Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5641
Peer-review started: May 24, 2019
First decision: June 16, 2019
Revised: July 12, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: October 7, 2019
Processing time: 129 Days and 21.4 Hours
Robotic surgery has been considered to be significantly better than laparoscopic surgery for complicated procedures.
To explore the short-term effect of robotic and laparoscopic spleen-preserving splenic hilar lymphadenectomy (SPSHL) for advanced gastric cancer (GC) by Huang’s three-step maneuver.
A total of 643 patients who underwent SPSHL were recruited from April 2012 to July 2017, including 35 patients who underwent robotic SPSHL (RSPSHL) and 608 who underwent laparoscopic SPSHL (LSPSHL). One-to-four propensity score matching was used to analyze the differences in clinical data between patients who underwent robotic SPSHL and those who underwent laparoscopic SPSHL.
In all, 175 patients were matched, including 35 patients who underwent RSPSHL and 140 who underwent LSPSHL. After matching, there were no significant differences detected in the baseline characteristics between the two groups. Significant differences in total operative time, estimated blood loss (EBL), splenic hilar blood loss (SHBL), splenic hilar dissection time (SHDT), and splenic trunk dissection time were evident between these groups (P < 0.05). Furthermore, no significant differences were observed between the two groups in the overall noncompliance rate of lymph node (LN) dissection (62.9% vs 60%, P = 0.757), number of retrieved No. 10 LNs (3.1 ± 1.4 vs 3.3 ± 2.5, P = 0.650), total number of examined LNs (37.8 ± 13.1 vs 40.6 ± 13.6, P = 0.274), and postoperative complications (14.3% vs 17.9%, P = 0.616). A stratified analysis that divided the patients receiving RSPSHL into an early group (EG) and a late group (LG) revealed that the LG experienced obvious improvements in SHDT and length of stay compared with the EG (P < 0.05). Logistic regression showed that robotic surgery was a significantly protective factor against both SHBL and SHDT (P < 0.05).
RSPSHL is safe and feasible, especially after overcoming the early learning curve, as this procedure results in a radical curative effect equivalent to that of LSPSHL.
Core tip: The safety and oncologic efficacy of spleen-preserving splenic hilar lymphadenectomy (SPSHL) are being evaluated, but the data are limited, especially for robotic surgery. What’s more, few previous studies have compared robotic SPSHL (RSPSHL) with laparoscopic SPSHL (LSPSHL) for gastric cancer. Huang’s three-step maneuver constitutes a set of effective surgical procedures for the SPSHL, which was proposed previously by our department. Thus, we offer a single-institution investigation on the comparative safety, feasibility, and oncologic efficacy of robotic vs laparoscopic SPSHL. It is worth noting that this is the first study that compared RSPSHL and LSPSHL using Huang’s three-step maneuver for advanced gastric cancer.
- Citation: Wang JB, Liu ZY, Chen QY, Zhong Q, Xie JW, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Que SJ, Zheng CH, Huang CM, Li P. Short-term efficacy of robotic and laparoscopic spleen-preserving splenic hilar lymphadenectomy via Huang's three-step maneuver for advanced upper gastric cancer: Results from a propensity score-matched study. World J Gastroenterol 2019; 25(37): 5641-5654
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5641
Since 1991, when Kitano[1] first reported the application of laparoscopic radical gastrectomy in early gastric cancer (GC), minimally invasive surgery has increasingly been used to treat GC. In subsequent years, many scholars have consistently confirmed that laparoscopic gastrectomy is a viable option for the treatment of GC. For D2 lymph node (LN) dissection in patients with advanced upper GC, some surgeons with extensive experience in laparoscopic surgery recommend spleen-preserving splenic hilar lymphadenectomy (SPSHL), which is the most challenging part of surgery for GC[2-4].
For successful surgical intervention in the splenic hilar area, our department proposed Huang's three-step maneuver in 2012. Huang's three-step maneuver divided this complicated surgical procedure into the dissection of LNs in the inferior pole region of the spleen (first step), the dissection of LNs in the region of the splenic artery trunk (second step), and the dissection of LNs in the superior pole region of the spleen (third step). Huang’s three-step maneuver constitutes a set of effective surgical procedures for SPSHL. Compared with traditional surgical methods, Huang’s three-step maneuver has better short-term surgical outcomes[5-7]. However, the splenic hilar region has a complicated and variable anatomy. Due to the characteristics of the splenic hilar region of interest, surgeons, especially novices, often find it difficult to maneuver in the narrow space during surgery, and thus the broad application of this procedure is limited.
Since 2003, when robotic distal gastrectomy was first reported[8], its safety and feasibility have been repeatedly confirmed. Due to its flexibility, the extensive range of motion beyond the human body, and the clear field of vision compared with traditional laparoscopy, robotic surgery has been considered in some preliminary studies to be significantly better than laparoscopic surgery for complicated procedures[8,9]. However, the safety and efficacy of robotic SPSHL (RSPSHL) in patients with advanced upper GC have rarely been reported. Therefore, this study aimed to retrospectively analyze the clinical and pathological data of patients with advanced upper GC in our department to compare the short-term effects of RSPSHL and laparoscopic SPSHL (LSPSHL) using propensity score matching.
From April 2012 to July 2017, we retrospectively analyzed the clinicopathological data of 643 patients who underwent SPSHL for GC at the Department of Gastric Surgery of Fujian Medical University Union Hospital. These patients were all treated by the same group of surgeons. The exclusion criteria (Supplemental Figure 1) were as follows: (1) Non-total gastrectomy; (2) Non-D2 radical surgery; (3) Non-No. 10 LN dissection; (4) American Society of Anesthesiologists (ASA) class IV; (5) cT1 or cT4b; (6) cTNM stage IV; and (7) incomplete data. Age, sex, body mass index (BMI), ASA class, cT, and cN were used as the matching factors. The two groups were matched by one-to-four propensity score matching. Finally, 175 patients were enrolled, including 35 who underwent RSPSHL and 140 who underwent LSPSHL. This retrospective study was approved by the Ethics Committee of Fujian Medical University Union Hospital. All patients signed informed consent forms before surgery. The clinical procedure in our department is described below. First, nasal feeding is performed through a nasal jejunal tube. If no abnormal conditions are present, an oral fluid and soft diet are provided in a stepwise manner. The drain tube is then removed based on the patient’s condition.
All patients underwent routine preoperative examinations, including upper gastrointestinal endoscopy and upper gastrointestinal imaging, to assess the tumor location. In addition to chest radiography, total abdominal computed tomography (CT), abdominal ultrasonography, positron emission tomography (PET)-CT, and bone scans were performed as needed to assess the preoperative clinical stage. Patients with upper GC, defined as a tumor located in the upper or middle region of the stomach, cardia, or gastroesophageal junction, received confirmation of the preoperative diagnosis of tumor invasion into or beyond the muscle layers. These patients were eligible for spleen-preserving total gastrectomy. The extent of LN dissection was based on the Japanese Gastric Cancer Treatment Guidelines in 2010[10]. Preoperative comorbidity was assessed using the Charlson score. pTNM staging was performed using the 2010 edition of the 7th edition of the Union for International Cancer Control (UICC) staging system. All robotic gastrectomies were performed with the Da Vinci surgical system. Each patient was given detailed information about the instruments, sizes of the incisions, oncological risks, and the cost of the operation for both the robotic and laparoscopic approaches. The decision to receive laparoscopic vs robotic treatment was made by the patient after an informed discussion about both approaches because of the additional expenses for robotic surgery[11]. A portion of the fee for laparoscopic surgery was covered by medical insurance, but the extra charges associated with robotic surgery were not included in the medical insurance coverage.
Operative procedures considered Huang’s three-step maneuver were the same as previously described[5-7,12,13] and included the following three steps: the dissection of the LNs in the inferior pole region of the spleen; the dissection of the LNs in the region of the trunk of the splenic artery (SpA); and the dissection of the LNs in the superior pole region of the spleen. The first step begins with the separation of the gastrosplenic ligament using an ultrasonic scalpel and ends with the dissection of the lymphatic tissue along the vessels distally up to the splenic hilar region. The second step involves using the ultrasonic scalpel to denude the trunk of the SpA along the latent anatomic space on its surface toward the splenic hilar area until the fork of the splenic lobar arteries is reached. The third step begins with the dissection of the fork of the splenic lobar arteries and ends with the division of the last short gastric vessel (SGV). This procedure was used in both RSPSHLs and LSPSHLs. Furthermore, the method of RSPSHL was similar to that of LSPSHL. A previous report[12] provided the details of the laparoscopic procedure. Each step starts with the initial transection of the tissues and ends with dividing the tissues in the subsequent step of the maneuver using an ultrasonic scalpel. The photographs show the relevant steps of RSPSHL (Supplemental Figure 2A and B).
The total operative time consisted of the docking time and operation time (OR time) during robotic surgery. OR time was defined as the time between the trocar insertion and the closure of the abdomen, but the extra time required to incorporate the robotic system was not included. Estimated blood loss (EBL) was determined by calculating the amount of gauze and the amount of blood in the aspirator. Splenic hilar dissection time (SHDT) began when the gastrosplenic ligament was cut and ended when the last SGV was divided. Splenic hilar blood loss (SHBL) during the SHDT was determined by calculating the amount of gauze used and the amount of blood in the aspirator. The amount of extra fluid pooled around the spleen after Huang’s three-step maneuver was completed was not included in the SHBL calculation. Two types of SpA were found, namely, the concentrated type and the distributed type. The concentrated type was present when the SpA divides into its terminal branches less than 2 cm from the splenic hilum. If the distance is equal to or greater than 2 cm, the artery was considered the distributed type. Vascular injury was defined as bleeding caused by intraoperative vascular injury that required a titanium clip for clamping or electrocoagulation[6,14]. Each individual case parameter was evaluated routinely after surgery as soon as possible. Noncompliance was defined as patients with more than one empty LN station, as described in the protocol of D2 LN dissection from the Japanese GC Association[10]. Complications were graded according to the Clavien–Dindo classification. According to the references[15,16], the patients in the robotic cohort were divided into an "early group" (EG) if surgery occurred before January 2017 (n = 20) and a "late group" (LG) if surgery occurred after January 2017 (n = 15).
Propensity score matching was performed using R software (3.4.2, the statistical packages MatchIt and Foreign, http://www.r-project.org). All data were statistically analyzed using the SPSS 20.0 (SPSS Inc. Chicago, IL, United States). Continuous variables were analyzed using the Student's t-test or Mann-Whitney U test, and categorical variables were analyzed using the χ2 test or Fisher's exact test. The SHDT and the times for the first, second, and third steps of the maneuver were analyzed by binary class comparison using the medians as the cut-off values. Logistic regression analysis was performed to evaluate the effects of robotic surgery on the intraoperative and postoperative parameters. Two-sided P < 0.05 indicated a significant difference.
Before matching, significant differences in ASA, cT, and pTNM were evident between the two groups of patients (P < 0.05). The clinical data of the patients in the RSPSHL (n = 35) and LSPSHL (n = 140) groups were similar when matched for age, sex, BMI, ASA, cT, and cN (P > 0.05) (Table 1).
| Variable | All patients | Propensity score matching | ||||||||
| RSPSHL | LSPSHL | P-value | RSPSHL | LSPSHL | P-value | |||||
| (n = 35) | % | (n = 608) | % | (n = 35) | % | (n = 140) | % | |||
| Age, yr1 | 55.3 ± 10.4 | 56.9 ± 10.6 | 0.424 | 55.3 ± 10.4 | 55.1 ± 11.5 | 0.925 | ||||
| Sex | 0.345 | 0.615 | ||||||||
| Female | 6 | 17.1 | 156 | 25.7 | 6 | 17.1 | 17 | 12.1 | ||
| Male | 29 | 82.9 | 452 | 74.3 | 29 | 82.9 | 123 | 87.9 | ||
| BMI, kg/m2[1] | 23.0 ± 2.7 | 22.2 ± 3.0 | 0.152 | 23.0 ± 2.7 | 23.1 ± 3.0 | 0.858 | ||||
| ASA score | 0.014 | 0.944 | ||||||||
| I | 4 | 11.4 | 186 | 30.6 | 4 | 11.4 | 19 | 13.6 | ||
| II | 28 | 80.0 | 401 | 65.9 | 28 | 80.0 | 109 | 77.8 | ||
| III | 3 | 8.6 | 21 | 3.5 | 3 | 8.6 | 12 | 8.6 | ||
| cT classification | 0.037 | 0.211 | ||||||||
| T2 | 10 | 28.6 | 100 | 16.4 | 10 | 28.6 | 22 | 15.7 | ||
| T3 | 15 | 42.9 | 207 | 34.1 | 15 | 42.9 | 69 | 49.3 | ||
| T4a | 10 | 28.6 | 301 | 49.5 | 10 | 28.6 | 49 | 35.0 | ||
| cN classification | 0.483 | 0.541 | ||||||||
| N0 | 13 | 37.1 | 212 | 34.8 | 13 | 37.1 | 57 | 40.7 | ||
| N1 | 14 | 40.0 | 187 | 30.6 | 14 | 40.0 | 39 | 27.9 | ||
| N2 | 4 | 11.4 | 122 | 20.0 | 4 | 11.4 | 21 | 15.0 | ||
| N3 | 4 | 11.4 | 89 | 14.6 | 4 | 11.4 | 23 | 16.4 | ||
| cTNM stage | 0.094 | 0.137 | ||||||||
| IB | 2 | 5.7 | 55 | 9.0 | 2 | 5.7 | 13 | 9.3 | ||
| IIA | 7 | 20.0 | 106 | 17.4 | 7 | 20.0 | 18 | 12.9 | ||
| IIB | 13 | 37.1 | 104 | 17.1 | 13 | 37.1 | 31 | 22.1 | ||
| IIIA | 7 | 20.0 | 137 | 22.5 | 7 | 20.0 | 22 | 15.7 | ||
| IIIB | 3 | 8.6 | 109 | 17.9 | 3 | 8.6 | 29 | 20.7 | ||
| IIIC | 3 | 8.6 | 97 | 16.0 | 3 | 8.6 | 27 | 19.3 | ||
| Depth of invasion | 0.201 | 0.617 | ||||||||
| pT1a | 5 | 14.3 | 31 | 5.1 | 5 | 14.3 | 10 | 7.1 | ||
| pT1b | 4 | 11.4 | 52 | 8.6 | 4 | 11.4 | 24 | 17.1 | ||
| pT2 | 2 | 5.7 | 55 | 9 | 2 | 5.7 | 10 | 7.1 | ||
| pT3 | 16 | 45.7 | 283 | 46.5 | 16 | 45.7 | 57 | 40.7 | ||
| pT4a | 8 | 22.9 | 187 | 30.8 | 8 | 22.9 | 39 | 27.9 | ||
| Metastatic LNs | 0.308 | 0.649 | ||||||||
| N0 | 16 | 45.7 | 194 | 31.9 | 16 | 45.7 | 54 | 38.6 | ||
| N1 | 6 | 17.1 | 174 | 28.7 | 6 | 17.1 | 20 | 14.3 | ||
| N2 | 6 | 17.1 | 120 | 19.7 | 6 | 17.1 | 23 | 16.4 | ||
| N3 | 7 | 20.0 | 120 | 19.7 | 7 | 20.0 | 43 | 30.7 | ||
| pTNM stage | 0.038 | 0.102 | ||||||||
| IA | 7 | 20.0 | 44 | 7.2 | 7 | 20.0 | 29 | 20.7 | ||
| IB | 3 | 8.6 | 45 | 7.4 | 3 | 8.6 | 8 | 5.7 | ||
| IIA | 6 | 17.1 | 91 | 15.0 | 6 | 17.1 | 23 | 16.4 | ||
| IIB | 6 | 17.1 | 93 | 15.3 | 6 | 17.1 | 16 | 11.4 | ||
| IIIA | 6 | 17.1 | 122 | 20.1 | 6 | 17.1 | 8 | 5.7 | ||
| IIIB | 2 | 5.7 | 149 | 24.5 | 2 | 5.7 | 31 | 22.1 | ||
| IIIC | 5 | 14.3 | 64 | 10.5 | 5 | 14.3 | 25 | 17.9 | ||
| Charlson score | 0.467 | 0.132 | ||||||||
| 0 | 25 | 71.5 | 439 | 72.2 | 25 | 71.5 | 90 | 64.3 | ||
| 1 | 6 | 17.1 | 129 | 21.2 | 6 | 17.1 | 43 | 30.7 | ||
| ≥2 | 4 | 11.4 | 40 | 6.6 | 4 | 11.4 | 7 | 5.0 | ||
| Primary site | 0.458 | 0.335 | ||||||||
| Upper | 21 | 60.0 | 402 | 66.1 | 21 | 60.0 | 96 | 68.6 | ||
| Middle | 14 | 40.0 | 206 | 33.9 | 14 | 40.0 | 44 | 31.4 | ||
No conversion to open surgery occurred in the LSPSHL group, and no conversion to open or laparoscopic surgery occurred in the RSPSHL group. After matching, the total OR time was significantly longer in the RSPSHL group than in the LSPSHL group, while the EBL, SHDT, SHBL, and time for the second step were lower in the RSPSHL group than in the LSPSHL group (P < 0.05). The two groups were similar in terms of OR time, first step time, third step time, splenic injury, vascular injury, and anatomy (e.g., SGVs, the PGA, and the terminal branches of the SpA) (P > 0.05). The No. 10 LN metastasis rate was 10.9% (19/175), and the rates were similar between the RSPSHL (11.4%, 4/35) and LSPSHL (10.7%, 15/140) groups (P = 0.855) (Table 2). The overall noncompliance rates for patients undergoing RSPSHL and LSPSHL were 62.9% (22/35) and 60% (84/140), respectively (P = 0.757). Subgroup analysis by clinical stage or BMI revealed no significant differences in the noncompliance rates of LN dissection between the two groups (P > 0.05) (Table 3).
| Variable | RSPSHL (n = 35) | % | LSPSHL (n = 140) | % | P-value |
| Total operative time, min1 | 221.3 ± 40.3 | 189.1 ± 43.8 | <0.001 | ||
| Docking time, min2 | 30 (26-34) | - | |||
| OR time, min1 | 186.0 ± 35.3 | 189.1 ± 43.8 | 0.698 | ||
| EBL, mL1 | 13.7 ± 4.3 | 62.4 ± 29.3 | <0.001 | ||
| SHDT, min1 | 20.4 ± 4.5 | 24.1 ± 8.9 | 0.018 | ||
| First step, min1 | 8.4 ± 3.5 | 8.7 ± 4.0 | 0.685 | ||
| Second step, min1 | 6.7 ± 2.6 | 9.8 ± 5.6 | 0.002 | ||
| Third step, min1 | 5.2 ± 2.1 | 5.6 ± 3.0 | 0.458 | ||
| SHBL, mL1 | 2.2 ± 1.9 | 10.0 ± 4.5 | <0.001 | ||
| No. of SGVs1 | 3.5 ± 1.2 | 3.6 ± 1.1 | 0.637 | ||
| No. of PGAs (yes) | 22 | 62.9 | 81 | 57.9 | 0.591 |
| No. of SUPAs (yes) | 4 | 11.4 | 20 | 14.3 | 0.870 |
| No. of SLPAs (yes) | 1 | 2.9 | 7 | 5 | 0.928 |
| Terminal branches of SpA | 0.754 | ||||
| Concentrated type | 23 | 65.7 | 88 | 62.9 | |
| Distributed type | 12 | 34.3 | 52 | 37.1 | |
| Splenic injury | 3 | 8.6 | 19 | 13.6 | 0.608 |
| Vascular injury | 7 | 20 | 17 | 12.1 | 0.350 |
| No.10 metastatic LN | 4 | 11.4 | 15 | 10.7 | 0.855 |
| No.10 retrieved LN1 | 3.1 ± 1.4 | 3.3 ± 2.5 | 0.650 | ||
| Total retrieved LNs1 | 37.8 ± 13.1 | 40.6 ± 13.6 | 0.274 |
| Variable | RSPSHL | LSPSHL | P-value | ||
| Compliant | Noncompliant | Compliant | Noncompli-ant | ||
| cT classification | |||||
| cT2 | 3 | 7 | 6 | 16 | 0.791 |
| cT3 | 6 | 9 | 31 | 39 | 0.761 |
| cT4 | 4 | 6 | 19 | 29 | 0.741 |
| cN classification | |||||
| cN0 | 4 | 9 | 22 | 36 | 0.868 |
| cN1 | 6 | 8 | 15 | 23 | 0.825 |
| cN2 | 2 | 2 | 11 | 10 | 0.647 |
| cN3 | 1 | 3 | 8 | 15 | 0.848 |
| cTNM stage | |||||
| I | 1 | 1 | 5 | 8 | 1.000 |
| II | 9 | 16 | 18 | 31 | 0.950 |
| III | 3 | 5 | 33 | 45 | 0.909 |
| BMI, kg/m2 | |||||
| < 25 | 11 | 14 | 38 | 68 | 0.449 |
| ≥ 25 | 2 | 8 | 18 | 16 | 0.139 |
| Total | 13 | 22 | 56 | 84 | 0.757 |
Significant differences were evident in the times to liquid diet intake and nasojejunal tube removal, which were shorter in the RSPSHL group than in the LSPSHL group (P < 0.05). However, the times to ambulation, flatus passage, soft diet intake, and drain removal, and the length of stay (LOS) were similar between the groups (P > 0.05) (Table 4). No significant differences were evident in the overall postoperative complications or in grades I-II and III-IV complications between the two groups. Surgical complications (including intraperitoneal hemorrhage and infection) in both groups were resolved with conservative treatments. No deaths occurred in the hospital or within 30 d after surgery.
| Variable | RSPSHL (n = 35) | % | LSPSHL (n = 140) | % | P-value |
| Time to ambulation, d1 | 2.4 ± 0.9 | 2.1 ± 1.0 | 0.107 | ||
| Flatus passage, d1 | 3.2 ± 0.9 | 3.5 ± 1.3 | 0.199 | ||
| Liquid diet, d1 | 4.8 ± 1.1 | 5.4 ± 1.7 | 0.049 | ||
| Soft diet, d1 | 7.0 ± 2.4 | 7.5 ± 1.5 | 0.125 | ||
| Drain removal, d1 | 9.2 ± 2.1 | 9.6 ± 2.0 | 0.296 | ||
| Nasojejunal tube removal, d1 | 3.9 ± 1.6 | 4.8 ± 2.5 | 0.044 | ||
| LOS, d1 | 14.7 ± 13.4 | 14.4 ± 11.5 | 0.894 | ||
| Overall complications | 5 | 14.3 | 25 | 17.9 | 0.616 |
| Grade I–II | 3 | 8.6 | 17 | 12.1 | 0.632 |
| Anastomotic fistula | 2 | 5.7 | 3 | 2.1 | |
| Digestive hemorrhage | - | 1 | 0.7 | ||
| Lymphatic fistula | - | 6 | 4.3 | ||
| Intestinal obstruction | - | 1 | 0.7 | ||
| Intraperitoneal infection | 1 | 2.9 | 4 | 2.9 | |
| Wound infection | - | 1 | 0.7 | ||
| Fat liquefaction | - | 1 | 0.7 | ||
| Grade III-IV | 2 | 5.7 | 8 | 5.7 | 1 |
| Anastomotic fistula | - | 1 | 0.7 | ||
| Digestive hemorrhage | 1 | 2.9 | 3 | 2.1 | |
| Lymphatic fistula | - | 2 | 1.4 | ||
| Intestinal obstruction | - | 1 | 0.7 | ||
| Intraperitoneal infection | 1 | 2.9 | 1 | 0.7 | |
| 30-day mortality | 0 | 0 | |||
| In-hospital mortality | 0 | 0 |
The SHDT, first step time, second step time, and LOS were longer in the EG than in the LG (P < 0.05). However, the OR time, EBL, third step time, SHBL, number of retrieved No. 10 LNs, total number of retrieved LNs, and postoperative complications were similar between the groups (P > 0.05) (Supplemental Table 1).
Table 5 shows that the first step time was longer than the second step time for both RSHPSH (8.4 ± 3.5 vs 6.7 ± 2.6, P = 0.024) and EG (10.0 ± 3.4 vs 7.5 ± 2.6, P = 0.013). In the LG, the first step time was similar to the second step time (6.3 ± 2.3 vs 5.8 ± 2.2, P = 0.548).
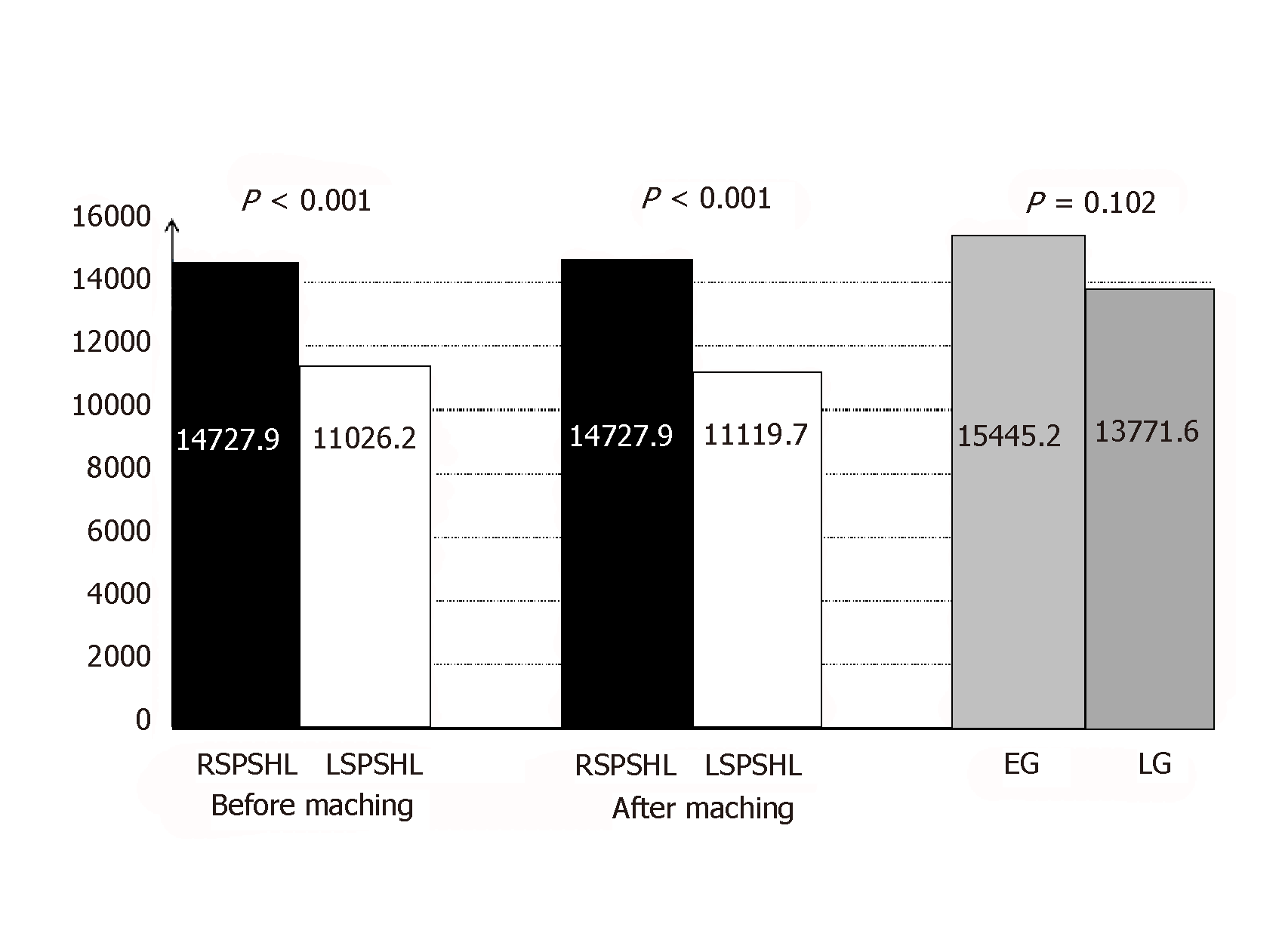
As Figure 1 shows, cost analyses of the inpatient cases revealed that RSPSHL had a higher mean total cost than LSPSHL before matching ($14727.9 vs $11026.2, P < 0.001) and after matching ($15445.2 vs $10397.2, P < 0.001). However, no difference was observed in the mean total cost between the EG and LG ($15445.2 vs $13771.6, P = 0.102).
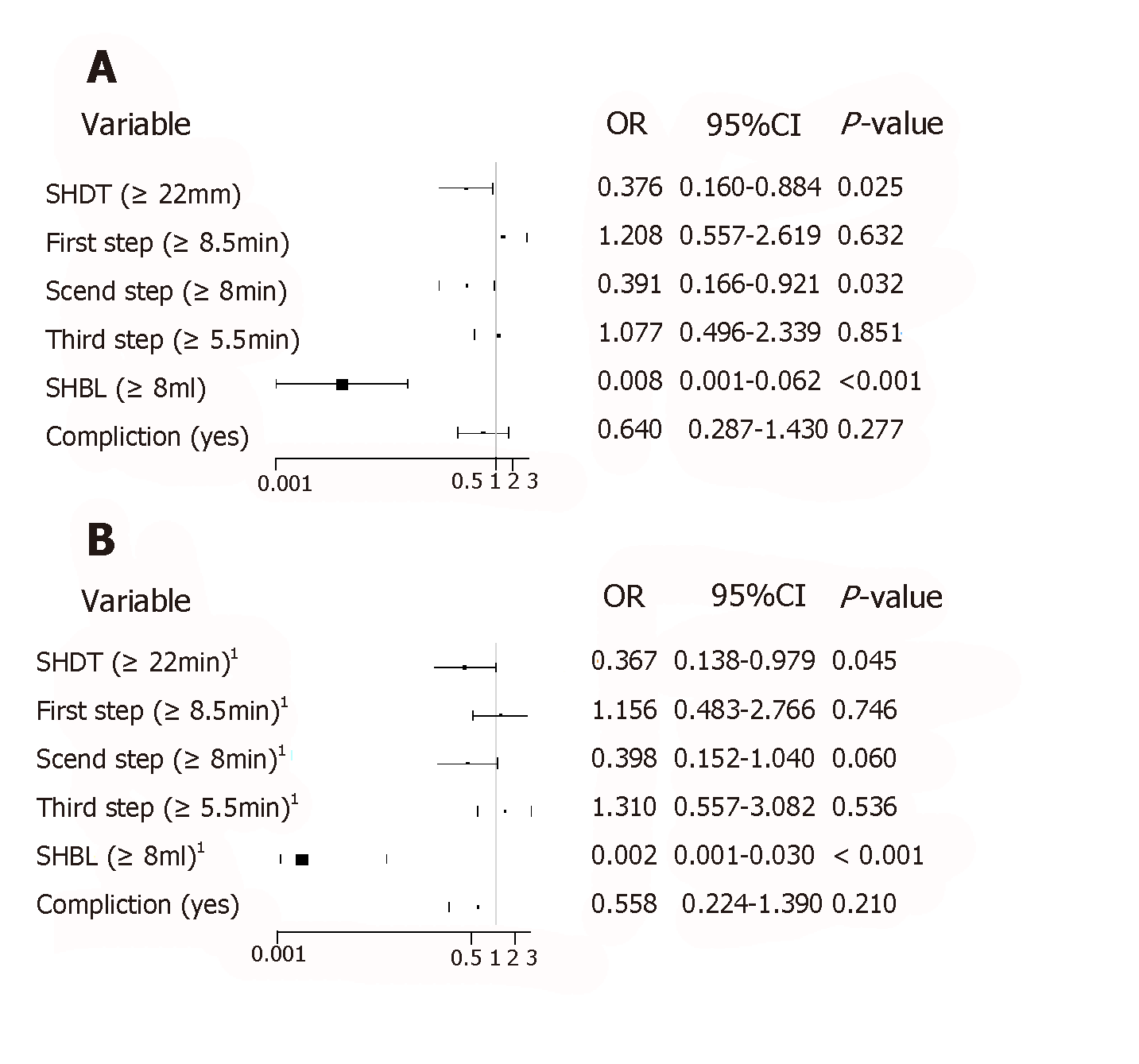
The unadjusted logistic analysis showed that robotic surgery was a positive factor affecting shorter SHDT and second step time and less SHBL. When robotic surgery was adjusted for age, sex, BMI, ASA, cT and cN stages, and several anatomical factors, such as the PGA, SGVs, splenic upper or lower pole arteries, and the terminal branches of the SpA, we found that the effects of robotic surgery were protective factors resulting in a shorter SHDT and less SHBL, both before and after the adjustments (P < 0.05) (Figure 2A and B).
In 2008, Hyung et al[17] first reported LSPSHL and confirmed that laparoscopic spleen-preserving surgery is safe and feasible[5,18-22]. However, traditional laparoscopic surgery is still associated with multiple shortcomings, including a restricted range of motion, amplified physiologic tremors, two-dimensional visualization, and inflexible instruments. These shortcomings have deterred novice surgeons from performing complicated operations, such as LSPSHL[23]. Due to its high stability and precision, the robotic system can overcome the technical limitations of conventional laparoscopic surgery and therefore has great potential for use in complicated surgeries[8,24].
The initial purpose of robotic or laparoscopic surgery was adherence to minimally invasive principles. For postoperative outcomes, a meta-analysis[25] performed in 2012 of robotic versus laparoscopic gastrectomy for GC showed no differences in the incidence of postoperative complications or the LOS between robotic and laparoscopic surgery. However, robotic surgery and laparoscopy could both reduce the risk of postoperative complications and shorten patients’ LOS compared with open surgery[24,26]. Our study also found that RSPSHL is safe. RSPSHL has a postoperative recovery similar to that of LSPSHL, and the related intraoperative and postoperative occurrences of complications were not increased compared with LSPSHL. In the field of GC treatment, the Da Vinci surgical system is expected to overcome the disadvantages of traditional laparoscopic gastrectomy. Most surgeons who recommend laparoscopic gastrectomy have confirmed that robotic gastrectomy (RG) is safe and feasible[27-29]. In 2015, Suda et al[27] demonstrated that the morbidity (overall complication) rate of RG was approximately one-fifth that of laparoscopic gastrectomy and that the incidence of postoperative pancreatic fistula was reduced by RG. RG showed significantly better short-term postoperative outcomes. Moreover, a multi-institutional prospective single-arm study conducted in Japan in 2018 also confirmed that the morbidity rate (Clavien–Dindo grade ≥ IIIa) within 30 d after surgery was significantly lower in the RG group than in the laparoscopic gastrectomy group (RG vs laparoscopic gastrectomy = 2.45% vs 6.4%, P = 0.0018)[30]. Through a cost analysis, we determined that the average total cost of robotic surgery was higher than that of laparoscopic surgery. The results are consistent with those reported by Keller et al[31]. Despite the high economic burden of robotic surgery[32,33], these results suggested that RG would likely exhibit improved cost-effectiveness if the surgical cost per procedure was reduced to approximately the same amount as that for laparoscopic gastrectomy[30]. Consequently, we believe that the application of RG will increase with the decreasing of cost of robotic systems and the government's policy regarding gradual expansion of the range of the medical insurance system.
RSPSHL resulted in a shorter SHDT and less SHBL than LSPSHL. Logistic analysis showed that robotic surgery was a protective factor for a shorter SHDT and less SHBL. We believe that the microscopic images from the robotic system are more vivid and allow easier identification of the spatial relationship between the tissue of the splenic hilar region and the operating instruments than the laparoscopic view[2]. Successful performance of this maneuver in laparoscopy requires close cooperation among the surgeon, assistant, and camera operator. However, if the assistant cannot generate good coordinates while pulling the spleen, intraoperative complications such as splenic envelope laceration can easily occur. Huang’s three-step robotic maneuver uses a multi-arm robotic system with which the surgeon can complete the main pull alone and greatly minimize pulling by the assistant compared with that needed for traditional laparoscopic surgery. Additionally, the mechanical arm can more stably and reliably pull the adipose connective tissue. Both the surgeon and the assistant can avoid errors based on the advantages of this system. The advantages of this system, including the high flexibility of the operative instruments and the elimination of natural hand trembling, result in less intraoperative damage. The stratified analysis showed that with an increasing number of surgical cases and techniques, the LG exhibited significant improvements in SHDT and length of stay compared with the EG. Notably, although shorter SHDT and less SHBL were significantly different between robotic surgery and laparoscopic surgery, it seems that the difference does not exist in the clinical setting.
We believe that Huang's three-step maneuver using the robotic system can help surgeons maintain fluent surgical thinking and body-motor memory, thus improving the response speed of novice surgeons. Even as a novice, the operator can operate freely. The three-step maneuver can reduce the impact of the complex anatomy of the splenic hilar area on the performance of the surgeon. The literature[16,34,35] indicates that the robotic surgery learning curve is shorter than that for laparoscopic surgery. We also propose that learning robotic surgery will be substantially easier for surgeons who already have some basic experience with laparoscopic surgery. A learning curve for Huang’s three-step robotic maneuver also exists, and patients' intraoperative outcomes will significantly improve if surgeons complete this learning curve.
With the application of robotic surgery for GC, minimally invasive advantages have gradually been acknowledged. However, a primary concern of most scholars is whether RSPSHL can achieve equal or better perioperative and postoperative outcomes than laparoscopic or open surgery. The number of retrieved LNs is an important factor in evaluating the efficacy of surgical techniques. After retrospectively analyzing 36 robotic procedures and 65 laparoscopic procedures, Yoon et al[36] found that the total number of retrieved LNs in robotic total gastrectomy (39.4 ± 13.4 vs 42.8 ± 12.7) was similar to that in laparoscopic total gastrectomy (P = 0.209). Obama et al[37] retrospectively analyzed 313 robotic procedures and 524 laparoscopic procedures and found similar outcomes regarding the total number of retrieved LNs between robotic and laparoscopic surgery. In our study, no significant differences between RSPSHL and LSPSHL were observed regarding the total number of retrieved LNs or the number of retrieved No. 10 LNs. This result is consistent with the results of the study by Kazutaka Obama. Moreover, this article introduces noncompliance as an objective measure of LN dissection in robotic surgery and laparoscopy[38-40]. To our knowledge, it is the first study that compares oncological outcomes between RSPSHL and LSPSHL through the LN noncompliance rate. Our study showed that the overall noncompliance rates were similar between the two groups. Further subgroup analysis revealed that the noncompliance rate was also similar after stratification by clinical stage and BMI. However, this conclusion requires more prospective data for validation. SPSHL is usually performed along the particularly intricate and variable vessels in the splenic hilar area. Due to the well-spaced depth of the field and the clear and vivid view provided by the robotic system, robotic surgery can easily identify the anatomy of the splenic hilar vessels and LNs. High-definition three-dimensional magnification imaging can better show small anatomical structures and can be used to more clearly visualize the splenic vasculature[41]. This method can reduce the difficulty of LN dissection and bleeding. The seven degrees of directional freedom of the mechanical wrist in the robotic system allow complex dissections in the narrow space of the splenic hilum, which greatly improves the flexibility of the operation and makes the procedure easier and more convenient than common laparoscopic surgery[2,42]. Moreover, we stress that the robotic system is substantially easier to use and more accurate than laparoscopy in terms of LN exposure, discrimination, and collection in practice. Therefore, robotic surgery has similar outcomes as laparoscopic surgery when the complex process of SPSHL is performed, and robotic surgery is considered as effective as laparoscopy[43,44].
Our study also had several limitations. First, this study was a retrospective study from a single center, and large-sample, prospective, multicenter data are required to validate our findings. Second, the number of robotic cases was very small in this study. Third, we did not address the long-term outcomes of Huang’s three-step robotic maneuver because robotic surgery was implemented relatively recently at our center. Despite these shortcomings, this is the first study in an Eastern population to identify the differences between RSPSHL and LSPSHL using propensity-matched scores to reduce the selection bias.
In conclusion, this study shows that robotic surgery using Huang’s three-step maneuver can be performed to complete SPSHL. The safety of robotic surgery is equivalent to that of laparoscopy, especially after overcoming the early learning curve. Therefore, this procedure is worth promoting to most surgeons and patients. However, its long-term outcomes require further confirmation.
Due to its high stability and precision, the robotic system can overcome the technical limitations of conventional laparoscopic surgery and therefore has great potential for use in complicated surgeries.
Robotic surgery has been considered to be significantly better than laparoscopic surgery for complicated procedures.
The aim of this study was to explore the short-term effect of robotic and laparoscopic spleen-preserving splenic hilar lymphadenectomy (SPSHL) for advanced gastric cancer by Huang’s three-step maneuver.
A total of 643 patients who underwent SPSHL were recruited from April 2012 to July 2017, including 35 patients who underwent robotic SPSHL (RSPSHL) and 608 who underwent laparoscopic SPSHL (LSPSHL). One-to-four propensity score matching was used to analyze the differences in clinical data between groups.
In all, 175 patients were matched, including 35 patients who underwent RSPSHL and 140 who underwent LSPSHL. After matching, there were no significant differences detected in the baseline characteristics between the two groups. Significant differences in total operative time, estimated blood loss (EBL), splenic hilar blood loss (SHBL), splenic hilar dissection time (SHDT), and splenic trunk dissection time were evident between these groups (P < 0.05). Furthermore, no significant differences were observed between the two groups in the overall noncompliance rate of lymph node (LN) dissection (62.9% vs 60%, P = 0.757), number of retrieved No. 10 LNs (3.1 ± 1.4 vs 3.3 ± 2.5, P = 0.650), total number of examined LNs (37.8 ± 13.1 vs 40.6 ± 13.6, P = 0.274), and postoperative complications (14.3% vs 17.9%, P = 0.616). A stratified analysis that divided the patients receiving RSPSHL into an early group (EG) and a late group (LG) revealed that the LG experienced obvious improvements in SHDT and length of stay compared with the EG (P < 0.05). Logistic regression showed that robotic surgery was a significantly protective factor against both SHBL and SHDT (P < 0.05).
The safety of robotic surgery is equivalent to that of laparoscopy, especially after overcoming the early learning curve. Therefore, this procedure is worth promoting to most surgeons and patients. However, its long-term outcomes require further confirmation.
We hope to perform a multi-center prospective study on the long-term outcomes of Huang’s three-step robotic maneuver in order to provide a high-level scientific theoretical basis and clinical experience.
The authors thank all the medical staff who contributed to the maintenance of the medical record database.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fiori E, Tanabe S S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Zhou BX
| 1. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Son SY, Shin DJ, Park YS, Oo AM, Jung DH, Lee CM, Ahn SH, Park DJ, Kim HH. Spleen-preserving lymphadenectomy versus splenectomy in laparoscopic total gastrectomy for advanced gastric cancer. Surg Oncol. 2017;26:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Huang CM, Chen T, Lin JX, Chen QY, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Cao LL, Lin M, Tu RH. The effects of laparoscopic spleen-preserving splenic hilar lymphadenectomy on the surgical outcome of proximal gastric cancer: a propensity score-matched, case-control study. Surg Endosc. 2017;31:1383-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Huang CM, Zhang JR, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY. A 346 case analysis for laparoscopic spleen-preserving no.10 lymph node dissection for proximal gastric cancer: a single center study. PLoS One. 2014;9:e108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Huang CM, Huang ZN, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Jun L, Chen QY, Cao LL, Lin M, Tu RH. Huang's three-step maneuver shortens the learning curve of laparoscopic spleen-preserving splenic hilar lymphadenectomy. Surg Oncol. 2017;26:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Huang ZN, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH. Learning Curve of the Application of Huang Three-Step Maneuver in a Laparoscopic Spleen-Preserving Splenic Hilar Lymphadenectomy for Advanced Gastric Cancer. Medicine (Baltimore). 2016;95:e3252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83:1429-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 11. | Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Huang CM, Zheng CH. A Propensity Score-Matched Comparison of Robotic Versus Laparoscopic Gastrectomy for Gastric Cancer: Oncological, Cost, and Surgical Stress Analysis. J Gastrointest Surg. 2018;22:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW. Huang's three-step maneuver for laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer. Chin J Cancer Res. 2014;26:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 13. | Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Yang XT. Laparoscopic spleen-preserving no. 10 lymph node dissection for advanced proximal gastric cancer using a left approach. Ann Surg Oncol. 2014;21:2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zheng CH, Xu M, Huang CM, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M. Anatomy and influence of the splenic artery in laparoscopic spleen-preserving splenic lymphadenectomy. World J Gastroenterol. 2015;21:8389-8397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, Marsh W, Reddy SK, Bartlett DL. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 16. | Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer. 2012;12:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Li P, Huang CM, Zheng CH, Xie JW, Wang JB, Lin JX. [Laparoscopic spleen-preserving splenic hilar lymph node dissection for proximal gastric cancer]. Zhonghua Wai Ke Za Zhi. 2011;49:795-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Shin SH, Jung H, Choi SH, An JY, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Clinical significance of splenic hilar lymph node metastasis in proximal gastric cancer. Ann Surg Oncol. 2009;16:1304-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Yamashita K, Watanabe M. Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Kosuga T, Ichikawa D, Okamoto K, Komatsu S, Shiozaki A, Fujiwara H, Otsuji E. Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer. 2011;14:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Chikara K, Hiroshi S, Masato N, Hirotoshi A, Goro M, Hidetaka O. Indications for pancreaticosplenectomy in advanced gastric cancer. Hepatogastroenterology. 2001;48:908-912. [PubMed] |
| 23. | Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 332] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Ballantyne GH. Telerobotic gastrointestinal surgery: phase 2--safety and efficacy. Surg Endosc. 2007;21:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Lee JH, Nam BH, Ryu KW, Ryu SY, Kim YW, Park YK, Kim S. Comparison of the long-term results of patients who underwent laparoscopy versus open distal gastrectomy. Surg Endosc. 2016;30:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Isogaki J, Haruta S, Man-I M, Suda K, Kawamura Y, Yoshimura F, Kawabata T, Inaba K, Ishikawa K, Ishida Y, Taniguchi K, Sato S, Kanaya S, Uyama I. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology. 2011;78:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, Ehara K, Obama K, Kuwabara S, Okabe H, Terashima M. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer. 2019;22:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 31. | Keller DS, Senagore AJ, Lawrence JK, Champagne BJ, Delaney CP. Comparative effectiveness of laparoscopic versus robot-assisted colorectal resection. Surg Endosc. 2014;28:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 33. | Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, Ryu KW, Kim YW, Lee JH. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg. 2012;99:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Park EJ, Kim CW, Cho MS, Kim DW, Min BS, Baik SH, Lee KY, Kim NK. Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer?: a comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine (Baltimore). 2014;93:e109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc. 2012;26:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ, Rho JY. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Obama K, Kim YM, Kang DR, Son T, Kim HI, Noh SH, Hyung WJ. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. 2018;21:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 38. | Lee JH, Kim YW, Ryu KW, Lee JR, Kim CG, Choi IJ, Kook MC, Nam BH, Bae JM. A phase-II clinical trial of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer patients. Ann Surg Oncol. 2007;14:3148-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, Jeong O, Yoon KY, Lee JH, Lee SE, Yu W, Jeong SH, Kim T, Kim S, Nam BH; COACT group. Laparoscopy-assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg. 2018;267:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 40. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 960] [Reference Citation Analysis (0)] |
| 41. | Coratti A, Annecchiarico M, Di Marino M, Gentile E, Coratti F, Giulianotti PC. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J Surg. 2013;37:2771-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg. 2004;188:19S-26S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 43. | Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, Kim JH, Park SH, Mok YJ, Park SS. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |