Published online Oct 14, 2019. doi: 10.3748/wjg.v25.i38.5850
Peer-review started: July 24, 2019
First decision: August 17, 2019
Revised: September 5, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 14, 2019
Processing time: 82 Days and 1.9 Hours
Thiopurine-induced leukopenia (TIL) is a life-threatening toxicity and occurs with a high frequency in the Asian population. Although nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) variants significantly improve the predictive sensitivity of TIL, more than 50% of cases of this toxicity cannot be predicted by this mutation. The potential use of the 6-thioguanine nucleotide (6TGN) level to predict TIL has been explored, but no decisive conclusion has been reached. Can we increase the predictive sensitivity based on 6TGN by subgrouping patients according to their NUDT15 R139C genotypes?
To determine the 6TGN cut-off levels after dividing patients into subgroups according to their NUDT15 R139C genotypes.
Patients’ clinical and epidemiological characteristics were collected from medical records from July 2014 to February 2017. NUDT15 R139C, thiopurine S-methyltransferase, and 6TGN concentrations were measured.
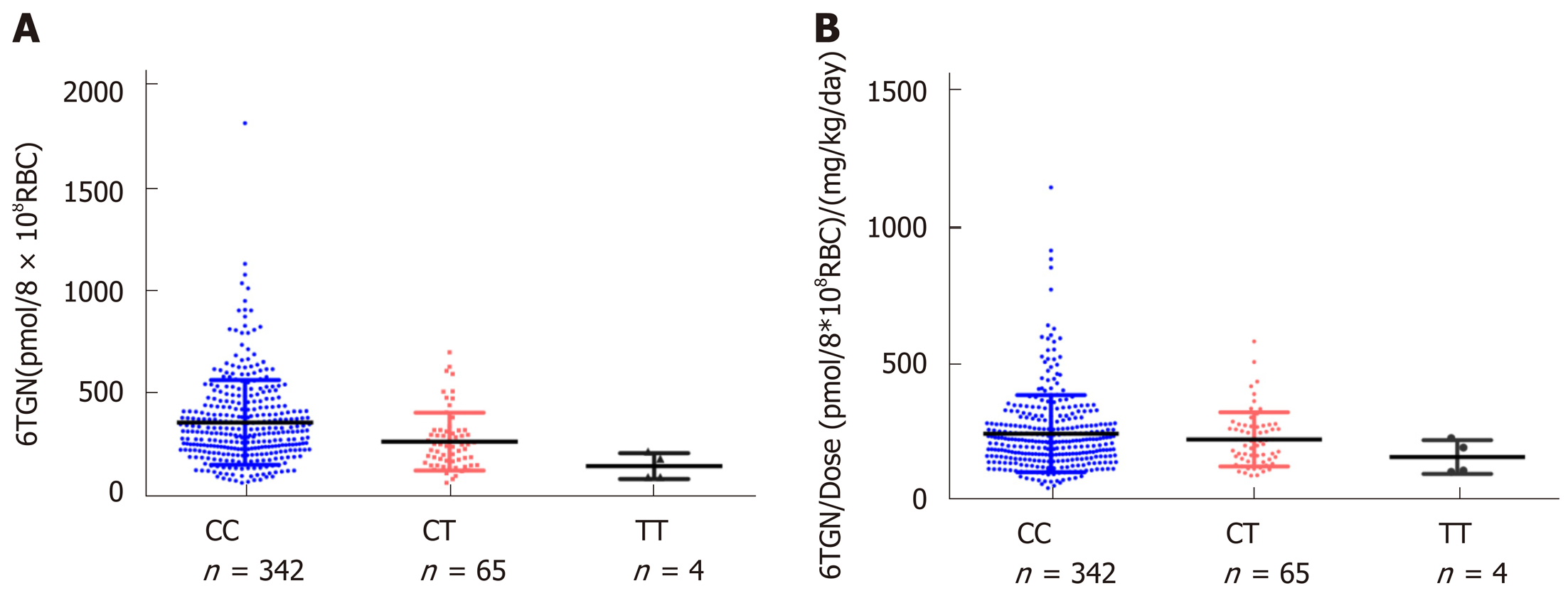
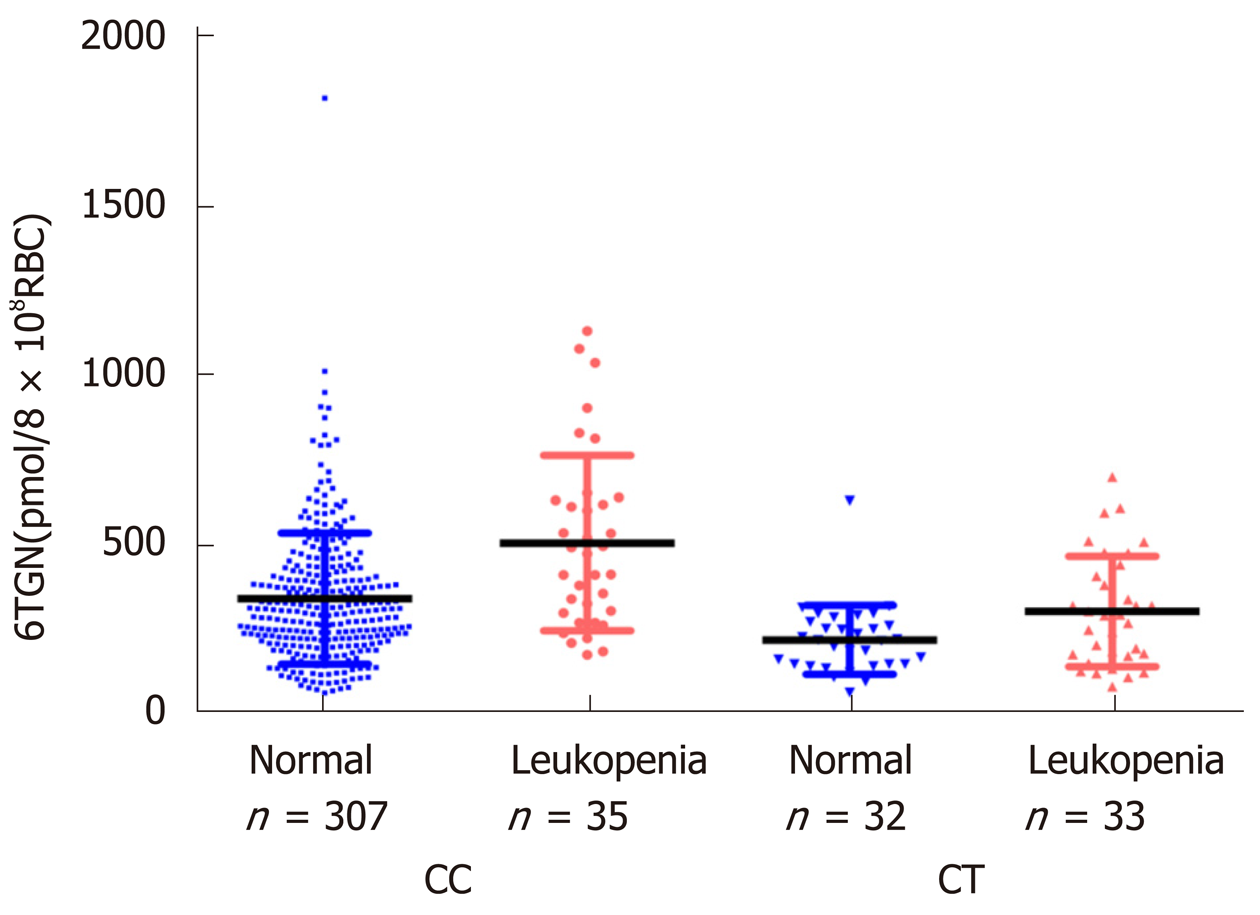
A total of 411 Crohn’s disease patients were included. TIL was observed in 72 individuals with a median 6TGN level of 323.4 pmol/8 × 108 red blood cells (RBC), which was not different from that of patients without TIL (P = 0.071). Then, we compared the 6TGN levels based on NUDT15 R139C. For CC (n = 342) and CT (n = 65) genotypes, the median 6TGN level in patients with TIL was significantly higher than that in patients without (474.8 vs 306.0 pmol/8 × 108 RBC, P = 9.4 × 10-5; 291.7 vs 217.6 pmol/8 × 108 RBC, P = 0.039, respectively). The four TT carriers developed TIL, with a median 6TGN concentration of 135.8 pmol/8 × 108 RBC. The 6TGN cut-off levels were 411.5 and 319.2 pmol/8 × 108 RBC for the CC and CT groups, respectively.
The predictive sensitivity of TIL based on 6TGN is dramatically increased after subgrouping according to NUDT15 R139C genotypes. Applying 6TGN cut-off levels to adjust thiopurine therapies based on NUDT15 is strongly recommended.
Core tip: Thiopurine-induced leukopenia (TIL), a life-threatening toxicity in inflammatory bowel disease, occurs with a high frequency in Asia. Although nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) variants significantly increase the prediction sensitivity of TIL, more than 50% of cases cannot be predicted by this mutation. The potential use of steady-state thioguanine nucleotide (6TGN) levels to predict TIL has been explored for decades, but no decisive conclusion has been reached. Can we increase the predictive sensitivity based on 6TGN by subgrouping patients according to their NUDT15 genotypes? Yes! According to our research, applying 6TGN levels to adjust thiopurine therapies based on NUDT15 is strongly recommended.
- Citation: Zhu X, Chao K, Li M, Xie W, Zheng H, Zhang JX, Hu PJ, Huang M, Gao X, Wang XD. Nucleoside diphosphate-linked moiety X-type motif 15 R139C genotypes impact 6-thioguanine nucleotide cut-off levels to predict thiopurine-induced leukopenia in Crohn’s disease patients. World J Gastroenterol 2019; 25(38): 5850-5861
- URL: https://www.wjgnet.com/1007-9327/full/v25/i38/5850.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i38.5850
Thiopurines, including mercaptopurine (MP) and azathioprine (AZA), are immunosuppressive drugs that have been regularly used to maintain remission in patients with Crohn’s disease (CD)[1,2]. Although thiopurine therapy is clinically effective, up to 10%-30% of patients discontinue treatment due to adverse reactions[3,4], among which thiopurine-induced leukopenia (TIL) is the most common and life-threatening toxicity, especially in Asian populations[5,6].
Neither AZA nor MP has an intrinsic activity. They must undergo extensive metabolic transformation into the active metabolites 6-thioguanine nucleotides (6TGN) and 6-methylmercaptopurine ribonucleotides (6MMPR), to exert their pharmacological effect or cytotoxicity[7,8]. The potential use of a steady-state 6TGN level above 450 pmol/8 × 108 red blood cells (RBC) to predict toxicity is still controversial[9-14]. Several studies have reported that 6TGN levels are not significantly correlated with TIL, and no applicable therapeutic range for 6TGN has been determined[15-18]. For example, a prospective study found that only three out of ten Japanese patients with TIL exhibited high 6TGN levels (> 450 pmol/8 × 108 RBC)[17]. In addition, a few groups have reported that extremely elevated 6MMPR concentrations are associated with TIL[12,19]. Therefore, further investigation of the exact relationship between the 6TGN and 6MMPR levels and TIL is warranted, especially in the Asian population, since Asians are more sensitive than Caucasians to thiopurine toxicity[6,20,21].
Nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) has been considered as the key gene which can highly predict thiopurine toxicity in Asian that is comparable to thiopurine S-methyltransferase (TPMT) in Europe[22-24]. TPMT variants with deficient enzyme activity were observed to have a preferential accumulation of 6TGN in TIL, so the guidelines have suggested adjusting the starting doses of thiopurine for variants based on the level of 6TGN[24]. However, NUDT15 variants have been discovered to develop TIL with normal or even lower 6TGN levels, suggesting that NUDT15 has an unfavorable effect on thiopurine metabolism[25,26]. NUDT15 is an enzyme responsible for catalyzing deoxy-6-thioguanine triphosphate (dTGTP) and 6-thioguanine triphosphate (TGTP) into their inactive monophosphates, preventing TGTP and dTGTP from incorporating into DNA[27]. Moriyama et al[28,29] discovered that NUDT15 R139C variants CT and TT were associated with a much higher TGTP/TGMP ratio and DNA-incorporated thioguanine (DNA-TG)/6TGN ratio in children with acute lymphoblastic leukemia (ALL). According to this mechanism, NUDT15 R139C variants are assumed to cause cytotoxicity with TGTP and dTGTP accumulation independent of the total 6TGN levels due to decreases in 6-thioguanine monophosphate (TGMP) and deoxy-6-thioguanine monophosphate (dTGMP). Thus, the guidelines applied to TPMT variants would not be suitable for patients with NUDT15 variants. Can we determine 6TGN cut-off levels thereafter dividing patients into subgroups according to NUDT15 R139C genotypes?
In this study, we investigated the relationship between the levels of 6TGN and 6MMPR and thiopurine-induced TIL based on patients’ NUDT15 R139C genotypes and obtained specific 6TGN cut-off levels to guide thiopurine therapy.
Patients diagnosed with CD according to the criteria of Lennard-Jones and prescribed thiopurines at a steady dose for more than 1 mo were recruited at the Sixth Affiliated Hospital, Sun Yat-sen University from July 1, 2014 to February 1, 2017. The exclusion criteria included blood transfusion or administration of cyclosporine or methotrexate; insufficient function of the heart, liver, or kidney; active infection; and pregnancy.
Weight-based thiopurine dosing was provided in a step-wise approach (the initial dose of AZA was 1.0 mg/kg daily or 0.5 mg/kg daily for MP and gradually increased to the target dose of 2.0 mg/kg or 1.0 mg/kg, respectively). If patients developed adverse effects, such as leukopenia [white blood cell count (WBC) < 3.5 × 109⁄L], gastric intolerance, hepatotoxicity, flu-like symptoms, pancreatitis, rash, or others, the dose was reduced. If the laboratory abnormalities did not subside, the treatment was discontinued.
A total of 2 mL of venous blood samples (EDTA anticoagulation) were obtained for determination of the erythrocyte 6TGN/6MMPR concentrations and NUDT15 R139C and TPMT*3C genotypes at the Institute of Clinical Pharmacology, Sun Yat-sen University[30,31].
We used allele-specific polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) to test the genotypes of NUDT15 R139C (rs116855232) and TPMT*3C (rs1142345). The sequences of the primers for NUDT15 R139C are: forward, 5-AGCTTACCCAAATAAACACCCT-3 and reverse, 5-TGGGGGATACAT TAAGAGACTGC-3, and those for TPMT*3C are: forward, 5-AAGTGTTGGGATTA CAGGTG-3 and reverse, 5-TCCTCAAAAACATGTCAGTGTG-3. PCR amplification started at 94 °C for 5 min, and continued with 30 cycles of 94 °C for 60 s, 59 °C for 30 s, and 72 °C for 30 s. Final extension was performed at 72 °C for 10 min. The PCR product was digested with restriction enzyme HpyCH4III (New England Biolabs, Hertfordshire, United Kingdom) for NUDT15 R139C testing and the enzyme AccI (New England Biolabs, Hertfordshire, United Kingdom) for TPMT*3C testing.
6TGN and 6MMPR concentrations in patients’ erythrocyte lysates were determined using the Dervieux et al’s method[32] by high performance liquid chromatography. First, 6TGN were hydrolyzed (100 °C for 45 min) into their bases (6-thioguanie) and 6MMPR were converted into their derivatives with perchloric acid. Then, they were separated using a C18 column with the mobile phase consisting of methanol-20 mmol/L potassium dihydrogenphosphate-triethylamine (pH adjusted to 3.2 using phosphoric acid) (5:95:0.1) at a flow rate of 1.0 mL/min. Finally, the wavelength for detection was set at 345 nm for 6TGN and at 303 nm for 6MMPR.
The present study was approved by the Ethics Committee of the Sixth Affiliated Hospital of the Sun Yat-sen University.
Statistical analyses and calculations were performed with SPSS 21.0 (SPSS, Inc., Chicago, IL, United States) and Prism 6 (GraphPad Software, La Jolla, CA, United States). Quantitative variables are expressed as medians and ranges. Categorical variables are summarized as frequencies. Data such as sex, NUDT15 R139C and TPMT*3C diplotypes, disease location, and co-medication were compared using the X2 method or Fisher’s test. Data such as age and thiopurine dose were compared using the nonparametric Kruskal-Wallis H-test. The nonparametric Kruskal-Wallis H-test was also used to evaluate the relationship between the 6TGN level and TIL. A receiver operating characteristic (ROC) curve was obtained to calculate the sensitivity and specificity of various 6TGN concentrations to predict the development of TIL. Multivariate logistic regression was used to identify the variables associated with TIL. P-values less than 0.05 were considered statistically significant.
A total of 411 patients with CD were included in this study. Among them, 100 (24.3%) patients had adverse effects during the AZA maintenance treatment, including TIL (n = 72, 17.5%), gastric intolerance (n = 21, 5.1%), flu-like symptoms (n = 6, 1.5%), hepatitis (n = 5, 1.2%), pancreatitis (n = 3, 0.7%), rash (n = 2, 0.5%), and others (n = 3, 0.7%). The characteristics of the patients are summarized in Table 1. The TIL rates were higher in patients carrying the NUDT15 R139C allele T (CT + TT) compared with those carrying the CC genotype (P = 9.04 × 10-20, OR = 8.04, 95%CI: 4.84-13.34). The median dosage was significantly lower for patients with TIL than for those without TIL (1.5 mg/kg per day vs 1.7 mg/kg per day, P = 0.03). Overall, there were no significant differences in gender, age, disease location, or co-medication between individuals with or without TIL (P > 0.05) (Table 1). No significant association of TPMT*3C with TIL was observed. Neither NUDT15 R139C nor TPMT*3C had significant associations with the other adverse effects (P > 0.05).
| Characteristic | All patients | With leukopenia | Without leukopenia | P value |
| No. of subjects (%) | 411 | 72 (17.5) | 339 (82.5) | - |
| Gender | ||||
| Male, n (%) | 305 (74.0) | 52 (17.0) | 253 (83.0) | 0.180 |
| Female, n (%) | 106 (26.0) | 20 (18.9) | 86 (81.1) | |
| NUDT15 R139C | ||||
| CC, n (%) | 342 (80.4) | 35 (10.2) | 307 (89.8) | 2.40 × 10-15 |
| CT, n (%) | 65 (17.9) | 33 (50.2) | 32 (49.8) | |
| TT, n (%) | 4 (1.7) | 4 (100) | 0 | |
| TPMT*3C | 0.26 | |||
| AA, n (%) | 398 (96.8) | 68 (17.1) | 330 (82.9) | |
| AG, n (%) | 13 (3.2) | 4 (30.8) | 9 (69.2) | |
| Age in years, median (range) | 28 (12-70) | 26(12-70) | 27(12-60) | 0.734 |
| Medication | ||||
| Azathiopurine | 337 (82.0) | 57 (17.3) | 273 (82.7) | 0.113 |
| Mercaptopurine | 74 (18.0) | 14 (18.9) | 60 (81.1) | |
| Thiopurines dose, mg/kg per day, median (range) | 1.7 (0.2-3.1) | 1.5 (0.5-2.6) | 1.7 (0.2-3.1) | 0.030 |
| CD | ||||
| Ileal L1 | 34 | 6 | 28 | 0.495 |
| Colorectal L2 | 21 | 6 | 15 | |
| Ileocolonic L3 | 292 | 48 | 244 | |
| Upper gastrointestinal L4 | 61 | 12 | 49 | |
| Co-medication | 211 | 45 | 166 | 0.74 |
| Corticosteroids | 79 | 15 | 64 | |
| Thalidomide | 51 | 13 | 38 | |
| Anti-TNF agent | 64 | 13 | 51 | |
| 5-ASA | 4 | 0 | 4 | |
| Allopurinol | 13 | 4 | 9 |
For NUDT15 R139C, the median 6TGN was 312.4 pmol/8 × 108 RBC in the CC group, 240.5 pmol/8 × 108 RBC in the CT group, and 135.8 pmol/8 × 108 RBC in the TT group. The level of 6TGN was much higher in the NUDT CC group than in the CT and TT groups (P = 9.0 × 10-6) (Figure 1), while the median level of 6MMPR was also significantly higher in CC patients (P = 0.030). To assess the levels of 6TGN and 6MMPR with equal doses of thiopurine, the ratios of the levels of 6TGN and 6MMPR to the thiopurine dose were compared across groups. There was no difference in the ratios between these three groups (P= 0.29, P= 0.58) (Figure 1). For TPMT*3C, the patients carrying TPMT*3C variants had excessively higher 6TGN (P = 2.0 × 10-6) and lower 6MMPR levels (P = 3.7 × 10-4), which could not be offset by the thiopurine dose (P = 4.8 × 10-5, P = 7.7 × 10-5) (Supplemental Figure 1).
In this study, neither the concentration of 6TGN nor 6MMPR was significantly different between patients with or without TIL (P = 0.071, P = 0.95). According to the NUDT15 R139C genotypes, the samples were divided into three subgroups. In the CC group (n = 342), the median 6TGN concentration was significantly higher in patients who developed TIL than in patients who did not [P = 9.4 × 10-5, 474.8 (174.2-1133.6) pmol/8 × 108 RBC vs 306.0 (62.2-1823.0) pmol/8 × 108 RBC] (Figure 2). In the CT genotype (n = 65), the level of 6TGN was significantly higher in patients who developed TIL [P = 0.039, 291.7 (80.6-701.5) pmol/8 × 108 RBC vs 217.6 (62.9-631.0) pmol/8 × 108 RBC] (Figure 2). All patients with the TT genotype (n = 4) developed TIL, with a median 6TGN concentration of 135.8 (90.0-291.3) pmol/8 × 108 RBC. No correlations between the 6-MMPR concentrations and TIL were found in the subgroups of CC (P = 0.55) and CT (P = 0.30), respectively.
Based on ROC analysis, we defined the cut-off level of 6TGN to be 411.5 pmol/8 × 108 RBC in the CC group and 319.2 pmol/8 × 108 RBC in CT group. After considering the level of 6TGN in different subgroups, the area under the curve (AUC) was increased from 0.57 to 0.65 and 0.70. Moreover, in the CT group, the specificity was as high as 96.9%, with a sensitivity of 42.4%, and in the CC group the specificity was 73.3%, with a high sensitivity of 60.0%, compared with the values in the total samples (Table 2).
| NUDT15 genotype (No. of patients) | 6TGNs (pmol/8 × 108 RBC) | P value | AUC | 95%CI | Sensitivity (%) | Specificity (%) |
| CT + CC | ≥ 474.7 | 0.071 | 0.57 | (0.49-0.65) | 34.7 | 82.6 |
| CC (342) | < 474.7 | 9.4 × 10-5 | (0.61-0.79) | 60.0 | 73.3 | |
| ≥ 411.5 | 0.70 | |||||
| < 411.5 | ||||||
| CT (65) | ≥ 319.2 | 0.039 | 0.65 | (0.51-0.79) | 42.4 | 96.9 |
| < 319.2 |
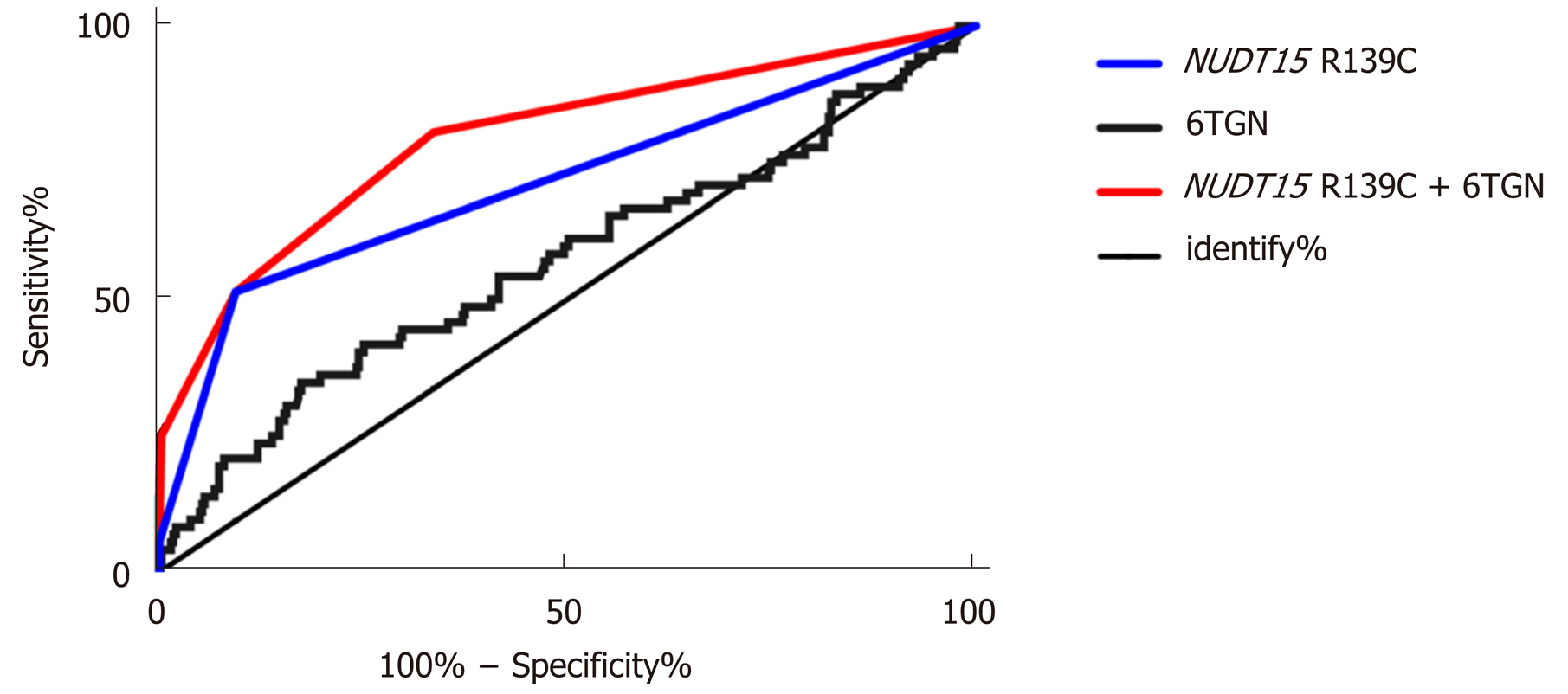
After performing the multivariate regression analysis, we found that the NUDT15 R139C genotypes and 6TGN concentration were relevant determinants for the development of TIL. Table 3 presents the prevalence of TIL and the odds of developing it for each category compared with the reference group of patients with the CC genotype and a 6TGN level below 411.5 pmol/8 × 108 RBC. Patients carrying the NUDT15 TT allele, irrespective of the 6TGN concentration, were at the highest risk, as all of them developed TIL in this study (Table 3). Patients with the CT genotype and a 6TGN concentration above the cut-off value of 319.2 pmol/8 × 108 RBC were at the second highest risk of developing TIL [OR 225.0 (95%CI: 27.6-1836.2)], followed by the CT genotype with a 6TGN concentration below 319.2 pmol/8 × 108 RBC [OR 9.9 (95%CI: 4.5-21.6)] and the CC genotype with a 6TGN concentration above 411.5 pmol/8 × 108 RBC [OR 4.1 (95%CI: 2.0-8.5)] (Table 3). The area under the ROC curve for the obtained predicted probabilities based on NUDT15 and the 6TGN level was 0.79 (95%CI: 0.76-0.92) (Figure 3).
| Category | Leukopenia | % of total | OR (95%CI) | P value | |
| Yes | No | ||||
| NUDT15 R139C TT | 4 | 0 | 4/4 (100%) | ↑↑↑ | - |
| NUDT15 R139C CT + 6TGN > 319.2 | 14 | 1 | 14/15 (93.3%) | 225.0 (27.6-1836.2) | 1.5 × 10-14 |
| NUDT15 R139C CT + 6TGN < 319.2 | 19 | 31 | 19/50 (38.0%) | 9.9 (4.5-21.6) | 8.1 × 10-11 |
| NUDT15 R139C CC + 6TGN > 411.5 | 21 | 82 | 21/103 (20.4%) | 4.1 (2.0-8.5) | 4.8 × 10-5 |
| NUDT15 R139C CC + 6TGN < 411.5 | 14 | 225 | 14/239 (5.9%) | Reference | - |
| Total | 72 | 339 | 72/411 (17.5%) | ||
In this retrospective study, for the first time, we found that the level of 6TGN level was significantly associated with thiopurine-induced TIL in different NUDT15 R139C genotypes. After combing NUDT15 R139C genotypes and different 6TGN cut-off levels, 80% of TIL cases could be explained by an ROCAUC of 0.79.
The incidence of thiopurine-induced TIL is more common in Asians than in Caucasians, even at the lower standard dose[6,33]. The discovery of NUDT15 R139C as a major genetic determinant predicting TIL in the Asian population was a milestone[22,23,34]. In our study, there was a strong relationship between NUDT15 R139C genotypes and TIL (P = 9.04 × 10-20, OR = 8.04, 95%CI: 4.84-13.34) (Table 1). However, in our study, no significant association of TPMT*3C with TIL was observed (Table 1), with only four (30.7%, 4/13) variants experiencing TIL, which is similar to our previous report[33]. Thus, in the Asian population, NUDT15 R139C might be a better biomarker to predict thiopurine toxicity.
NUDT15 is a gene that encodes the purine-specific Nudix hydrolase, which dephosphorylates the active metabolites TGTP and dTGTP into their inactive monophosphates, thus preventing TGTP from binding to Rac1 and dTGTP from incorporating into DNA[27,28]. When the function of NUDT15 is decreased, the excessive accumulation of TGTP and dTGTP may induce TIL, with decreased levels of TGMP and dTGMP[28]. Thus, the total 6TGN remains apparently unchanged. In our study, there were lower median levels of 6TGN in the CT and TT groups compared with the CC group (P = 9.0 × 10-6) (Figure 1). A study on Korean children with ALL also found that the level of 6TGN was negatively correlated with the number of NUDT15 risk alleles (P = 5.3 × 10-6), and the association remained significant after adjusting for the MP dosage (P = 1.7 × 10-6)[26]. However, in our study, this difference was counteracted by the thiopurine dosage (P = 0.29) (Figure 1), suggesting lower 6TGN and 6MMPR levels in patients carrying the T allele due to lower thiopurine administration. Contrary to the function of NUDT15, the patients carrying TPMT variants had excessively higher 6TGN and lower 6MMP levels, which could not be offset by the thiopurine dose (Supplemental Figure 1). These data suggested different mechanisms of TPMT and NUDT15, which made us believe that considering the 6TGN level alone may overlook NUDT15-deficient Asian patients who are prone to TIL[14,26,27]. In our study, we found no difference in the 6TGN concentration between patients with and without TIL in the total samples (P = 0.071). Thus, we subgrouped the entire sample based on patients’ NUDT15 R139C genotypes.
Few studies have analyzed the relationship of the level of 6TGN with TIL according to patients’ NUDT15 diplotypes[31,26]. Based on our previous research, we found that the concentration of 6TGN was significantly correlated with TIL in patients with the CC genotype, while no correlation was found in patients with the CT genotype due to a small sample size (24 patients). However, in this study, which had a larger sample size (n = 65), the level of 6TGN was significantly associated with TIL in the CC and CT groups. In the CC group (n = 342), the median 6TGN concentration was significantly higher in patients who developed TIL than in those who did not [P = 9.4 × 10-5, 474.8 (174.2-1133.6) pmol/8 × 108 RBC vs 306.0 (62.2-1823.0) pmol/8 × 108 RBC] (Figure 2). Similarly, in the CT genotype (n = 65), the level of 6TGN was higher in patients who developed TIL than in patients who did not [P = 0.039, 291.7 (80.6-701.5) pmol/8 × 108 RBC vs 217.6 (62.9-631.0) pmol/8 × 108 RBC] (Figure 2). According to ROC analysis, we defined the cut-off level of 6TGN to be 319.2 pmol/8 × 108 RBC in the CT group, which was significantly lower than that in the CC group (411.5 pmol/8 × 108 RBC). Moriyama et al[28,29] reported that all patients with deficient NUDT15 diplotypes had a higher DNA-TG/dosage ratio, even for the lower level of 6TGN. Thus, in our cohort, CT individuals might have a higher percentage of active dTGTP incorporated into their DNA (DNA-TG), even with a lower TGN level compared with that of CC genotype individuals, which needs to be further investigated.
Although NUDT15 genotype screening can inform the initial drug dosage of thiopurine, therapeutic drug monitoring of its metabolites still serves as a valuable tool for further fine-tuning of the treatment, especially to reduce the incidence of TIL. Dubinsky et al[9] reported that excessive 6TGN levels (> 450 pmol/8 × 108 RBC) were associated with a higher risk of TIL in Caucasians. However, in our study, the sensitivity of 450 pmol/8 × 108 RBC to predict TIL was 34.7% (25/72), as 79% of patients in the NUDT15 CT group and all patients in the TT group developed TIL with a 6TGN level far below 450 pmol/8 × 108 RBC, which indicated that NUDT15 deficient Asian patients were more sensitive to thiopurines. Feng et al[20] reported that the cut-off level for 6TGN in Chinese IBD was from 180-355 pmol/8 × 108 RBC, but patients’ with NUDT15 genotypes were not incorporated and the sample size of TIL was small (n = 12), indicating that the result was not conclusive. Based on our study including 411 patients, the sensitivity of the 6TGN cut-off level to TIL was increased to 42.4% in the CT group and 60.0% in the CC group compared with the threshold values in the studies of Dubinsky and Feng. Thus, different 6TGN cut-off levels should be considered in different NUDT15 genotypes in CD patients.
Finally, multivariable regression analysis showed that both the NUDT15 genotypes and 6TGN level were associated with TIL. Based on the combinations of these two parameters, patients can be categorized to assess their degree of risk for the development of TIL. Patients with the TT genotype, irrespective of the 6TGN level, developed TIL. Patients with the CT genotype and a 6TGN level above 319.2 pmol/8 × 108 RBC exhibited the highest risk of TIL [OR = 225.0 (95%CI: 27.6-1836.2)], followed by patients carrying either the CT genotype (< 319.2 pmol/8 × 108 RBC) or the CC genotype (> 411.5 pmol/8 × 108 RBC). In a Japanese report, the AUC of the model based on NUDT15 to predict TIL was 0.71[23]. Cao et al[33] reported a similar AUC value of 0.69 in a Chinese multicenter study. However, in our study, the AUC of the combination model to predict TIL was increased to 0.79, and 80% of TIL cases could be predicted. Based on this model, we can distinguish the thiopurine refractoriness if the patient has the steady-state 6TGN levels above the cut-off values without efficacy, which suggests that the thiopurine dose should not be increased and the alternative therapeutic agent should be chose to avoid the incidence of TIL.
There were some limitations in our research. The concentration of DNA-TG was not detected in our study. DNA-TG, the final active metabolite of thiopurines, has been reported to be a new biomarker to predict relapse and is correlated with the concentration of 6TGN in childhood acute lymphoblastic TIL based on a prospective study[35]. Curffari et al[36] also discovered that the levels of DNA-TG metabolites were correlated with the erythrocyte 6TGN level, but not the total leukocyte level. However, a recent study performed by Coulthard et al[37] showed contradictory results, indicating that the exact function of DNA-TG still requires further validation in IBD. On the other hand, we performed this retrospective research to determine the cut-off levels of 6TGN for predicting TIL, which need to be validated in further prospective studies.
In conclusion, this study found that the level of 6TGN was significantly correlated with thiopurine-induced TIL in Chinese CD patients with different NUDT15 genotypes, and specific cut-off values were determined. It is strongly recommended that these specific 6TGN cut-off levels be applied to adjust thiopurine therapy to ensure the therapeutic effects and decrease the incidence of TIL.
Thiopurine-induced leukopenia (TIL) is life-threatening and occurs with a high frequency in Asia. Although nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) variants improve the predictive sensitivity of TIL, more than 50% of TIL cannot be predicted by this mutation. The potential use of the 6-thioguanine nucleotide (6TGN) level to predict TIL is controversial.
Can we increase the predictive sensitivity based on 6TGN by subgrouping patients according to their NUDT15 R139C genotypes?
To obtain a better model with combination of 6TGN levels and NUDT15 R139C genotypes to predict thiopurine-induced TIL and improve the safety for the thiopurine treatment.
A total of 411 patients diagnosed with Crohn’s disease at the Sixth Affiliated Hospital of the Sun Yat-sen University were included in this study. Peripheral blood from patients was collected to detect the NUDT15 R139C/TPMT*3C genotypes and 6TGN concentrations at School of Pharmaceutical Sciences, Sun Yat-sen University. The X2 method or Fisher’s exact test was used to check the association of TIL with NUDT15 R139C/TPMT*3C diplotypes. A receiver operating characteristic (ROC) curve was used to obtain 6TGN cut-off levels to predict the development of TIL.
TIL was observed in 72 individuals with a median 6TGN level of 323.4 pmol/8 × 108 red blood cells (RBC), which was not different from that of patients without TIL (P = 0.071). After comparing the 6TGN levels based on NUDT15 R139C, for CC (n = 342) and CT (n = 65), the median 6TGN level in patients with TIL was significantly higher than that in patients without (P = 9.4 × 10-5, 474.8 pmol/8 × 108 RBC vs 306.0 pmol/8 × 108 RBC and P = 0.039, 291.7 pmol/8 × 108 RBC vs 217.6 pmol/8 × 108 RBC, respectively). The four TT carriers developed TIL, with a median 6TGN concentration of 135.8 pmol/8 × 108 RBC. The 6TGN cut-off levels were 411.5 and 319.2 pmol/8 × 108 RBC for the CC and CT groups, respectively. The area under the ROC curve for the obtained predicted probabilities based on NUDT15 R139C and the 6TGN level was 0.79 (95%CI: 0.76-0.92).
The predictive sensitivity of TIL based on 6TGN is dramatically increased after subgrouping patients according to NUDT15 R139C genotypes.
Applying these specific 6TGN cut-off levels to adjust thiopurine therapies based on NUDT15 R139C is strongly recommended during the thiopurine treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kee BP, Mattar MC S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1448] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 2. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnar T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F European Crohn´s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 875] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 3. | Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, Gomollón F, García-Planella E, Merino O, Gutiérrez A, Esteve M, Márquez L, Garcia-Sepulcre M, Hinojosa J, Vera I, Muñoz F, Mendoza JL, Cabriada JL, Montoro MA, Barreiro-de Acosta M, Ceña G, Saro C, Aldeguer X, Barrio J, Maté J, Gisbert JP. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Shi HY, Chan FK, Leung WK, Li MK, Leung CM, Sze SF, Ching JY, Lo FH, Tsang SW, Shan EH, Mak LY, Lam BC, Hui AJ, Chow WH, Wong MT, Hung IF, Hui YT, Chan YK, Chan KH, Loo CK, Ng CK, Lao WC, Harbord M, Wu JC, Sung JJ, Ng SC. Low-dose azathioprine is effective in maintaining remission in steroid-dependent ulcerative colitis: results from a territory-wide Chinese population-based IBD registry. Therap Adv Gastroenterol. 2016;9:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Lee KM, Kim YS, Seo GS, Kim TO, Yang SK; IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015;13:193-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Moon W, Loftus EV. Review article: recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2016;43:863-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | de Boer NKH, Peyrin-Biroulet L, Jharap B, Sanderson JD, Meijer B, Atreya I, Barclay ML, Colombel JF, Lopez A, Beaugerie L, Marinaki AM, van Bodegraven AA, Neurath MF. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J Crohns Colitis. 2018;12:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 722] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 10. | Gupta P, Gokhale R, Kirschner BS. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Armstrong L, Sharif JA, Galloway P, McGrogan P, Bishop J, Russell RK. Evaluating the use of metabolite measurement in children receiving treatment with a thiopurine. Aliment Pharmacol Ther. 2011;34:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Wong DR, Coenen MJ, Vermeulen SH, Derijks LJ, van Marrewijk CJ, Klungel OH, Scheffer H, Franke B, Guchelaar HJ, de Jong DJ, Engels LG, Verbeek AL, Hooymans PM; TOPIC recruitment team. Early Assessment of Thiopurine Metabolites Identifies Patients at Risk of Thiopurine-induced Leukopenia in Inflammatory Bowel Disease. J Crohns Colitis. 2017;11:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Lim SZ, Chua EW. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front Pharmacol. 2018;9:1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Ooi CY, Bohane TD, Lee D, Naidoo D, Day AS. Thiopurine metabolite monitoring in paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Hanai H, Iida T, Takeuchi K, Arai O, Watanabe F, Abe J, Maruyama Y, Oohata A, Ikeya K, Kageoka M, Miwa I, Yoshirou S, Hosoda Y, Kubota T. Thiopurine maintenance therapy for ulcerative colitis: the clinical significance of monitoring 6-thioguanine nucleotide. Inflamm Bowel Dis. 2010;16:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Odahara S, Uchiyama K, Kubota T, Ito Z, Takami S, Kobayashi H, Saito K, Koido S, Ohkusa T. A Prospective Study Evaluating Metabolic Capacity of Thiopurine and Associated Adverse Reactions in Japanese Patients with Inflammatory Bowel Disease (IBD). PLoS One. 2015;10:e0137798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | van Gennep S, Konté K, Meijer B, Heymans MW, D'Haens GR, Löwenberg M, de Boer NKH. Systematic review with meta-analysis: risk factors for thiopurine-induced leukopenia in IBD. Aliment Pharmacol Ther. 2019;50:484-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Feng R, Guo J, Zhang SH, Qiu Y, Chen BL, He Y, Zeng ZR, Ben-Horin S, Chen MH, Mao R. Low 6-thioguanine nucleotide level: Effective in maintaining remission in Chinese patients with Crohn's disease. J Gastroenterol Hepatol. 2019;34:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Komiyama T, Yajima T, Kubota R, Iwao Y, Sakuraba A, Funakoshi S, Negishi K, Minami I, Tanaka Y, Mae H, Hibi T. Lower doses of 6-mercaptopurine/azathioprine bring enough clinical efficacy and therapeutic concentration of erythrocyte 6-mercaptopurine metabolite in Japanese IBD patients. J Crohns Colitis. 2008;2:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, Haritunians T, Ye BD, Kim KJ, Park SH, Park SK, Yang DH, Dubinsky M, Lee I, McGovern DP, Liu J, Song K. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 23. | Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, Nishida A, Nakagawa S, Miura M, Toyonaga T, Onodera K, Shinozaki M, Ishiguro Y, Mizuno S, Takahara M, Yanai S, Hokari R, Nakagawa T, Araki H, Motoya S, Naito T, Moroi R, Shiga H, Endo K, Kobayashi T, Naganuma M, Hiraoka S, Matsumoto T, Nakamura S, Nakase H, Hisamatsu T, Sasaki M, Hanai H, Andoh A, Nagasaki M, Kinouchi Y, Shimosegawa T, Masamune A, Suzuki Y; MENDEL study group. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53:1065-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, Moyer AM, Evans WE, Klein TE, Antillon-Klussmann FG, Caudle KE, Kato M, Yeoh AEJ, Schmiegelow K, Yang JJ. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin Pharmacol Ther. 2019;105:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 25. | Yi ES, Choi YB, Choi R, Lee NH, Lee JW, Yoo KH, Sung KW, Lee SY, Koo HH. NUDT15 Variants Cause Hematopoietic Toxicity with Low 6-TGN Levels in Children with Acute Lymphoblastic Leukemia. Cancer Res Treat. 2018;50:872-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Valerie NC, Hagenkort A, Page BD, Masuyer G, Rehling D, Carter M, Bevc L, Herr P, Homan E, Sheppard NG, Stenmark P, Jemth AS, Helleday T. NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to Mediate the Anticancer Efficacy of 6-Thioguanine. Cancer Res. 2016;76:5501-5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K, Hofmann U, Komada Y, Kato M, McCorkle R, Li L, Koh K, Najera CR, Kham SK, Isobe T, Chen Z, Chiew EK, Bhojwani D, Jeffries C, Lu Y, Schwab M, Inaba H, Pui CH, Relling MV, Manabe A, Hori H, Schmiegelow K, Yeoh AE, Evans WE, Yang JJ. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 29. | Moriyama T, Nishii R, Lin TN, Kihira K, Toyoda H, Jacob N, Kato M, Koh K, Inaba H, Manabe A, Schmiegelow K, Yang JJ, Hori H. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017;27:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Ding L, Zhang FB, Liu H, Gao X, Bi HC, Wang XD, Chen BL, Zhang Y, Zhao LZ, Zhong GP, Hu PJ, Chen MH, Huang M. Hypoxanthine guanine phosphoribosyltransferase activity is related to 6-thioguanine nucleotide concentrations and thiopurine-induced leukopenia in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Zhu X, Wang XD, Chao K, Zhi M, Zheng H, Ruan HL, Xin S, Ding N, Hu PJ, Huang M, Gao X. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther. 2016;44:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998;44:551-555. [PubMed] |
| 33. | Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, Yang H, Liang J, Lin L, Huang Z, Zhang Y, Huang Y, Sun Y, Xue X, Huang M, Hu P, Lan P, Gao X. Combined Detection of NUDT15 Variants Could Highly Predict Thiopurine-induced Leukopenia in Chinese Patients with Inflammatory Bowel Disease: A Multicenter Analysis. Inflamm Bowel Dis. 2017;23:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, Pei D, Chen Y, Crews KR, Kornegay N, Wong FL, Evans WE, Pui CH, Bhatia S, Relling MV. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 332] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 35. | Nielsen SN, Grell K, Nersting J, Abrahamsson J, Lund B, Kanerva J, Jónsson ÓG, Vaitkeviciene G, Pruunsild K, Hjalgrim LL, Schmiegelow K. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol. 2017;18:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Cuffari C, Li DY, Mahoney J, Barnes Y, Bayless TM. Peripheral blood mononuclear cell DNA 6-thioguanine metabolite levels correlate with decreased interferon-gamma production in patients with Crohn's disease on AZA therapy. Dig Dis Sci. 2004;49:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Coulthard SA, Berry P, McGarrity S, McLaughlin S, Ansari A, Redfern CPF. Azathioprine with Allopurinol: Lower Deoxythioguanosine in DNA and Transcriptome Changes Indicate Mechanistic Differences to Azathioprine Alone. Inflamm Bowel Dis. 2017;23:946-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |