Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3334
Peer-review started: March 21, 2019
First decision: April 4, 2019
Revised: May 5, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: July 14, 2019
Processing time: 120 Days and 18.2 Hours
Choledochal cysts (CCs) are rare bile duct dilatations, intra-and/or extrahepatic, and have higher prevalence in the Asian population compared to Western populations. Most of the current literature on CC disease originates from Asia where these entities are most prevalent. They are thought to arise from an anomalous pancreaticobiliary junction, which are congenital anomalies between pancreatic and bile ducts. Some similarities in presentation between Eastern and Western patients exist such as female predominance, however, contemporary studies suggest that Asian patients may be more symptomatic on presentation. Even though CC disease presents with an increased malignant risk reported to be more than 10% after the second decade of life in Asian patients, this risk may be overstated in Western populations. Despite this difference in cancer risk, management guidelines for all patients with CC are based predominantly on observations reported from Asia where it is recommended that all CCs should be excised out of concern for the presence or development of biliary tract cancer.
Core tip: Choledochal cysts (CCs) are rare, predominantly occurring in Asian populations. While current global guidelines for their management are based on studies on Asian populations, there may be relevant differences between Western and Asian populations. For example, the risk of malignancy in Western populations may be lower than that reported in Asian populations. Although studies on CC disease in Western populations have been conducted, further evidence is still needed to properly elucidate this disease in the West and design appropriate clinical guidelines. Continued efforts in this regard are paramount, especially with the evolving field of hepatobiliary surgery that has recently seen the adoption of minimally invasive techniques.
- Citation: Baison GN, Bonds MM, Helton WS, Kozarek RA. Choledochal cysts: Similarities and differences between Asian and Western countries. World J Gastroenterol 2019; 25(26): 3334-3343
- URL: https://www.wjgnet.com/1007-9327/full/v25/i26/3334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i26.3334
Choledochal cysts (CCs), congenital dilatations of the biliary tree that may be either extrahepatic and/or intrahepatic, are uncommon with an incidence that ranges from 1 in 100000-150000 live births in Western populations, to 1 in 1000 in some Asian populations[1-7]. Most of what is known about CCs comes from Asia[2,6,8-10], although there have been a few large Western cohorts reported[2,6,11-13]. Some clinicians postulate that CC disease in the West is different from CC disease in the East and different management strategies should be employed. Therefore, we present an overview of CC disease in the West and East noting the similarities and differences in presentation, diagnosis, and management.
CCs are typically classified according to the Todani classification, modified from the Alonso-Lej classification[14-16]. This classification outlines five types of CCs: Type I, the most common, described as a solitary extrahepatic cyst; type II is an extrahepatic supraduodenal diverticulum; type III CC is an intraduodenal cyst, which is sometimes referred to as a choledochocele; type IV is comprised of both extrahepatic and intrahepatic cysts; and type V, comprised of multiple intrahepatic cysts, and often referred to as Caroli’s disease[5,16] (Figure 1A-E and Table 1). In both Eastern and Western populations, type I CCs have been noted to be most common, ranging from 65%-84% in Eastern cohorts and 67%-73% in Western cohorts. Type IV CCs are the next most common with 6%-30% in the East compared to 18%-19% in Western cohorts (Table 2).
| Todani classification | |
| Type I | Solitary extrahepatic cyst |
| Type II | Extrahepatic supraduodenal diverticulum |
| Type III | Intraduodenal cyst |
| Type IV | Both extrahepatic and intrahepatic cysts |
| Type V | Multiple intrahepatic cysts |
| Western | Eastern | Aggregate | |||||||||||
| Moslim et al[6] | Edil et al[2] | Soares et al[11] | Singham et al[12] | Baison et al (unpublished data) | Shi et al[8] | She et al[9] | Woon et al[10] | He et al[25] | Lee et al[24] | Aggrega-te East | Aggrega-te West | ||
| Single-center or multi-center | Single | Single | Multiple | Single | Single | Multiple | Single | Single | Single | Multiple | 3-Single2-Multi | 4-Single1-Multi | |
| Total number | 67 | 92 | 394 | 70 | 103 | 108 | 83 | 32 | 214 | 808 | 32-808 | 67-394 | |
| Adults | 78% | 79% | 66% | 73% | 100% | 84% | 0% | 100% | - | 100% | 0-100% | 66%-100% | |
| Study period | 1984 - 2014 | 1976-2006 | 1972-2014 | 1985-2002 | 1998-2018 | 1980-2000 | 1978-2008 | 1991-2005 | 1968-2013 | 1990-2007 | 1968-2013 | 1972-2018 | |
| APBJ | - | - | 8% (cancer patients only) | - | 57.3% | 93.4% | - | - | 81.2% | 71.4% | 71.4%-93.4% | 8%-57.3% | |
| Female:Male ratio | 4:01 | 9:01 | 3.2:1 | 4.2:1 | 4.7:1 | 3.7:1 | 2.6:1 | 3.5:1 | 3.7:1 | 3.8:1 | 2.6:1-3.8:1 | 3.2:1-9:1 | |
| Age (yr) | 46 (55.6-34.3) | - | 45 (SD 15.2) | - | 44.53 (18-74) | 27.8 (3-68) | 4 (0-16) | 41 (18-74) | 36.2 (1-78) | 42 (18-82) | 4-42 | 44-46 | |
| Symp-tomatic | 72% | - | 84.5% | - | 83% | - | - | 91% | 98% | 88% | 88%-91% | 72%-85% | |
| Presen-tation | Abdom-inal pain only | 51% | 91% | 61% | 88% | 44% | 57% | 47% | 91% | 78% | 75% | 47%-91% | 44%-91% |
| Pancrea-titis (recurr-ent) | - | 31% | 19% | - | 9% | - | 22% | - | 13% | - | 13%-22% | 9%-31% | |
| Choleli-thiasis | - | 58% | - | - | 8% | - | - | - | 22% | - | 22 -- | 8%-58% | |
| Jaundice | 15% | 34% | 19% | 39% | 6% | 71% | 42% | 41% | 25% | 21% | 21%-71% | 6%-39% | |
| Cholecy-stitis | - | - | - | - | 6% | - | - | - | 28% | - | 28% -- | 6 -- | |
| Cholan-gitis (recurr-ent) | - | - | - | - | 3% | 56% | - | - | - | - | 56% -- | 3 -- | |
| Choled-ocholith-iasis | - | - | - | - | 3% | - | - | - | 29% | 28% | 28%-29% | 3 -- | |
| Weight loss, Bloating | - | 8% | - | - | 2% | - | - | - | - | - | - - - | 2%-8% | |
| Other | - | 2% | - | - | 2% | - | - | 9% | - | - | 9% -- | 2%-2% | |
| Acute pancrea-titis | 13% | - | - | - | 1% | - | - | - | - | - | - - - | 1%-13% | |
| Fever/ chills | - | 26% | - | 39% | - | 57% | - | 34% | 19% | - | 19%-57% | 26%-39% | |
| Nausea/vomiting | - | 47% | - | 63% | - | - | 31% | 38% | - | - | 31%-38% | 47%-63% | |
| Abdom-inal mass | - | - | - | - | - | - | 22% | 25% | 4% | - | 4%-25% | - - - | |
| Classic triad | - | - | - | - | - | - | 2% | 13% | - | - | 2%-13% | - - - | |
| CC type | I | 73% | 67% | 70% | 33% | 71% | 69% | 68% | 84% | 65% | 68% | 65%-84% | 67%-73% |
| II | 1.5% | 7% | 5% | 6% | 3% | - | 5% | 6% | 1.4% | 0.9% | 0.9%-6% | 3%-7% | |
| III | - | 4% | 2% | 2% | 5% | 0.9% | 3% | - | 0.5% | 0.5% | 0.5%-3% | 2%-5% | |
| IV | 13.4% | 19% | 18% | 55% | 18% | 22% | 19% | 6% | 25% | 30% | 6%-30% | 18%-19% | |
| V | 12% | 2% | 4.9% | 4% | 4% | 5.60% | 5% | 3% | 8% | 0.7% | 0.7%-8% | 2%-12% | |
| Prior cholec-ystec-tomy | - | 38% | 32% | - | 28% | - | - | 3% | 31% | 7% | 3%-31% | 28%-38% | |
| Surgery | 100% | 100% | 98.9% | 100% | 70% | 100% | 90% | 84% | 92% | 100% | 84%-100% | 70%-100% | |
| Biliary Malig-nancy | On presen-tation | 7.5% | 5.4% | 3% | 7.8% | 7% | 17% | 0% | 3% | 5% | 10% | 0-17% | 3-8% |
| On follow-up/re-currence | 1.5% | 3.2% | 3.30% | - | 0% | 10% | 0% | 0% | 2.3% | 3% | 0-10% | 0-3% | |
| Overall mortal-ity | 7.5% | 7.6% | 4.50% | 0% | 2% | 9% | 0% | 0% | 6% | 4% | 0-9% | 0-8% | |
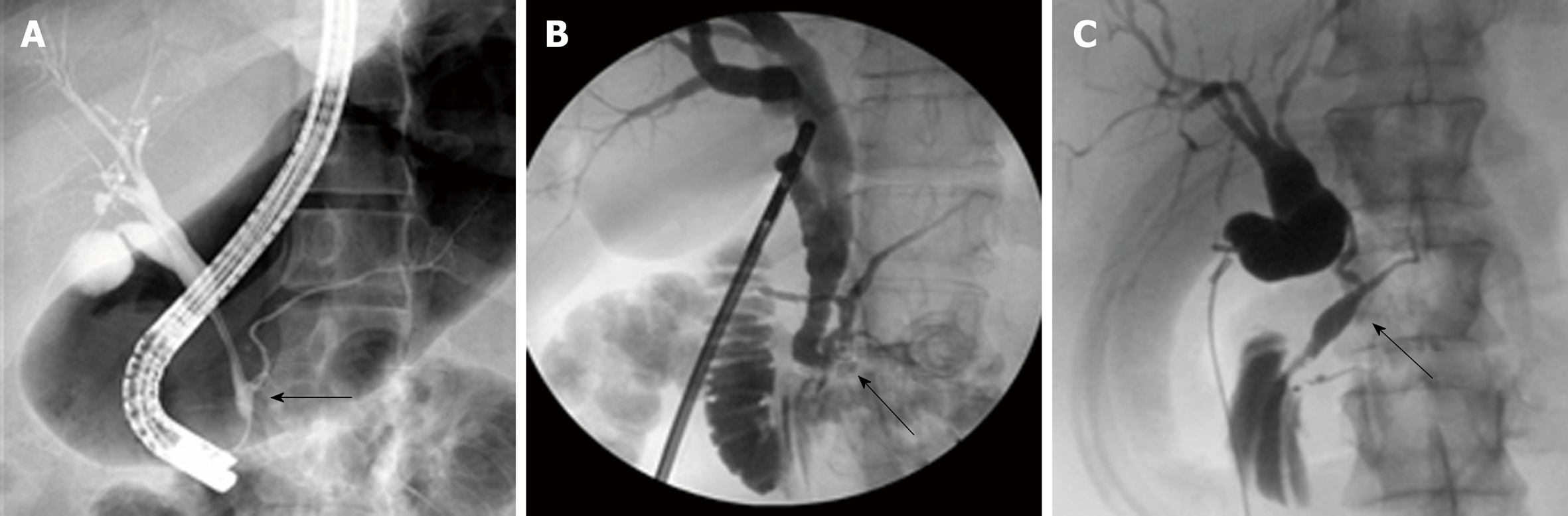
The similar distribution in CC types between Eastern and Western cohorts is likely related to the similar etiology of CCs. Babbitt’s theory is the most commonly proposed theory and states that CCs result from an anomalous pancreaticobiliary junction (APBJ) where the pancreatic duct and the bile duct connect 1-2 cm proximal to the sphincter of Oddi[3,4,17-22]. The clinical entity of APBJ has become widely accepted to be etiologic in the pathogenesis of biliary carcinogenesis in patients with CC[8,11,23-25]. There are several proposed classifications for APBJ, but one common classification is the simplified Komi’s classification, so-called Association Française de Chirurgie classification that includes three subtypes: Type I (C–P type), type II (P–C type), and type III (complex type) with ‘‘anse-de-seau’’ (Figure 2A-C)[22].
The long common channel associated with APBJ facilitates reflux of pancreatic juice into the biliary tree, causing increased pressure and possible proteolysis within the CBD culminating in ductal dilation[4,26]. High amylase levels have been noted in CC bile, adding evidence to this theory[27]. This reflux of pancreatic juices also leads to biliary tree inflammation, epithelial breakdown, mucosal dysplasia, and, potentially, malignancy[21,27]. Furthermore, it has been suggested that the inflammation and bile duct breakdown are exacerbated by high trypsinogen and phospholipase A2 levels in bile of patients with CCs[28]. However, some authors have questioned this theory because an APBJ is found in only 50%-80% cases of CCs, there is no pancreatic juice reflux in antenatally detected CCs, and neonatal acini do not secrete sufficient pancreatic enzymes[29]. Animal studies suggest that obstruction of the distal CBD may contribute to the development of CCs, particularly sphincter of Oddi dysfunction, which also causes pancreatic juice reflux into the biliary system[30]. Kusunoki et al[31] proposed a pure congenital theory in which fewer ganglion cells are seen in distal CBD in patients with CCs resulting in proximal dilation in the same manner as achalasia of the esophagus or Hirschsprung’s disease[1,31].
The demographics and clinical presentation of CC are similar between Eastern and Western populations, especially the female to male preponderance, ranging from 4:1 to 3:1[1,2,6]. Most CCs are diagnosed in children, although about 25% are discovered in adults[1]. In adults, the presentation is usually nonspecific and vague, with non-specific abdominal pain being the most common symptom[1], but when the symptoms are more specific, they are typically acute biliary tract and/or pancreatic in nature[2]. While some patients present with the classic triad of symptoms, abdominal pain, palpable abdominal mass, and jaundice, it is observed in only 25% of adults, although 85% of children have at least two features of this classic triad[4,6,32]. Furthermore, recent literature suggests increased rates of CC disease in adults[2,7]. While the trend in common presentation symptoms may be similar between the East and West, the literature suggests that associated biliary conditions such as cholecystitis, cholangitis, and choledocholithiasis may be more prevalent in Eastern patients on presentation[24,25] (Table 2). Indeed, further investigation is necessary to delineate if this is a true difference between the two populations.
CC disease can be diagnosed using imaging modalities such as ultrasound, computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), and endoscopic ultrasonography, with MRCP and ERCP considered the diagnostic methods of choice[6]. MRCP has been reported to have a 90%-100% sensitivity for detecting CCs[33]. However, MRCP has a much lower sensitivity for delineating the pancreatic duct and common pancreaticobiliary channel of only 46%, which is lower when compared with CT cholangiopancreatography which has a reported sensitivity of 64%[33]. With recent advances in cross-sectional imaging, more asymptomatic cases are being discovered incidentally[2,6]. Though current practice on imaging and diagnosis is similar across continents, Asian patients are marginally more symptomatic on presentation; 88%-91% of Asian patients present with symptoms compared to 72%-85% of Western patients. Various reasons may account for this including potential difference in the use of imaging modalities in clinical practice between the East and West, such as difference in CC disease presentation or selection bias in various studies.
CC disease is associated with various complications including obstructive jaundice, symptomatic choledocholithiasis, pancreatitis, cholangitis, spontaneous cyst rupture, secondary biliary cirrhosis, and cholangiocarcinoma[1]. Of particular concern is the increased risk of malignancy[6,18,34,35], which varies from 2.5% to 17.5%, with some reports as high as 21%[13,36-39]. Malignant risk in Western cohorts is likely overstated; the risk of malignancy may be higher in Eastern cohorts compared to Western cohorts, particularly in adults (Table 2). This high malignant risk in Asian cohorts has been the main driver for the current approach to CC disease management.
The risk of malignancy increases with age, reported to be lowest in the first decade of life at 0.7% and exceeding 10% after the second decade of life, suggesting that early diagnosis and treatment lead to a more favorable outcome[1,36,40]. Some studies have noted malignancy in up to 50% of patients with CC over the age of 60 years[24,25]. Not only is malignancy associated with age, but CC type. Todani et al[16] observed that of the patients who develop biliary malignancy, 68% occurred in type I, 5% in type II, 1.6% in type III, 21% in type IV, and 6% in type V CCs[16]. Additionally, the malignant risk due to the presence of an APBJ has been well established in both Western and Eastern patients. At our institution, we have observed a 4-fold increase in malignancy in patients with CC and an APBJ when compared to those with CC without an APBJ[41]. Imazu et al[29] showed that regurgitation and stasis of pancreatic fluid into the biliary system not only cause inflammation, but lead to carcinogenesis. Histologic findings by Katabi et al[42] further corroborate Imazu et al’s[29] findings that biliary cancer due to CC disease is a progression from inflammation, to dysplasia, metaplasia, and finally malignancy.
A small subset of patients, from 0.7%-3%, develop cholangiocarcinoma after surgical resection, notably in patients with type I and IV cysts[11,15,24,41]. This indicates that the risk of malignancy does not return to baseline after CC resection in this group of patients[11,42]. The etiology behind these delayed malignancies remains unclear. Incomplete resection of the cyst or a field defect within the biliary epithelium leading to an increased inherent susceptibility have been suggested as possible etiologies for this persistent cancer risk[36,40]. Liu et al[40] observed a 33.3% risk of malignancy in patients with incomplete cyst resection compared with 6% after complete cyst resection. Many studies, both Western and Eastern, do not have follow-up intervals long enough to detect this persistent risk since it can take more than 3 decades to manifest. Some have therefore suggested careful postoperative lifelong follow-up, especially in cases where there is a high suspicion for incomplete resection[24,43,44]. The utility of life-long follow-up in Western patients where recurrence rates are reportedly as low as 0-3% has yet to be determined.
Malignancy in CC disease takes the form of both gallbladder and bile duct cancers. Lee et al[24] reported biliary tract malignancies in 9.9% of CCs, of which 50% were cholangiocarcinoma and 44% gallbladder carcinoma. A large-scale survey of 2561 patients with CC in Japan showed that in those patients who had cancer, 62.3% had gallbladder cancer, 32.1% had bile duct cancer, and 4.7% had both, which is similar to other reports[1,34]. Of note, gallbladder carcinoma is found in 5% of CC disease patients with APBJ[45], usually in patients in their sixth or seventh decade of life, when adult cohorts are investigated[4]. Thus, it is perceived that the likelihood of the development of both bile duct cancer and gallbladder cancer increases in patients with CC and APBJ, whereas there is a significant predilection for gallbladder cancer in APBJ patients without CC[18,20,46-48].
The demographics of the population being treated must be taken into consideration when managing CC disease. While Western populations present a mix of racial diversity, Eastern cohorts are almost exclusive of Asian descent. The effect of race on the natural history of CC has not been fully explored. Our institution’s experience with CC disease suggests that presentation and malignant risk may be different between Asian and Caucasian patients, with malignant risk being higher in Asian patients[41]. Therefore, it is essential to further understand the risks posed by CC disease in Western patients in order to design appropriate management guidelines as it may represent a separate entity in the West compared to Eastern patients.
Currently, practice guidelines are based almost exclusively on data derived from the Asian literature. At present, there is no conclusive evidence to suggest that there is over-treatment of Western patients by using Asian guidelines, but the lower malignant risk in these patients necessitates further investigation into Western treatment outcomes. Current evidence suggests similar efficacy in treatment options between the East and West, however, this remains an area of limited data. This continues to evolve, especially as minimally invasive techniques are now being adopted for CC disease management[49-52]. More multi-institutional studies such as the one by Soares et al[11] and ideally international registries are needed to further understand CC disease in the West and devise management protocols.
The current standard of care for most CCs is complete excision of the cyst, specifically, resection from the bifurcation of lobar[4,6] hepatic ducts into the parenchyma of the pancreas near the junction of the pancreatic duct[1,3,20], coupled with cholecystectomy. Biliary tract continuity can be restored either by means of Roux-en-Y hepaticojejunostomy (HJ), hepaticoduodenostomy (HD), or jejunal interposition HD[6]. However, this approach does not take into consideration various types of CC. Management should be tailored to the type of CC and the presence or absence of APBJ. Furthermore, advocating for resection in all patients may potentially be overly aggressive therapy, especially in Western patients where the risk of malignancy appears to be potentially overstated.
Surgical resection has been espoused to be the ideal strategy for type I CCs. There may, however, be a subset of patients in Western cohorts who are at low risk for developing cancer, in whom long term surveillance as opposed to surgical resection be considered. This includes patients who are asymptomatic, have undergone previous cholecystectomy, are older with medical comorbidity, and have no APBJ since their risk of malignancy appears to be quite low. However, there are no reports of patients managed by long term observation. Hence, long term risk of cancer and other biliary complications in such patients is currently unknown. Until such data become available, excision of all type I CC should be recommended to all Western as well as Eastern patients who can tolerate a major operation and are willing to do so.
Type II CCs are very rare and can be managed by simple excision. Usually these cysts are ligated at the neck and excised without the need for bile duct reconstruction[17]. However, occasionally extrahepatic bile duct excision is necessary. This is sometimes encountered in patients where associated inflammation causes intimate adhesion of the diverticulum to the extra- or intrahepatic biliary tree, depending on disease location, leading to technical challenges in liberating the diverticulum from the bile duct[5].
Type III CCs, sometimes referred to as choledochoceles, are discrete from other CC diseases and their origin has been long debated[6,53]. One hypothesis is that these cysts may originate from a rudimentary inferior embryonic bud of the ampulla of Vater, or an acquired anomaly as a result of sphincter of Oddi dysfunction or obstruction[53]; thus, choledochoceles may not represent a true form of CC. Most patients with type III CCs present to interventional gastroenterologists rather than surgeons. Type III CCs were historically treated via transduodenal excision and sphincteroplasty. Currently endoscopic sphincterotomy with cyst de-roofing is regarded as the treatment of choice for type III cysts[54,55]. Regardless, these patients should undergo periodic endoscopic surveillance since malignancy has occasionally been reported in choledochoceles[17].
Management, especially the operative approach, in type IV CC remains con-troversial. Visser et al[13] suggested excising the extrahepatic component of the cyst alone with concomitant HJ. However, hepatic resection in patients with unilobar disease[1] and liver transplantation in patients with bilobar disease should be considered, especially in patients with extensive intrahepatic dilation associated with complications, such as stones, cholangitis, or biliary cirrhosis[13,56,57]. We have found that for most patients, extrahepatic resection has been sufficient, although we concur that hepatic resection and transplantation should remain as tools in the surgeon’s armamentarium when dealing with type IV CCs.
The management of type V CCs (Caroli’s disease) is complex and requires a multi-disciplinary approach that includes endoscopy, interventional radiology, and surgery. Surgical treatment options include segmental or lobar hepatic resection and liver transplantation depending on the extent of the disease and presence of impaired hepatic function[6]. When the intrahepatic CC disease is localized without congenital hepatic fibrosis, segmental hepatectomy should be considered[58]. Percutaneous or endoscopic drainage and stents are used for palliative therapy. For diffuse disease with life-threatening complications, liver transplantation is the most viable option[1].
CC disease has a distinct presentation in the Eastern population yet shares some commonality with Western patients. However, some reported differences in presentation, malignancy risk, and patient demographics between Western and Eastern populations should spur further investigation into CC disease in Western patients to understand this disease and tailor management guidelines to Western populations. For now, the management of CC disease continues to be driven by the Asian literature. Large multi-institutional studies in the West with decades of follow-up are necessary to better understand the natural history of CC disease , and in particular the risk of biliary tract cancer, in Western patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujino Y, Hu B S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Bhavsar MS, Vora HB, Giriyappa VH. Choledochal cysts: A review of literature. Saudi J Gastroenterol. 2012;18:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Edil BH, Cameron JL, Reddy S, Lum Y, Lipsett PA, Nathan H, Pawlik TM, Choti MA, Wolfgang CL, Schulick RD. Choledochal cyst disease in children and adults: A 30-year single-institution experience. J Am Coll Surg. 2008;206:1000-5; discussion 1005-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;394:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Ishibashi H, Shimada M, Kamisawa T, Fujii H, Hamada Y, Kubota M, Urushihara N, Endo I, Nio M, Taguchi T, Ando H; Japanese Study Group on Congenital Biliary Dilatation (JSCBD). Japanese clinical practice guidelines for congenital biliary dilatation. J Hepatobiliary Pancreat Sci. 2017;24:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Ouaïssi M, Kianmanesh R, Belghiti J, Ragot E, Mentha G, Adham M, Troisi RI, Pruvot FR, Dugué L, Paye F, Ayav A, Nuzzo G, Falconi M, Demartines N, Mabrut JY, Gigot JF; Working Group of the French Surgical Association. Todani Type II Congenital Bile Duct Cyst: European Multicenter Study of the French Surgical Association and Literature Review. Ann Surg. 2015;262:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Moslim MA, Takahashi H, Seifarth FG, Walsh RM, Morris-Stiff G. Choledochal Cyst Disease in a Western Center: A 30-Year Experience. J Gastrointest Surg. 2016;20:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Nicholl M, Pitt HA, Wolf P, Cooney J, Kalayoglu M, Shilyansky J, Rikkers LF. Choledochal cysts in western adults: Complexities compared to children. J Gastrointest Surg. 2004;8:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Shi LB, Peng SY, Meng XK, Peng CH, Liu YB, Chen XP, Ji ZL, Yang DT, Chen HR. Diagnosis and treatment of congenital choledochal cyst: 20 years' experience in China. World J Gastroenterol. 2001;7:732-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | She WH, Chung HY, Lan LC, Wong KK, Saing H, Tam PK. Management of choledochal cyst: 30 years of experience and results in a single center. J Pediatr Surg. 2009;44:2307-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Woon CY, Tan YM, Oei CL, Chung AY, Chow PK, Ooi LL. Adult choledochal cysts: An audit of surgical management. ANZ J Surg. 2006;76:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Soares KC, Kim Y, Spolverato G, Maithel S, Bauer TW, Marques H, Sobral M, Knoblich M, Tran T, Aldrighetti L, Jabbour N, Poultsides GA, Gamblin TC, Pawlik TM. Presentation and Clinical Outcomes of Choledochal Cysts in Children and Adults: A Multi-institutional Analysis. JAMA Surg. 2015;150:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Singham J, Schaeffer D, Yoshida E, Scudamore C. Choledochal cysts: Analysis of disease pattern and optimal treatment in adult and paediatric patients. HPB (Oxford). 2007;9:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 13. | Visser BC, Suh I, Way LW, Kang SM. Congenital choledochal cysts in adults. Arch Surg. 2004;139:855-60; discussion 860-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | ALONSO-LEJ F, REVER WB, PESSAGNO DJ. Congenital choledochal cyst, with a report of 2, and an analysis of 94, cases. Int Abstr Surg. 1959;108:1-30. [PubMed] |
| 15. | Watanabe Y, Toki A, Todani T. Bile duct cancer developed after cyst excision for choledochal cyst. J Hepatobiliary Pancreat Surg. 1999;6:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Todani T, Watanabe Y, Fujii M, Toki A, Uemara S, Koike Y, Inoue T. Carcinoma arising from the bile duct in choledochal cyst and anomalous arrangement of the pancreatobiliary ductal union (in Japanese). Tan to Sui (Biliary Tract Pancreas). 1985;6:525-535. |
| 17. | Babbitt DP. [Congenital choledochal cysts: New etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb]. Ann Radiol (Paris). 1969;12:231-240. [PubMed] [DOI] [Full Text] |
| 18. | García Cano J, Godoy MA, Morillas Ariño J, Pérez García JI. [Pancreatobiliary maljunction. Not only an Eastern disease]. An Med Interna. 2007;24:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Kamisawa T, Honda G, Kurata M, Tokura M, Tsuruta K. Pancreatobiliary disorders associated with pancreaticobiliary maljunction. Dig Surg. 2010;27:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kim Y, Hyun JJ, Lee JM, Lee HS, Kim CD. Anomalous union of the pancreaticobiliary duct without choledochal cyst: Is cholecystectomy alone sufficient? Langenbecks Arch Surg. 2014;399:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Matsumoto Y, Fujii H, Itakura J, Matsuda M, Nobukawa B, Suda K. Recent advances in pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 2002;9:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ragot E, Mabrut JY, Ouaïssi M, Sauvanet A, Dokmak S, Nuzzo G, Halkic N, Dubois R, Létoublon C, Cherqui D, Azoulay D, Irtan S, Boudjema K, Pruvot FR, Gigot JF, Kianmanesh R; Working Group of the French Surgical Association. Pancreaticobiliary Maljunctions in European Patients with Bile Duct Cysts: Results of the Multicenter Study of the French Surgical Association (AFC). World J Surg. 2017;41:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Kinoshita H, Nagata E, Hirohashi K, Sakai K, Kobayashi Y. Carcinoma of the gallbladder with an anomalous connection between the choledochus and the pancreatic duct. Report of 10 cases and review of the literature in Japan. Cancer. 1984;54:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Lee SE, Jang JY, Lee YJ, Choi DW, Lee WJ, Cho BH, Kim SW; Korean Pancreas Surgery Club. Choledochal cyst and associated malignant tumors in adults: A multicenter survey in South Korea. Arch Surg. 2011;146:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | He XD, Wang L, Liu W, Liu Q, Qu Q, Li BL, Hong T. The risk of carcinogenesis in congenital choledochal cyst patients: An analysis of 214 cases. Ann Hepatol. 2014;13:819-826. [PubMed] |
| 26. | Iwai N, Yanagihara J, Tokiwa K, Shimotake T, Nakamura K. Congenital choledochal dilatation with emphasis on pathophysiology of the biliary tract. Ann Surg. 1992;215:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Sugiyama M, Haradome H, Takahara T, Izumisato Y, Abe N, Masaki T, Mori T, Hachiya J, Atomi Y. Biliopancreatic reflux via anomalous pancreaticobiliary junction. Surgery. 2004;135:457-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Okada A, Hasegawa T, Oguchi Y, Nakamura T. Recent advances in pathophysiology and surgical treatment of congenital dilatation of the bile duct. J Hepatobiliary Pancreat Surg. 2002;9:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Imazu M, Iwai N, Tokiwa K, Shimotake T, Kimura O, Ono S. Factors of biliary carcinogenesis in choledochal cysts. Eur J Pediatr Surg. 2001;11:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Ponce J, Garrigues V, Sala T, Pertejo V, Berenguer J. Endoscopic biliary manometry in patients with suspected sphincter of Oddi dysfunction and in patients with cystic dilatation of the bile ducts. Dig Dis Sci. 1989;34:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Levy AD, Rohrmann CA, Murakata LA, Lonergan GJ. Caroli's disease: Radiologic spectrum with pathologic correlation. AJR Am J Roentgenol. 2002;179:1053-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL. Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994;220:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 222] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Lee YS, Seo DW, Won HJ, Kim MY. Can MRCP replace the diagnostic role of ERCP for patients with choledochal cysts? Gastrointest Endosc. 2005;62:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Mori H, Iida H, Maehira H, Kitamura N, Shimizu T, Tani M. Synchronous primary gallbladder and pancreatic cancer associated with congenital biliary dilatation and pancreaticobiliary maljunction. Surg Case Rep. 2017;3:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Rossi RL, Silverman ML, Braasch JW, Munson JL, ReMine SG. Carcinomas arising in cystic conditions of the bile ducts. A clinical and pathologic study. Ann Surg. 1987;205:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Bismuth H, Krissat J. Choledochal cystic malignancies. Ann Oncol. 1999;10 Suppl 4:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Kumamoto T, Tanaka K, Takeda K, Nojiri K, Mori R, Taniguchi K, Matsuyama R, Ueda M, Sugita M, Ichikawa Y, Nagashima Y, Endo I. Intrahepatic cholangiocarcinoma arising 28 years after excision of a type IV-A congenital choledochal cyst: Report of a case. Surg Today. 2014;44:354-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Zheng X, Gu W, Xia H, Huang X, Liang B, Yang T, Yang S, Zeng J, Dong J. Surgical treatment of type IV-A choledochal cyst in a single institution: Children vs adults. J Pediatr Surg. 2013;48:2061-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Morine Y, Shimada M, Takamatsu H, Araida T, Endo I, Kubota M, Toki A, Noda T, Matsumura T, Miyakawa S, Ishibashi H, Kamisawa T, Shimada H. Clinical features of pancreaticobiliary maljunction: Update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci. 2013;20:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Liu YB, Wang JW, Devkota KR, Ji ZL, Li JT, Wang XA, Ma XM, Cai WL, Kong Y, Cao LP, Peng SY. Congenital choledochal cysts in adults: Twenty-five-year experience. Chin Med J (Engl). 2007;120:1404-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Ng WT. Bile duct carcinogenesis after excision of extrahepatic bile ducts in pancreaticobiliary maljunction. Surgery. 2000;128:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Ohashi T, Wakai T, Kubota M, Matsuda Y, Arai Y, Ohyama T, Nakaya K, Okuyama N, Sakata J, Shirai Y, Ajioka Y. Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cysts. J Gastroenterol Hepatol. 2013;28:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Takeshita N, Ota T, Yamamoto M. Forty-year experience with flow-diversion surgery for patients with congenital choledochal cysts with pancreaticobiliary maljunction at a single institution. Ann Surg. 2011;254:1050-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Kobayashi S, Asano T, Yamasaki M, Kenmochi T, Nakagohri T, Ochiai T. Risk of bile duct carcinogenesis after excision of extrahepatic bile ducts in pancreaticobiliary maljunction. Surgery. 1999;126:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Chijiiwa K, Kimura H, Tanaka M. Malignant potential of the gallbladder in patients with anomalous pancreaticobiliary ductal junction. The difference in risk between patients with and without choledochal cyst. Int Surg. 1995;80:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Kamisawa T, Ando H, Shimada M, Hamada Y, Itoi T, Takayashiki T, Miyazaki M. Recent advances and problems in the management of pancreaticobiliary maljunction: Feedback from the guidelines committee. J Hepatobiliary Pancreat Sci. 2014;21:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Kimura W. Congenital dilatation of the common bile duct and pancreaticobiliary maljunction: Clinical implications. Langenbecks Arch Surg. 2009;394:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, Shimada H, Takamatsu H, Miyake H, Todani T; Committee for Registration of the Japanese Study Group on Pancreaticobiliary Maljunction. Pancreaticobiliary maljunction: Retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Han JH, Lee JH, Hwang DW, Song KB, Shin SH, Kwon JW, Lee YJ, Kim SC, Park KM. Robot resection of a choledochal cyst with Roux-en-y hepaticojejunostomy in adults: Initial experiences with 22 cases and a comparison with laparoscopic approaches. Ann Hepatobiliary Pancreat Surg. 2018;22:359-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Lee H, Kwon W, Han Y, Kim JR, Kim SW, Jang JY. Comparison of surgical outcomes of intracorporeal hepaticojejunostomy in the excision of choledochal cysts using laparoscopic versus robot techniques. Ann Surg Treat Res. 2018;94:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Madadi-Sanjani O, Petersen C, Ure B. Minimally Invasive Hepatobiliary Surgery. Clin Perinatol. 2017;44:805-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Talini C, DE-Carvalho BCN, Antunes LA, Schulz C, Sabbaga CC, Avilla SGA, Garbers JC, DE-Aguiar LRF, Telles LG, DE-Almeida GC, Amado FAB, E-Silva EM. Choledochal cyst in the pediatric population: Experience of 13 laparoscopic procedures in two years at a single institution. Rev Col Bras Cir. 2018;45:e1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Law R, Topazian M. Diagnosis and treatment of choledochoceles. Clin Gastroenterol Hepatol. 2014;12:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Elton E, Hanson BL, Biber BP, Howell DA. Dilated common channel syndrome: Endoscopic diagnosis, treatment, and relationship to choledochocele formation. Gastrointest Endosc. 1998;47:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Samavedy R, Sherman S, Lehman GA. Endoscopic therapy in anomalous pancreatobiliary duct junction. Gastrointest Endosc. 1999;50:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Xia HT, Dong JH, Yang T, Zeng JP, Liang B. Extrahepatic cyst excision and partial hepatectomy for Todani type IV-A cysts. Dig Liver Dis. 2014;46:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Cerwenka H. Bile duct cyst in adults: Interventional treatment, resection, or transplantation? World J Gastroenterol. 2013;19:5207-5211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Hussain SZ, Bloom DA, Tolia V. Caroli's disease diagnosed in a child by MRCP. Clin Imaging. 2000;24:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |