) expression.
Protein expression was evaluated by immunoblot. Samples were run in a PAGErTM Mini-gel Chamber (Lonza, Milan, Italy) using 10% acrylamide gels with a Tris-Glycine buffer and subsequently semi-dry blotted for 2 h with 50 mA current on PVDF membrane. After blocking for 1 h with 5% not-fat milk in Tween/Tris buffered salt solution (T-TBS), membranes were incubated overnight at 4 °C with primary antibodies (anti-actin 1:10000, anti-TTP 1:1000 Millipore, Milan, Italy, anti-14-3-3 1:1000 Abcam, Cambridge, United Kingdom and anti-TNF-α 1:6000, Inflectra, Pfizer, New York, United States). Membranes were then washed in T-TBS and incubated for 1 h at 37 °C with anti-rabbit HRP-conjugated secondary antibody 1:50000 (Millipore, Milan, Italy) and anti-human HRP-conjugated secondary antibody 1:10000 (Sigma-Aldrich, St. Louis, MO, United States). Chemiluminescence was developed using LiteAblot® TURBO (EuroClone®, Milan, Italy) and exposed on Kodak Biomax film (Sigma-Aldrich, St. Louis, MO, United States). Protein expression was quantified on immunoblot images using the ImageJ software and are reported in percentage respect to actin.
Statistical analyses were performed using GraphPad Prism version 6.00e. Mann-Whitney test and t-test were used for the analysis of protein expression. Wilcoxon signed rank test and t-test were used for gene expression analysis in colon biopsies of IBD patients. The results are provided as means and standard errors.
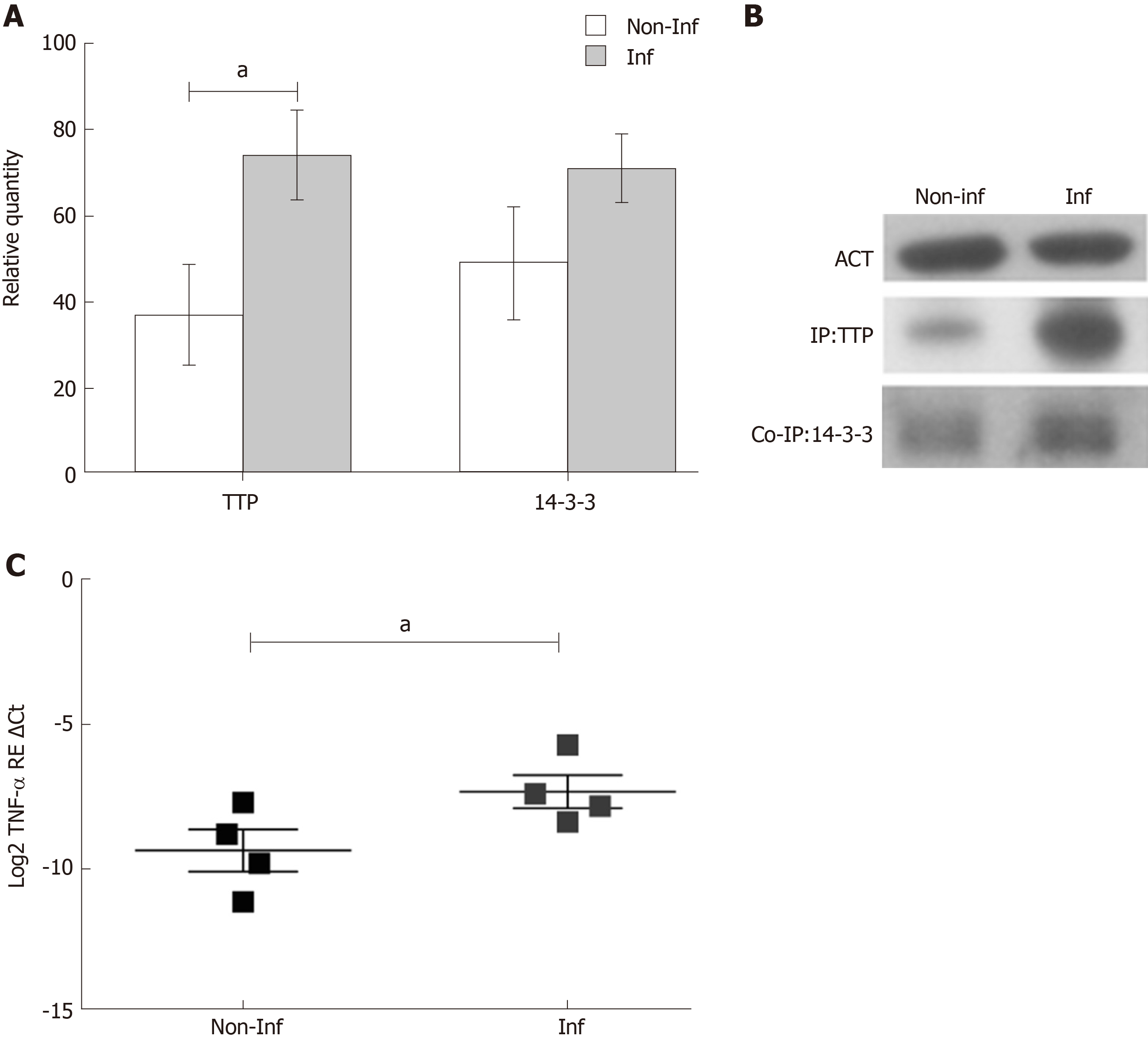
TTP gene expression analysis was performed in inflamed and non-inflamed biopsies of thirty-four paediatric IBD patients. After normalization with RPLP0, no differences were observed between the two groups (Log2 TTP RE ΔCt, non-inflamed = -4.384 ± 0.20 and inflamed = -4.682 ± 0.22, P > 0.05). Therefore, TTP was analysed at the protein level, since the protein undergoes extensive post-translational modifications, particularly phosphorylations, that can influence its stability and activity. Immunoprecipitation analysis was used to enrich TTP levels in tissue extracts. The experiments were conducted on four IBD paediatric patients in active phase of the disease. Quantification of the protein, performed with immunoblot analysis, showed an increased expression of TTP in the inflamed mucosa in comparison to the non-inflamed (fold change inflamed/non-inflamed = 2; relative quantity of TTP expression, non-inflamed = 37 ± 11.69 and inflamed = 74 ± 10.44, P < 0.05) (Figure 1A and 1B).
Since the 14-3-3 protein forms a complex with the phospho-TTP, the 14-3-3 protein expression was also evaluated. 14-3-3 levels exhibited the same trend of TTP expression, suggesting the presence of a phospho-TTP/14-3-3 complex. Interesting TNF-α mRNA levels were more expressed in IBD inflamed in comparison to non-inflamed biopsies (Log2 TNF-α RE Ct, non-inflamed = -9.373 ± 0.73 and inflamed = -7.331 ± 0.58, P < 0.05) indicating a potential role of TTP in its regulation (Figure 1C).
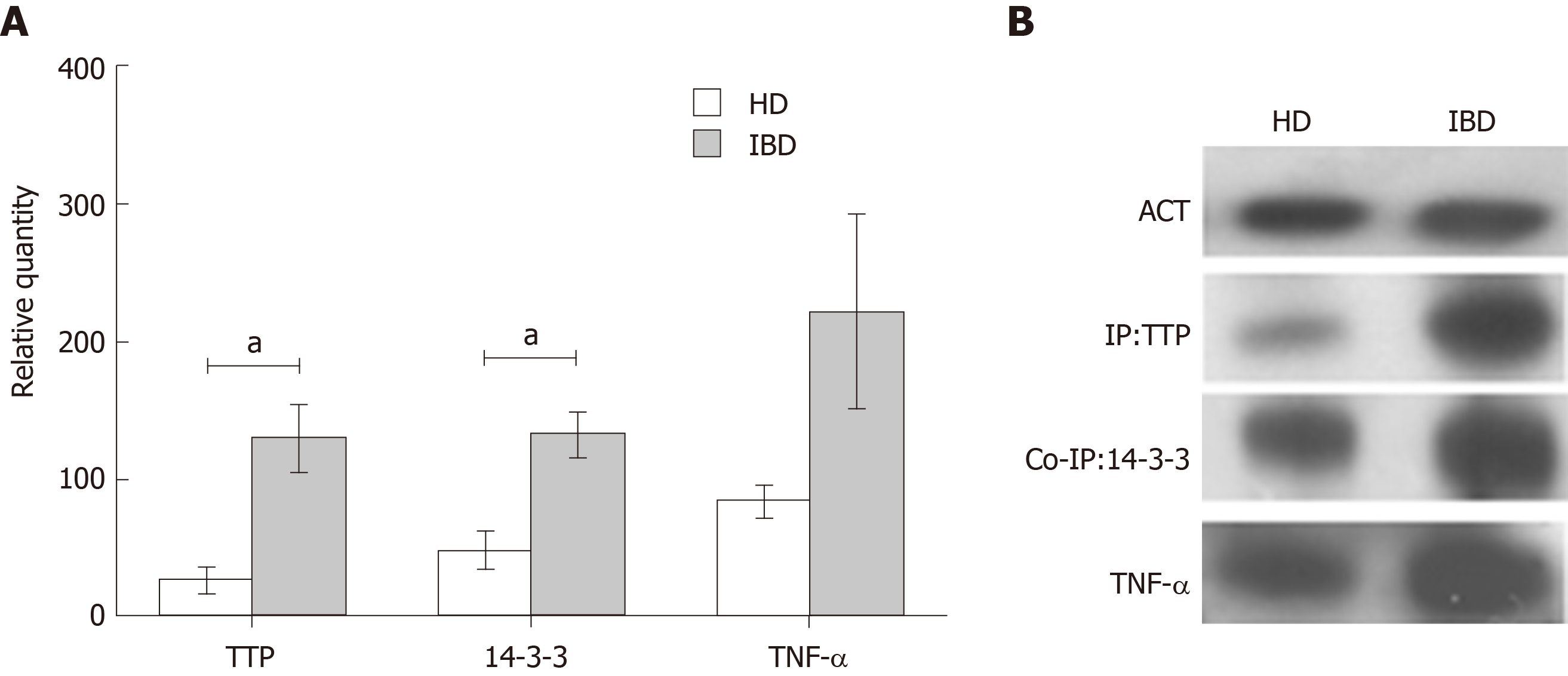
Macrophages were differentiated from PBMCs of four IBD paediatric patients with active disease and four HD. Immunoprecipitation, co-immunoprecipitation and immunoblot analyses were used to detect and quantify TTP, 14-3-3 and TNF-α. TTP and 14-3-3 showed a significantly higher complex formation in IBD compared to HD macrophages (fold change TTP IBD/HD = 4.8; relative quantity TTP, HD= 27 ± 10.29 and IBD = 130 ± 24.48, TTP, P < 0.05. Fold change 14-3-3 IBD/HD = 2.7 14-3-3; relative quantity HD = 49 ± 13.34 and IBD = 133 ± 16.72, P < 0.05). For TNF-α, a higher expression was observed in IBD in comparison to HD samples, however the difference was not significant (fold change TNF-α IBD/HD = 2.6; relative quantity TNF-α, HD = 85 ± 12.00 and IBD = 221 ± 70.44, P > 0.05) (Figure 2).
TTP expression in terms of mRNA and proteins has been studied in in vitro cell line models such as fibroblasts and macrophages under inflammatory conditions, but little is known on the regulation of TTP in patients with inflammatory diseases[9,20]. We have studied TTP mRNA expression in inflamed and non-inflamed colonic mucosa of paediatric IBD patients and no appreciable differences were evident. These results are in line with data obtained by Suzuki and collaborators on TTP mRNA expression in the synovium of patients with rheumatoid arthritis[21].
We speculate that the activity of TTP is under the control of post-translational modifications such as phosphorylation. Indeed, in terms of protein expression, in the present work we describe different TTP levels in inflamed tissues of IBD paediatric patients compared to the non-inflamed tract; moreover, the same trend of expression was evident for 14-3-3 protein expression after a co-immunoprecipitation assay. Since the phosphorylation process promotes the binding of TTP protein with the 14-3-3 protein[14,15], these data suggest indirectly the presence of the phospho-TTP/14-3-3 complex. These results are in agreement with what was observed by other authors; indeed, Ross and collaborators, using an immunostaining approach, demonstrated an increased TTP expression in synovial tissues of patients with RA compared with non-inflamed controls. The authors evaluated the involvement of phosphorylation through the study of the kinases cascade that involves the MAPK p38 and MK2. In detail, also in this case, the phosphorylation is described indirectly thanks to the co-localization of TTP and MK2 which induces the phosphorylation of TTP[11,12]. The phosphorylation process promotes the binding of TTP with 14-3-3[14,15] and the expression of pro-inflammatory cytokines[16,17] as demonstrated by the TNF-α mRNA expression observed in our samples.
The role of macrophages in TTP production is known, and most studies were conducted on immortalized or primary mouse macrophages; however, little is known about TTP expression in human macrophages differentiated from patients affected by inflammatory diseases[7]. Macrophages of IBD paediatric patients showed a higher complex formation of TTP and 14-3-3 in comparison to HD. Hence, levels of TTP protein in IBD patients would be indicative of active inflammation, and the increase of 14-3-3 protein indirectly supports the hypothesis of TTP phosphorylation.
These preliminary results describe, for the first time, the involvement of TTP/14-3-3 complex in IBD. Further studies are needed in a larger court of patients to clarify the role of TTP phosphorylation and this finding could open new perspectives in the study of IBDs and in the investigation of new target therapies based on the modulation of TTP phosphorylation.
Inflammatory bowel disease (IBD) is an immune-mediated condition of the gastrointestinal tract characterized by chronic, relapsing or progressive inflammatory condition. Tristetraprolin (TTP) is a zinc finger protein belonging to the group of mRNA-binding proteins. TTP is able to interact with pro-inflammatory cytokines mRNAs, influencing their stability. The function of TTP is affected by a post-translational modification. The unphosphorylated TTP destabilizes pro-inflammatory cytokines mRNAs; on the contrary, the phosphorylation of TTP impairs protein activity.
The incidence of paediatric IBD is increasing, and an optimal treatment is far from being achieved. Studies are therefore needed to enlighten new mechanisms that can be targeted by novel therapies.
The aim of this project is to assess if TTP phosphorylation has a role in paediatric IBD.
A small cohort of IBD paediatric patients was enrolled for the study. For each patient, during a colonoscopy, inflamed and non-inflamed tissues were collected. Moreover, macrophages from the same patients and healthy donors were isolated. Co-immunoprecipitation assay and immunoblotting analyses were performed to evaluate the formation of the phospho-TTP/14-3-3 complex. TNF-α expression was also evaluated.
TTP and 14-3-3 protein expression was higher in inflamed colonic samples in comparison to the non-inflamed. Furthermore, TNF-α gene expression analysis showed the same pattern of expression. The protein analysis of TTP, co-immunoprecipitated 14-4-3 protein and TNF-α in macrophages of IBD patients and healthy donors demonstrated a higher expression of all the proteins in paediatric patients in comparison to controls.
These data demonstrated for the first time a role of TTP in IBD inflammation. Indeed, TTP is highly expressed in both inflamed colon tissues and in macrophages of IBD patients.
These preliminary results provide new information about the role of TTP in IBD, opening new perspectives in the investigation of a target therapy based on the modulation of TTP phosphorylation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin LJ, Vradelis S S-Editor: Wang J L-Editor: A E-Editor: Ma YJ
| 1. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 520] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 2. | Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ. 2017;357:j2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1973] [Article Influence: 179.4] [Reference Citation Analysis (1)] |
| 4. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn's disease in population-based cohorts. Inflamm Bowel Dis. 2011;17:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 498] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Taylor GA, Lai WS, Oakey RJ, Seldin MF, Shows TB, Eddy RL, Blackshear PJ. The human TTP protein: sequence, alignment with related proteins, and chromosomal localization of the mouse and human genes. Nucleic Acids Res. 1991;19:3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Brooks SA, Blackshear PJ. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829:666-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 399] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 965] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 10. | Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461-6469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Ross EA, Naylor AJ, O'Neil JD, Crowley T, Ridley ML, Crowe J, Smallie T, Tang TJ, Turner JD, Norling LV, Dominguez S, Perlman H, Verrills NM, Kollias G, Vitek MP, Filer A, Buckley CD, Dean JL, Clark AR. Treatment of inflammatory arthritis via targeting of tristetraprolin, a master regulator of pro-inflammatory gene expression. Ann Rheum Dis. 2017;76:612-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin: 14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 428] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR. Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol Cell Biol. 2006;26:2408-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Clark AR, Dean JL. The control of inflammation via the phosphorylation and dephosphorylation of tristetraprolin: a tale of two phosphatases. Biochem Soc Trans. 2016;44:1321-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | O'Neil JD, Ammit AJ, Clark AR. MAPK p38 regulates inflammatory gene expression via tristetraprolin: Doing good by stealth. Int J Biochem Cell Biol. 2018;94:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Bustos DM. The role of protein disorder in the 14-3-3 interaction network. Mol Biosyst. 2012;8:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Marchese FP, Aubareda A, Tudor C, Saklatvala J, Clark AR, Dean JL. MAPKAP kinase 2 blocks tristetraprolin-directed mRNA decay by inhibiting CAF1 deadenylase recruitment. J Biol Chem. 2010;285:27590-27600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Ross EA, Smallie T, Ding Q, O'Neil JD, Cunliffe HE, Tang T, Rosner DR, Klevernic I, Morrice NA, Monaco C, Cunningham AF, Buckley CD, Saklatvala J, Dean JL, Clark AR. Dominant Suppression of Inflammation via Targeted Mutation of the mRNA Destabilizing Protein Tristetraprolin. J Immunol. 2015;195:265-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 652] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 20. | Taylor GA, Thompson MJ, Lai WS, Blackshear PJ. Mitogens stimulate the rapid nuclear to cytosolic translocation of tristetraprolin, a potential zinc-finger transcription factor. Mol Endocrinol. 1996;10:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Suzuki E, Tsutsumi A, Sugihara M, Mamura M, Goto D, Matsumoto I, Ito S, Ikeda K, Ochiai N, Sato Y, Sumida T. Expression of TNF-alpha, tristetraprolin, T-cell intracellular antigen-1 and Hu antigen R genes in synovium of patients with rheumatoid arthritis. Int J Mol Med. 2006;18:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |