Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4261
Peer-review started: April 19, 2019
First decision: June 16, 2019
Revised: June 22, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: August 14, 2019
Processing time: 118 Days and 3.2 Hours
In recent years, increasing evidence of second neoplasms associated with gastrointestinal stromal tumors (GIST) has been found. Numerous case reports, mostly retrospective studies and a few reviews, have been published. To our knowledge, however, no systematic review or meta-analysis of the existing data has been performed so far.
To prepare a compilation, as complete as possible, of all reported second tumor entities that have been described in association with GIST and to systematically analyze the published studies with regard to frequency, localization, and types of GIST-associated neoplasms.
The MEDLINE and EBSCO databases were searched for a combination of the keywords GIST/secondary, synchronous, coincident/tumor, neoplasm, and relevant publications were selected by two independent authors.
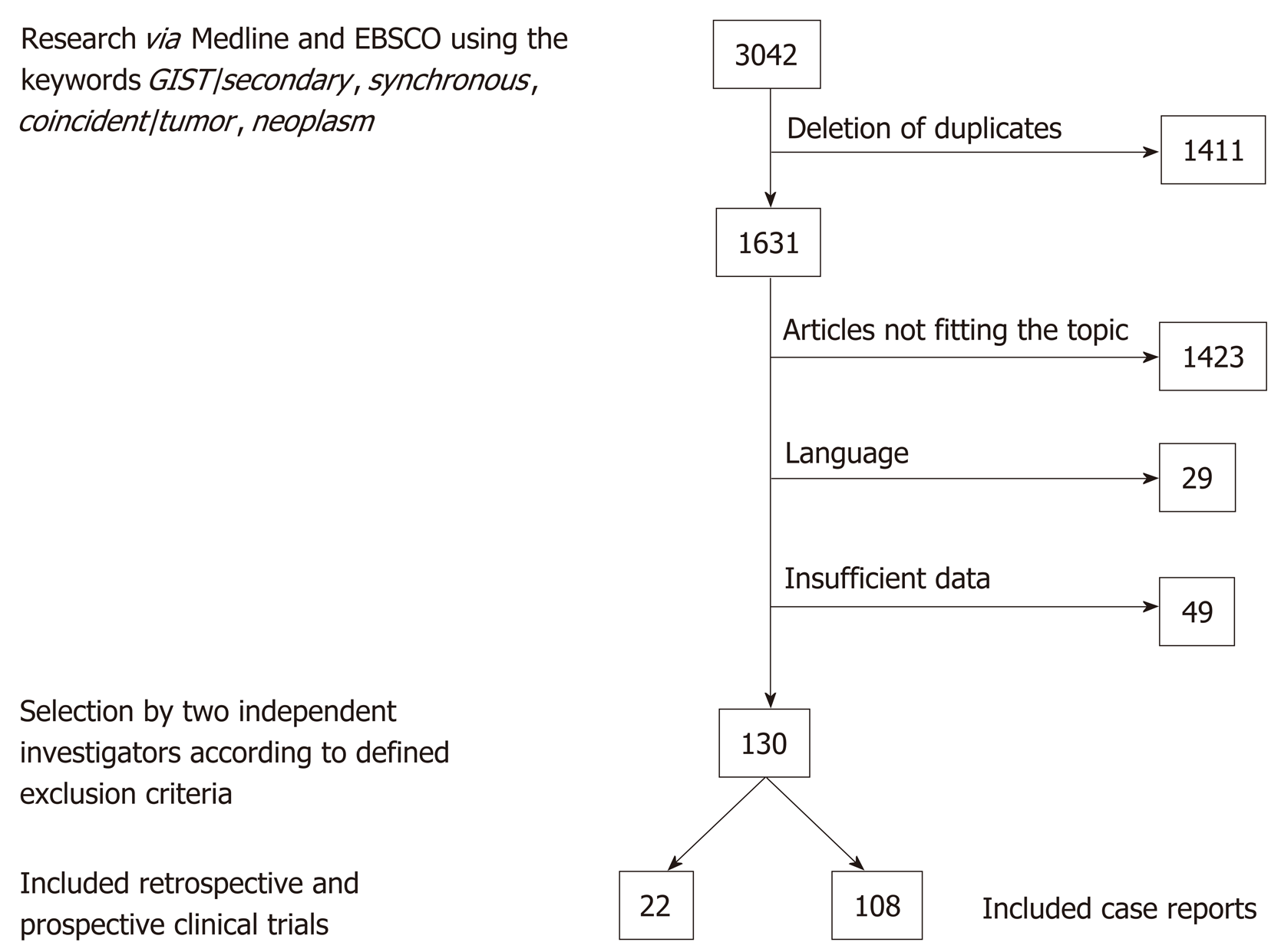
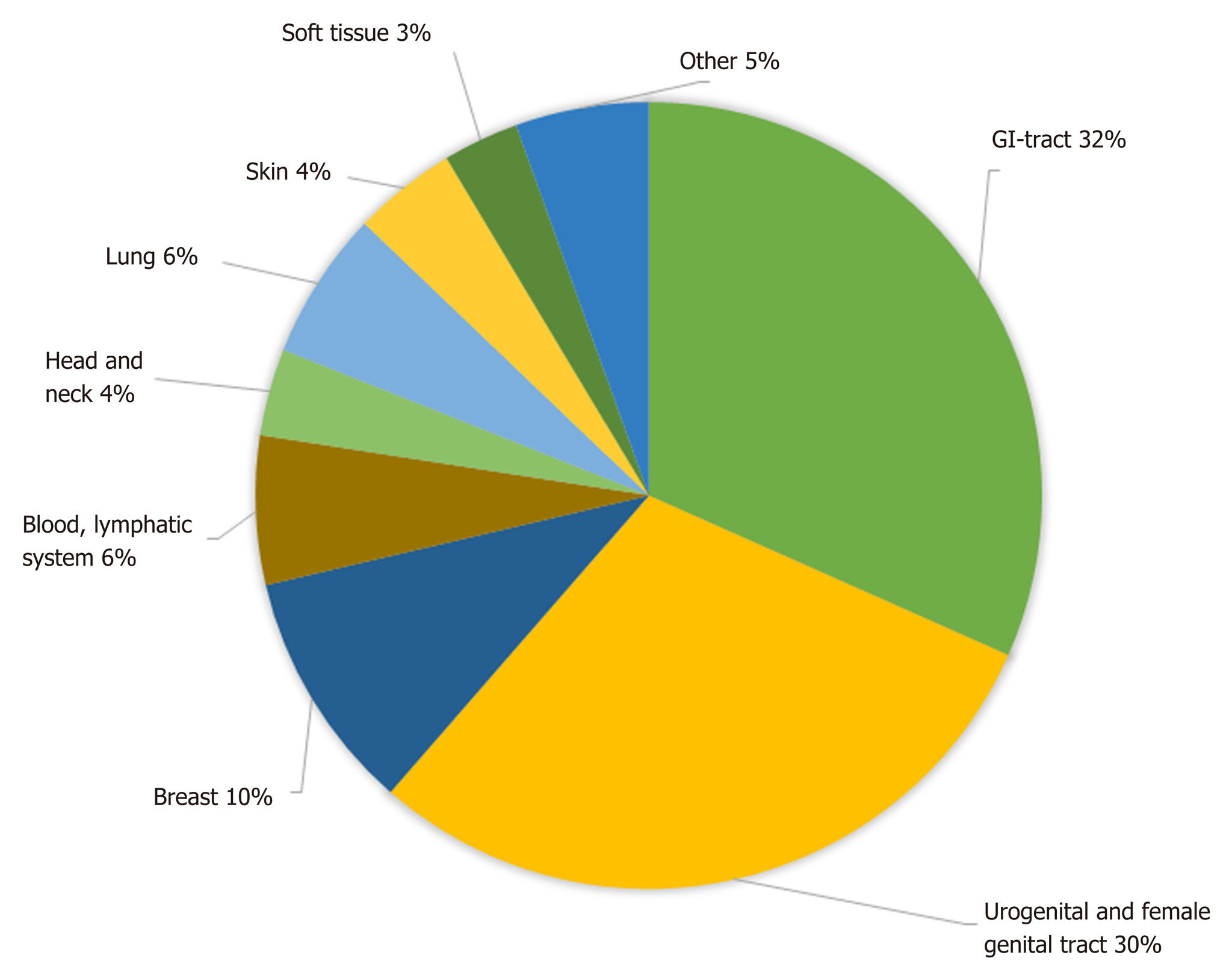
Initially, 3042 publications were found. After deletion of duplicates, 1631 remained, and 130 papers were selected; 22 of these were original studies with a minimum of 20 patients, and 108 were case reports. In the 22 selected studies, comprising a total number of 12050 patients, an overall rate of GIST-associated neoplasias of 20% could be calculated. Most second neoplasias were found in the gastrointestinal tract (32%) and in the male and female urogenital tract (30%). The specific risk scores of GISTs associated with other tumors were significantly lower than those without associated neoplasias.
In this first systematic review, we could confirm previously reported findings of a more than coincidental association between GIST and other neoplasias. The question whether there is an underlying causal association will need further investigation. Our data suggest that even GIST with a very low risk of disease progression should prompt screening for second neoplasia and subsequent frequent controls or extended staging.
Core tip: Gastrointestinal stromal tumors (GIST) associated neoplasms have been previously reported to occur with a more than coincidental frequency. Numerous case reports, mostly retrospective studies and a few reviews have been published on this topic. In this, to our knowledge, first systematic review we analyzed 108 case reports and 22 retrospective and prospective studies with a total of 12050 patients. An overall rate of GIST-associated neoplasias of 20% could be calculated. Most second neoplasias were found in the gastrointestinal tract (32%) and in the male and female urogenital tract (30%).
- Citation: Waidhauser J, Bornemann A, Trepel M, Märkl B. Frequency, localization, and types of gastrointestinal stromal tumor-associated neoplasia. World J Gastroenterol 2019; 25(30): 4261-4277
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4261
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the digestive tract. Yearly incidence rates vary from 4.3 to 22 per million in different geographic regions, which may at least in part be caused by changing and improving diagnostic criteria and a lack of established GIST registries. In most studies, however, the yearly incidence is indicated as 10 to 15 per million[1], fulfilling the criterion of a rare disease. Median age at diagnosis is reported to be between 60 and 69 years in most studies[1,2]. GIST are considered to develop from interstitial cells of Cajal (ICC), which play an important role in autonomous gastrointestinal movement[3]. The most common localization of GIST is the stomach, followed by other gastrointestinal tract localizations[2,4].
Several driver mutations have been identified as playing an essential role in the development of GIST. The most frequent mutation can be found in the tyrosine kinase receptor c-kit (c-KIT), which accounts for 70%-80% of GIST[5-7] and is nowadays the most important target of medical tumor therapy in GIST patients. Other relevant mutations can be observed in the platelet-derived growth factor receptor alpha (PDGFR-α) in 5%-10%[5-7] and, in rare cases, in other genes such as neurofibromin 1 (NF1), succinate dehydrogenase (SDH), or BRAF[6,8].
GIST can occur in the setting of genetic syndromes such as neurofibromatosis 1[9], Carney triad[10], or familial GIST[11] and, in these cases, frequently come along with other benign or malignant neoplasias. In recent years, though, there has been increasing evidence of second neoplasia in patients with sporadic GIST[12-15]. Several retrospective studies and case series have been published on this topic, complemented by many case reports and a few reviews, but so far to our knowledge, no meta-analysis or systematic reviews have been conducted. The aim of this first systematic review is to prepare a compilation, as complete as possible, of all reported second tumor entities that have been described in association with GIST and to systematically analyze the published studies with regard to frequency, localization, and types of GIST-associated neoplasms.
We performed a literature search in the MEDLINE and EBSCO databases, using the keywords GIST/secondary, synchronous, coincident/tumor, neoplasm. All results were transferred to the citation manager Endnote® and duplicates were deleted. The remaining results were screened by two authors with regard to suitable topic, language, and publication standard. Discrepancies were resolved after discussion with a senior third author. Case reports and case series/studies in English or German were included. Only data that was fully published was eligible. Syndromic settings as familial GIST or neurofibromatosis were excluded as well as cases involving children. Studies had to include at least 20 patients, and studies that investigated only one specific kind of second neoplasm were excluded. Second neoplasms were considered regardless of the time frame between their occurrence and the occurrence of GIST. Malignant as well as benign second neoplasms were selected. In addition, the bibliographies of all selected papers that were published between 2016 and 2019 were screened for suitable references, as were the six published reviews on this topic.
For statistical calculations, we used SigmaPlot 13.0 (Systat, Erkrath, Germany) and Microsoft Excel (Microsoft Office 16). The chi-squared test was performed for testing the relationship between two categorical variables. A aP-value of < 0.05 was considered significant.
Before starting the literature research, a registration of this systematic review in the international Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42019122784) was performed.
The literature search revealed a total of 3042 publications before February 2019. After deletion of duplicates, 1631 papers remained. Screening by the two authors (Waidhauser J and Bornemann A) resulted in 126 eligible papers. In addition, one study on 188 GIST patients that was performed at our institute by Mayr et al[15] and had not been published by the time of the literature search was included. Of the 130 selected publications, 108 were case reports and 22 were case series or retrospective and prospective studies (Figure 1).
All additional neoplasms that were reported in the case reports are listed in Table 1. The most frequent types were gastric and colorectal adenocarcinomas.
| Tumor entity | Ref. |
| Gastrointestinal Tract | |
| Gastric adenocarcinoma | [29-55] |
| Colorectal adenocarcinoma | [56-71] |
| Gastric high grade IEN | [72] |
| Esophagus SCC | [48,59,73] |
| Esophagus Small Cell Carcinoma | [39] |
| Gastro-esophageal junction adenocarcinoma | [74,75] |
| Duodenum adenocarcinoma | [76] |
| Papilla Vateri NET | [77] |
| Gastric Lipoma | [78] |
| Gastric NET | [79-82] |
| Doudenum NET | [67] |
| Ileum NET | [83] |
| Gastric Schwannoma | [84] |
| Colon NET | [85] |
| Jejunal Sarcomatoid Carcinoma | [86] |
| Pancreas adenosquamous carcinoma | [87,88] |
| Pancreas adenocarcinoma | [89,90] |
| Pancreas NET | [91-95] |
| Hepatocellular carcinoma | [96-99] |
| Cholangiocellular Carcinoma | [100,101] |
| Perivascular Epitheloid Cell Tumor Liver | [102] |
| Urogenital Tract | |
| Renal Cell Carcinoma | [103,104] |
| Renal Chromophobe Cell Carcinoma | [105] |
| Prostate Adenocarcinoma | [40,69,82,106,107] |
| Transitional Cell Carcnimona Bladder | [86] |
| Female Genitale Tract | |
| Ovarian Carcinoma | [108] |
| Ovarian Serous Adenocarcinoma | [109,110] |
| Uterus Leiomyoma | [91] |
| Breast | |
| Sarcoma | [111] |
| Invasive Ductal Carcinoma | [55,61,63,92] |
| Blood/ Lymphatic | |
| Acute Myeloid Leukemia | [112-114] |
| Acute Biphenotypic Leukemia | [100] |
| Chronic Lymphoid Leukemia | [115] |
| Low Grade B Cell Lymphoma Stomach | [116] |
| Extranodal DLBCL | [117,118] |
| Burkitt Lymphoma | [119] |
| Mantle Cell Lymphoma Appendix | [120] |
| MALT Lymphoma | [50,121] |
| Multiple Myeloma | [122] |
| Head and Neck | |
| Oral Melanoma | [123] |
| Papillary Thyroid Carcinoma | [124] |
| Lung | |
| Adenocarcinoma | [125-127] |
| SCC | [107] |
| NSCLC | [106] |
| Skin | |
| Melanoma | [128,129] |
| Squamous Cell Carcinoma | [130] |
| Merkel Cell Carcinoma | [131] |
| Soft Tissue | |
| Myxofibrosarcoma | [82] |
| Dermatofibrosarcoma | [132] |
| Ewing Sarkoma | [133] |
| Others | |
| Adrenocortical Carcinoma | [134] |
| Peritoneal Mesothelioma | [135] |
| Mesenterial Fibromatosis | [136] |
| Granular cell tumor | [55] |
Among the 22 retrospective and prospective studies, a total of 12050 patients were included. Basic information on these studies is summarized in Table 2. The number of patients in which an additional tumor to the GIST diagnosis was found was 2426 (20.1%). The median age at the diagnosis of GIST was 63 years in the total study population and 68 years in those patients with an additional tumor. The male-to-female ratio was 1.1:1 in the total population and 1.4:1 in the GIST with secondary neoplasia group. The chi-squared test revealed a significant difference for the sex distribution of P < 0.001 with a predominance of male gender in cases with associated neoplasia (Table 3).
| Patient characteristics | Organ systems affected by associated NPL | ||||||||||||||||||
| Au-thor | Year | Date of diag-nosis | N | Age average (median) | Sex m/f | Nu-mber (rate) of pati-ents with ass. NPL | GI-tract | Liver/ gall bla-dder/ pan-creas | Uro geni-tal tract | Fem-ale geni-tal tract | Bre-ast | Blo-od/ lymp-hatic sys-tem | Head and neck | Lung | Skin/ mela noma | Soft | Other | ||
| Total pop-ula-tion | Pat. with ass. NPL | Total pop-ula-tion | Pat. with ass. NPL | ||||||||||||||||
| Ad-im et al[137] | 2011 | 1997-2009 | 78 | 62 | 9/4 | 13 (17%) | 9 | 2 | 1 | 1 | |||||||||
| Aga-imy et al[14] | 2005 | 1997-2004 | 97 | 65 | 72 | 42/55 | 6/12 | 18 (17%) | 6 | 3 | 2 | 3 | 2 | 2 | 2 | ||||
| Agh-das-si et al[138] | 2018 | 1993-2011 | 104 | 67 | 54/50 | 44 (42%) | 16 | 4 | 8 | 7 | 2 | 1 | |||||||
| Am-aad-our et al[139] | 2013 | 1998-2006 | 43 | 62 | 65 | 15/28 | 1/5 | 6 (14%) | 5 | 1 | |||||||||
| Fer-nan-dez et al[140] | 2018 | 1999-2016 | 104 | 64 | 62/42 | 22/10 | 32 (31%) | 9 | 4 | 7 | 3 | 3 | 2 | 2 | 1 | 1 | |||
| Ferr-eira et al[141] | 2010 | 1998-2006 | 43 | 62 | 61 | 15/28 | 1/5 | 6 (14%) | 5 | 1 | |||||||||
| Giu-liani et al[142] | 2012 | 2002-2010 | 24 | 66 | 69 | 12/12 | 5/3 | 8 (33%) | 6 | 1 | 1 | ||||||||
| Gon-alves et al[143] | 2010 | 1998-2008 | 101 | 68 | 5/9 | 14 (14%) | 8 | 2 | 2 | 1 | 1 | ||||||||
| Hec-htm-ann et al[144] | 2015 | 2009-2013 | 260 | 65 | 142/118 | 30/20 | 50 (19%) | 2 | 21 | 1 | 8 | 8 | 7 | 1 | 3 | 3 | 6 | ||
| Kra-mer et al[17] | 2015 | 2006-2011 | 836 | 68 | 69 | 423/413 | 148/119 | 422 (51%) | 118 | 20 | 60 | 22 | 26 | 23 | 6 | 8 | 23 | 2 | 9 |
| Lai et al[145] | 2016 | 1995-2015 | 749 | 68 | 77/59 | 136 (18%) | 64 | 27 | 12 | 10 | 7 | 1 | 9 | 13 | 4 | 3 | 10 | ||
| Lis-zka et al[146] | 2007 | 1989-2006 | 82 | 64 | 38/44 | 12/10 | 22 (27%) | 17 | 5 | 1 | |||||||||
| Ma-yr et al[15] | 2019 | 1998-2017 | 188 | 69 | 70 (37%) | 23 | 1 | 17 | 9 | 5 | 5 | 2 | 5 | 3 | |||||
| Mur-phy et al[12] | 2015 | 2001-2011 | 6112 | 3252/2860 | 727/481 | 1047 (17%) | 208 | 34 | 432 | 144 | 80 | 54 | 99 | 52 | 48 | 72 | |||
| Pan-dur-eng-an et al[147] | 2010 | 1995-2007 | 783 | 57 | 444/339 | 91/62 | 153 (20%) | 40 | 8 | 48 | 14 | 15 | 12 | 1 | 10 | 9 | 9 | 20 | |
| Ponti et al[148] | 2010 | 1988-2007 | 141 | 66 | 67 | 77/64 | 20/26 | 46 (33%) | 18 | 1 | 7 | 2 | 6 | 1 | 2 | 1 | 2 | 3 | |
| Ric-hter et al[149] | 2008 | 1993-2005 | 54 | 65 | 28/26 | 13 (20%) | 7 | 3 | 1 | 1 | 1 | ||||||||
| Rod-riq-uenz et al[150] | 2016 | 2002-2014 | 128 | 65 | 68 | 59/69 | 19/27 | 46 (34%) | 24 | 5 | 3 | 4 | 2 | 1 | 1 | 3 | 3 | 1 | |
| Rub-io-Cas-ade-vall et al[151] | 2015 | 1996-2006 | 132 | 65 | 67/53 | 30 (23%) | 8 | 2 | 3 | 1 | 3 | 3 | |||||||
| Sevi-nc et al[152] | 2011 | 2002-2009 | 200 | 67 | 20/12 | 32 (16%) | 16 | 5 | 1 | 2 | 1 | 2 | 1 | 4 | |||||
| Smi-th et al[13] | 2016 | 2001-2009 | 1705 | 63 | 69 | 885/820 | 95/86 | 181 (11%) | |||||||||||
| Vas-sos et al[153] | 2014 | 2000-2009 | 86 | 66 | 70 | 50/36 | 27/10 | 37 (43%) | 19 | 7 | 5 | 4 | 3 | 3 | 1 | ||||
| 12050 | 63 | 68 | 1.1 : 1 | 1.4 : 1 | 2426 (20,1%) | 751 (32%) | 706 (30%) | 236 (10%) | 145 (6%) | 85 (4%) | 145 (6%) | 101 (4%) | 74 (3%) | 130 (5%) | |||||
| Total study population | GIST with associated neoplasia | No. of patients available for calculation | |
| Age (median) | 63 | 68 | 4176/1139 |
| Sex male: Female | 1.1:1 | 1.4:1 | 10444/2080 |
Of 2248 patients, for whom the respective data were available, 253 benign (11%) and 1995 (89%) malignant neoplasias were reported, with the restriction that in some studies, only patients with malignant second neoplasias were included. Chronological considerations revealed that 50% (366 of 732) of second neoplasias occurred synchronously to GIST, 26% (187 of 732) occurred before GIST, and 24% (179 of 732) were diagnosed after GIST. Focusing on synchronous second neoplasias, a rate of 6% (366 of 5131) was detected among all GIST patients. Of these synchronous second neoplasias 77% (177 of 230) occurred in the GI-Tract and 7% (16 of 230) in the male and female urogenital tract. The distribution of different histological subtypes (spindle vs epithelioid vs mixed) revealed no differences between the GIST-only patients and the patients with another neoplasm (spindle: 78% vs 80%; epithelioid: 8% vs 6%; mixed: 14% vs 14%) (Table 4).
| Parameter | Quantification | No. of patients available for calculation | |
| Designtion of associated NPL, n (%) | Benigne 253 (11) | 2248 | |
| Malignant 1995 (89) | |||
| Chronological presentation, n (%) | Synchoronous 366 (50) | 732 | |
| GIST first 179 (24) | |||
| Ass. NPL first 187 (26) | |||
| Histological subtypes of GIST, n (%) | Spindle | Total population 409 (78) | 525; 185 |
| GIST + ass. NPL 149 (80) | |||
| Epitheloid | Total population 43 (8) | ||
| GIST + ass. NPL 11 (6) | |||
| Mixed | Total population 73 (14) | ||
| GIST + ass. NPL 25 (14) | |||
Figure 2 gives an overview of the different localizations of the GIST-associated neoplasias. The most common manifestation was seen in the gastrointestinal tract (32%), followed by urogenital and female genital tract (30%); 10% of additional tumors were found in the breast and 6% each in the lung and in the blood and lymphatic system.
Regarding the risk scores for disease progression or recurrence of GIST, there was a significantly (P < 0.001) higher proportion of very low- and low-risk GIST in patients with an additional tumor (65%) compared to the GIST-only group (35%), whereas in this latter group, the portion of intermediate and high-risk patients was higher (69% vs 31%). For calculation, we used the risk scores as they were applied in the different studies, which were most frequently those according to Fletcher et al[4] or Miettinen et al[16] (Table 5).
| Risk score | GIST without associated neoplasia | GIST with associated neoplasia | Total |
| Low and very low | 123 (35) | 230 (65) | 353 |
| Intermediate and high | 373 (69) | 165 (31) | 538 |
| Total | 496 | 395 | 891 |
The mutational status of driver genes in patients with GIST and associated neoplasias was reported in only four of the 22 studies, with a total of 167 patients. These patients with GIST and second tumors showed mutations in exon 11 of the KIT gene in 69%, non-exon-11 mutations of the KIT gene in 6%, mutations in the PDGFR-α gene in 13%, and a “wildtype” status in 13%.
Data on follow-up was very heterogeneously reported or not available in most of the included studies, which is why even a descriptive analysis was not feasible.
In our systematic review, we detected a rate of 20.1% of second neoplasias in GIST patients, with the most frequent localization of associated tumors in the gastrointestinal tract and in the urogenital and female genital tract. Previously described rates of GIST-associated neoplasias varied between 11% and 50%[13,17]. The general probability of being diagnosed with cancer twice in a lifetime is estimated between 2% and 17% (syndromic settings or familial predisposition included)[18] or in other words with a chance of 1:9[12,19]. Compared to this number the rate of second neoplasias we found in GIST patients is obviously higher than expected. Several reasons can be considered accountable for the development of multiple tumors in one patient, for example, similar risk factors, environmental factors, or genetic predisposition, but also the higher likelihood of detection of another tumor within the examinations for staging or follow-up. In cases of sporadic GIST there are no definitely confirmed intrinsic risk factors or environmental factors. Genetic factors play a role in syndromes such as neurofibromatosis type I or Carney triad, but these patients were excluded in our study, and only patients with sporadic GIST were included. The occurrence of GIST-specific mutations such as in the c-KIT or PDGFRα gene that we found in the group of patients with GIST and second neoplasms were similar to those reported for GIST in general before[5-7].
The localization of GIST-associated neoplasias with the highest frequency in the gastrointestinal tract, the urogenital, and female genital tract is consistent with previously reported findings[20,21]. In addition to a possible common underlying predisposition, the probability of detecting even small GIST during staging examinations for gastrointestinal tract tumors might be higher than in cases of, for example, lung or head and neck tumors. This is might also be the explanation for the high rate of 77% of GI-tract localization in synchronous second neoplasias.
The median age of the total study population compared to the group of patients who developed a second tumor showed a difference of five years at the time-point of GIST diagnosis, with the higher median age in the GIST with associated neoplasm group. A possible explanation for this finding could be the age-dependent risk increase for cancer in general[14]. Previously performed reviews on the topic of GIST and associated neoplasms mostly concentrated on the occurrence rate and localization of the associated tumors and, in some cases, on the outcome and follow-up[20-24]. Analysis of age or sex distribution have not been performed on larger numbers of patients. We found a significantly higher number of male patients who were diagnosed with GIST and a second neoplasia than in the total population of GIST patients. An equal sex distribution for GIST patients in general has been reported in the literature before[1]. Regarding the overall incidence of cancer worldwide, the sex distribution of patients diagnosed with cancer in 2018 is estimated at a male-to-female ratio of 1.1:1 (9.5 million new cases in men and 8.6 million new cases in women). Among gastrointestinal neoplasias, which were the most frequent GIST-associated neoplasias in our review, there is a difference in the worldwide incidence between men and women with a higher rate of gastrointestinal (GI) tumors diagnosed in men in 2018 (2.7 million cases in men vs 1.4 million cases in women)[25]. Whether there is a difference of distribution of the second neoplasias between sexes in our review population remains unclear. In most analyzed studies, the reported data was not sufficient to answer this question.
Several risk classification systems are used to assess the risk of disease progression or recurrence of GIST[4,16]. Most of them use the size, localization, and mitotic rate of GIST and are therefore, at least in part, comparable. By summarizing the risk categories in two groups (very low and low vs intermediate and high), we could find a significant difference between the GIST-only patients and those having GIST and another neoplasia by using the chi-squared test. The patients with GIST-associated neoplasias had lower risk scores, which might be due to a higher detection rate of even small GISTs with low risk scores in the setting of another neoplastic disease.
Since the establishment of targeted therapies for GIST with imatinib or second-line tyrosine kinase inhibitors, the prognosis even of advanced GIST has significantly improved[26]. On the other side, there is growing interest in the question of elevated risk of developing secondary neoplasia under the treatment with imatinib. Phan et al. found a higher rate of secondary tumors among GIST patients in the imatinib era than in the pre-imatinib era[27], although the most likely reason for this is the prolonged survival even of patients with advanced GIST, as it has been described by different authors[28]. Another point that relativizes the impact of imatinib on the development of secondary tumors can be seen in our review: About 75% of associated neoplasias were diagnosed either before GIST or synchronously with GIST; furthermore, not all patients with a GIST diagnosis in advance were treated with imatinib.
In summary, in this, to our knowledge first, systematic review on the topic of secondary neoplasia in patients with GIST, we confirm the previously described elevated number of associated neoplasms and the most common localizations of this neoplasms. We found a higher median age in the GIST with second neoplasia group and significantly more male patients who developed associated tumors, whereas the risk scores of GIST in this group were significantly lower. We conclude that even very low- and low-grade GISTs should be a reason to consider frequent controls or extended staging for early detection of second neoplasias, especially in the gastrointestinal and urogenital tract. To understand whether there is an underlying genetic cause for the elevated rates of GIST-associated neoplasias, further studies will be needed.
In recent years, numerous case reports, mostly retrospective studies and a few reviews on the topic of second neoplasias associated with gastrointestinal stromal tumors (GIST) have been published. To our knowledge, however, this is the first systematic review of the existing data.
The aim of this review was to prepare a compilation, as complete as possible, of all reported second tumor entities that have been described in association with GIST, and to systematically analyze the published studies with regard to frequency, localization, and types of GIST-associated neoplasms.
The main focus of this review was on frequency, localization, dependence of gender, age and risk classification of GIST associated neoplasias. Summarizing the data of a large number of patients could especially help in the daily clinical work with GIST patients.
The MEDLINE and EBSCO databases were searched for a combination of the keywords GIST/secondary, synchronous, coincident/tumor, neoplasm, and relevant publications were selected by two independent authors. All case reports were summarized according to the reported tumor entity and included clinical studies were analyzed with regard to the previously mentioned topics.
Of the initially found 3042 publications, 130 papers were selected; 22 of these were original studies, and 108 were case reports. In the 22 selected studies, comprising a total number of 12050 patients, an overall rate of GIST-associated neoplasias of 20% could be calculated. Most second neoplasias were found in the gastrointestinal tract (32%) and in the male and female urogenital tract (30%). The male-to-female ratio revealed a predominance of male gender in cases with associated neoplasia. The specific risk scores of GISTs associated with other tumors were significantly lower than those of GIST without associated neoplasias. The question if there are specific genetic mutations that occur with a higher frequency in GIST patients with second tumors could not be answered and would be an interesting topic for future research.
GISTs are associated with other neoplasias with a rate of 20% and occur most frequently in the gastrointestinal and urogenital tract. This confirms previous findings on a larger number of patients. GIST associated neoplasias occur with a higher likelihood in older, male patients with low grade GIST. 50% of GIST associated neoplasias are detected synchronously. Our findings should be a reason to consider frequent controls or extended staging for early detection of second neoplasias, especially in the gastrointestinal and urogenital tract.
If there is a causal relation between GIST and second tumors remains unclear. As data on genetic mutations of the GIST were reported very heterogeneously focusing on this topic could be an interesting point for future research.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Laghi A, Wilkinson MJ S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1178] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 3. | Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumors. Pathol Int. 2006;56:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 6. | Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 623] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 7. | Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 478] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Rubin BP, Heinrich MC. Genotyping and immunohistochemistry of gastrointestinal stromal tumors: An update. Semin Diagn Pathol. 2015;32:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Carney JA. Carney triad. Front Horm Res. 2013;41:92-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Li FP, Fletcher JA, Heinrich MC, Garber JE, Sallan SE, Curiel-Lewandrowski C, Duensing A, van de Rijn M, Schnipper LE, Demetri GD. Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in a kindred. J Clin Oncol. 2005;23:2735-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Murphy JD, Ma GL, Baumgartner JM, Madlensky L, Burgoyne AM, Tang CM, Martinez ME, Sicklick JK. Increased risk of additional cancers among patients with gastrointestinal stromal tumors: A population-based study. Cancer. 2015;121:2960-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Smith MJ, Smith HG, Mahar AL, Law C, Ko YJ. The impact of additional malignancies in patients diagnosed with gastrointestinal stromal tumors. Int J Cancer. 2016;139:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Agaimy A, Wuensch PH. Gastrointestinal stromal tumours in patients with other-type cancer: a mere coincidence or an etiological association? A study of 97 GIST cases. Z Gastroenterol. 2005;43:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Mayr P, Märkl B, Agaimy A, Kriening B, Dintner S, Schenkirsch G, Schneider-Stock R. Malignancies associated with GIST: a retrospective study with molecular analysis of KIT and PDGFRA. Langenbecks Arch Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 17. | Kramer K, Wolf S, Mayer B, Schmidt SA, Agaimy A, Henne-Bruns D, Knippschild U, Schwab M, Schmieder M. Frequence, spectrum and prognostic impact of additional malignancies in patients with gastrointestinal stromal tumors. Neoplasia. 2015;17:134-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 370] [Article Influence: 46.3] [Reference Citation Analysis (1)] |
| 19. | Rheingold SR, Neugut AI, Meadows AT. Secondary cancers: incidence, risk factors, and management. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Gansler TS, Holland JF, Frei E III, editor. Holland-Frei Cancer Medicine. 6th edition. Hamilton: BC Decker, 2003: Chapter 159. |
| 20. | Agaimy A, Wünsch PH, Sobin LH, Lasota J, Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Fernández Hernández JÁ, Olivares Ripoll V, Parrilla Paricio P. [Additional primary malignancies in patients with gastrointestinal stromal tumors. Proposal for a new classification]. Med Clin (Barc). 2016;147:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Núñez-Martín R, Cubedo Cervera R, Provencio Pulla M. Gastrointestinal stromal tumour and second tumours: A literature review. Med Clin (Barc). 2017;149:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 23. | Burgoyne AM, Somaiah N, Sicklick JK. Gastrointestinal stromal tumors in the setting of multiple tumor syndromes. Curr Opin Oncol. 2014;26:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Giuliani J, Bonetti A. The Occurrence of Gastrointestinal Stromal Tumors and Second Malignancies. J Gastrointest Cancer. 2015;46:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55843] [Article Influence: 7977.6] [Reference Citation Analysis (132)] |
| 26. | Artinyan A, Kim J, Soriano P, Chow W, Bhatia S, Ellenhorn JD. Metastatic gastrointestinal stromal tumors in the era of imatinib: improved survival and elimination of socioeconomic survival disparities. Cancer Epidemiol Biomarkers Prev. 2008;17:2194-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Phan K, Martires K, Kurlander DE, Gaddipati1 K, Xavier M. The incidence of second primary malignancies after gastrointestinal stromal tumor before and after the introduction of imatinib mesylate. TCR. 2013;3:152-159. [DOI] [Full Text] |
| 28. | Giuliani J, Bonetti A. Gastrointestinal stromal tumors and second primary malignancies before and after the introduction of imatinib mesylate. Chin J Cancer Res. 2013;25:486-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Cinar H, Topgul K, Kesicioglu T, Can B, Koca B, Karabicak I, Ozbalci GS. Synchronous primary adenocarcinoma and gastrointestinal stromal tumor in the stomach. Haseki Tip Bulteni. 2014;52:50-52. |
| 30. | Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, Jung EJ, Ju YT, Jeong CY, Ha WS. Synchronous Adenocarcinoma and Gastrointestinal Stromal Tumor of the Stomach Treated by a Combination of Laparoscopy-assisted Distal Gastrectomy and Wedge Resection. J Gastric Cancer. 2011;11:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Kleist B, Lasota J, Miettinen M. Gastrointestinal stromal tumor and gastric adenocarcinoma collision tumors. Hum Pathol. 2010;41:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Kountourakis P, Arnogiannaki N, Stavrinides I, Apostolikas N, Rigatos G. Concomitant gastric adenocarcinoma and stromal tumor in a woman with polymyalgia rheumatica. World J Gastroenterol. 2008;14:6750-6752. [PubMed] |
| 33. | Kycler W, Teresiak M, Lozinski C, A case of multi-focus gastric adenocarcinoma occurring synchronously with an associated gastric GIST. Rep Pract Oncol Radiother. 2006;11:97-100. |
| 34. | Lee FY, Jan YJ, Wang J, Yu CC, Wu CC. Synchronous gastric gastrointestinal stromal tumor and signet-ring cell adenocarcinoma: a case report. Int J Surg Pathol. 2007;15:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Moran M, Bilgic I, Dilektasli E, Albayrak L, Oruc MT, Ozmen MM. Synchronous tumors of the stomach: A case report of mixed gastrointestinal stromal tumor and adenocarcinoma. Turk Geriatri Dergisi. 2010;13:125-128. |
| 36. | Munekage E, Namikawa T, Kawanishi Y, Munekage M, Maeda H, Kitagawa H, Sakamoto K, Obatake M, Kobayashi M, Hanazaki K. Synchronous large gastrointestinal stromal tumor and adenocarcinoma in the stomach treated with imatinib mesylate followed by total gastrectomy. J Gastroenterol Hepatol (Australia). 2016;31 Suppl 3:106-107. |
| 37. | Narasimhamurthy MS, Vallachira GP, Mahadev PS. Synchronous adenocarcinoma and gastrointestinal stromal tumor in the stomach. Saudi J Gastroenterol. 2010;16:218-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Athanassiou E, Vamvakopoulou DN, Zacharoulis D, Paroutoglou G, Sioutopoulou D, Tepetes K, Nomikos I, Vamvakopoulos NC. Immunophenotypic evaluation of DNA mismatch repair markers in 2 cases of synchronous histomorphologically distinct gastric adenocarcinomas with gastrointestinal stromal tumors of the proximal small bowel. Appl Immunohistochem Mol Morphol. 2010;18:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Fan H, Lu P, Xu L, Qin Y, Li J. Synchronous occurrence of hereditary gastric adenocarcinoma, gastrointestinal stromal tumor, and esophageal small cell and squamous carcinoma in situ: an extremely rare case report. BMC Cancer. 2017;17:720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Kalender ME, Sevinc A, Kucukdurmaz Z, Balik A, Sari I, Camci C. Gastric and prostate adenocarcinoma in a patient with metastatic gastrointestinal stromal tumor. Onkologie. 2007;30:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Ozgun YM, Ergul E, Sisman IC, Kusdemir A. Gastric adenocarcinoma and GIST (collision tumors) of the stomach presenting with perforation; first report. Bratisl Lek Listy. 2009;110:504-505. [PubMed] |
| 42. | Sista F, Abruzzese V, Schietroma M, Amicucci G. Concomitant gastrointestinal stromal tumor of the stomach and gastric adenocarcinoma in a patient with billroth 2 resection. Case Rep Surg. 2013;2013:583856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Telugu RB, Pushparaj M, Masih D, Pulimood A. Synchronous Appearance of Adenocarcinoma and Gastrointestinal Stromal Tumour (GIST) of the Stomach: A Case Report. J Clin Diagn Res. 2016;10:ED16-ED18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Theodosopoulos T, Dellaportas D, Psychogiou V, Gennatas K, Kondi-Pafiti A, Gkiokas G, Papaconstantinou I, Polymeneas G. Synchronous gastric adenocarcinoma and gastrointestinal stromal tumor (GIST) of the stomach: a case report. World J Surg Oncol. 2011;9:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Uchiyama S, Nagano M, Takahashi N, Hidaka H, Matsuda H, Nagaike K, Maehara N, Hotokezaka M, Chijiiwa K. Synchronous adenocarcinoma and gastrointestinal stromal tumors of the stomach treated laparoscopically. Int J Clin Oncol. 2007;12:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Villias C, Gourgiotis S, Veloudis G, Sampaziotis D, Moreas H. Synchronous early gastric cancer and gastrointestinal stromal tumor in the stomach of a patient with idiopathic thrombocytopenic purpura. J Dig Dis. 2008;9:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Vogel Y, Müller C, Uhl W, Tannapfel A. [Coexistence of multifocal gastric adenocarcinoma with signet-ring cell morphology and a gastrointestinal stromal tumour in a stomach with hp-associated gastritis]. Z Gastroenterol. 2011;49:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Zhou Y, Wu XD, Shi Q, Jia J. Coexistence of gastrointestinal stromal tumor, esophageal and gastric cardia carcinomas. World J Gastroenterol. 2013;19:2005-2008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Salemis NS, Gourgiotis S, Tsiambas E, Karameris A, Tsohataridis E. Synchronous occurrence of advanced adenocarcinoma with a stromal tumor in the stomach: a case report. J Gastrointestin Liver Dis. 2008;17:213-215. [PubMed] |
| 50. | Kaffes A, Hughes L, Hollinshead J, Katelaris P. Synchronous primary adenocarcinoma, mucosa-associated lymphoid tissue lymphoma and a stromal tumor in a Helicobacter pylori-infected stomach. J Gastroenterol Hepatol. 2002;17:1033-1036. [PubMed] |
| 51. | Yamamoto D, Hamada Y, Tsubota Y, Kawakami K, Yamamoto C, Yamamoto M. Simultaneous development of adenocarcinoma and gastrointestinal stromal tumor (GIST) in the stomach: case report. World J Surg Oncol. 2012;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Lin YL, Tzeng JE, Wei CK, Lin CW. Small gastrointestinal stromal tumor concomitant with early gastric cancer: a case report. World J Gastroenterol. 2006;12:815-817. [PubMed] |
| 53. | Chen JH, Chen CC, Tzeng LM, Tsay SH, Chiang JH, Lu CC, Chang FY, Lee SD. Resection of triple synchronous tumors--gastric adenocarcinoma, gallbladder adenocarcinoma and stromal tumor of the stomach. Zhonghua Yi Xue Za Zhi (Taipei). 2001;64:655-660. [PubMed] |
| 54. | Bircan S, Candir O, Aydin S, Başpinar S, Bülbül M, Kapucuoğlu N, Karahan N, Ciriş M. Synchronous primary adenocarcinoma and gastrointestinal stromal tumor in the stomach: a report of two cases. Turk J Gastroenterol. 2004;15:187-191. [PubMed] |
| 55. | Sailors JL, French SW. The unique simultaneous occurrence of granular cell tumor, gastrointestinal stromal tumor, and gastric adenocarcinoma. Arch Pathol Lab Med. 2005;129:e121-e123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Chiu HH, Huang TC, Liu YW, Ko TL, Lu NK. Synchronous ileal stromal tumor (GIST) and colonic adenocarcinoma. J Int Med Taiwan. 2009;20:260-263. |
| 57. | Dimitroulopoulos D, Fotopoulou A, Xinopoulos D, Arnogiannaki N, Korkolis D, Tsamakidis K, Kypreos D, Bassioukas S, Patsavela S, Paraskevas E. Synchronous occurrence of colorectal adenocarcinoma and colonic gastrointestinal stromal tumor (GIST). A case report. Ann Gastroenterol. 2009;22:197-200. |
| 58. | Efstathios P, Athanasios P, Papaconstantinou I, Alexandros P, Frangisca S, Sotirios G, Evangelos F, Athanasios G. Coexistence of gastrointestinal stromal tumor (GIST) and colorectal adenocarcinoma: A case report. World J Surg Oncol. 2007;5:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Firat O, Yazici P, Makay O, Aydin A, Tuncyurek M, Ersin S, Guler A. Co-existence of gastrointestinal stromal tumors with malign epithelial tumors: a report of two cases. Acta Chir Belg. 2009;109:629-632. [PubMed] |
| 60. | Gavriilidis P, Nikolaidou A. Colon Adenocarcinoma Associated with Synchronous Extramural Gastrointestinal Stromal Tumor (GIST) of the Ileum. Am J Case Rep. 2015;16:837-839. [PubMed] |
| 61. | Jafferbhoy S, Paterson H, Fineron P. Synchronous gist, colon and breast adenocarcinoma with double colonic polyp metastases. Int J Surg Case Rep. 2014;5:523-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Kosmidis C, Efthimiadis C, Levva S, Anthimidis G, Baka S, Grigoriou M, Tzeveleki I, Masmanidou M, Zaramboukas T, Basdanis G. Synchronous colorectal adenocarcinoma and gastrointestinal stromal tumor in Meckel's diverticulum; an unusual association. World J Surg Oncol. 2009;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Marković M, Jurišić V, Petrović M, Dagović A, Stanković V, Mitrović S. Appearance of ductal breast and colon carcinoma with gastrointestinal stromal tumor (GIST) in a female patient: an extremely rare case. Rom J Morphol Embryol. 2018;59:613-617. [PubMed] |
| 64. | Melis M, Choi EA, Anders R, Christiansen P, Fichera A. Synchronous colorectal adenocarcinoma and gastrointestinal stromal tumor (GIST). Int J Colorectal Dis. 2007;22:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Nemes C, Rogojan L, Surdea-Blaga T, Seicean A, Dumitrascu DL, Ciuce C. Gastrointestinal stromal tumor (GIST) associated with synchronous colon adenocarcinoma - a case report. J Gastrointestin Liver Dis. 2012;21:101-103. [PubMed] |
| 66. | Dragan R, Dejan S, Nebojsa M, Vinka V, Predrag S, Dragos S, Vladan Z, Dragoljub B. Synchronous appearance of gastric gastrointestinal stromal tumors and colorectal adenocarcinoma:a case report. Hepatogastroenterology. 2011;58:2171-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Kaur R, Bhalla S, Nundy S, Jain S. Synchronous gastric gastrointestinal stromal tumor (GIST) and other primary neoplasms of gastrointestinal tract: report of two cases. Ann Gastroenterol. 2013;26:356-359. [PubMed] |
| 68. | Seshadri RA, Singh SS, Ratnagiri R. Synchronous jejunal gastrointestinal stromal tumor and primary adenocarcinoma of the colon. Indian J Surg. 2012;74:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Suzuki T, Suwa K, Hanyu K, Okamoto T, Fujita T, Yanaga K. Large gastrointestinal stromal tumor and advanced adenocarcinoma in the rectum coexistent with an incidental prostate carcinoma: A case report. Int J Surg Case Rep. 2014;5:640-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Trajkovska E, Janevska V, Spasevska L, Janevski V, Zhivadinovik J, Petrushevska G, Dukova B. Synchronous occurrence of ileal stromal tumor (GIST) and colonic adenocarcinoma: a case report. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36:219-223. [PubMed] |
| 71. | Kumar K, Rowsell C, Law C, Ko YJ. Coexistence of gastrointestinal stromal tumour and colorectal adenocarcinoma: Two case reports. J Gastrointest Oncol. 2011;2:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 72. | Mou YP, Xu XW, Xie K, Zhou W, Zhou YC, Chen K. Laparoscopic wedge resection of synchronous gastric intraepithelial neoplasia and stromal tumor: a case report. World J Gastroenterol. 2010;16:5005-5008. [PubMed] |
| 73. | Spinelli GP, Miele E, Tomao F, Rossi L, Pasciuti G, Zullo A, Zoratto F, Nunnari J, Pisanelli GC, Tomao S. The synchronous occurrence of squamous cell carcinoma and gastrointestinal stromal tumor (GIST) at esophageal site. World J Surg Oncol. 2008;6:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Alkaaki A, Abdulhadi B, Aljiffry M, Nassif M, Al-Maghrabi H, Maghrabi AA. Coexistence of Primary GEJ Adenocarcinoma and Pedunculated Gastric Gastrointestinal Stromal Tumor. Case Rep Surg. 2018;2018:4378368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Grigor'eva VD, Guliaeva EN. [The combined use of dry-air carbon dioxide baths and applications of peloids at low temperatures in the rehabilitation of patients with psoriatic arthritis]. Vopr Kurortol Fizioter Lech Fiz Kult. 1998;25-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Blandamura S, Alessandrini L, Bertorelle R, Simonato F, Guzzardo V, Valentini E, Angriman I, Fassina A. Multiple sporadic gastrointestinal stromal tumors concomitant with ampullary adenocarcinoma: a case report with KIT and PDGFRA mutational analysis and miR-221/222 expression profile. Pathol Res Pract. 2014;210:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Koçer NE, Kayaselçuk F, Calişkan K, Ulusan S. Synchronous GIST with osteoclast-like giant cells and a well-differentiated neuroendocrine tumor in Ampula Vateri: coexistence of two extremely rare entities. Pathol Res Pract. 2007;203:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Al-Brahim N, Radhi J, Gately J. Synchronous epithelioid stromal tumour and lipoma in the stomach. Can J Gastroenterol. 2003;17:374-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Cirillo F. Neuroendocrine tumors and their association with rare tumors: observation of 4 cases. Eur Rev Med Pharmacol Sci. 2010;14:577-588. [PubMed] |
| 80. | Ding J, Sun P, Cai XY, Fei SH, Wu J, Qi YK, Liu ZB, Yuan L, He YJ, Song H, Chen WX. Synchronous poorly-differentiated neuroendocrine carcinoma and gastrointestinal stromal tumor of the stomach: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA. Int J Clin Exp Pathol. 2014;7:9076-9080. [PubMed] |
| 81. | Samaras VD, Foukas PG, Triantafyllou K, Leontara V, Tsapralis D, Tsompanidi EM, Machairas A, Panayiotides IG. Synchronous well differentiated neuroendocrine tumour and gastrointestinal stromal tumour of the stomach: a case report. BMC Gastroenterol. 2011;11:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Tan E, Friedman M, Coppola D. Occurrence of Multiple Tumors in a Patient. Cancer Control. 2015;22:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 83. | Buragas M, Kidd M, Modlin IM, Cha C. Multiple gastrointestinal stromal tumors and synchronous ileal carcinoids. Nat Clin Pract Oncol. 2005;2:166-70; quiz 1 p following 170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Cho H, Watanabe T, Aoyama T, Hayashi T, Yamada T, Ogata T, Yoshikawa T, Tsuburaya A, Sekiguchi H, Nakamura Y, Sakuma Y, Kameda Y, Miyagi Y. Small bud of probable gastrointestinal stromal tumor within a laparoscopically-resected gastric schwannoma. Int J Clin Oncol. 2012;17:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Amoruso M, Papagni V, Picciariello A, Pinto VL, D'Abbicco D, Margari A. Intestinal occlusion by stenotic neuroendocrine tumours of left colon and concomitant association with small bowel gastrointestinal stromal tumours: A case report. Int J Surg Case Rep. 2018;53:182-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Pata F, Sengodan M, Tang CB, Kadirkamanathan SS, Harvey M, Zaitoun A, Petkar M, Rotundo A. Concomitant jejunal sarcomatoid carcinoma and gastric GIST in patient with polymyalgia rheumatica: A case report. Int J Surg Case Rep. 2013;4:449-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Dasanu CA, Mesologites T, Trikudanathan G. Synchronous tumors: adenosquamous carcinoma of pancreas and GIST of stomach. J Gastrointest Cancer. 2011;42:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | He JJ, Ding KF, Zheng L, Xu JH, Li J, Wu YL, Sun LF, Zhou DE, Zheng S. Adenosquamous carcinoma of the uncinate process of the pancreas with synchronous gastrointestinal stromal tumor of the stomach: Case report and review of the literature. Oncol Lett. 2012;4:1191-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Fiore M, de Stefano G, Coppola N, Giorgio A. Synchronous and metachronous gastric gist with pancreatic adenocarcinoma: report of 2 cases and a review of literature. Gastroenterol Hepatol Bed Bench. 2015;8:298-301. [PubMed] |
| 90. | Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK, Yang XD, Jiang KW. Carcinosarcoma of pancreas with liver metastasis combined with gastrointestinal stromal tumour of the stomach: is there a good prognosis with the complete resection? Eur J Cancer Care (Engl). 2010;19:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Arabadzhieva E, Yonkov A, Bonev S, Bulanov D, Taneva I, Vlahova A, Dikov T, Dimitrova V. A rare case with synchronous gastric gastrointestinal stromal tumor, pancreatic neuroendocrine tumor, and uterine leiomyoma. World J Surg Oncol. 2016;14:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Tavares AB, Viveiros FA, Cidade CN, Maciel J. Gastric GIST with synchronous neuroendocrine tumour of the pancreas in a patient without neurofibromatosis type 1. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Ueda K, Hijioka M, Lee L, Igarashi H, Niina Y, Osoegawa T, Nakamura K, Takahashi S, Aishima S, Ohtsuka T, Takayanagi R, Ito T. A synchronous pancreatic neuroendocrine tumor and duodenal gastrointestinal stromal tumor. Intern Med. 2014;53:2483-2488. [PubMed] |
| 94. | Alabraba E, Bramhall S, O'Sullivan B, Mahon B, Taniere P. Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World J Surg Oncol. 2009;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Haugvik SP, Røsok BI, Edwin B, Gladhaug IP, Mathisen Ø. Concomitant Nonfunctional Pancreatic Neuroendocrine Tumor and Gastric GIST in a Patient Without Neurofibromatosis Type 1. J Gastrointest Cancer. 2012;43 Suppl 1:S171-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Felekouras E, Athanasios P, Vgenopoulou S, Papaconstantinou I, Prassas E, Giannopoulos A, Griniatsos J. Coexistence of hepatocellular carcinoma (HCC) and c-Kit negative gastrointestinal stromal tumor (GIST): a case report. South Med J. 2008;101:948-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Ferreira E Mora H, Pinto de Sousa J, Devesa V, Barbosa J, Costa J, Portugal R, Costa Maia J. Gastrointestinal stromal tumor of the stomach and hepatocellular carcinoma: An unusual association. Int J Surg Case Rep. 2015;12:75-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Yamashita K, Baba Y, Kurashige J, Iwatsuki M, Imai K, Hashimoto D, Sakamoto Y, Chikamoto A, Yoshida N, Beppu T, Baba H. Co-occurrence of liver metastasis of gastrointestinal stromal tumor and hepatocellular carcinoma: a case report. Surg Case Rep. 2016;2:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Jaworski R, Jastrzebski T, Swierblewski M, Drucis K, Kobierska-Gulida G. Coexistence of hepatocellular carcinoma and gastrointestinal stromal tumor: a case report. World J Gastroenterol. 2006;12:665-667. [PubMed] |
| 100. | Mrzljak A, Košuta I, Škrtić A, Kardum-Skelin I, Vrhovac R. Metachronous gastrointestinal stromal tumor and acute leukemia after liver transplantation for cholangiocellular carcinoma: is there a link? Case Rep Oncol. 2013;6:163-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Nam SJ, Choi HS, Kim ES, Keum B, Jeen YT, Chun HJ. Synchronous occurrence of gastrointestinal stromal tumor and intrahepatic cholangiocarcinoma: A case report. Oncol Lett. 2015;9:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Paiva CE, Moraes Neto FA, Agaimy A, Custodio Domingues MA, Rogatto SR. Perivascular epithelioid cell tumor of the liver coexisting with a gastrointestinal stromal tumor. World J Gastroenterol. 2008;14:800-802. [PubMed] |
| 103. | Gundes E, Kucukkartallar T, Colak MH, Kartal A, Esen HH. Gastrointestinal stromal tumor in the stomach co-existent with renal cell carcinoma. Erciyes Tip Dergisi. 2014;36:47-50. |
| 104. | Torous VF, Su A, Lu DY, Dry SM. Adult Patient with Synchronous Gastrointestinal Stromal Tumor and Xp11 Translocation-Associated Renal Cell Carcinoma: A Unique Case Presentation with Discussion and Review of Literature. Case Rep Urol. 2015;2015:814809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 105. | Jiang Q, Zhang Y, Zhou YH, Hou YY, Wang JY, Li JL, Li M, Tong HX, Lu WQ. A novel germline mutation in SDHA identified in a rare case of gastrointestinal stromal tumor complicated with renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:12188-12197. [PubMed] |
| 106. | Comandini D, Damiani A, Pastorino A. Synchronous GISTs associated with multiple sporadic tumors: a case report. Drugs Context. 2017;6:212307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Rebegea LF, Pătraşcu A, Miron D, Dumitru ME, Firescu D. Metachronous gastrointestinal stromal tumor associated with other neoplasia - case presentation. Rom J Morphol Embryol. 2016;57:1429-1435. [PubMed] |
| 108. | Favero G, Pfiffer T, Riedlinger WF, Chiantera V, Schneider A. Uncommon synchronous association between ovarian carcinoma and gastrointestinal stromal tumor: a case study and literature review. Tumori. 2013;99:e70-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 109. | Li W, Wu X, Wang N, Yin D, Zhang SL. Gastrointestinal stromal tumor with synchronous isolated parenchymal splenic metastasis of ovarian cancer. Chin Med J (Engl). 2011;124:4372-4375. [PubMed] |
| 110. | Damiani GR, Coco L, Monfreda L, Gaetani M, Barnaba M, Germinario S, Loverro G. A rare case of coexistence of ovarian cancer and gastrointestinal stromal tumor. Giornale Italiano di Ostetricia e Ginecologia. 2011;33:255-258. |
| 111. | Sharma M BK, Barad AK, Padu K, Singh K S, Singh Th SC. Spontaneous Perforation as a First Presentation of Ileal Gastrointestinal Stromal Tumour (GIST) with Synchronous Breast Sarcoma. J Clin Diagn Res. 2014;8:ND07-ND09. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 112. | Joo YB, Choi SH, Kim SK, Shim B, Kim MS, Kim YJ. Synchronous development of KIT positive acute myeloid leukemia in a patient with gastrointestinal stromal tumor. Korean J Hematol. 2010;45:66-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | Gao NA, Guo NJ, Yu WZ, Wang XX, Sun JR, Yu N, Liu RT, Liu XD, Liu ZY, Feng R. Synchronous occurrence of gastrointestinal stromal tumor and acute myeloid leukemia: A case report and review of the literature. Oncol Lett. 2016;11:2977-2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Sonmez M, Arslan M, Cobanoglu U, Kavgaci H, Ozbas HM, Aydin F, Ovali E, Omay SB. Association of gastrointestinal stromal tumor and acute myeloid leukemia preceded by myelodysplastic syndrome with refractory anemia. Tumori. 2009;95:240-242. [PubMed] |
| 115. | Herbers AH, Keuning JJ. Staging for CLL-type non-Hodgkin's lymphoma reveals a gastrointestinal stromal tumour. Neth J Med. 2005;63:74-75. [PubMed] |
| 116. | Pamukçuoglu M, Budakoğlu B, Han O, Tad M, Oksüzoğlu B, Abali H, Zengin N. An extraordinary case in whom gastrointestinal stromal tumor and low-grade malignant lymphoma are seen together in the stomach. Med Oncol. 2007;24:351-353. [PubMed] |
| 117. | Ludmir EB, Gutschenritter T, Pinnix CC, Gunther JR, Nastoupil LJ, Khoury JD, Medeiros LJ, Dabaja BS, Milgrom SA. Coincident primary breast lymphoma and gastrointestinal stromal tumor: case series and molecular mechanisms. Onco Targets Ther. 2018;11:8937-8942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 118. | Takahashi T, Maruyama Y, Saitoh M, Itoh H, Yoshimoto M, Tsujisaki M, Nakayama M. Synchronous Occurrence of Diffuse Large B-cell Lymphoma of the Duodenum and Gastrointestinal Stromal Tumor of the Ileum in a Patient with Immune Thrombocytopenic Purpura. Intern Med. 2016;55:2951-2956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Au WY, Wong WM, Khoo US, Liang R. Challenging and unusual cases: Case 2. Concurrent gastrointestinal stromal tumor and Burkitt's lymphoma. J Clin Oncol. 2003;21:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 120. | Rahimi K, Gologan A, Haliotis T, Lamoureux E, Chetty R. Gastrointestinal stromal tumor with autonomic nerve differentiation and coexistent mantle cell lymphoma involving the appendix. Int J Clin Exp Pathol. 2008;2:608-613. [PubMed] |
| 121. | Salar A, Ramón JM, Barranco C, Nieto M, Prats M, Serrano S, Besses C. Double diagnosis in cancer patients and cutaneous reaction related to gemcitabine: CASE 1. Synchronous mucosa-associated lymphoid tissue lymphoma and gastrointestinal stromal tumors of the stomach. J Clin Oncol. 2005;23:7221-7223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 122. | Tzilves D, Gatopoulou A, Zervas K, Katodritou E, Patakiouta F, Tarpagos A, Katsos I. Development of multiple myeloma in a patient with gastrointestinal stromal tumor treated with imatinib mesylate: a case report. World J Gastroenterol. 2007;13:2011-2013. [PubMed] |
| 123. | Nagai K, Matsumura Y, Nomura J, Inui M, Tagawa T. A case of double cancer involving oral malignant melanoma and gastrointestinal stromal tumor (GIST). Int J Oral Maxillofac Surg. 2005;34:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Arnogiannaki N, Martzoukou I, Kountourakis P, Dimitriadis E, Papathanasaki A, Nastoulis E, Gazalidou M, Fida A, Apostolikas N, Agnantis NJ. Synchronous presentation of GISTs and other primary neoplasms: a single center experience. In Vivo. 2010;24:109-115. [PubMed] |
| 125. | Miyoshi T, Mori R, Amano S, Sumitomo H, Aoyama M, Inoue S, Hino N, Wada D. Efficacy of erlotinib and imatinib in a patient with a rectal gastrointestinal stromal tumor and synchronous pulmonary adenocarcinoma: A case report. J Med Invest. 2016;63:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Jiang MJ, Weng SS, Cao Y, Li XF, Wang LH, Xu JH, Yuan Y. Metachronous Primary Adenocarcinoma of Lung During Adjuvant Imatinib Mesylate Therapy for Gastrointestinal Stromal Tumor of Stomach: A Case Report. Medicine (Baltimore). 2015;94:e1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Wada Y, Koizumi T, Yokoyama T, Urushihata K, Yamamoto H, Hanaoka M, Kubo K. Synchronous gastrointestinal stromal tumor and primary lung adenocarcinoma. Intern Med. 2012;51:2407-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Brummel N, Awad Z, Frazier S, Liu J, Rangnekar N. Perforation of metastatic melanoma to the small bowel with simultaneous gastrointestinal stromal tumor. World J Gastroenterol. 2005;11:2687-2689. [PubMed] |
| 129. | Su YY, Chiang NJ, Wu CC, Chen LT. Primary gastrointestinal stromal tumor of the liver in an anorectal melanoma survivor: A case report. Oncol Lett. 2015;10:2366-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Inayat F, Saif MW. New Drug and Possible New Toxicity - Squamous Cell Carcinoma Following Imatinib in Patients with Gastrointestinal Stromal Tumors. Anticancer Res. 2016;36:6201-6204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 131. | Wollina U, Hansel G, Zimmermann F, Schönlebe J, Nowak A. Merkel cell carcinoma of the lower leg with retroperitoneal GIST: a very rare association. Wien Klin Wochenschr. 2015;127:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 132. | McCarthy CJ, O'Brien GC, Cummins RJ, Kay EW, Broe PJ. GIST with a twist--upregulation of PDGF-B resulting in metachronous gastrointestinal stromal tumor and dermatofibrosarcoma protuberans. J Gastrointest Surg. 2010;14:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 133. | Kondo S, Yamaguchi U, Sakurai S, Ikezawa Y, Chuman H, Tateishi U, Furuta K, Hasegawa T. Cytogenetic confirmation of a gastrointestinal stromal tumor and ewing sarcoma/primitive neuroectodermal tumor in a single patient. Jpn J Clin Oncol. 2005;35:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 134. | Kovecsi A, Jung I, Bara T, Bara T, Azamfirei L, Kovacs Z, Gurzu S. First Case Report of a Sporadic Adrenocortical Carcinoma With Gastric Metastasis and a Synchronous Gastrointestinal Stromal Tumor of the Stomach. Medicine (Baltimore). 2015;94:e1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | de la Torre J, Banerjee S, Baumgartner J, Lin GY, Burgoyne AM, Kirane A, Sicklick J. Tumor Symbiosis: Gastrointestinal Stromal Tumor as a Host for Primary Peritoneal Mesothelioma. J Gastrointest Surg. 2019;23:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Lee CK, Hadley A, Desilva K, Smith G, Goldstein D. When is a GIST not a GIST? A case report of synchronous metastatic gastrointestinal stromal tumor and fibromatosis. World J Surg Oncol. 2009;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | Adim SB, Filiz G, Kanat O, Yerci O. Simultaneous occurrence of synchronous and metachronous tumors with gastrointestinal stromal tumors. Bratisl Lek Listy. 2011;112:623-625. [PubMed] |
| 138. | Aghdassi A, Christoph A, Dombrowski F, Döring P, Barth C, Christoph J, Lerch MM, Simon P. Gastrointestinal Stromal Tumors: Clinical Symptoms, Location, Metastasis Formation, and Associated Malignancies in a Single Center Retrospective Study. Dig Dis. 2018;36:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 139. | Amaadour L, Oualla K, Benbrahim Z, Najib R, Benhammane H, Chad A, Arifi S, Mellas N, El Mesbahi O. Synchronous gastrointestinal stromaltumors and other primary cancers: Case series of a single institution experience. Ann Oncol. 2013;4:iv73. |
| 140. | Fernández JA, Olivares V, Gómez-Ruiz AJ, Ferri B, Frutos MD, Soria T, Torres G, Parrilla P. Additional malignancies in patients with gastrointestinal stromal tumors (GIST): incidence, pathology and prognosis according to a time of occurrence-based classification. Clin Transl Oncol. 2019;21:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 141. | Ferreira SS, Werutsky G, Toneto MG, Alves JM, Piantá CD, Breunig RC, Brondani da Rocha A, Grivicich I, Garicochea B. Synchronous gastrointestinal stromal tumors (GIST) and other primary cancers: case series of a single institution experience. Int J Surg. 2010;8:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 142. | Giuliani J, Marzola M, Indelli M, Aliberti C, Sartori S, Lanza G, Lelli G, Frassoldati A. Gastrointestinal stromal tumors and other malignancies: a case series. J Gastrointest Cancer. 2012;43:634-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Gonçalves R, Linhares E, Albagli R, Valadão M, Vilhena B, Romano S, Ferreira CG. Occurrence of other tumors in patients with GIST. Surg Oncol. 2010;19:e140-e143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 144. | Hechtman JF, DeMatteo R, Nafa K, Chi P, Arcila ME, Dogan S, Oultache A, Chen W, Hameed M. Additional Primary Malignancies in Patients with Gastrointestinal Stromal Tumor (GIST): A Clinicopathologic Study of 260 Patients with Molecular Analysis and Review of the Literature. Ann Surg Oncol. 2015;22:2633-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 145. | Lai BR, Wu YT, Kuo YC, Hsu HC, Chen JS, Chen TC, Wu RC, Chiu CT, Yeh CN, Yeh TS. Targeted ultra-deep sequencing unveils a lack of driver-gene mutations linking non-hereditary gastrointestinal stromal tumors and highly prevalent second primary malignancies: random or nonrandom, that is the question. Oncotarget. 2016;7:83270-83277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 146. | Liszka Ł, Zielińska-Pajak E, Pajak J, Gołka D, Huszno J. Coexistence of gastrointestinal stromal tumors with other neoplasms. J Gastroenterol. 2007;42:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 147. | Pandurengan RK, Dumont AG, Araujo DM, Ludwig JA, Ravi V, Patel S, Garber J, Benjamin RS, Strom SS, Trent JC. Survival of patients with multiple primary malignancies: a study of 783 patients with gastrointestinal stromal tumor. Ann Oncol. 2010;21:2107-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 148. | Ponti G, Luppi G, Martorana D, Rossi G, Losi L, Bertolini F, Sartori G, Pellacani G, Seidenari S, Boni E, Neri TM, Silini E, Tamburini E, Maiorana A, Conte PF. Gastrointestinal stromal tumor and other primary metachronous or synchronous neoplasms as a suspicion criterion for syndromic setting. Oncol Rep. 2010;23:437-444. [PubMed] |
| 149. | Richter KK, Schmid C, Thompson-Fawcett M, Settmacher U, Altendorf-Hofmann A. Long-term follow-up in 54 surgically treated patients with gastrointestinal stromal tumours. Langenbecks Arch Surg. 2008;393:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 150. | Rodriquenz MG, Rossi S, Ricci R, Martini M, Larocca M, Dipasquale A, Quirino M, Schinzari G, Basso M, D'Argento E, Strippoli A, Barone C, Cassano A. Gastrointestinal stromal tumors (GISTs) and second malignancies: A novel "sentinel tumor"? A monoinstitutional, STROBE-compliant observational analysis. Medicine (Baltimore). 2016;95:e4718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Rubió-Casadevall J, Borràs JL, Carmona-García MC, Ameijide A, Gonzalez-Vidal A, Ortiz MR, Bosch R, Riu F, Parada D, Martí E, Miró J, Sirvent JJ, Galceran J, Marcos-Gragera R. Correlation between mutational status and survival and second cancer risk assessment in patients with gastrointestinal stromal tumors: a population-based study. World J Surg Oncol. 2015;13:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | Sevinc A, Seker M, Bilici A, Ozdemir NY, Yildiz R, Ustaalioglu BO, Kalender ME, Dane F, Karaca H, Gemici C, Gumus M, Buyukberber S. Co-existence of gastrointestinal stromal tumors with other primary neoplasms. Hepatogastroenterology. 2011;58:824-830. [PubMed] |
| 153. | Vassos N, Agaimy A, Hohenberger W, Croner RS. Coexistence of gastrointestinal stromal tumours (GIST) and malignant neoplasms of different origin: prognostic implications. Int J Surg. 2014;12:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |