Published online Sep 21, 2019. doi: 10.3748/wjg.v25.i35.5257
Peer-review started: July 2, 2019
First decision: August 2, 2019
Revised: August 8, 2019
Accepted: August 24, 2019
Article in press: August 2, 2019
Published online: September 21, 2019
Processing time: 81 Days and 21.1 Hours
Minimal hepatic encephalopathy (MHE) represents the mildest type of hepatic encephalopathy (HE). MHE is considered as a preclinical stage of HE and is part of a wide spectrum of typical neurocognitive alterations characteristic of patients with liver cirrhosis, particularly involving the areas of attention, alertness, response inhibition, and executive functions. MHE can be detected by testing the patients’ psychometric performance, attention, working memory, psychomotor speed, and visuospatial ability, as well as by means of electrophysiological and other functional brain measures. MHE is very frequent, affecting from 20% up to 80% of patients tested, depending of the diagnostic tools used. Although subclinical, MHE is considered to be clinically relevant. In fact, MHE has been related to the patients’ falls, fitness to drive, and working ability. As a consequence, MHE affects the patients and caregivers lives by altering their quality of life and even their socioeconomic status. Recently sarcopenia, a very common condition in patients with advanced liver disease, has been shown to be strictly related to both minimal and overt HE. Aim of this review is to summarize the most recently published evidences about the emerging relationship between sarcopenia and cognitive impairment in cirrhotic patients and provide suggestions for future research.
Core tip: Minimal hepatic encephalopathy (MHE) represents the mildest type of hepatic encephalopathy, is very frequent, affecting from 20% up to 80% of patients tested, depending of the diagnostic tools used and has been related to the patients’ falls, fitness to drive, and working ability. As a consequence, MHE affects the patients and caregivers lives by altering their quality of life and even their socioeconomic status. Sarcopenia has been recently proposed as a risk factor for both minimal and overt Hepatic Encephalopathy. Aim of this review is to summarize the most recently published evidences about the emerging relationship between sarcopenia and cognitive impairment.
- Citation: Nardelli S, Gioia S, Faccioli J, Riggio O, Ridola L. Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy. World J Gastroenterol 2019; 25(35): 5257-5265
- URL: https://www.wjgnet.com/1007-9327/full/v25/i35/5257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i35.5257
Hepatic encephalopathy (HE) is a complex neurological syndrome caused by liver failure and shunting of the portal blood into the systemic circulation. HE produces a wide and complex spectrum of nonspecific neurological and psychiatric manifestations[1]. In its milder expression, the so called minimal HE (MHE)[2,3], the manifestations are subclinical and detectable only by means of psychometric testing as well as electrophysiological and other functional brain measures[4,5]. Depending on the population studied and the diagnostic tool used, MHE ranges between 20% and 80% and thus, may be considered as the most complication of liver cirrhosis[6-11]. Although subclinical, MHE involves the areas of attention, alertness, response inhibition, and executive functions as well as the working memory, psychomotor speed, and visuospatial ability[12-15].
Depending on the population studied and the diagnostic tool used, MHE can be detected in 20%-80% of patients with cirrhosis, MHE is considered as a pre-clinical stage of HE and is part of a wide spectrum of typical neurocognitive alterations in liver cirrhosis, particularly involving the areas of attention, alertness, response inhibition, and executive functions. Although these typical characteristics of MHE reduce the safety and quality of life of patients with cirrhosis together with their caregivers, they are difficult to detect from the clinical point of view. The optimal diagnostic criteria for MHE remain controversial, also because an essential condition for MHE diagnosis is the correct standardization of the tests used by age, education and also employment of the patient. To overcome the difficulties in the execution of complex psychometric batteries, which may require specialist staff for their administration, are time and money consuming, other techniques have been proposed, such as the critical flicker frequency, the smooth pursuit eye movement, and the use of cognitive evoked potentials. Computerized psychometric tests, including the scan test and the inhibitory control test (ICT), which may be more specific and repeatable, have also been proposed. An electroencephalogram is also useful for detecting conditions MHE, especially if a spectral analysis is carried out. A comprehensive summary on the clinical manifestations and diagnosis of MHE has been recently published by our group[12,16].
Low quality of life, falls, sleep disorders, erectile dysfunction in males, impairment in fitness to drive and car accident incidence have been found to be more frequent in patients with MHE than in those without. Moreover, MHE by reducing the patients’ working capacity, may affect their socioeconomic status[16,17]. Finally, MHE is a clear risk factor for the development of overt HE and for its recurrence, leading to frequent hospitalization of the patients. In summary, MHE, despite subclinical is a burden for the patients and their caregivers, which results in the use of more health care resource than other manifestations of liver disease[6,18-21]. Recently, a strong statistical correlation between muscle alterations, sarcopenia and myosteatosis, and MHE was found[22-25]. A first consequence of this observation is that muscle alterations should be investigated in all patients with cirrhosis and cognitive impairment.
In cirrhotic patients, sarcopenia has been assessed by different techniques such as anthropometry (Triceps-Skinfold-Thickness or Mid-Arm-Muscle-Circumference), the bioelectrical impedance analysis and the dual-energy X-ray. The functional consequence of sarcopenia is estimable by the Hand Grip or other more composite tests such the six-minute walk test[26-30]. Moreover, some evidences showed that patients with cirrhosis may develop simultaneously loss of skeletal muscle and gain of intermuscular and intramuscular fat, denominated ‘myosteatosis’[31].
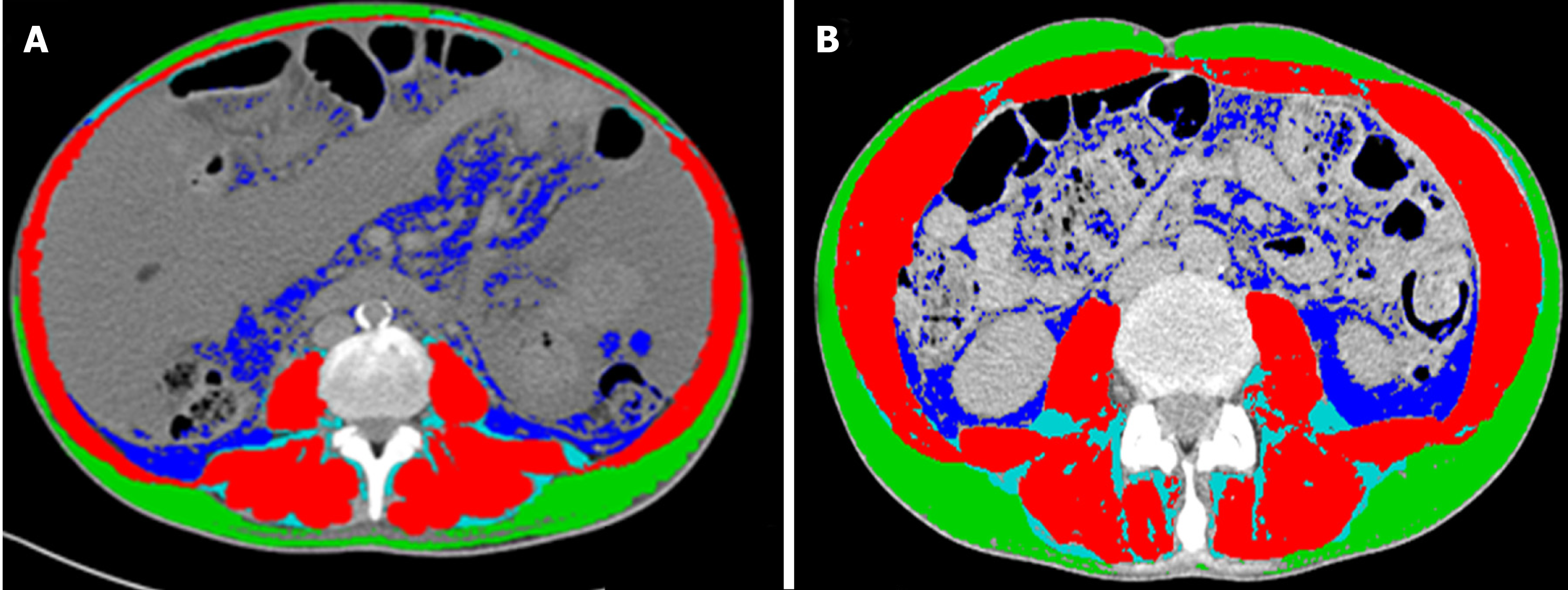
The gold standard to quantify muscle mass is to date represented by the computed tomography (CT) and magnetic resonance image (MRI) analysis[32-34]. The CT scan, by using muscle attenuation, is indirectly able to measure muscle fat infiltration and to add informations not only on quantity but also on the quality of muscle tissue[31,35]. MRI is able to quantify the muscle mass and the fat free muscle mass[36]. These diagnostic tools are certainly limited by cost, radiation exposure and logistic concerns[37]. Nevertheless, a CT or MR scan is often performed in cirrhotic patient for clinical reasons, such as the suspicion of hepatocellular carcinoma or portal vein thrombosis. In these patients, sarcopenia can be easily assessed by validated softwares such as SliceOmatic V4.2 software (Tomovision, Montreal, Quebec, Canada), which enables specific tissue demarcation by using previously reported Hounsfield unit (HU) thresholds[38], as shown in Figure 1. Skeletal muscle is identified and quantified by HU thresholds of -29 to +150 as previously described[34] and with these specific HU thresholds, measurements of the SMI are not influenced by the presence of ascites. Cross-sectional areas (cm2) were automatically computed by summing tissue pixels and multiplying by pixel surface area. Muscle cross-sectional area was normalized for stature (cm2/m2) to obtain the L3 Skeletal Muscle Index (L3 SMI). Sarcopenia was defined according to previously validated cutoff values in cirrhotic patients: L3 SMI: < 39 cm2/m2 for females and < 50 cm2/m2 for males[26]. The following HU thresholds were used for adipose tissues: -190 to -30 for subcutaneous and intermuscular adipose tissue[39], and -150 to -50 for visceral adipose tissue[40].
Since muscle attenuation indirectly measures fat infiltration in muscles, mean muscle attenuation in HU was reported for the entire muscle area from the same image used to calculate L3 SMI. To define myosteatosis, we used cutoff values that have been previously associated with mortality: < 41 HU in patients with a body mass index (BMI) up to 24.9, and < 33 in those with a BMI ≥ 25[27,31].
Using these cut off, sarcopenia, with a prevalence ranging between 65 and 90%, has to be considered a very common condition in patients with advanced liver disease[41]. The severity and prevalence of sarcopenia correlates with Child-Pugh’s Class[42] and, when added to model for end-stage liver disease (MELD) score, sarcopenia has been shown to improve the prediction of the patients’ survival[22,23]. Sarcopenia contributes to fatigue, limits exercise tolerance and may have a heavy burden on performance status and activities of daily living[24]. Multiple factors are involved in sarcopenia development. In liver disease dietary intake may be inadequate to the energy expenditure; nutrient absorption compromised and substrate utilization impaired[25]. Moreover, malabsorption, altered metabolism, hormonal factors, hyperammonemia, may also play a role.
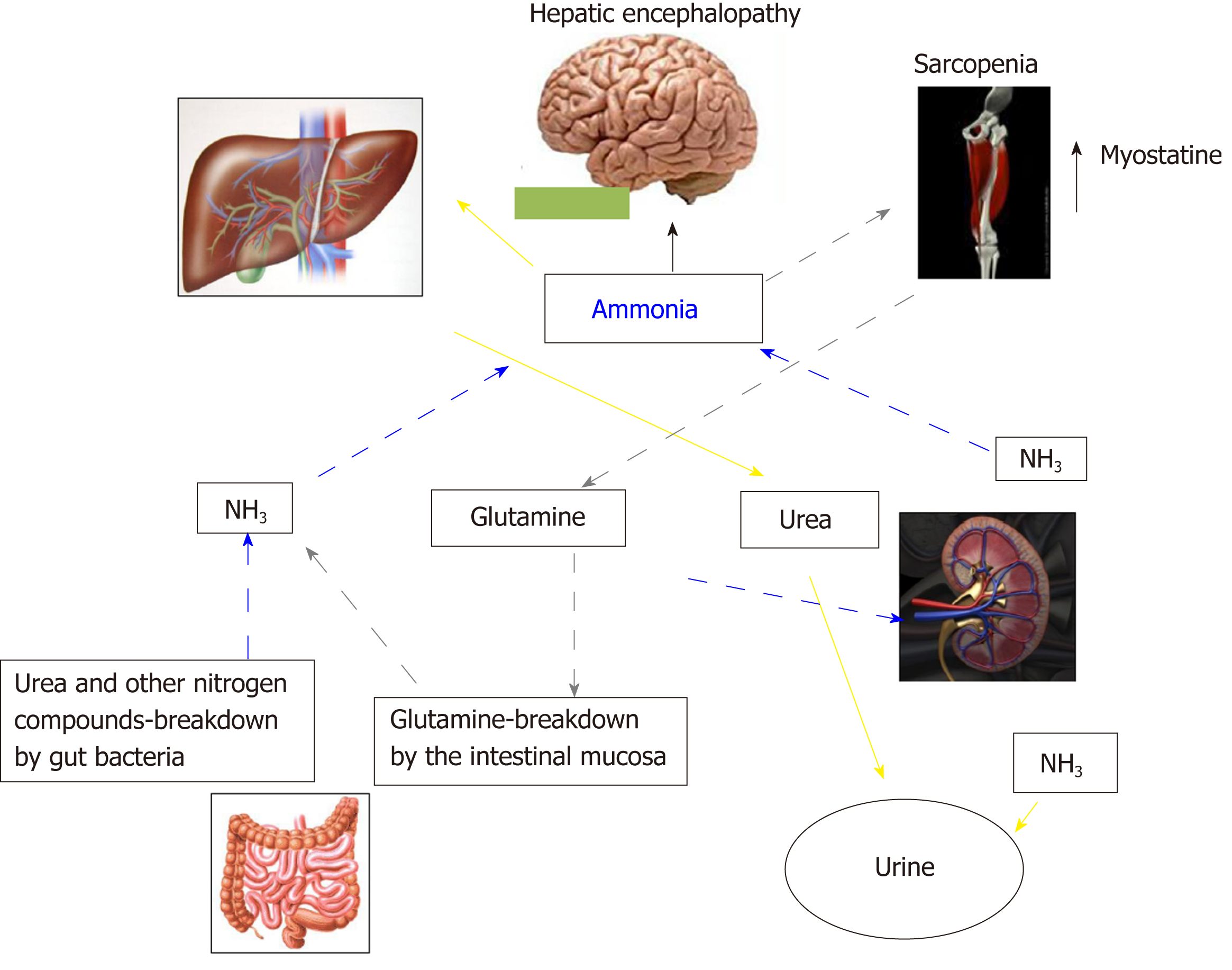
In Addition to the prognostic impact[32,43,44], sarcopenia is related to several complications of cirrhosis[45-49], including overt HE[22-24,38,50]. The pathophysiological background supporting the relationship between muscle depletion and HE origins from the involvement of muscle tissue in ammonia metabolism and trafficking (Figure 2). Ammonia plays a central role in the pathogenesis of cognitive impairment of cirrhosis. Its concentrations is increased because of the inability of the impaired liver in removing ammonia through urea synthesis due to liver failure and porto-systemic shunts. Skeletal muscle may play a compensatory role in ammonia clearance[49,50] through glutamine-synthase, which metabolize ammonia into glutamine. Consequently, a muscle depletion may favor the ammonia accumulation. Furthermore, any catabolic increases the glutamine release from muscle. Glutamine metabolism in the small intestine and the kidney to glutamic acid and ammonia, may contribute to the appearance of ammonia in portal circulation where, due to liver failure and the shunts may lead the whole-body ammonia availability[49,51]. Moreover, on the other hand, ammonia impairs muscle protein synthesis in part through the up regulation of myostatin production[52], that is upregulated because skeletal muscle removes large quantities of ammonia from the circulation[53]. Finally, the mitochondrial dysfunction increases reactive oxygen species bringing to autophagy and muscle damage. Therefore, hyperammonemia may be considered the trigger of a vicious circle: By one side the occurrence of hyperammonemia is a consequence of sarcopenia due to the inability of the depleted muscle to metabolize ammonia, on the other side, the increase in ammonia concentrations, through the above processes, induces further muscle wasting.
The studies in which the possible association between muscle alterations and MHE was evaluated are summarized in Table 1. In the first one[47], conducted on 300 hospitalized cirrhotic patients, muscle depletion was evaluated with anthropometry, muscle function with handgrip strength and minimal HE with psychometric tests. At multivariate analysis, muscle depletion was significantly and independently associated with MHE. In another study MHE was found to be higher in patients with sarcopenia than in those without and sarcopenia was an independent predictor of MHE at multivariate analysis[54]. In a further study, a multivariate analysis showed that the time needed to perform number connection test A (one of the tests used to detect MHE) was independently correlated to age, Child Pugh class, malnutrition and diabetes[55]. None of the above studies used the CT scan to determine muscle quantity and quality. This limitation was recently filled by a prospective study which used CT scan for the quantitative estimation of sarcopenia and myosteatosis and the Phychometric Hepatic Encephalopathy Score (PHES) for the estimation of the cognitive impairment in order to investigate the link between muscle quantitative alteration and MHE. In this study both myosteatosis and sarcopenia were strongly related to presence of MHE as well as to the development of overt HE during the follow-up[46]. In particular, a direct correlation was found between the muscle quantity estimated by the SMI and the psychometric performance estimated by the PHES (more muscle = better psychometric performance) while SMI was inversely correlated to ammonia levels. These correlations support the possibility that a reduced muscle mass (and muscle quality) is able at least to contribute to higher ammonia levels and to the cognitive impairment observable in cirrhotic patients. A further evidence on the importance of muscle alteration on both ammonia level and HE is provided by a recently published paper on the modification of muscle mass and the evolution of HE after a transjugular intrahepatic portosystemic shunt (TIPS)[56,57]. TIPS can ameliorate the muscle mass, at least in some patients. In this setting we described that the patients with a muscle wasting amelioration after TIPS placement obtained an improvement of MHE and a lower number of episodes of overt HE in the follow up supporting a causal relationship between muscle alterations and cognitive impairment. Ammonia was also reduced in the patients with muscle mass amelioration and a significant correlation between muscle and ammonia modifications after TIPS was observed. The last study, recently published by Tapper et al[58] evaluated the cognitive impairment with the ICT, a computerized test previously validated on cirrhotic patients. In this study, hand grip correlated strongly with skeletal muscle area and mildly with ICT performance.
| First author (year) | Number of patients | Methods to identify sarcopenia and/or myosteatosis | Tests to detect minimal hepatic encephalopathy | Results |
| Merli et al[23], (2013) | 300 hospitalized cirrhotics | Anthropometric measurements (MAMC) and hand grip strenghth | PHES battery (NCT-A, NCT-B, LT, SDT, LTT): 5 paper pencil tests | MHE prevalence was higher in pts with malnutrition compared to those without (49% vs 30%, P < 0.001). At multivariate analysis, only protein malnutrition (OR 2.15, 95%CI: 1.1-4.1, P = 00.02) and hyponatremia (OR 4.6, 95%CI: 1.9-9, P = 00.01) were independent predictors of MHE. Venous blood ammonia levels resulted significantly higher in patients with vs those without muscle depletion (85 ± 64 vs 61 ± 46 µg/dL, P = 0.025) and in patients with vs those without a decreased muscle strength (81 ± 62 µg/dL vs 63 ± 45 µg/dL, P = 0.047) |
| Hanai et al[56], (2017) | 120 cirrhotic patients | Bioelectrical impedance analysis and hand grip strenght | NCT-A, NCT-B, DST, BDT | The prevalence of MHE was higher in patients with sarcopenia than in those without sarcopenia (P = 0.01). In the multivariate analysis, serum BCAA levels (OR = 2.98, 95%CI: 1.08-8.34, P = 0.03) and sarcopenia (OR 3.31, 95%CI: 1.19-9.42, P = 0.02) were found to be associated with MHE. Ammonia levels were similar in sarcopenic and non-sarcopenic patients |
| Kalaitzakis et al[57], (2007) | 128 cirrhotic patients | BMI, weight loss, MAMC and triceps skinfold | NCT-A, NCT-B | Multivariate analysis showed that the time needed to perform number connection test was independently associated to age, the Child–Pugh score, diabetes and malnutrition (P < 0.05). Plasma ammonium ion was also related to BMI (r = 0.26, P = 0.006) and to muscle mass expressed as mid-arm muscle circumference (r = 0.28, P = 0.003) but not to fat mass expressed as triceps skin-fold thickness (r = 0.02, NS) |
| Nardelli et al[22], (2019) | 89 cirrhotic patients | CT scan to evaluate sarcopenia and myosteatosis | PHES battery (NCT-A, NCT-B, LT, SDT, LTT): 5 paper pencil tests | Both myosteatosis (62.5% vs 12.5%, P < 0.001) and sarcopenia (84% vs 31%, P < 0.001) were more frequent in patients with MHE. At multivariate analysis, the variables independently associated to the presence of MHE were: sarcopenia, previous overt HE and myosteatosis. Venous ammonia was significantly higher in patients with sarcopenia (62.6 ± 17.7 μg/dL vs 41.4 ± 16.1 μg/dL, P < 0.001) and in patients with myosteatosis (65.2 ± 19.2 μg/dL vs 46.7 ± 17.1 μg/dL, P < 0.001) and inversely correlated to both parameters |
| Gioia et al[25], (2019) | 27 cirrhotic patients submitted to TIPS | CT scan to evaluate sarcopenia and myosteatosis before and after TIPS | PHES battery (NCT-A, NCT-B, LT, SDT, LTT): 5 paper pencil tests | PHES and ammonia significantly improved in the patients with amelioration in Skeletal Muscle Index (SMI) > 10% (n = 16) and not in those without (n = 11) (PHES: -1.6 ± 2 vs -4.8 ± 2.1, P = 0.0005; ammonia: 48.5 ± 28.7 μg/dL vs 96 ± 31.5 μg/dL, P = 0.0004). Moreover, the prevalence of minimal HE (12.5% vs 73%, P = 0.001) was significantly reduced in patients with muscle improvement |
| Tapper et al[58], (2019) | 106 cirrhotic patients | Anthropometric measurements (MAMA), hand grip strenght and CT scan to evaluate muscle assesment | ICT | Hand grip correlated strongly with skeletal muscle area (correlation coefficient 0.64, P < 0.001) and mildly with ICT performance (0.34, P = 0.002) |
Given the above background, a possible hypothetical point of view is that at least some of the clinical effects attributed until now to the presence of MHE may be partially or totally explained by the contemporary presence of sarcopenia. The falls, which have been shown to be more frequent in the cirrhotic patients with MHE could be due to sarcopenia and to their functional consequences. The same could be hypothesized for the reduced driven capacity and car accidents. Even the reduced working capacity could be considered as an outcome of muscle alterations. This hypothesis should be supported by studies in which the contemporary assessment of the quantity, quality and functionality of the muscle mass are coupled with the assessment of the cognitive impairment. Multivariate analysis should be used to distinguish, at least statistically, the relative role of these two components, which are expected to covariate. This point of view, which must be supported by adequate observations could open a new perspective on the evaluation of the patients’ risk.
Moreover, recent studies showed an improvement of the nutritional assessment after physical exercise in patients with cirrhosis[59-61], but no study investigated the modifications of cognitive impairment after physical activity. Thus, the management of cirrhotic patients could be also seen from a new perspective. In fact, the amelioration of nutritional status may be a possible goal to decrease the prevalence of MHE and its clinical consequences.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li LJ, Lv Y, Shi YJ S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 2. | Gitlin N, Lewis DC, Hinkley L. The diagnosis and prevalence of subclinical hepatic encephalopathy in apparently healthy, ambulant, non-shunted patients with cirrhosis. J Hepatol. 1986;3:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Lockwood AH. "What's in a name?" Improving the care of cirrhotics. J Hepatol. 2000;32:859-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | McCrea M, Cordoba J, Vessey G, Blei AT, Randolph C. Neuropsychological characterization and detection of subclinical hepatic encephalopathy. Arch Neurol. 1996;53:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB, Bell DE, Gilles H, Morton K, Noble N, Puri P, Sanyal AJ. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Saxena N, Bhatia M, Joshi YK, Garg PK, Tandon RK. Auditory P300 event-related potentials and number connection test for evaluation of subclinical hepatic encephalopathy in patients with cirrhosis of the liver: A follow-up study. J Gastroenterol Hepatol. 2001;16:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Sharma P, Sharma BC, Puri V, Sarin SK. Critical flicker frequency: Diagnostic tool for minimal hepatic encephalopathy. J Hepatol. 2007;47:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Bajaj JS. Management options for minimal hepatic encephalopathy. Expert Rev Gastroenterol Hepatol. 2008;2:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ridola L, Cardinale V, Riggio O. The burden of minimal hepatic encephalopathy: From diagnosis to therapeutic strategies. Ann Gastroenterol. 2018;31:151-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Bajaj JS, Saeian K, Verber MD, Hischke D, Hoffmann RG, Franco J, Varma RR, Rao SM. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Ford JM, Gray M, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia: Event-related brain potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2004;61:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Schiff S, Vallesi A, Mapelli D, Orsato R, Pellegrini A, Umiltà C, Gatta A, Amodio P. Impairment of response inhibition precedes motor alteration in the early stage of liver cirrhosis: A behavioral and electrophysiological study. Metab Brain Dis. 2005;20:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ridola L, Nardelli S, Gioia S, Riggio O. Quality of life in patients with minimal hepatic encephalopathy. World J Gastroenterol. 2018;24:5446-5453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Bajaj JS, Riggio O, Allampati S, Prakash R, Gioia S, Onori E, Piazza N, Noble NA, White MB, Mullen KD. Cognitive dysfunction is associated with poor socioeconomic status in patients with cirrhosis: An international multicenter study. Clin Gastroenterol Hepatol. 2013;11:1511-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Rakoski MO, McCammon RJ, Piette JD, Iwashyna TJ, Marrero JA, Lok AS, Langa KM, Volk ML. Burden of cirrhosis on older Americans and their families: Analysis of the health and retirement study. Hepatology. 2012;55:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Kappus MR, Bajaj JS. Covert hepatic encephalopathy: Not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10:1208-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Bajaj JS, Hafeezullah M, Hoffmann RG, Varma RR, Franco J, Binion DG, Hammeke TA, Saeian K. Navigation skill impairment: Another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 397] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 22. | Nardelli S, Lattanzi B, Merli M, Farcomeni A, Gioia S, Ridola L, Riggio O. Muscle Alterations Are Associated With Minimal and Overt Hepatic Encephalopathy in Patients With Liver Cirrhosis. Hepatology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 23. | Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: Results of a prospective study. Metab Brain Dis. 2013;28:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, Riggio O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin Gastroenterol Hepatol. 2017;15:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 25. | Gioia S, Merli M, Nardelli S, Lattanzi B, Pitocchi F, Ridola L, Riggio O. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int. 2019;39:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, Dunn MA; Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 27. | Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1512] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 28. | Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, Lucidi C, Di Martino M, Catalano C, Merli M. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Dazzani F, Micati M, Caraceni P, Drago GM, Domenicali M, Pacilli P, Tomassetti V, Gelonesi E, Trevisani F, Bernardi M. Transthoracic electrical bioimpedance: A non-invasive technique for the evaluation of the haemodynamic alterations in patients with liver cirrhosis. Dig Liver Dis. 2005;37:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 31. | Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 32. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 609] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 33. | Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97:2333-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1270] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 34. | Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 1126] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 35. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8456] [Article Influence: 563.7] [Reference Citation Analysis (0)] |
| 36. | Praktiknjo M, Book M, Luetkens J, Pohlmann A, Meyer C, Thomas D, Jansen C, Feist A, Chang J, Grimm J, Lehmann J, Strassburg CP, Abraldes JG, Kukuk G, Trebicka J. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67:1014-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 37. | Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 431] [Article Influence: 47.9] [Reference Citation Analysis (1)] |
| 38. | Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, Montano-Loza AJ. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 39. | Kvist H, Sjöström L, Tylén U. Adipose tissue volume determinations in women by computed tomography: Technical considerations. Int J Obes. 1986;10:53-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord. 1996;20:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF, Berloco P, Rossi M. Nutritional status: Its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 43. | Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 44. | Kang SH, Jeong WK, Baik SK, Cha SH, Kim MY. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. 2018;9:860-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 45. | Musumeci G. Sarcopenia and exercise “The State of the Art”. J Funct Morphol Kinesiol. 2017;2:40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 47. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 48. | Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 49. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 50. | Lattanzi B, D'Ambrosio D, Merli M. Hepatic Encephalopathy and Sarcopenia: Two Faces of the Same Metabolic Alteration. J Clin Exp Hepatol. 2019;9:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Ganda OP, Ruderman NB. Muscle nitrogen metabolism in chronic hepatic insufficiency. Metabolism. 1976;25:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: The basis of current and future therapies. Liver Int. 2011;31:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 54. | Dasarathy S. Myostatin and beyond in cirrhosis: All roads lead to sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8:864-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 55. | Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, McDonald C, Almasan A, Hazen SL, Naga Prasad SV, Dasarathy S. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983-E993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 56. | Hanai T, Shiraki M, Watanabe S, Kochi T, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res. 2017;47:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 57. | Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Björnsson E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Tapper EB, Derstine B, Baki J, Su GL. Bedside Measures of Frailty and Cognitive Function Correlate with Sarcopenia in Patients with Cirrhosis. Dig Dis Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, Tandon P. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920-1926.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 60. | Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 61. | Reuter B, Shaw J, Hanson J, Tate V, Acharya C, Bajaj JS. Nutritional Assessment in Inpatients with Cirrhosis can be Improved after Training and is Associated with Lower Readmissions. Liver Transpl. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |