Published online Jul 28, 2019. doi: 10.3748/wjg.v25.i28.3684
Peer-review started: February 27, 2019
First decision: April 30, 2019
Revised: June 13, 2019
Accepted: July 1, 2019
Article in press: July 3, 2019
Published online: July 28, 2019
Processing time: 152 Days and 7.1 Hours
Acute kidney injury (AKI) is a common complication of liver cirrhosis and is of the utmost clinical and prognostic relevance. Patients with cirrhosis, especially decompensated cirrhosis, are more prone to develop AKI than those without cirrhosis. The hepatorenal syndrome type of AKI (HRS–AKI), a spectrum of disorders in prerenal chronic liver disease, and acute tubular necrosis (ATN) are the two most common causes of AKI in patients with chronic liver disease and cirrhosis. Differentiating these conditions is essential due to the differences in treatment. Prerenal AKI, a more benign disorder, responds well to plasma volume expansion, while ATN requires more specific renal support and is associated with substantial mortality. HRS–AKI is a facet of these two conditions, which are characterized by a dysregulation of the immune response. Recently, there has been progress in better defining this clinical entity, and studies have begun to address optimal care. The present review synopsizes the current diagnostic criteria, pathophysiology, and treatment modalities of HRS–AKI and as well as AKI in other chronic liver diseases (non-HRS–AKI) so that early recognition of HRS–AKI and the appropriate management can be established.
Core tip: Acute kidney injury following advanced liver disease is a very common syndrome in clinical practice. Recent evidence from both basic research on pathophysiology and clinical studies has revealed a complex association between the liver and kidney through the vascular microenvironment and related immune mediators. These connections may play roles in promising new treatments of acute kidney injury on top of chronic liver disease. Furthermore, non-cell-based liver support systems have yielded promising preliminary data on the attenuation of the mortality rate of these conditions of dual organ failure.
- Citation: Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J Gastroenterol 2019; 25(28): 3684-3703
- URL: https://www.wjgnet.com/1007-9327/full/v25/i28/3684.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i28.3684
Acute kidney injury (AKI) superimposed on chronic liver disease and cirrhosis is common and consists of varying phenotypes. Prerenal renal dysfunction caused by severe hypoalbuminemia is the most common clinical syndrome in patients with advanced liver disease. Although prerenal azotemia seems to be the first phase of AKI, it is difficult to differentiate from hepatorenal syndrome (HRS) and acute tubular necrosis (ATN). Once the onset of AKI occurs in chronic liver disease, a consequence of its complications is an increased morbidity and mortality rate. Accordingly, the characteristics of renal dysfunctions in both noncirrhotic and chronic liver diseases, such as prerenal HRS-ATN, require not only earlier recognition but also precise diagnosis with optimal management. Recently, there have been great advances, including in classification, nomenclature, and pathophysiology, in identifying the connection between chronic liver disease and acute renal dysfunction in patients with cirrhosis. Indeed, acute-on-chronic liver failure (ACLF) was first recognized in the early 2000s as a new classification that represents the distinct characteristics of chronic liver failure (or decompensated cirrhosis) with rapid deterioration leading to hepatic and extrahepatic multiorgan failure[1,2]. Since then, many efforts in natural history and pathophysiology have been made[3]. Substantial advancements have been made not only in the field of hepatology but also in the understanding of the significance of renal dysfunction in chronic liver disease, as suggested by the international guideline that all acute renal dysfunction in patients with cirrhosis requires the same clinician attention as AKI[4].
Since the first description by Hecker and Sherlock in the 1960s, renal dysfunction in patients with ascites and advanced cirrhosis has typically been called HRS[2], which refers to a syndrome of decreased renal function mainly resulting from the systemic hemodynamic effects of advanced portal hypertension. However, advanced chronic liver disease can impact renal function with a wide range of complications, including bile acid nephropathy, coagulopathy-induced bleeding from ischemic ATN, related glomerular diseases (e.g., immunoglobulin, a nephropathy, hepatitis B-related glomerulonephritis, hepatitis C-related glomerulonephritis, cryoglobulinemia, membranoproliferative glomerulonephritis), and other comorbid diseases such as inherited cystic diseases. Herein, we review the updated information, including the etiology, pathophysiology, and therapeutic aspects, of renal dysfunction in advanced chronic liver disease.
The clinical spectrum of advanced liver disease is currently recognized as follows:
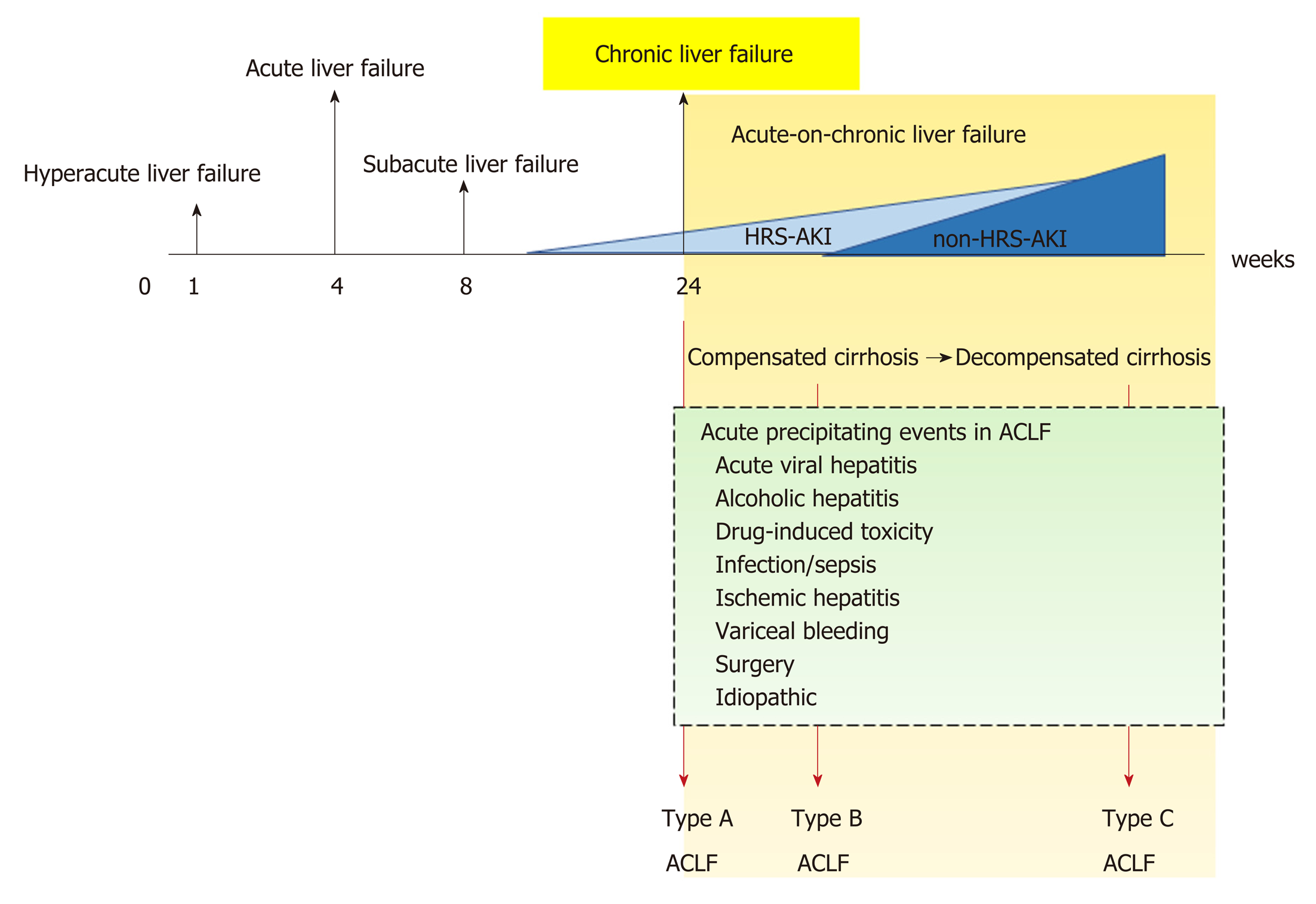
Chronic liver disease is a progressive process of destruction and regeneration of liver parenchyma resulting in fibrosis and cirrhosis over a period of 24 wk (Figure 1) caused by inflammation, infection, abnormal metabolism, or malignancy. The functional classification of chronic liver disease can be divided into compensated and decompensated liver disease.
ACLF is liver failure with one or more extrahepatic organ failures, leading to an increased 28-d mortality rate within 3 mo of disease onset[5]. However, there are currently at least 4 different definitions for ACLF in clinical practice: (1) The Asian Pacific Association for the Study of Liver (APASL); (2) The American Association for the Study of Liver Diseases (AASLD); (3) The European Association for the Study of the Liver (EASL) or AASLD-EASL; and (4) The World Gastroenterology Organization (WGO) definition (Table 1).
| Criteria | APSAL | AASLD-EASL | WGO |
| Preexisting or underlying chronic liver disease | Noncirrhotic chronic liver disease, compensated cirrhosis | Cirrhotic chronic liver disease, cirrhosis with prior decompensation | Noncirrhotic chronic liver disease, compensated cirrhosis, decompensated cirrhosis |
| Precipitating causes | Alcohol, drugs, hepatotropic viruses, surgery, trauma | Alcohol, drugs, hepatotropic viruses, surgery, trauma, variceal bleeding, infection/sepsis | Alcohol, drugs, hepatotropic viruses, surgery, trauma, variceal bleeding, infection/sepsis |
| Duration between acute liver injury and ACLF | 4 wk | NA | NA |
| Organ failure | Hepatic failure | Extrahepatic organ failure | Hepatic failure, extrahepatic organ failure |
The first ACLF definition, given by the APASL in 2009, was “acute hepatic insult manifesting as jaundice, and coagulopathy complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease”[6]. Then, in 2014, the revised ACLF definition included the presentation of a high mortality rate within 28 d of disease onset (short-term mortality)[7]. Later, the AASLD-EASL workgroup defined ACLF as “acute deterioration of preexisting chronic liver disease usually related to a precipitating event and associated with increased mortality at 3 mo due to multisystem organ failure”, based on the data from large prospective multicenter studies[8,9]. After that, the WGO workgroup announced an improved ACLF definition: “a syndrome in patients with chronic liver disease with or without previously diagnosed cirrhosis which is characterized by acute hepatic decompensation resulting in liver failure (jaundice and prolonged international normalized ratio) and one or more extrahepatic organ failure that is associated with increased mortality within a period of 28 d and up to 3 mo from onset”[5]. Interestingly, while the timeframe between the onset of acute liver injury and liver failure development (4 wk) is clear in the APASL definition, time intervals are not mentioned in the AASLD-EASL or the WGO definition.
In addition, there are some differences in ACLF definition regarding the underlying liver conditions of the patients. Patients with cirrhosis and non-cirrhosis (i.e., pre-cirrhotic) are included in the APASL and WGO but not in the AASLD-EASL definition. Patients with hepatic decompensation (previously or currently) are not included in the APASL definition, while they are included in the AASLD-EASL definition (which does not include noncirrhotic chronic liver disease). In parallel, the WGO definition includes both cirrhosis (compensated and decompensated) and noncirrhotic chronic liver disease.
Because ACLF can develop from: (1) Noncirrhotic liver disease (e.g., hepatitis B, alcohol-related chronic liver disease, and nonalcoholic fatty liver disease (NAFLD); (2) Compensated cirrhosis with precipitating factors; and (3) Decompensated cirrhosis, the WGO suggested that all patients with chronic liver disease (no cirrhosis, compensated cirrhosis, or decompensated cirrhosis) should be included in the ACLF definition and divided into types A (noncirrhotic chronic kidney disease), B (compensated cirrhosis), and C (decompensated cirrhosis)[5] (Figure 1).
Historically, AKI in patients with cirrhosis was often defined as HRS type 1 and type 2. HRS type 1 referred to AKI in cirrhosis with a relatively rapid progressive deterioration of renal function with serum creatinine (SCr) higher than 2.5 mg/dL for more than 2 wk, and HRS type 2 referred to AKI with slowly progressing renal function deterioration in cirrhosis with refractory ascites (SCr level of 1.5-2.5 mg/dL)[10]. Because SCr is a less sensitive biomarker of AKI, with several pathophysiological limitations due to sarcopenia caused by hepatic injury (discussed later), the International Club of Ascites (ICA) recently proposed a new diagnostic criterion for HRS in 2015[4]. Accordingly, the SCr > 2.5 mg/dL criterion was removed along with recommending HRS as another kind of AKI, called HRS–AKI. In addition, the 2-wk threshold for the diagnosis of HRS and its subtypes was removed. The ultimate changes to the diagnostic criteria from HRS to HRS-AKI are now in the context of “cirrhotic patients who develop AKI by detecting a change in absolute SCr level of ≥ 0.3 mg/dL or by an increase in SCr ≥ 50% from baseline within 48 h with a lack of volume expansion response without evidence of shock, recent exposure to nephrotoxic agents or preexisting structural renal disease”[4] (Table 2).
| International Club of Ascites diagnostic criteria for hepatorenal syndrome |
| Diagnosis of cirrhosis and ascites |
| Diagnosis of acute kidney injury (AKI) according to ICA-AKI criteria (Table 3) |
| No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion administration with albumin at 1 g/kg of body weight |
| Absence of shock |
| No current or recent use of nephrotoxic agents |
| No signs of structural kidney injuries, defined as the following: |
| Absence of proteinuria (> 500 mg/day or equivalent) |
| Absence of microscopic hematuria (> 50 red blood cells per high-power field) |
| Normal findings on renal ultrasonography |
| Diagnosis | Definition |
| Acute kidney injury (AKI) | Rise in serum creatinine (SCr) of ≥ 50% from baseline or a rise in SCr by ≥ 0.3 mg/dL (26.5 μmol/L) in < 48 h. Hepatorenal syndrome (HRS) type 1 is a specific form of AKI |
| Stage 1: Increase in serum creatinine (SCr) ≥ 0.3 mg/dL (26.5 μmol/L) or an increase in SCr 1.5-fold to 2-fold from baseline | |
| Stage 2: Increase in SCr > 2-fold to 3-fold from baseline | |
| Stage 3: Increase in SCr > 3-fold from baseline or an increase in SCr ≥ 4.0 mg/dL (353.6 μmol/L) with an acute increase ≥ 0.3 mg/dL (26.5 μmol/L) or initiation of renal replacement therapy | |
| Chronic kidney disease (CKD) | Glomerular filtration rate (GFR) of < 60 mL/min for > 3 mo, calculated using the MDRD6 formula. HRS type 2 is a specific form of CKD |
| Acute-on-chronic kidney disease | Rise in SCr of ≥ 50% from baseline or a rise of SCr by ≥ 0.3 mg/dL (26.5 μmol/L) in < 48 h in a patient with cirrhosis whose GFR is < 60 mL/min for > 3 mo, calculated using the MDRD6 formula |
In 2007, the Acute Kidney Injury Network (AKIN) proposed the guidelines for AKI-defining criteria[11], which were integrated into the ICA and the Acute Dialysis Quality Initiative (ADQI) in 2011[12] (Table 3), because several validation studies on the AKIN criteria show the independent mortality association depending on the stage of renal injury[13-16]. The most recently proposed guideline from Huelin et al[17] suggests that stage 1 AKI should be divided into 2 phases, stage 1A (SCr < 1.5 mg/dL) and stage 1B (SCr > 1.5 mg/dL), because stage 1 AKI with SCr > 1.5 mg/dL shows a similar mortality rate as stage 2 AKI. However, further validation study is needed due to the unclear differences in baseline characteristics between stage 1A and stage 1B AKI, where the more severe liver dysfunction is shown in the latter group.
The new definitions of advanced liver disease and AKI improve the understanding of the underlying pathophysiologic mechanisms in the liver and kidneys. HRS is currently not the only form of AKI in chronic liver disease. Indeed, a vast majority of AKI in chronic liver disease is not characterized as HRS, particularly in ACLF (Table 4).
| Hepatorenal syndrome | Non-hepatorenal syndrome |
| Splanchnic vasodilatation | Acute-on-chronic liver failure |
| Inflammation | Inflammation |
| Adrenal insufficiency | Bacterial translocation |
| Cardiac dysfunction | Bile acid |
| Worsening portal hypertension | |
| Worsening cardiac output |
The progression of advanced chronic liver disease into liver cirrhosis (liver architecture disruption) results from splanchnic vasodilatation in response to increased intrahepatic resistance (portal hypertension) caused by several mediators, including nitric oxides, prostacyclin, carbon monoxide, epoxyeicosatrienoic acids, glucagon, endogenous cannabinoids, and adrenomedullin[18-24]. In advanced cirrhosis, the loss of liver compensation that slows disease progression in the liver is due to progressive splanchnic vasodilatation (loss of the counteract vasoconstriction), leading to a decrease in the effective circulatory volume, reduced renal blood flow and AKI. This phenomenon simulates the renin-angiotensin-aldosterone system (RAAS) and vasopressin, which in turn cause more severe renal vasoconstriction and worsening renal hypoperfusion[25]. Evidence of their functional nature was illustrated by measuring copeptin, a 39-amino-acid glycopeptide released from the neu-rohypophysis, and arginine vasopressin (AVP). The higher copeptin level is, the greater the risk of AKI and the worse the outcomes of decompensated cirrhosis[26]. In addition, the deterioration of renal perfusion is caused by the alteration of renal blood flow autoregulation[27,28]. Together, the current treatment concepts of HRS-AKI are reversing the physiological responses by improving the hemodynamic vascular bed using volume expansion (such as with albumin) together with splanchnic vaso-constrictors.
Systemic inflammation is one of the mechanisms responsible for decompensated cirrhosis because increased inflammatory cytokines are demonstrated in HRS-AKI (the late stage of cirrhosis). Advanced liver disease with spontaneous bacterial peritonitis (SBP) and renal insufficiency is characterized by high tumor necrosis factor (TNF)-α and interleukin (IL)-6 compared with advanced liver disease with normal renal function[29]. Furthermore, patients with acute decompensated cirrhosis and difficult-to-treat HRS-AKI (persistent AKI) demonstrate higher interferon-inducible protein-10 and vascular cell adhesion molecule-1 compared to patients with treatment-responsive HRS-AKI[30]. In addition, the growing evidence afforded by systems biology analysis has demonstrated a similar nature of inflammation in HRS-AKI in comparison with AKI in chronic non-hepatic conditions, such as lupus nephritis[30].
Relative adrenal insufficiency is found in over 25%-30% of decompensated cirrhosis patients. Of note, adrenal insufficiency contributes to cardiomyopathy in cirrhosis patients via the downregulation of β-adrenergic receptors as well as the alteration of catecholamines’ effects on the systemic vascular tone[31]. Furthermore, the presence of adrenal insufficiency in patients with stable decompensated cirrhosis is associated with poorer clinical characteristics, such as circulatory dysfunction, previous history of SBP, and worse survival rate[32].
The impacts of advanced chronic liver disease on cardiac function and the systemic circulatory system are well known. Cirrhotic cardiomyopathy is a diminished cardiac contractile function with electrophysiological abnormalities in response to stress stimuli without preexisting cardiac disease[33]. The emerging data reflect a liver-heart-kidney interaction. Cirrhotic cardiomyopathy arises from sustained portal hypertension, which increases the risk of bacterial translocation and portosystemic shunt. Bacterial translocation stimulates the production of systemic inflammatory cytokines and eventually causes endothelial dysfunction[34], while an increased portosystemic shunt decreases systemic vascular resistance by increasing nitric oxide, adenosine, bradykinin, and endocannabinoids[35]. In addition, portal hypertension and splanchnic dilation activate the sympathetic nervous system and several neurohormones, including AVP, renin, and angiotensin, which affect heart function by increasing afterload and left ventricular end-diastolic pressure[36]. Decreased cardiac output and increased right atrial pressure cause renal tissue hypoxia and venous congestion, respectively, leading to kidney injury and lower glomerular filtration rate[37]. Accordingly, cardiac dysfunction in advanced chronic liver disease deteriorates renal function in HRS through a liver–heart–kidney connection, which is possibly related to a new model of therapeutic approaches to HRS[38].
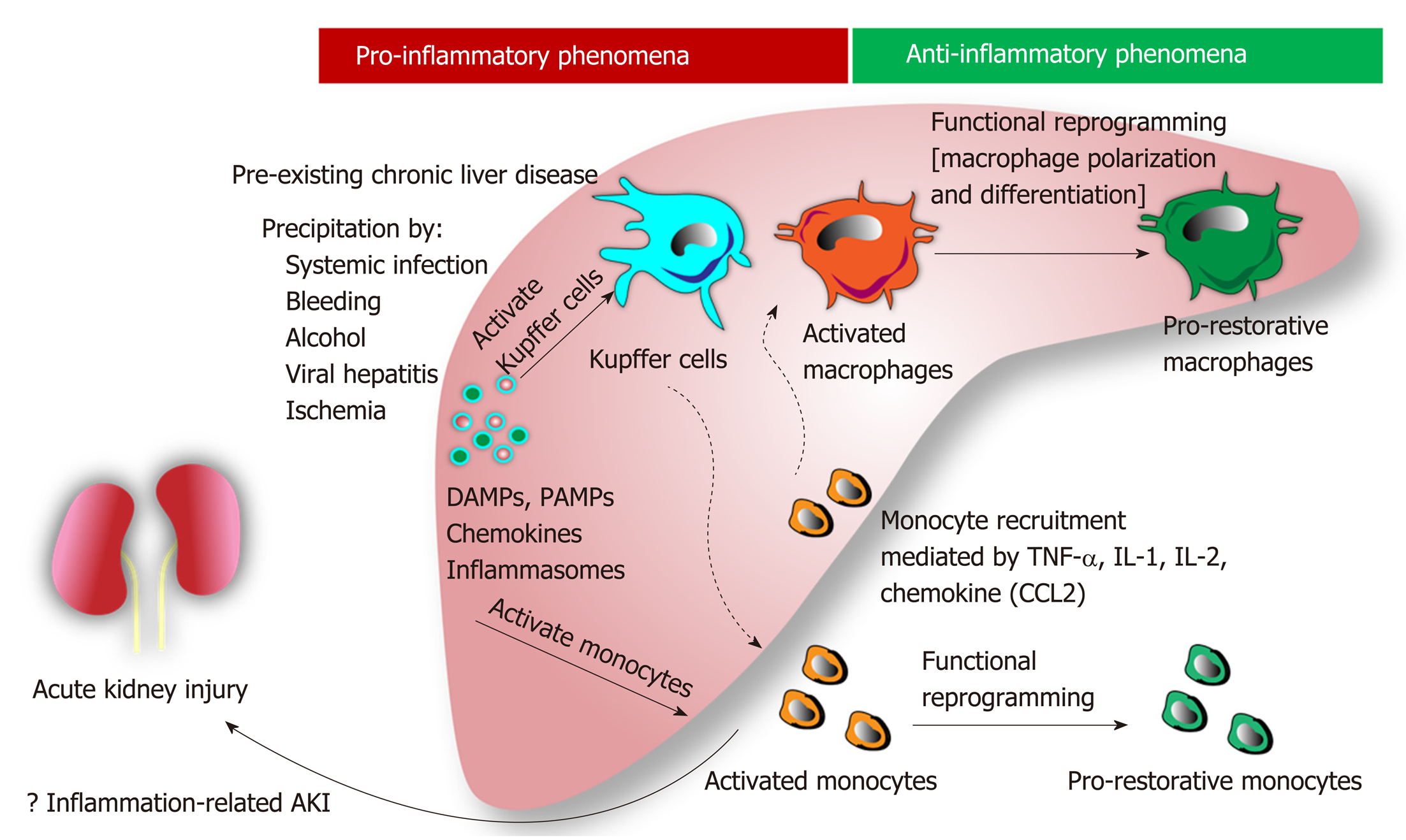
Role of inflammation, apoptosis, and cell death: Hepatic inflammation is a novel major component for the initiation and progression of liver injury[39]. In chronic liver disease, hepatic inflammation is activated by several risk factors (i.e., systemic infection, gastrointestinal bleeding, alcohol, viral infection, etc.) resulting in (Figure 1): (1) Hepatocyte damage that delivering several damage-associated molecular patterns (DAMPs) from liver cells; and (2) Gut immunity impairment that enhancing the translocation of pathogen-associated molecular pattern (PAMPs) from gut organisms[40]. Subsequently, both DAMPs and PAMPs enhance the more severe liver damage (ALF, ACLF, and liver cirrhosis). Several liver associated DAMPs including IL-1, IL-33, high-mobility group box-1, and bile acid[41] are recognized by several receptors [e.g., toll-like receptors (TLR)-4, TLR-9 and the receptor for advanced glycation end-products] on Kupffer cells (liver macrophages)[42] resulting in the more enhanced hepatic damages (Figure 2).
In addition, the imbalance of inflammatory response in ACLF (a local inflammatory response) contributes to the systemic inflammatory response syndrome (SIRS) in different severity and subsequently turns into the compensatory anti-inflammatory response syndrome (CARS)[43]. While SIRS relates to the excessive liver inflammation and extrahepatic organ dysfunction, CARS is a counter regulatory mechanism against the inappropriate hyper-inflammatory process. Overwhelming of activated monocyte function may contribute to AKI, compatible with those in septic AKI[44]. In addition, Because macrophage polarization is related to pro- versus anti- inflammation, SIRS and CARS in ACLF might be associated with M1 and M2 macrophage polarization, respectively, of Kupffer cells in liver[45]. Moreover, bone marrow-derived macrophage that was recruited into liver by the inflammatory-induced CCL2 and CCR5 chemokine expression might also be important in liver injury[45]. Macrophage polarization and macrophage origin may be beneficial to the understanding in the inflammatory phase of liver injury (i.e., initiation, propagation and resolution)[46]. The inhibition of Kupffer cells, the prevention of monocyte recruitment into liver, and the promotion of proper macrophage polarization might be the novel strategies for liver injury attenuation[47].
Systemic oxidative stress and several inflammatory cytokines (human non-mercaptalbumin 2, IL-6, and IL-8) are also higher in patients with ACLF compared to non-ACLF conditions[48]. Similarly, the severity of ACLF is associated with apoptosis as determined by the apoptosis index [plasma caspase-cleaved keratin 18 (cK18; an apoptosis biomarker)/keratin 18 (K18; an indicator of total cell death)][49], implying the influence of systemic inflammation and apoptosis in ACLF. Indeed, persistent infection (inflammatory activation) is an important risk factor in cirrhotic patients on the basis of the score of chronic liver failure-organ failure. Therefore, AKI superimposed in ACLF is associated with a 20% increase in mortality depending on AKI severity (more severe with SCr > 1.5 mg/dL)[9].
Role of bile acid: The limited responsiveness of terlipressin plus albumin treatment in HRS type 1 with high serum bilirubin (≥ 10 mg/dL) suggests the influence of bile acid in chronic liver disease[50]. Indeed, the markedly elevated serum bilirubin in chronic liver disease induces bile cast nephropathy, a common renal pathology of AKI with severe liver dysfunction diagnosed from the presentation of intratubular bile casts by Hall histochemical staining, which are associated with irreversible AKI in chronic liver disease[51-53]. In the less severe form of renal injury, bile acid accumulation in cirrhosis induces proximal tubulopathy mimicking Fanconi syndrome (low uric acid, low phosphate but high bile acid in serum) without bile cast nephropathy[51-53]. Although serum bilirubin is an independent predictor of the therapeutic response of HRS, the precise role of bile cast nephropathy is still unclear.
Worsening portal hypertension: Markedly increased intrahepatic resistance is common in progressive ACLF patients and usually results in increased portal hypertension[54]. This, in turn, may potentiate hepatorenal reflex disturbance, causing progressive AKI[27].
Worsening cardiac output: ACLF worsens cardiac functions through arterial vas-odilation in the splanchnic area and peripheral circulation, which can be demonstrated in all stages of liver injury, from the early compensated stage to progressive liver decompensation to the late stage of HRS. The low cardiac output due to excessive vascular dilatation is counteracted by: (1) Several vasoconstriction mediators (including the RAAS, vasopressin, and the sympathetic nervous system); and (2) Vigorous salt and water balancing from renal homeostasis[55]. Once cardiac function fails to compensate for arterial vasodilation, as determined by the low cardiac output and reduced systolic function, the more severe liver injury condition (late HRS) will develop due to the decreased effective circulating volume[56].
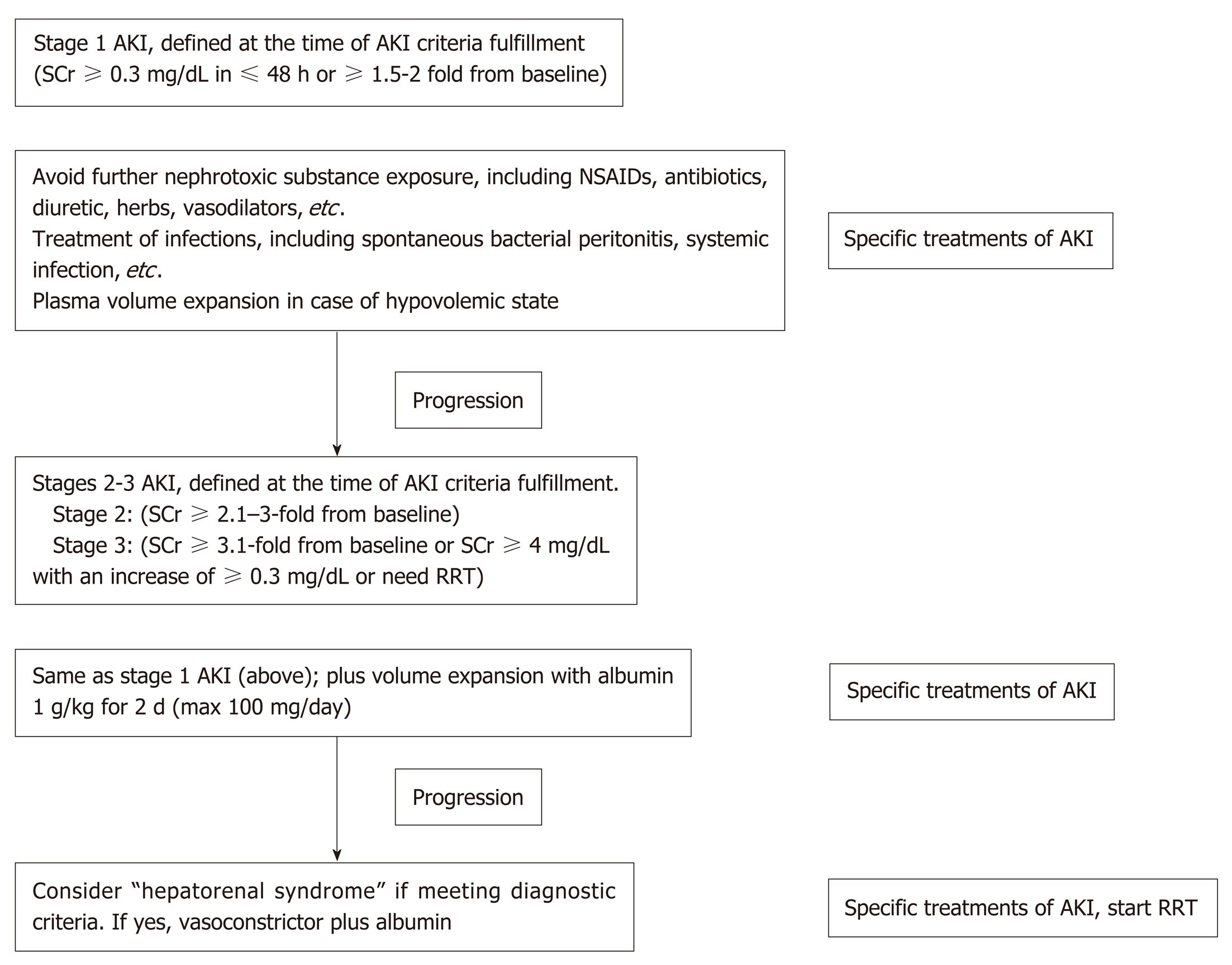
As discussed above, the points on the continuum of AKI disease in liver cirrhosis, i.e., prerenal azotemia-HRS-ATN (prerenal-HRS-ATN), are difficult to differentiate clearly. AKI management in a certain setting should focus on early recognition with an understanding of the individual patient’s clinical course (Figure 3). The disturbance of hemodynamics in cirrhosis patients is the principal cause of AKI that is precipitated by infection, abrupt onset of severe hyperbilirubinemia, gastrointestinal bleeding, over-diuresis, or nephrotoxic agents. All of these factors must be immediately identified and corrected in patients with advanced cirrhosis to prevent subsequent renal complications. An intravascular volume status assessment is an initial step for any AKI etiologies. Volume status assessment is challenging, particularly in cirrhosis, because the patients usually also have hyperdynamic circulation between a total-body hypervolemic state and a low effective circulatory volume status. Unfortunately, there is no effective monitoring tool for these patients. Central venous pressure monitoring not only has a poor correlation with the intravascular volume due to confounding effects from ascites but also is too invasive for the coagulopathy that is commonly found in chronic liver disease. Echocardiography for intravascular volume assessment depends solely on the determination of inferior vena cava size and variability, which relies mostly on operator expertise[57].
Although there is no advantage in mortality attenuation or AKI incidence when comparing albumin and crystalloids[58], albumin is usually the recommended volume expander in chronic liver disease due to albumin’s pleiotropic effects (anti-inflammatory and antioxidant properties)[59,60]. Hydroxyethyl starch is contraindicated due to its associations with increased AKI and mortality rates[61]. In addition, intravenous albumin administration at 1-1.5 and 1 g/kg on days 1 and 3, respectively, with antibiotics attenuates the mortality of SBP and the AKI incidence[62]. In parallel, SBP prophylaxis with oral quinolones (norfloxacin 400 mg twice a day for 7 d) is recommended in the high-risk group (low protein in ascites fluid and previous history of SBP)[63], while intravenous ceftriaxone (1 g/d for 7 d) is more proper than oral quinolones in patients with active gastrointestinal bleeding[64]. Further, patients with high serum bilirubin (> 20 mg/dL) (with an abrupt onset or long duration) may develop nephropathy due to jaundice or bile cast induction[52,65], and selective bilirubin removal might be beneficial[66].
Intravascular volume depletion due to excessive diuretic treatment (diuretic-induced AKI) is common. Furthermore, electrolyte disturbance, such as hyperkalemia from aldosterone antagonists or other potassium-sparing diuretics, may necessitate urgent renal replacement therapy, particularly in those with renal impairment. Hypokalemia, a precipitating factor of hepatic encephalopathy, is a frequent diuretic complication. Accordingly, diuretic administration should be reserved for patients with a hypervolemic state or marked ascites and should be avoided in those vulnerable to intravascular volume depletion. All nephrotoxic agents, such as radiological contrasts, need to be prescribed with caution. Sepsis is also a common condition that is found with decompensated cirrhosis[67]. Organ failures such as AKI and acute respiratory distress syndrome may be a result of a high production of sepsis-induced proinflammatory cytokine production[67]. Cirrhosis patients with AKI should undergo a full septic workup and be treated with empirical antibiotics. Measuring the appropriate markers for early sepsis might be beneficial, as mentioned in the setting of resuscitation guided by serum lactate[68,69]. However, this approach remains controversial in advanced liver disease because of: (1) The poor lactic acid metabolism in liver diseases; and (2) The weak association between hyperlactatemia and tissue hypoperfusion[70].
If renal function does not improve after adequate volume expansion, HRS-AKI and non-HRS-AKI must be in line with the differential diagnosis and be further assessed with simultaneous treatment. The goal of HRS–AKI treatment is to optimize the cardiac output and mean arterial blood pressure (MAP). Although there was no significant difference in outcomes between targeting the higher MAPs (80-85 mmHg) compared with the lower MAPs (65-75 mmHg) in septic AKI[71], the most recent study revealed that AKI incidence was lowest among those whose postresuscitation MAP was closest to or higher than their preadmission MAP[72]. Perhaps an individualized target MAP acts as a key marker for HRS-AKI. To increase the MAP and cardiac output, intravenous albumin administration along with systemic vasoconstrictors has shown promising outcomes. A number of systemic vasoconstrictors are used to counter splanchnic vasodilation, including a vasopressin analog (terlipressin)[73,74], an α-adrenergic agonist (norepinephrine)[75,76], and a combination of α-adrenergic agonist (midodrine) and somatostatin analog (octreotide)[77]. The most recent meta-analysis demonstrates that: (1) The treatment of HRS type 1 with terlipressin plus albumin attenuates the short-term mortality; and (2) Terlipressin plus albumin or noradrenaline plus albumin is superior to triple therapy with midodrine, octreotide and albumin[78]. Regarding AKI outcome, terlipressin infusion gives an earlier and stronger response than noradrenaline, with a higher reversal rate of HRS (40% vs 17%) and a lower rate of renal support requirement (57% vs 80%)[79]. Interestingly, the effectiveness of albumin depends on the cumulative administration dose, as a 100 g increase in cumulative albumin (max dose of 600 g albumin) increases the survival rate with a hazard ratio of 1.15 and a 95% confidence interval of 1.02-1.31[80]. Because most HRS-AKI develops in an ACLF setting and is associated with inflammatory mediators, therapeutic strategies not only for hemodynamic restoration but also for the attenuation of systemic inflammation might represent a paradigm shift in the treatment, as demonstrated by the balancing of monocyte function[81]. Recently, simvastatin, a well-known lipid-lowering agent, has demonstrated beneficial effects in an ACLF rat model with advanced chronic liver disease through the improvement of hepatic hemodynamics, microvascular dysfunction, and endotoxemia[82].
Compared with noncomplicated HRS-AKI, non-HRS-AKI and HRS-AKI in advanced ACLF have more morbidity and mortality because of their poor response to terlipressin and albumin. ACLF with ≥ 2 organ failures (ACLF grade 2-3) is associated with a 60%-75% rate of 28-d mortality[9], and therapies such as plasmapheresis that potentially ameliorate the ACLF severity by modulating the immune system may be an option. Currently, both renal and liver support in clinical studies have failed to have any survival advantage[83,84]. Regarding the mode of hemodialysis, continuous renal replacement therapy (CRRT) does not improve mortality in comparison with intermittent hemodialysis; however, CRRT might be well tolerated in patients with unstable conditions, including fulminant hepatic failure, as it does not raise intracranial pressure[85]. Indeed, the ADQI group recommends renal support only in cases with acute reversible components. Otherwise, renal support is not reco-mmended in AKI-superimposed chronic liver disease, which is similar to the recommendation of limited utilization of liver support in liver transplantation[85]. However, extracorporeal albumin dialysis may improve outcomes based on the clearance of excess bilirubin, bile acid, inflammatory cytokines, and endotoxins in systemic circulation[84]. The extracorporeal liver support is currently divided into 2 kinds of systems.
Cell-based liver support systems (bioartificial liver support systems): The data on bioartificial liver support systems benefit in patients with ACLF are still limited and from uncontrolled studies with limited numbers of patients.
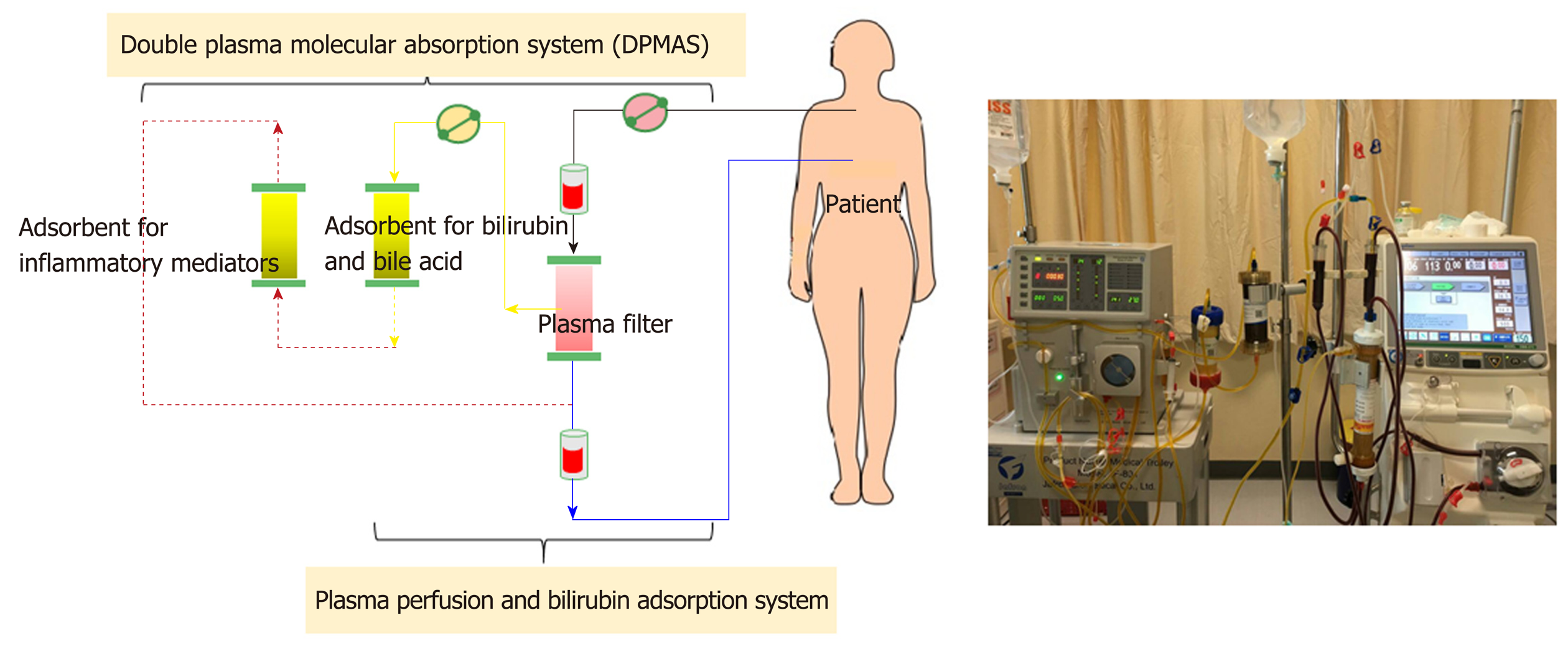
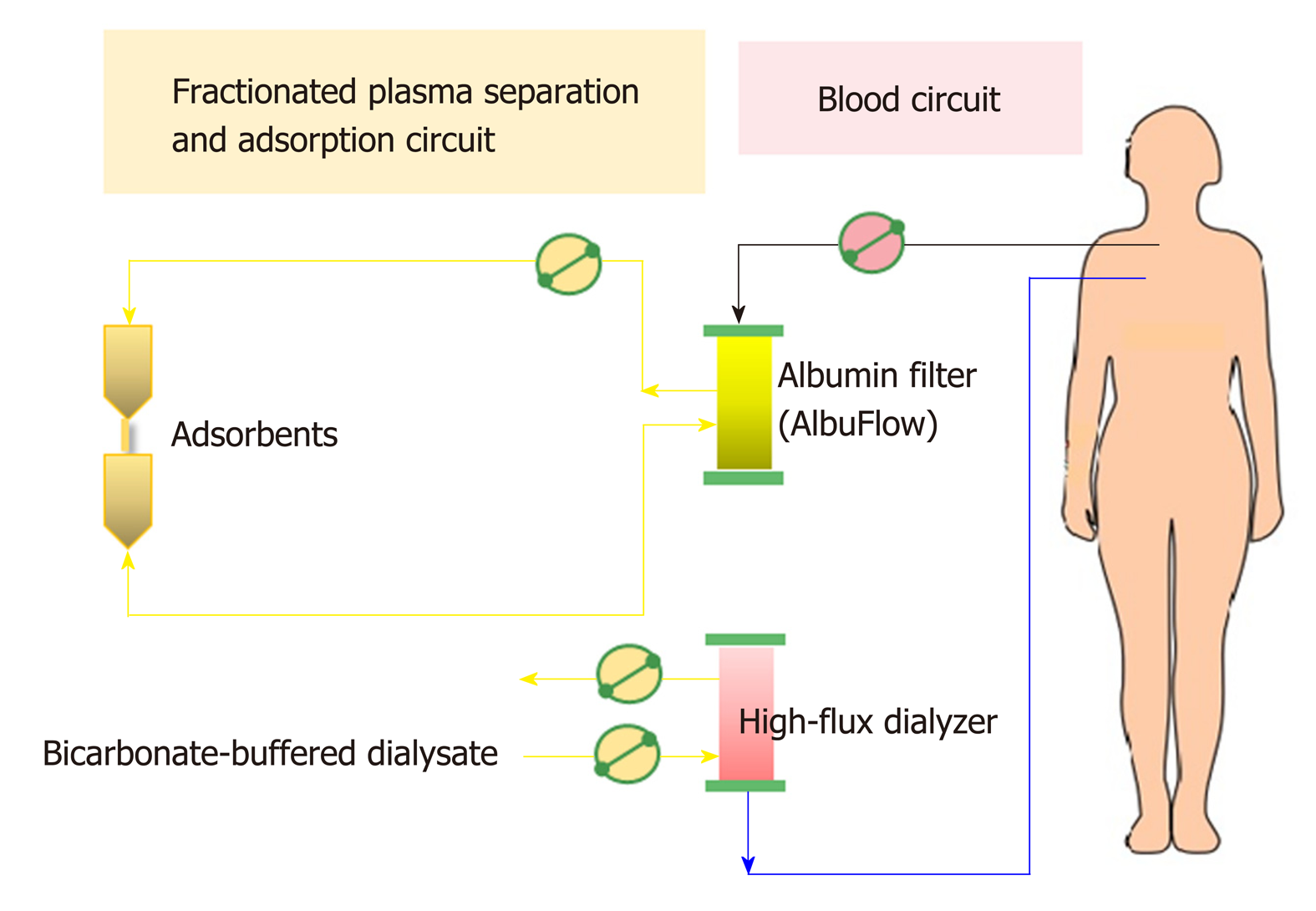
Non-cell-based liver support systems: There are two major modalities regarding to the non-cell-based liver support systems-plasma therapy and albumin dialysis. Plasma therapy comprises 4 subtypes of techniques: (1) Standard plasma exchange is a simple circuit (Figure 4) that enhances the elimination of inflammatory cytokines and endotoxins by using 1.2 L of plasma as fluid replacement. However, it has so far failed to show a confirmed survival advantage; (2) High-volume plasma exchange (HVP). Unlike standard plasma exchange, the HVP procedure uses a large amount of fresh-frozen plasma (approximately > 10 L of fresh-frozen plasma or 15%-20% of ideal body weight) as replacement fluid. The established technique was first used for immunologically driven disorders in the early 1990s[86]. HVP decreases the vasopressor dose requirement in resuscitation and improves hepatic encephalopathy symptoms by decreasing blood ammonia and urea[87]. A recent prospective, randomized study was conducted in 183 patients with acute liver failure, with approximately 2.4 treatments per patient and 9 h per treatment, and it demonstrated a slightly improved survival by an intention-to-treat analysis (59% vs 48%) and reduced circulating proinflammatory mediators [IL-6, TNF-α, DAMPs[88] and soluble B7 (CD80/CD89)[89]]. Unfortunately, there is no evidence on using HPV in ACLF so far. Further prospective studies are required to confirm these results in patients with acute liver failure and might be extended to patients with ACLF in the near future; (3) Plasma perfusion and bilirubin adsorption system and double plasma molecular absorption system, as shown in Figure 5. The fundamental mechanism in the plasma perfusion and bilirubin adsorption system is the separation of plasma that passes through an anion-exchange column (adsorbent), which has an adsorption effect on specific molecules (such as bilirubin, bile acid, and related similar molecular structures). Data from several studies showed a safe decrease in the plasma bilirubin concentration of approximately 18%-50% from baseline after one session[90,91]. The double plasma molecular absorption system is more sophisticated than plasma perfusion and bilirubin adsorption, combining both with an adsorbent for the reduction in inflammatory mediators, drugs or toxins (Figure 5). Although the rationale for using these systems seems to be that they will improve our understanding of ACLF pathophysiology, most studies on the double plasma molecular absorption system are in patients with hepatitis B-related liver failure[92-94]. In our experience, the double plasma molecular absorption system decreased bilirubin and ammonia 25%-30% from baseline after one treatment session in patients with ACLF and cancer (unpublished data). The reduction in hyperbilirubinemia in patients with sepsis-related ACLF was also demonstrated in our case series (unpublished data); (4) Fractionated plasma separation and adsorption (FPSA) (Prometheus®). FPSA, first introduced in 1999, is fractionated through an albumin-permeable filter with a cutoff of 250 kDa. Albumin and other plasma proteins cross the membrane and pass across 2 columns in a series-an anion-exchange column and a neutral resin adsorber. The cleansed albumin/plasma is returned to the standard blood pool circuit, where it is then treated by conventional high-flux hemodialysis (Figure 6). Clinical studies have evaluated the effect of FPSA in ACLF patients, with favorable outcomes, indicating that the use of FPSA is well tolerated and decreases the circulating levels of serum bilirubin, bile acids, and ammonia[95-100]. However, the improvement of neurological status and hemodynamics following FPSA treatment is controversial[96,98]. A prospective, randomized clinical trial (HELIOS study)[100] conducted in 145 ACLF patients demonstrated that FPSA decreased serum bilirubin with nondifferent 28-d survival rates (66% vs 63%). The secondary endpoint showed a significant improvement in survival in ACLF patients using the cutoff of Model for End-Stage Liver Disease (MELD) score > 30. It should be emphasized that there is no further confirmatory study or any study in an acute liver failure setting so far.
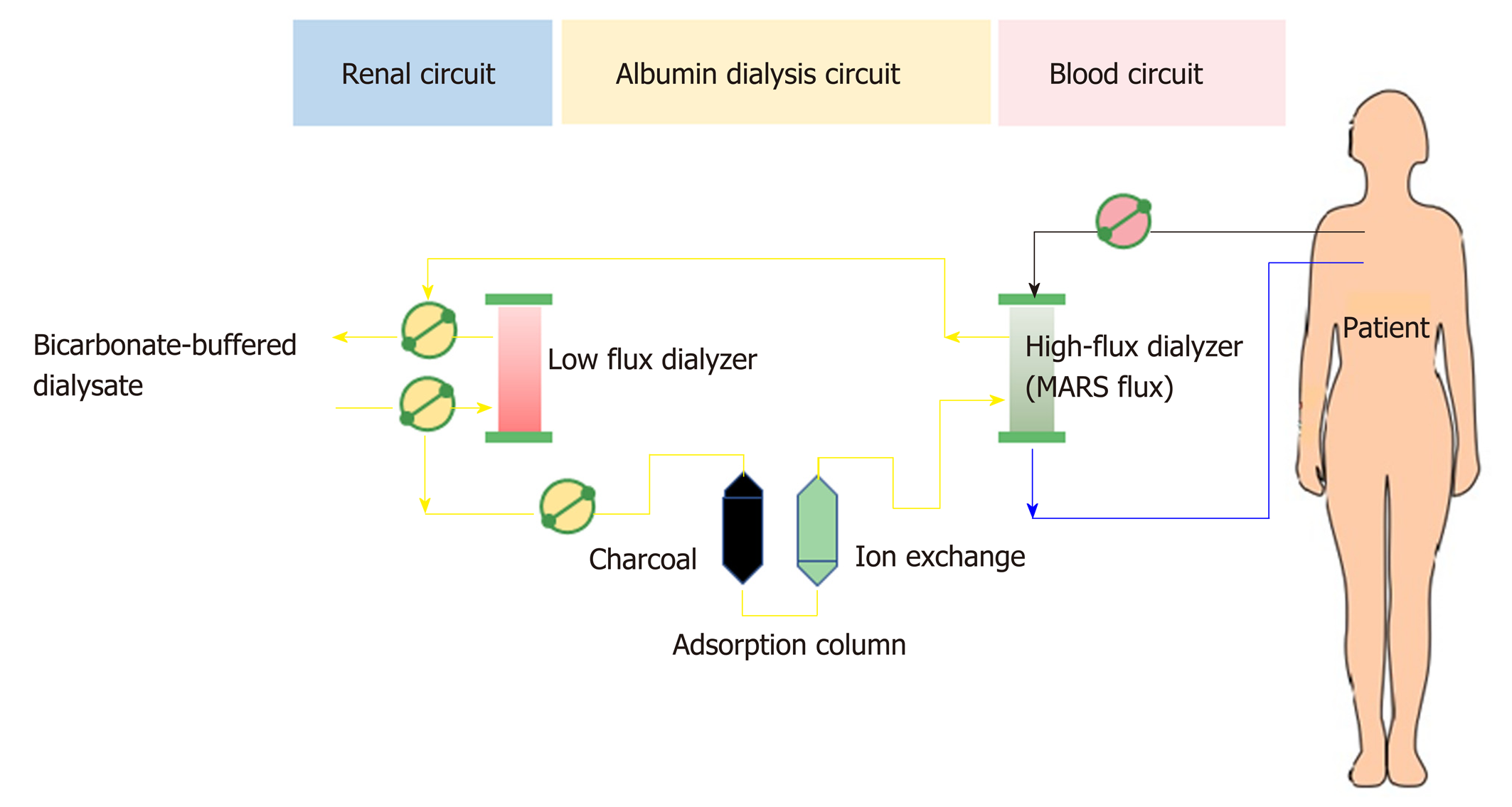
Albumin dialysis has been classified as molecular adsorbent recycling system (MARS) and single plasma albumin dialysis (SPAD). Technically, MARS consists of 3 main circuits of the blood delivery system, albumin flow, and routine hemodialysis system (Figure 7). In the MARS circuit, blood is dialyzed through an albumin-saturated high-flux dialyzer with 20% human albumin (600 mL) and is used as the dialysate for carrying the albumin-bound toxins into the dialysate, where the water-soluble and albumin-bound toxins will be removed from albumin through 2 sequential adsorbent columns containing activated charcoal and anion-exchange resin. Then, the toxin-free albumin will be circulated in the circuit. The high-molecular-weight toxins (> 50 kDa) are not removed by MARS due to the dialyzer pore size limitation. The RELIEF study is the largest randomized trial using MARS in ACLF, with 189 patients from 19 European centers, and it demonstrated that undergoing up to ten 6-h to 8-h sessions of MARS was associated with the improvement of hepatic encephalopathy (from grade 2-4 to grade 0-1; 63% vs 38%, respectively; P = 0.07) without a 28-d survival benefit[101]. Similarly, the FULMAR trial was a randomized controlled study using MARS in 102 patients with acute liver failure fulfilling transplant criteria and demonstrated no significant differences in 6-month survival between the MARS (85%) and standard medical treatment (76%) groups (P = 0.28)[102]. However, the major confounder in the study was the short transplant waiting time [median 16.2 h, mostly within 24 h (75%)], which might be too short to see the benefit of liver support. Interestingly, neither MARS nor FPSA showed significant decreases in inflammatory markers (IL-6, IL-8, IL-10, and TNF-α) after the treatment session in a small study[103].
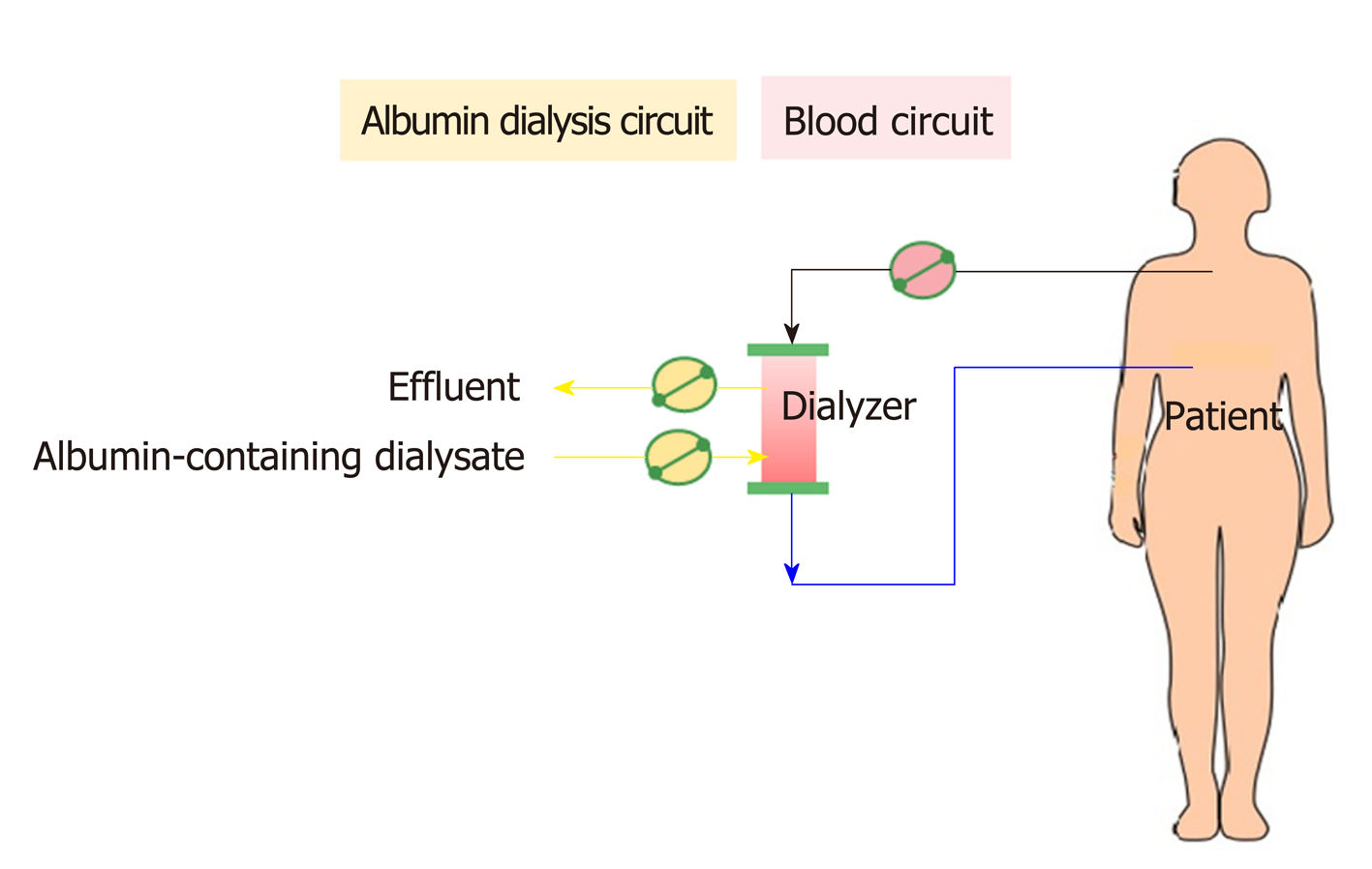
In contrast to MARS, SPAD uses a standard CRRT system without any additional columns (Figure 8). After a SPAD cycle is run, water-soluble toxins (ammonia, creatinine, urea, uric acid) are removed almost completely. Blood is dialyzed against a standard dialysis solution with the addition of albumin to the dialysate. The most effective albumin concentration was 3%. A further increase in the albumin concentration to 4% did not lead to a significant increase in the detoxification[104]. However, SPAD increased the detoxification efficiency of albumin-bound substances from 350 mL/h to 700 mL/h (for bilirubin) or 1000 mL/h (for bile acids) of the dialysate flow in SPAD for the first time[104]. Unfortunately, SPAD has been evaluated in a case-control fashion mostly in drug overdose-related liver failure, with controversial outcomes[105-107].
So far, liver transplantation is the only treatment modality for the reversal of either HRS-AKI or non-HRS-AKI in an ACLF setting. Recently, a large retrospective study of liver transplantation in patients with either HRS-AKI or non-HRS-AKI in ACLF demonstrated that 1-year and 5-year survival rates were 91% and 77%, respectively. However, those with a renal injury background of either diabetes or hypertension showed a lower survival rate (1-year and 5-year survival at 87% and 71%, respectively)[108]. Pretransplant AKI severity contributed as an important factor for renal function recovery at 4-6 wk posttransplantation. Thus, simultaneous liver-kidney transplantation should be considered in those with predicted renal recovery longer than 6 wk[85].
Regarding the high rate of mortality and limited treatment options for ACLF and AKI following ACLF; novel, robust therapies are needed.
Targeting liver macrophage: So far, unfortunately, none of proven macrophage-directed therapies has been recommended in ACLF although a number of immunomodulators including N-acetylcysteine, albumin and glucocorticoids are approved for other immune-related liver diseases[46]. The limitation of knowledge is probably due to the limitation in retrieving human liver tissue and the differentiation process for liver macrophage in human[46].
Targeting inhibition of Kupffer cell activation: Inhibition of progression of the early phase of SIRS in ACLF could be attenuate the signal induction for Kupffer cell activation especially targeting to inhibit released DAMPs. Recent study demonstrated potential beneficial of HMGB-1 blockade treatment in rat with ACLF model through alleviating inflammation in SIRS[109]. Likewise, the blocking responses against of histones (a DAMP) attenuated cytokine production and reduced liver injury severity[110]. In addition, the prevention and treatment of bacterial translocation by appropriate antibiotics is the most effective attenuation of initial innate immune activation leading to the subsequently Kupffer cell inhibition[111].
Targeting inhibition of monocyte recruitment into the liver: The recruitment of monocytes into liver is mediated mainly through chemokine system including; CCL2-CCR2 and CCL5-CCR5 for the recruitment of monocyte and lymphocyte, respectively[112]. As such, Cenicriviroc, an CCR2/CCR5 inhibitor, in a randomized, double-blind study in 289 patients with NAFLD and hepatic fibrosis demonstrated a reduction on fibrosis in treatment group (n = 145) compares with placebo (n = 144) (20% vs 10%) without the reduction of the primary endpoint (NAFLD activity score)[113] supporting the animal model data[112].
Targeting promotion of macrophage differentiation: An early promotion of macrophage differentiation from activated macrophages phenotype into a pro-restorative phenotype (such as by corticosteroids) might enhance the resolution of liver injury[111]. However, the data on macrophage differentiation treatment in ACLF is still too less. Corticosteroids might provides some advantages in the early phase of ACLF but possibly augment infectious complications in the late phase[114].
ACLF is mainly mediated through immune dysfunction and is also susceptible to the serious infections. Accordingly, treatment by hematopoietic growth factor to induce immune cells seems reasonable to restore immune homeostasis through improving impaired phagocytosis[115] and mobilization of CD34+ progenitor cells into liver[116]. The first randomized placebo-controlled study in 2012 using granulocyte colony-stimulating factor (G-CSF) in patients with ACLF demonstrated an improved 60-d survival rate and a reduction in multiorgan injury on the basis of clinical scores, including those of the MELD and Sequential Organ Failure Assessment (66% vs 26%)[117]. Likewise, G-CSF improved the 90-d mortality rate (78% vs 30%) in a randomized trial on 46 patients with ACLF[118]. The largest study, on 55 patients with hepatitis B-related ACLF randomized to receive standard medical treatment or G-CSF in addition to standard medical treatment, again showed a significant benefit in the 90-d survival rate in the G-CSF-treated group (48% vs 28%)[119]. Interestingly, the most recent randomized study in 32 patients with hepatitis B virus-ACLF showed that 5 μg/kg/day of G-CSF for six consecutive days in addition to the standard treatment, improves survival, facilitates clinical recovery, prevents renal failure and protects from hyponatremia[120].
AKI following advanced liver cirrhosis is a critical condition. The early recognition and rapid diagnosis of AKI in these patients may improve therapeutic outcomes. However, the understanding of the pathogenesis and the quality of the diagnostic tools for the simultaneous injury of the kidney and liver are still limited. A distinct diagnostic criterion for differentiating the points on the prerenal-HRS-ATN spectrum needs robust validation as well as an accurate distinction between HRS-AKI and non-HRS-AKI in ACLF. Inflammation is increasingly recognized as an important driver of AKI, particularly in patients with infection and multiorgan failure. As AKI in chronic liver disease is persistent and rapidly becomes irreversible by medical treatment as the condition prolongs, novel therapies and new approaches for either liver or renal support are required.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd-Elsalam S S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22 Suppl 2:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Jalan R, Williams R. Acute-on-chronic liver failure: Pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 3. | Olson JC, Kamath PS. Acute-on-chronic liver failure: Concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 5. | Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 6. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P, Lau GK, Liu Q, Madan K, Mohamed R, Ning Q, Rahman S, Rastogi A, Riordan SM, Sakhuja P, Samuel D, Shah S, Sharma BC, Sharma P, Takikawa Y, Thapa BR, Wai CT, Yuen MF. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 7. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O; APASL ACLF Working Party. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 500] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 8. | Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, Kamath PS. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 9. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2172] [Article Influence: 181.0] [Reference Citation Analysis (5)] |
| 10. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 11. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4989] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 12. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R, Ronco C, Genyk Y, Arroyo V. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Wong F, O'Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B, Thacker LR, Bajaj JS; North American Consortium for Study of End-Stage Liver Disease. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:1280-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR; TRIBE-AKI Consortium. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 15. | Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A, Angeli P. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 16. | Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, Graupera I, Ariza X, Pereira G, Alfaro I, Cárdenas A, Fernández J, Poch E, Ginès P. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 17. | Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, Pose E, Fasolato S, Fabrellas N, de Prada G, Pilutti C, Graupera I, Ariza X, Romano A, Elia C, Cárdenas A, Fernández J, Angeli P, Ginès P. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2017;15:438-445.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (33)] |
| 19. | Oberti F, Sogni P, Cailmail S, Moreau R, Pipy B, Lebrec D. Role of prostacyclin in hemodynamic alterations in conscious rats with extrahepatic or intrahepatic portal hypertension. Hepatology. 1993;18:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | De las Heras D, Fernández J, Ginès P, Cárdenas A, Ortega R, Navasa M, Barberá JA, Calahorra B, Guevara M, Bataller R, Jiménez W, Arroyo V, Rodés J. Increased carbon monoxide production in patients with cirrhosis with and without spontaneous bacterial peritonitis. Hepatology. 2003;38:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Di Pascoli M, Zampieri F, Verardo A, Pesce P, Turato C, Angeli P, Sacerdoti D, Bolognesi M. Inhibition of epoxyeicosatrienoic acid production in rats with cirrhosis has beneficial effects on portal hypertension by reducing splanchnic vasodilation. Hepatology. 2016;64:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Gomis R, Fernández-Alvarez J, Pizcueta P, Fernández M, Casamitjana R, Bosch J, Rodés J. Impaired function of pancreatic islets from rats with portal hypertension resulting from cirrhosis and partial portal vein ligation. Hepatology. 1994;19:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Domenicali M, Caraceni P, Giannone F, Pertosa AM, Principe A, Zambruni A, Trevisani F, Croci T, Bernardi M. Cannabinoid type 1 receptor antagonism delays ascites formation in rats with cirrhosis. Gastroenterology. 2009;137:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Kojima H, Tsujimoto T, Uemura M, Takaya A, Okamoto S, Ueda S, Nishio K, Miyamoto S, Kubo A, Minamino N, Kangawa K, Matsuo H, Fukui H. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J Hepatol. 1998;28:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Solà E, Ginès P. Renal and circulatory dysfunction in cirrhosis: Current management and future perspectives. J Hepatol. 2010;53:1135-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Solà E, Kerbert AJ, Verspaget HW, Moreira R, Pose E, Ruiz P, Cela R, Morales-Ruiz M, López E, Graupera I, Solé C, Huelin P, Navarro AA, Ariza X, Jalan R, Fabrellas N, Benten D, de Prada G, Durand F, Jimenez W, van der Reijden JJ, Fernandez J, van Hoek B, Coenraad MJ, Ginès P. Plasma copeptin as biomarker of disease progression and prognosis in cirrhosis. J Hepatol. 2016;65:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Stadlbauer V, Wright GA, Banaji M, Mukhopadhya A, Mookerjee RP, Moore K, Jalan R. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Snowdon VK, Lachlan NJ, Hoy AM, Hadoke PW, Semple SI, Patel D, Mungall W, Kendall TJ, Thomson A, Lennen RJ, Jansen MA, Moran CM, Pellicoro A, Ramachandran P, Shaw I, Aucott RL, Severin T, Saini R, Pak J, Yates D, Dongre N, Duffield JS, Webb DJ, Iredale JP, Hayes PC, Fallowfield JA. Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Med. 2017;14:e1002248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: Relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 295] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Acevedo J, Fernández J, Prado V, Silva A, Castro M, Pavesi M, Roca D, Jimenez W, Ginès P, Arroyo V. Relative adrenal insufficiency in decompensated cirrhosis: Relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology. 2013;58:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Cholongitas E, Goulis I, Pagkalidou E, Haidich AB, Karagiannis AKA, Nakouti T, Pipili C, Oikonomou T, Gerou S, Akriviadis E. Relative Adrenal Insufficiency is Associated with the Clinical Outcome in Patients with Stable Decompensated Cirrhosis. Ann Hepatol. 2017;16:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Møller S, Lee SS. Cirrhotic cardiomyopathy. J Hepatol. 2018;69:958-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | Nazar A, Guevara M, Sitges M, Terra C, Solà E, Guigou C, Arroyo V, Ginès P. LEFT ventricular function assessed by echocardiography in cirrhosis: Relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013;58:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 38. | Kazory A, Ronco C. Hepatorenal Syndrome or Hepatocardiorenal Syndrome: Revisiting Basic Concepts in View of Emerging Data. Cardiorenal Med. 2019;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 39. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 41. | Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 825] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 42. | Woolbright BL, Jaeschke H. The impact of sterile inflammation in acute liver injury. J Clin Transl Res. 2017;3:170-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 851] [Article Influence: 77.4] [Reference Citation Analysis (1)] |
| 44. | Chousterman BG, Boissonnas A, Poupel L, Baudesson de Chanville C, Adam J, Tabibzadeh N, Licata F, Lukaszewicz AC, Lombès A, Deterre P, Payen D, Combadière C. Ly6Chigh Monocytes Protect against Kidney Damage during Sepsis via a CX3CR1-Dependent Adhesion Mechanism. J Am Soc Nephrol. 2016;27:792-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Antoniades CG, Khamri W, Abeles RD, Taams LS, Triantafyllou E, Possamai LA, Bernsmeier C, Mitry RR, O'Brien A, Gilroy D, Goldin R, Heneghan M, Heaton N, Jassem W, Bernal W, Vergani D, Ma Y, Quaglia A, Wendon J, Thursz M. Secretory leukocyte protease inhibitor: A pivotal mediator of anti-inflammatory responses in acetaminophen-induced acute liver failure. Hepatology. 2014;59:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1004] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 47. | Triantafyllou E, Woollard KJ, McPhail MJW, Antoniades CG, Possamai LA. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front Immunol. 2018;9:2948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 48. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 49. | Macdonald S, Andreola F, Bachtiger P, Amoros A, Pavesi M, Mookerjee R, Zheng YB, Gronbaek H, Gerbes AL, Sola E, Caraceni P, Moreau R, Gines P, Arroyo V, Jalan R. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology. 2018;67:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, Solá E, Baccaro ME, Terra C, Arroyo V, Ginès P. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 51. | Adebayo D, Morabito V, Davenport A, Jalan R. Renal dysfunction in cirrhosis is not just a vasomotor nephropathy. Kidney Int. 2015;87:509-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 53. | Bairaktari E, Liamis G, Tsolas O, Elisaf M. Partially reversible renal tubular damage in patients with obstructive jaundice. Hepatology. 2001;33:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 55. | McConnell M, Iwakiri Y. Biology of portal hypertension. Hepatol Int. 2018;12:11-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 56. | Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018;38:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 57. | Huggins JT, Doelken P, Walters C, Rockey DC. Point-of-Care Echocardiography Improves Assessment of Volume Status in Cirrhosis and Hepatorenal Syndrome. Am J Med Sci. 2016;351:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1885] [Cited by in RCA: 1551] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 59. | Valerio C, Theocharidou E, Davenport A, Agarwal B. Human albumin solution for patients with cirrhosis and acute on chronic liver failure: Beyond simple volume expansion. World J Hepatol. 2016;8:345-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | O'Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, Newson J, Karra E, Winstanley A, Alazawi W, Garcia-Martinez R, Cordoba J, Nicolaou A, Gilroy DW. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 61. | Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, Fergusson DA. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: A systematic review and meta-analysis. JAMA. 2013;309:678-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 413] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 62. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 63. | Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, Forné M, Miranda ML, Llach J, Salmerón JM. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 387] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 64. | Fernández J, Ruiz del Arbol L, Gómez C, Durandez R, Serradilla R, Guarner C, Planas R, Arroyo V, Navasa M. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-56; quiz 1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 65. | Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: Cholemic nephrosis revisited. J Nephrol. 2006;19:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Esposito P, Rampino T, Sileno G, Dal Canton A. Selective bilirubin removal: A treatment of jaundice-related kidney injury? Kidney Int. 2013;84:624-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 68. | Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: A meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med. 2015;41:1862-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 69. | Lu Y, Zhang H, Teng F, Xia WJ, Sun GX, Wen AQ. Early Goal-Directed Therapy in Severe Sepsis and Septic Shock: A Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. J Intensive Care Med. 2018;33:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, Reinhart K, Selvakumar N, Levy MM. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 71. | Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Hervé F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P; SEPSISPAM Investigators. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 783] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 72. | Moman RN, Ostby SA, Akhoundi A, Kashyap R, Kashani K. Impact of individualized target mean arterial pressure for septic shock resuscitation on the incidence of acute kidney injury: A retrospective cohort study. Ann Intensive Care. 2018;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, Ganger D, Jamil K, Pappas SC; REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology. 2016;150:1579-1589.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 74. | Sanyal AJ, Boyer TD, Frederick RT, Wong F, Rossaro L, Araya V, Vargas HE, Reddy KR, Pappas SC, Teuber P, Escalante S, Jamil K. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther. 2017;45:1390-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: A prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 76. | Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 77. | Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 78. | Facciorusso A, Chandar AK, Murad MH, Prokop LJ, Muscatiello N, Kamath PS, Singh S. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: A systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 79. | Arora V, Maiwall R, Rajan V, Jindal A, Muralikrishna Shasthry S, Kumar G, Jain P, Sarin SK. Terlipressin Is Superior to Noradrenaline in the Management of Acute Kidney Injury in Acute on Chronic Liver Failure. Hepatology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 80. | Salerno F, Navickis RJ, Wilkes MM. Albumin treatment regimen for type 1 hepatorenal syndrome: A dose-response meta-analysis. BMC Gastroenterol. 2015;15:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Korf H, du Plessis J, van Pelt J, De Groote S, Cassiman D, Verbeke L, Ghesquière B, Fendt SM, Bird MJ, Talebi A, Van Haele M, Feio-Azevedo R, Meelberghs L, Roskams T, Mookerjee RP, Mehta G, Jalan R, Gustot T, Laleman W, Nevens F, van der Merwe SW. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity. Gut. 2018;pii:gutjnl-2018-316888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 82. | Tripathi DM, Vilaseca M, Lafoz E, Garcia-Calderó H, Viegas Haute G, Fernández-Iglesias A, Rodrigues de Oliveira J, García-Pagán JC, Bosch J, Gracia-Sancho J. Simvastatin Prevents Progression of Acute on Chronic Liver Failure in Rats With Cirrhosis and Portal Hypertension. Gastroenterology. 2018;155:1564-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 83. | Gonwa TA, Wadei HM. The challenges of providing renal replacement therapy in decompensated liver cirrhosis. Blood Purif. 2012;33:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 84. | Karvellas CJ, Subramanian RM. Current Evidence for Extracorporeal Liver Support Systems in Acute Liver Failure and Acute-on-Chronic Liver Failure. Crit Care Clin. 2016;32:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Nadim MK, Kellum JA, Davenport A, Wong F, Davis C, Pannu N, Tolwani A, Bellomo R, Genyk YS; ADQI Workgroup. Hepatorenal syndrome: The 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16:R23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 86. | Kondrup J, Almdal T, Vilstrup H, Tygstrup N. High volume plasma exchange in fulminant hepatic failure. Int J Artif Organs. 1992;15:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 87. | Larsen FS, Ejlersen E, Hansen BA, Mogensen T, Tygstrup N, Secher NH. Systemic vascular resistance during high-volume plasmapheresis in patients with fulminant hepatic failure: Relationship with oxygen consumption. Eur J Gastroenterol Hepatol. 1995;7:887-892. [PubMed] |
| 88. | Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W, Auzinger G, Shawcross D, Eefsen M, Bjerring PN, Clemmesen JO, Hockerstedt K, Frederiksen HJ, Hansen BA, Antoniades CG, Wendon J. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016;64:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (4)] |
| 89. | Khamri W, Abeles RD, Hou TZ, Anderson AE, El-Masry A, Triantafyllou E, Bernsmeier C, Larsen FS, Singanayagam A, Kudo N, Possamai LA, Lebosse F, Auzinger G, Bernal W, Willars C, Weston CJ, Lombardi G, Wendon J, Thursz M, Antoniades CG. Increased Expression of Cytotoxic T-Lymphocyte-Associated Protein 4 by T Cells, Induced by B7 in Sera, Reduces Adaptive Immunity in Patients With Acute Liver Failure. Gastroenterology. 2017;153:263-276.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Morimoto T, Matsushima M, Sowa N, Ide K, Sawanishi K. Plasma adsorption using bilirubin-adsorbent materials as a treatment for patients with hepatic failure. Artif Organs. 1989;13:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Ott R, Rupprecht H, Born G, Müller V, Reck T, Hohenberger W, Köckerling F. Plasma separation and bilirubin adsorption after complicated liver transplantation: A therapeutic approach to excessive hyperbilirubinemia. Transplantation. 1998;65:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Wan YM, Li YH, Xu ZY, Yang J, Yang LH, Xu Y, Yang JH. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: A prospective study. J Clin Apher. 2017;32:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Fan Z, EnQiang C, Yao DL, LiBo Y, Hong L, Lang B, Ping F, Hong T. Neutrophil-lymphocyte ratio predicts short term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure treated with an artificial liver support system. PLoS One. 2017;12:e0175332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Yao J, Li S, Zhou L, Luo L, Yuan L, Duan Z, Xu J, Chen Y. Therapeutic effect of double plasma molecular adsorption system and sequential half-dose plasma exchange in patients with HBV-related acute-on-chronic liver failure. J Clin Apher. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 95. | Rifai K, Ernst T, Kretschmer U, Bahr MJ, Schneider A, Hafer C, Haller H, Manns MP, Fliser D. Prometheus--a new extracorporeal system for the treatment of liver failure. J Hepatol. 2003;39:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 96. | Krisper P, Haditsch B, Stauber R, Jung A, Stadlbauer V, Trauner M, Holzer H, Schneditz D. In vivo quantification of liver dialysis: Comparison of albumin dialysis and fractionated plasma separation. J Hepatol. 2005;43:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 97. | Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 98. | Dethloff T, Tofteng F, Frederiksen HJ, Hojskov M, Hansen BA, Larsen FS. Effect of Prometheus liver assist system on systemic hemodynamics in patients with cirrhosis: A randomized controlled study. World J Gastroenterol. 2008;14:2065-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Oppert M, Rademacher S, Petrasch K, Jörres A. Extracorporeal liver support therapy with Prometheus in patients with liver failure in the intensive care unit. Ther Apher Dial. 2009;13:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van Vlierberghe H, Escorsell A, Hafer C, Schreiner O, Galle PR, Mancini E, Caraceni P, Karvellas CJ, Salmhofer H, Knotek M, Ginès P, Kozik-Jaromin J, Rifai K; HELIOS Study Group. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782-789.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 101. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V; RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 102. | Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, Barange K, Perrigault PF, Belnard M, Ichaï P, Samuel D. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: A randomized, controlled trial. Ann Intern Med. 2013;159:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 103. | Stadlbauer V, Krisper P, Aigner R, Haditsch B, Jung A, Lackner C, Stauber RE. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit Care. 2006;10:R169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Schmuck RB, Nawrot GH, Fikatas P, Reutzel-Selke A, Pratschke J, Sauer IM. Single Pass Albumin Dialysis-A Dose-Finding Study to Define Optimal Albumin Concentration and Dialysate Flow. Artif Organs. 2017;41:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Karvellas CJ, Bagshaw SM, McDermid RC, Stollery DE, Bain VG, Gibney RT. A case-control study of single-pass albumin dialysis for acetaminophen-induced acute liver failure. Blood Purif. 2009;28:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Chan WK, Hui WF. Sequential use of hemoperfusion and single-pass albumin dialysis can safely reverse methotrexate nephrotoxicity. Pediatr Nephrol. 2016;31:1699-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Churchwell MD, Pasko DA, Smoyer WE, Mueller BA. Enhanced clearance of highly protein-bound drugs by albumin-supplemented dialysate during modeled continuous hemodialysis. Nephrol Dial Transplant. 2009;24:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Cannon RM, Jones CM, Davis EG, Eckhoff DE. Effect of Renal Diagnosis on Survival in Simultaneous Liver-Kidney Transplantation. J Am Coll Surg. 2019;228:536-544.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Li X, Wang LK, Wang LW, Han XQ, Yang F, Gong ZJ. Blockade of high-mobility group box-1 ameliorates acute on chronic liver failure in rats. Inflamm Res. 2013;62:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Li X, Gou C, Yao L, Lei Z, Gu T, Ren F, Wen T. Patients with HBV-related acute-on-chronic liver failure have increased concentrations of extracellular histones aggravating cellular damage and systemic inflammation. J Viral Hepat. 2017;24:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Possamai LA, Thursz MR, Wendon JA, Antoniades CG. Modulation of monocyte/macrophage function: A therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 112. | Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, Heymann F, Kalthoff S, Lefebvre E, Eulberg D, Luedde T, Marx G, Strassburg CP, Roskams T, Trautwein C, Tacke F. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology. 2016;64:1667-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 113. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 534] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 114. | Bernsmeier C, Singanayagam A, Patel VC, Wendon J, Antoniades CG. Immunotherapy in the treatment and prevention of infection in acute-on-chronic liver failure. Immunotherapy. 2015;7:641-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Rolando N, Clapperton M, Wade J, Panetsos G, Mufti G, Williams R. Granulocyte colony-stimulating factor improves function of neutrophils from patients with acute liver failure. Eur J Gastroenterol Hepatol. 2000;12:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 116. | Rolando N, Clapperton M, Wade J, Wendon J. Administering granulocyte colony-stimulating factor to acute liver failure patients corrects neutrophil defects. Eur J Gastroenterol Hepatol. 2000;12:1323-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 117. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 118. | Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: A randomized pilot study. Am J Gastroenterol. 2014;109:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 119. | Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, Mao YL, Xin SJ, Hu JH. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (3)] |
| 120. | Saha BK, Mahtab MA, Akbar SMF, Noor-E-Alam SM, Mamun AA, Hossain SMS, Alam MA, Moben AL, Khondaker FA, Chowdhury FI, Raihan R, Rahman S, Choudhury AK; APASL ACLF working party. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: Increased survival and containment of liver damage. Hepatol Int. 2017;11:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |