Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3256
Peer-review started: April 19, 2019
First decision: May 9, 2019
Revised: May 20, 2019
Accepted: June 8, 2019
Article in press: June 8, 2019
Published online: July 7, 2019
Processing time: 78 Days and 5.8 Hours
Accurate detection of significant fibrosis (fibrosis stage 2 or higher on the METAVIR scale) is important especially for chronic hepatitis B (CHB) patients with high viral loads but with normal or mildly elevated alanine aminotransferase (ALT) levels because the presence of significant fibrosis is accepted as the indication for antiviral treatment. Liver biopsy is the reference standard for diagnosing significant fibrosis, but it is an invasive procedure. Consequently, noninvasive imaging-based measurements, such as magnetic resonance elastography (MRE) or two-dimensional shear-wave elastography (2D-SWE), have been proposed for the quantitative assessment of liver fibrosis.
To explore MRE and 2D-SWE to identify fibrosis stage, and to compare their performance with that of serum-based indices.
The study enrolled 63 treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels who underwent liver biopsy before a decision was made to initiate antiviral therapy. MRE and 2D-SWE were performed, and serum-based indices, such as FIB-4 and aspartate transaminase to platelet ratio index (APRI), were calculated. The diagnostic performances of MRE, 2D-SWE, FIB-4, and APRI for assessing significant fibrosis (≥ F2) and cirrhosis (F4) were evaluated with liver histology as the reference standard, using receiver operating characteristic analyses.
The liver fibrosis stage was F0/F1 in 19, F2 in 14, F3 in 14, and F4 in 16 patients, respectively. MRE significantly discriminated F2 from F0/1 (P = 0.022), whereas 2D-SWE showed a broad overlap in distinguishing those stages. MRE showed a higher correlation coefficient value with fibrosis stage than 2D-SWE with fibrosis stage (0.869 vs 0.649, Spearman test; P < 0.001). Multivariate linear regression analyses showed that fibrosis stage was the only factor affecting the values of MRE (P < 0.001), whereas body mass index (P = 0.042) and fibrosis stage (P < 0.001) were independent factors affecting 2D-SWE values. MRE performance for diagnosing significant fibrosis was better [area under the curve (AUC) = 0.906, positive predictive value (PPV) 97.3%, negative predictive value (NPV) 69.2%] than that of FIB-4 (AUC = 0.697, P = 0.002) and APRI (AUC = 0.717, P = 0.010), whereas the performance of 2D-SWE (AUC = 0.843, PPV 86%, NPV 65%) was not significantly different from that of FIB-4 or APRI.
Compared to SWE, MRE might be more precise non-invasive assessment for depicting significant fibrosis and for making-decision to initiate antiviral-therapy in treatment-naïve CHB patients with normal or mildly-elevated ALT levels.
Core tip: The present study investigated magnetic resonance elastography (MRE) and two-dimensional shear-wave elastography (2D-SWE) to identify significant fibrosis, and to compare their performance with that of serum-based indices in treatment-naïve chronic hepatitis B (CHB) patients with borderline-normal alanine aminotransferase levels, who should be considered for initiation of antiviral therapy depending on the presence of significant fibrosis. Our data demonstrated that MRE was a more accurate and noninvasive measurement for detecting significant fibrosis, compared to 2D-SWE as well as serum-based indices, and our results suggested that MRE could be used as a basis for anti-HBV treatment-decisions in treatment-naïve CHB patients.
- Citation: Park HS, Choe WH, Han HS, Yu MH, Kim YJ, Jung SI, Kim JH, Kwon SY. Assessing significant fibrosis using imaging-based elastography in chronic hepatitis B patients: Pilot study. World J Gastroenterol 2019; 25(25): 3256-3267
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3256.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3256
Hepatitis B virus (HBV) infection remains a major health problem, causing chronic liver disease. If left untreated, chronic HBV infection may potentially lead to complications such as cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC)[1]. Therefore, effective antiviral treatment in chronic hepatitis B (CHB) patients can reduce the disease progression towards HBV-related cirrhosis and the risk of HCC development[1,2].
Accurate staging of liver fibrosis in CHB patients is necessary not only for predicting the long-term clinical course but also for determining whether and when to begin antiviral therapy. Recent clinical guidelines have recommended that CHB patients with high serum HBV-DNA levels [hepatitis B e-antigen (HBeAg) positive patients with serum HBV-DNA levels > 20000 IU/mL or HBeAg-negative patients with serum HBV-DNA levels > 2000 IU/mL] and elevated alanine aminotransferase (ALT) levels of twice the upper limit of normal (ULN) or greater should be considered for antiviral treatment[3-5]. CHB patients with high viral loads and significant fibrosis (METAVIR scoring system ≥ F2) should also be considered for treatment even if the ALT level is normal or mildly elevated (less than 2 times) because long-term viral suppression reduces liver-related complications, such as decompensated cirrhosis or HCC, in these patients[3-5].
Liver biopsy is still considered the “gold standard” for the evaluation of significant fibrosis in CHB patients[6]. However, its utilization is often restricted because its invasiveness can cause life‐threatening complications[7]. Moreover, tissue obtained via biopsy represents approximately only 1/50000 of the liver volume, which may result in a sampling error and is associated with considerable interobserver variability in the microscopic evaluation. Furthermore, repeating the liver biopsy to monitor changes in liver fibrotic burden is generally not feasible in clinical practice[7,8]. To overcome these limitations of liver biopsy, noninvasive serum- and imaging-based measurements for staging liver fibrosis have been developed[9,10].
To date, noninvasive methods incorporating serum-based indices or imaging-based tests using elastography have been increasingly used to assess liver fibrosis[11]. A variety of serum-based indices have been evaluated to predict the degree of liver fibrosis[10,12]. Among those, aspartate transaminase (AST)-to-platelet ratio index (APRI) and fibrosis index based on four factors (FIB-4) are commonly used for identifying liver fibrosis and cirrhosis in CHB patients because they are easily calculated with routine laboratory tests, and they have successfully predicted liver fibrosis in large cohorts[13]. However, their main disadvantage is their low accuracy in detecting mild to intermediate stages of fibrosis[10,11,13]. Imaging-based methods of elastography estimate liver stiffness that is associated with the severity of fibrosis by applying mechanical waves and by measuring their propagation speed through tissue using imaging[14-16]. Elastographic modalities can be either ultrasound (US)-based or magnetic resonance imaging (MRI)-based. US-based elastography techniques include strain-based imaging, transient elastography (TE), and shear wave elastography (SWE)[17,18]. MRI measures tissue stiffness with magnetic resonance elastography (MRE)[19,20]. These techniques have been proven superior to conventional cross-sectional imaging for the evaluation of fibrosis and cirrhosis, especially in the precirrhotic stages[19-22]. Several studies comparing the diagnostic performance of serum-based indices and imaging-based elastographies have been published[17,23], but little is known regarding their diagnostic performances that can be used to inform the applicability of these modalities to whether and when to initiate antiviral therapy in treatment-naive CHB patients with high viral loads but with normal or mildly elevated ALT levels.
Therefore, the objective of this study was to evaluate the liver stiffness values of MRE and two-dimensional SWE (2D-SWE) to assess liver fibrosis and to compare their diagnostic performances with those of FIB-4 and APRI for the prediction of significant fibrosis, which is an indicator for initiating antiviral therapy in treatment-naïve CHB patients with high viral loads but with borderline-normal or mildly elevated ALT levels.
Between March 2013 and February 2018, 67 treatment-naïve CHB patients with high viral loads but borderline-normal or mildly elevated ALT levels who underwent liver biopsy at Konkuk University Medical Center before a decision was made to initiate antiviral therapy were recruited. The following inclusion criteria were applied: (1) Hepatitis B surface antigen (HBsAg) positivity more than 6 months, HBeAg positive patients with > 20000 IU/mL, or HBeAg-negative patients with > 2000 IU/mL, normal ALT values (our laboratory reference value was 40 IU/L), or less than two times ULN; (2) Absence of any previous or concomitant anti-HBV therapy; (3) No liver comorbidity, including hepatitis C virus (HCV) coinfection, chronic ethanol consumption (more than 20 g of alcohol per day), HIV coinfection, or autoimmune hepatitis; (4) Availability of liver histologic assessment after liver biopsy, and time interval between liver biopsy and MRE/ 2D-SWE within 2 wk; and (5) Availability of both MRE and 2D-SWE, and time interval between MRE and 2D-SWE within 3 d. Patients who have clinical features or complications of liver cirrhosis, including ascites, medium/large gastroesophageal varices, or moderate to severe thrombocytopenia (platelet counts < 80000/μL), were excluded because they should be considered for antiviral treatment without requiring liver biopsy for confirmation of liver cirrhosis. Patients under 35 years of age were also excluded because they might stay in the immune-tolerant phase of chronic HBV infection. Our Institutional Review Board approved this study, waiving informed consent because of its retrospective nature.
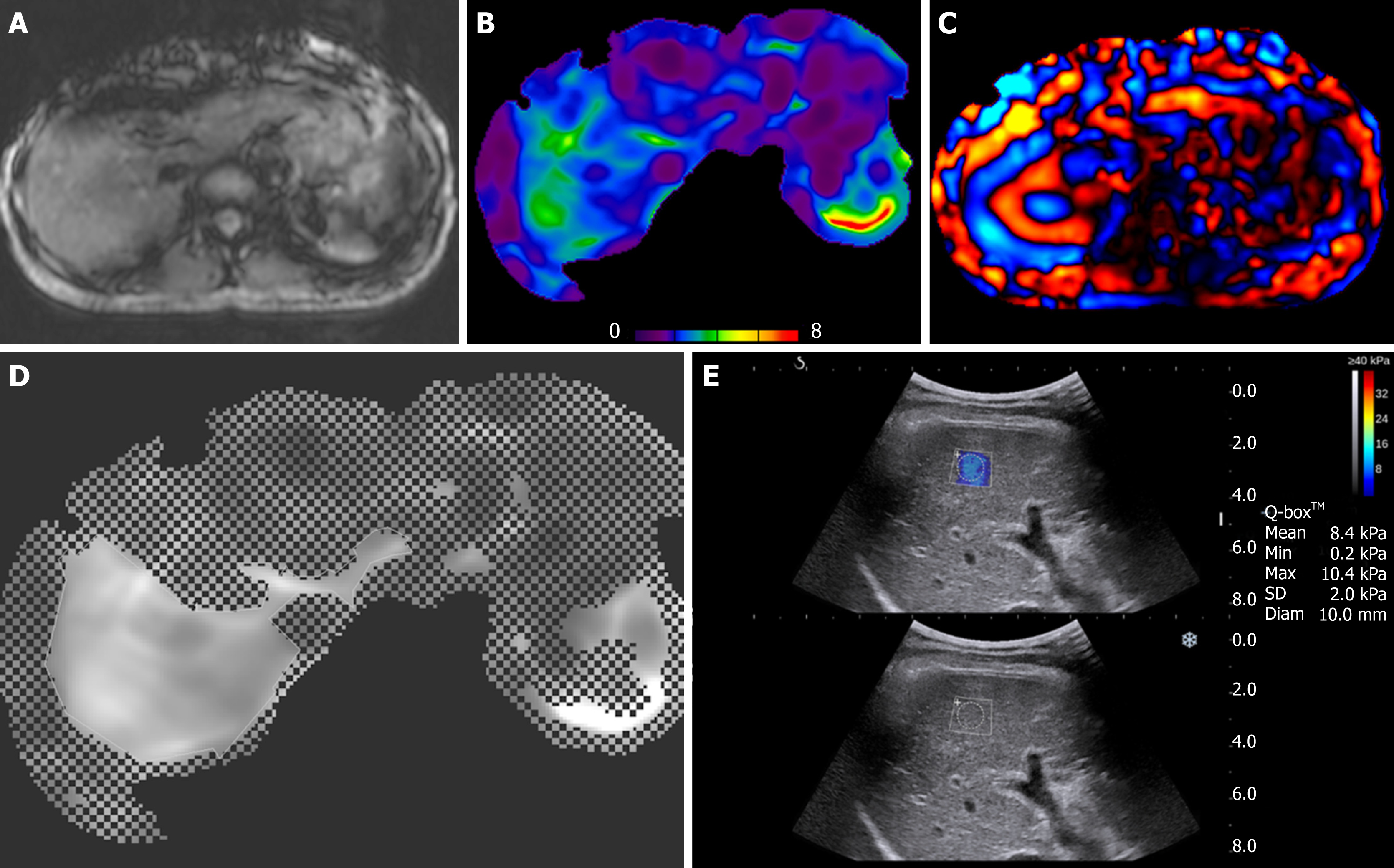
All MR examinations were performed using a 3-T MR unit (Magnetom Skyra, Siemens Medical Solutions, Erlangen, Germany). Patients were asked to hold their breath at the end-expiratory period to obtain a consistent position of the liver for each phase offset. When the acquisition was completed, wave images were automatically processed by the MR scanner, and images depicting tissue stiffness (elastograms) were generated (Figure 1A-D). These quantitative images represented shear stiffness in units of kilopascals (kPa). In addition, the elastogram was reviewed automatically by the intrinsic software for artifacts, such as significant wave interference and oblique wave propagation. Elastograms with 95% confidence mapping were produced by excluding the artifact area. MRE technical failure was considered when the following occurred: (1) Wave images showed no wave propagation; (2) Anatomic images showed severe respiratory motion artifact along the z-axis; or (3) Substantial loss of signal in the liver parenchyma suggesting an iron overload was present[16].
The mean shear stiffness of the liver was calculated by placing a manually specified region of interest (ROI) into the stiffness map of MRE images. The stiffness value of the liver parenchyma was calculated as the mean value in four ROIs (mean area, 4044.8 ± 1715.8 mm2) placed by one radiologist.
Measurements for 2D SWE were obtained by using an Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France) equipped with a broadband convex transducer (SC6-1). The operator was a single board-certified abdominal radiologist with more than 10 years of liver US experience and more than one year of clinical experience performing real-time elastography studies. SWE examinations were performed in the right lobe of the liver through the intercostal space. Liver stiffness measurements were obtained within an ROI of 10 mm2 in diameter at the area where the elasticity image was most homogeneously displayed. SWE measurement failure was considered when little or no signal was obtained in the SWE box, and an appropriate color-coded elasticity map was not acquired. Five consecutive acquisitions were obtained in the same location of the liver for each patient. Each measurement was performed during a separate breath hold. The system calculated the mean, maximum, minimum, and standard deviation of the elasticity value of each measurement in kPa (Figure 1E). The mean value of five liver stiffness measurements was calculated.
The FIB-4 values were calculated automatically using the formula [age (years) × AST (U/L)]/{platelets (109/L) × [ALT (U/L)]1/2}[24], in which the age of the patient was the age at the time of the liver biopsy. The APRI values were calculated using the formula (AST/upper limit of normal)/[platelet count (109/L)] × 100[25]. Our laboratory reference value of AST was 40 IU/L.
Biopsy specimens were fixed in formalin and embedded in paraffin. Thereafter, 4-mm-thick slices were cut and stained with hematoxylin-eosin. All specimens were analyzed by a pathologist who was blinded to the MRE results, SWE results, and the clinical data and who had 10 years of clinical experience interpreting liver pathologic examinations. The fibrosis stage and the degree of inflammation in the liver were assessed based on the METAVIR scoring system as shown below: F0, no fibrosis; F1, portal fibrosis; F2, periportal fibrosis; F3, septal fibrosis; and F4, cirrhosis[26]. In this study, a fibrosis stage of F2 or higher was considered to indicate significant fibrosis. Inflammatory activity was graded as A0 to A3: A0, no activity; A1, mild activity; A2, moderate activity; A3, severe activity.
Quantitative variables were expressed as the mean ± standard deviation (SD), which were analyzed with a t-test or a Mann-Whitney U-test, and categorical variables were demonstrated with numbers and percentages and compared using the Chi-squared method or Fisher’s exact test, when appropriate. Correlations between noninvasive methods and liver histological fibrosis stages were assessed using the Spearman correlation test. The strength of the correlation coefficients was classified as follows: 0.0-0.2, very weak; 0.2-0.4, weak; 0.4-0.7, moderate; 0.7-0.9, strong; and 0.9-1.0, very strong correlation. The difference between two dependent correlations was calculated by the Steiger test. Factors affecting liver stiffness values of the MRE or 2D-SWE were first analyzed with univariate testing, and those with P < 0.05 were subsequently included in a multivariate linear regression analysis. The diagnostic performance of noninvasive methods was assessed using receiver-operating characteristic (ROC) analysis; areas under the curve (AUCs) with 95% confidence intervals, sensitivity, specificity, and positive and negative predictive values were used for the classification of significant fibrosis (≥ F2) and cirrhosis (F4). AUCs were compared using the method of DeLong et al. A P value less than 0.05 was considered to indicate a sig-nificant difference. All statistical analyses were performed by using commercially available software programs (SPSS version 17, SPSS, Chicago, IL, United States; MedCalc, version 11.6, MedCalc Software, Mariakerke, Belgium).
Among 67 participants, MRE failed to provide liver stiffness values in one patient because there were no visible waves on MRE images due to overweight (BMI = 27.9) (technical failure rate, 1.5%). With regard to 2D-SWE, a proper elasticity map was not ade-quately displayed in three patients due to overweight (n = 2), or uncontrolled respiration (n = 1), yielding a 4.5% technical failure rate.
Finally, a total of 63 patients who could be successfully measured using both MRE and 2D-SWE were evaluated in this study. All 63 patients were treatment naïve and included 37 men and 26 women, with a median (range) age of 50 (30-68) years. The mean (± SD) levels of serum ALT were 44 ± 20.8 U/L. The median HBV-DNA levels of 35 HBeAg-positive CHB patients and 28 HBeAg-negative patients were 6.93 ± 1.25 log10 IU/mL and 4.35 ± 0.59 log10 IU/mL, respectively. Histopathologically, 3, 16, 14, 14, and 16 patients were diagnosed with fibrosis stage F0 to F4, respectively. The main characteristics of the patients are shown in Table 1.
| Characteristics (n = 63) | |
| Sex, male/female | 37/26 |
| Age, mean (± SD) yr | 50.8 (± 8.9) |
| Body mass index, mean (± SD) kg/m2 | 23.4 (± 3.4) |
| AST, mean (± SD), IU/L (normal 4-40 IU/L) | 43.5 (± 22.6) |
| ALT, mean (± SD), IU/L (normal 4-40 IU/L) | 44.0 (± 20.8) |
| Platelet counts, mean (± SD), × 103/mm3 | 163.5 (± 39.4) |
| Prothrombin time, mean (± SD), INR | 1.05 (± 0.08) |
| Total bilirubin, mean (± SD), mg/dL | 0.75 (± 0.47) |
| Albumin, mean (± SD), g/dL | 3.97 (± 0.24) |
| γ-glutamyl transferase, mean (± SD), U/L | 47.5 (± 31.0) |
| HBeAg status, positive/negative | 35/28 |
| HBV-DNA, mean (± SD), log10 IU/mL | 5.78 (± 1.64) |
| Grade of inflammatory activity (0/1/ 2/3) | 9/26/15/13 |
| Fibrosis stage (0/1/2/3 /4) | 3/16/14/ 14/16 |
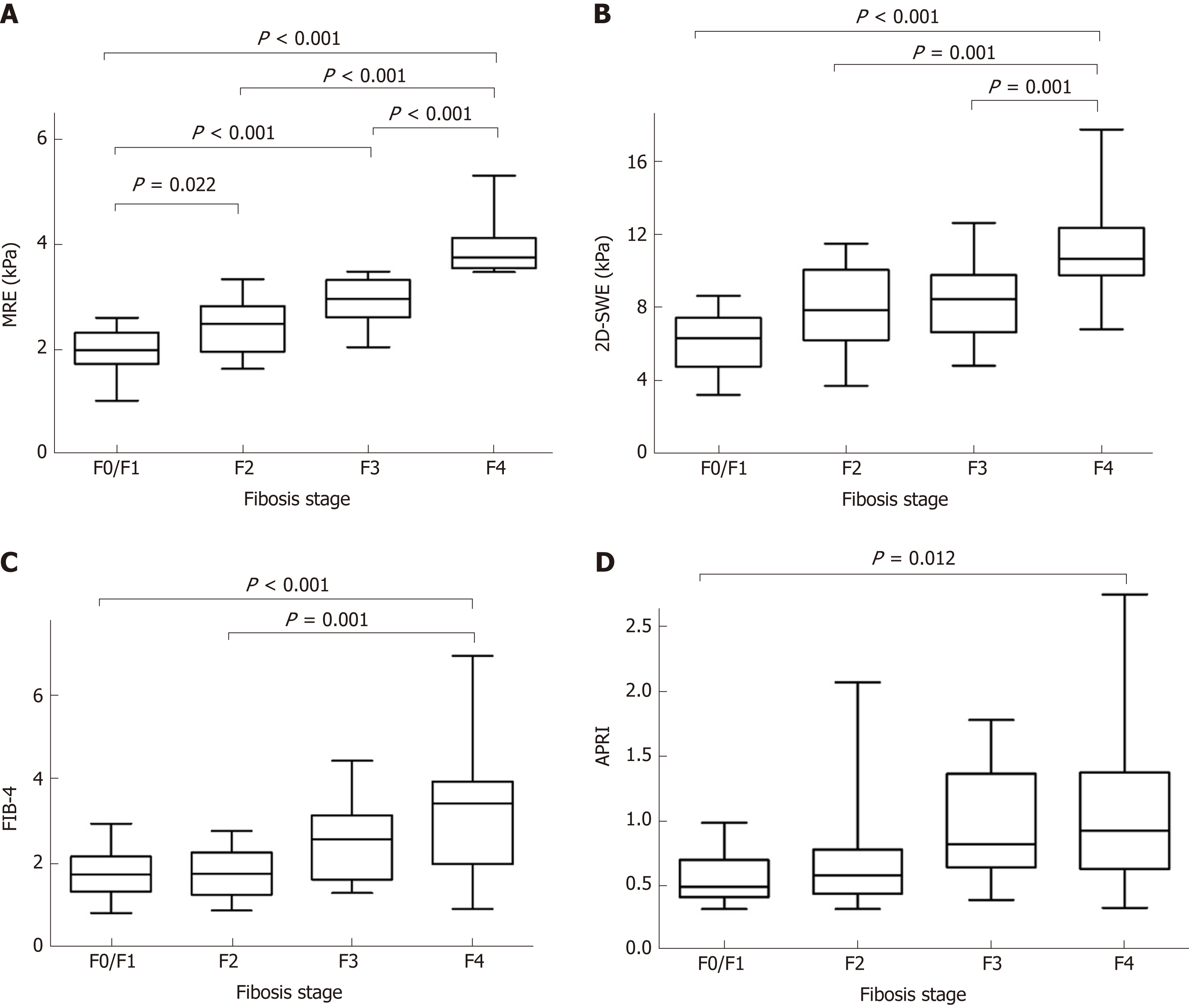
The measurements of MRE, 2D-SWE, FIB-4 and APRI for different fibrosis stages are shown in Table 2. All measurements increased as the fibrosis score increased (MRE, F = 50.642, aP < 0.001; 2D-SWE, F = 16.063, bP < 0.001; FIB-4, F = 8.608, cP < 0.001; APRI, F = 4.165, dP = 0.010). Distributions of the liver stiffness values of MRE, 2D-SWE, and the FIB-4 and APRI scores in comparison with the different fibrosis stages using METAVIR scores as the reference methods are shown in Figure 2. MRE revealed a statistical significance in distinguishing between F0/1 and F2 fibrosis stages (eP = 0.022), whereas 2D-SWE showed a broad overlap for those stages. Compared to MRE and 2D-SWE, large overlaps existed even with F4 fibrosis stage in FIB-4 and APRI, and they showed a wide range of readings (large SDs).
| F0/F1 (n = 19) | F2 (n = 14) | F3 (n = 14) | F4 (n = 16) | r | P value | |
| MRE | 1.96 ± 0.43 | 2.46 ± 0.54 | 2.91 ± 0.45 | 3.91 ± 0.50 | 0.869 | < 0.001 |
| 2D-SWE | 6.09 ± 1.58 | 8.05 ± 2.23 | 8.16 ± 2.08 | 11.39 ± 3.01 | 0.649 | < 0.001 |
| FIB-4 | 1.75 ± 0.61 | 1.76 ± 0.60 | 2.48 ± 0.91 | 3.21 ± 1.45 | 0.517 | < 0.001 |
| APRI | 0.58 ± 0.20 | 0.71 ± 0.46 | 0.95 ± 0.44 | 1.04 ± 0.58 | 0.431 | < 0.001 |
MRE showed strong correlations with fibrosis stage (MRE, r = 0.869, fP < 0.001; Spearman correlation), whereas 2D-SWE, FIB-4 and APRI scores showed a moderate correlation with fibrosis stage (SWE, r = 0.649, gP < 0.001; FIB-4, r = 0.517, hP < 0.001; APRI, r = 0.431, iP < 0.001: Spearman correlation). Using the Steiger test, the correlation coefficient between the liver stiffness values of MRE and liver fibrosis stage is significantly higher than that between the liver stiffness values of 2D-SWE and fibrosis stage (jP < 0.001). MRE and 2D-SWE measurements showed a moderate correlation with each other (MRE and 2D-SWE, r = 0.669, kP < 0.001), while there were moderate or weak correlations between radiology-based and serum-based measurements (MRE and FIB4, r = 0.465, lP < 0.001; MRE and APRI, r = 0.378, mP = 0.002; 2D-SWE and FIB4, r = 0.553, nP < 0.001; 2D-SWE and APRI, r = 0.396, oP = 0.001: Spearman correlation).
We investigated the factors that affect liver stiffness values by MRE and 2D-SWE. These parameters include sex, age, body mass index (BMI), platelet counts, total bilirubin, albumin, AST, ALT, γ-GT, prothrombin time, HBeAg status, HBV-DNA levels, inflammatory grade, and liver fibrosis stage (Table 3). Concerning MRE, a univariate analysis revealed correlations between liver stiffness values of MRE and platelet counts, inflammatory grade, and liver fibrosis stage, and a multivariate an-alysis showed that only the liver fibrosis stage was an independent factor affecting liver stiffness values of MRE. Concerning 2D-SWE, a univariate analysis revealed correlations between liver stiffness values of 2D-SWE and BMI, platelet counts, inflammatory grade, and liver fibrosis stage, and a multivariate analysis showed that not only the liver fibrosis stage but also BMI were independent factors affecting liver stiffness values of 2D-SWE.
| Parameters | Factors associated with liver stiffness values by MRE | Factors associated with liver stiffness values by 2D-SWE | ||||||
| Univariate | P value | Multivariate | P value | Univariate | P value | Multivariate | P value | |
| Sex, male/female | -0.054 (-0.508, 0.401) | 0.815 | 0.685 (-0.840, 2.210) | 0.372 | ||||
| Age, yr | 0.019 (-0.006, 0.044) | 0.132 | 0.067 (-0.017, 0.151) | 0.117 | ||||
| BMI, kg/m2 | -0.048 (-0.114, 0.018) | 0.149 | -0.251 (-0.469, -0.034) | 0.024 | -0.186 (-0.366, -0.007) | 0.042 | ||
| AST, U/L | 0.007 (-0.003, 0.017) | 0.157 | 0.032 (-0.001, 0.065) | 0.054 | ||||
| ALT, U/L | 0.002 (-0.009, 0.013) | 0.674 | 0.000 (-0.037, 0.037) | 0.995 | ||||
| PLT counts, × 103/mm3 | -0.007 (-0.012, -0.001) | 0.014 | 0.001 (-0.002, 0.005) | 0.530 | -0.027 (-0.045, -0.009) | 0.004 | -0.012 (-0.029, 0.004) | 0.136 |
| PT, INR | 1.312 (-1.595, 4.220) | 0.370 | 8.658 (-0.971, 18.288) | 0.077 | ||||
| Total bilirubin, mg/dL | 0.331 (-0.140, 0.802) | 0.165 | 0.098 (-1.517, 1.713) | 0.904 | ||||
| Albumin, g/dL | -0.540 (-1.510, 0.429) | 0.270 | -3.152 (-6.359, 0.055) | 0.054 | ||||
| γ-GT, U/L | 0.004 (-0.003, 0.011) | 0.302 | 0.012 (-0.012, 0.036) | 0.327 | ||||
| HBeAg status, +/- | 0.166 (-0.282, 0.615) | 0.461 | 1.052 (-0.445, 2.549) | 0.165 | ||||
| HBV-DNA, log10 IU/mL | -0.051 (-0.189, 0.086) | 0.456 | -0.076 (-0.541, 0.389) | 0.745 | ||||
| Inflammatory grade | 0.363 (0.153, 0.573) | 0.001 | 0.105 (-0.031, 0.241) | 0.129 | 0.903 (0.163, 1.644) | 0.018 | 0.220 (-0.411, 0.852) | 0.487 |
| Fibrosis stage | 0.626 (0.520, 0.732) | < 0.001 | 0.609 (0.487, 0.731) | < 0.001 | 1.616 (1.116, 2.116) | < 0.001 | 1.276 (0.690, 1.863) | < 0.001 |
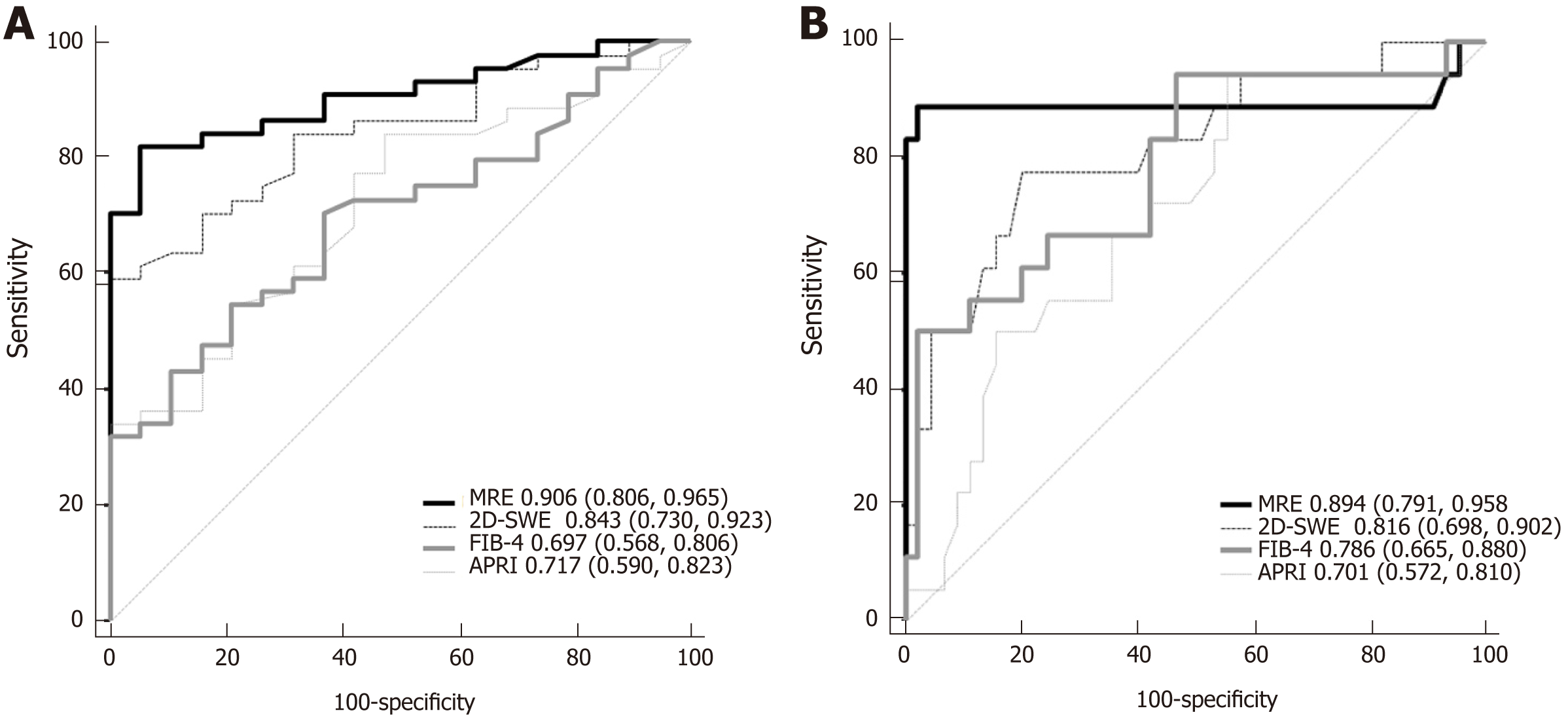
The areas under ROC curve (AUCs), cut-off values, sensitivity, specificity, positive predictive values, and negative predictive values for the diagnosis of significant fibrosis (≥ F2) and cirrhosis (F4) using radiology-based or serum-based measurement indices are shown in Table 4. The AUCs for MRE, 2D-SWE, FIB-4, and APRI scores were 0.906, 0.843, 0.697, and 0.717, respectively, for the diagnosis of significant fibrosis, and 0.894, 0.816, 0.786, and 0.701, respectively, for the diagnosis of cirrhosis.
| AUC (95%CI) | P value | Cutoff | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||
| MRE | ≥ F2 | 0.906 (0.806, 0.965) | < 0.001 | > 2.47 (kPa) | 81.8 | 94.7 | 97.3 | 69.2 |
| F4 | 0.894 (0.791, 0.958) | < 0.001 | > 3.46 (kPa) | 88.9 | 97.8 | 94.1 | 95.6 | |
| 2D-SWE | ≥ F2 | 0.843 (0.730, 0.923) | < 0.001 | > 6.73 (kPa) | 84.1 | 68.4 | 86.0 | 65.0 |
| F4 | 0.816 (0.698, 0.902) | < 0.001 | > 9.50 (kPa) | 77.8 | 80.0 | 60.9 | 90.0 | |
| FIB-4 | ≥ F2 | 0.697 (0.568, 0.806) | 0.003 | > 1.80 | 70.5 | 63.2 | 81.6 | 48.0 |
| F4 | 0.786 (0.665, 0.880) | < 0.001 | > 3.22 | 50.0 | 97.8 | 90.0 | 83.0 | |
| APRI | ≥ F2 | 0.717 (0.590, 0.823) | 0.001 | > 0.49 | 84.1 | 52.6 | 80.4 | 58.8 |
| F4 | 0.701 (0.572, 0.810) | 0.006 | > 0.96 | 50.0 | 84.4 | 562 | 80.9 |
The AUCs of the MRE and 2D-SWE for the diagnosis of significant fibrosis were more than 0.80, with no statistically significant differences between indicators. The performance of MRE for the diagnosis of significant fibrosis was significantly better than that of serum-based measurements by pairwise comparison of the ROC curves (MRE vs FIB-4, pP = 0.002; MRE vs APRI, qP = 0.010, respectively). In addition, the performance of SWE was not significantly different compared to FIB-4 or APRI for the diagnosis of significant fibrosis (Figure 3A).
The AUCs of the radiology-based measurements for the diagnosis of cirrhosis were more than 0.80, and their performance was not significantly different from that of serum-based measurements for the identification of cirrhosis (F4) (Figure 3B).
The accurate diagnosis of significant fibrosis is of particular clinical value for treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels because it is considered an indicator for antiviral treatment[3-5]. Among 63 patients analyzed in our study, 44 (69.8%) patients should need to initiate antiviral therapy because they were diagnosed with significant fibrosis. If they did not undergo liver biopsy, they did not fulfill the indications for antiviral therapy. Therefore, a main application of our research is intended to reduce the need for invasive liver biopsy by assessing and comparing noninvasive measurements for a precise diagnosis of significant fibrosis and, consequently, to assist in making antiviral treatment decisions. Our results showed that MRE was able to better discriminate significant fibrosis from normal or mild fibrosis than 2D-SWE. Furthermore, MRE showed a higher correlation coefficient value with fibrosis stage than that between 2D-SWE and fibrosis stage. Moreover, the performance of MRE for diagnosing sig-nificant fibrosis was better than that of FIB-4 and APRI, whereas the performance of SWE was not significantly different from that of FIB-4 or APRI. Furthermore, liver fibrosis stage was the only independent factor affecting the liver stiffness values of MRE, whereas BMI as well as liver fibrosis stage can affect the liver stiffness values of 2D-SWE. In addition, technical failure rate was lower in MRE (n = 1, 1.5%) than in 2D-SWE (n = 3, 4.5%). In our study, MRE could significantly discriminate between F0/1 and F2 fibrosis stage (P = 0.022), whereas 2D-SWE showed a broad overlap for those stages. The correlation coefficient between fibrosis stage and the liver stiffness values of MRE (r = 0.859) is higher than that between fibrosis stage and the values of 2D-SWE, FIB-4, and APRI (r = 0.647, r = 0.498, r = 0.442, respectively). These data suggest that MRE has a better diagnostic performance in the identification of significant fibrosis than 2D-SWE as well as FIB-4 and APRI, and this is similar to a previous study comparing MR-based and US-based elastographies[17,19]. The possible reason may be that MRE can measure a larger volume of liver, and therefore potentially assesses the stiffness of nearly the entire liver, whereas SWE is able to analyze a smaller volume of liver[27,28]. Thus, MRE is more representative of liver parenchyma with less sampling variability[29,30].
The AUCs in our study showed that MRE has excellent diagnostic accuracy in the assessment of significant fibrosis. The AUC of MRE was numerically higher than that of 2D-SWE but the difference was statistically insignificant (0.906 vs 0.843). The statistical insignificance might be explained by the homogeneity of the patients in our study, as our study selected only CHB patients with normal or mildly elevated ALT levels, who are borderline in terms of a decision to initiate antiviral treatment, whereas the previous studies, which showed MRE has statistically significant higher accuracy than US-based elastography, enrolled participants with a wide range of ALT values[16,27,28,31,32]. Compared to serum-based indices, the diagnostic performance of MRE for diagnosing significant fibrosis is better than those of FIB-4 and APRI, whereas the performance of 2D-SWE is not significantly different from those of FIB-4 and APRI. These data suggested that among MRE and 2D-SWE, only MRE might help identify CHB patients who may benefit from treatment compared to serum based indices, such as FIB-4 or APRI.
We also investigated the confounding factors affecting liver stiffness values by MRE and 2D-SWE, including sex, age, BMI, platelet counts, total bilirubin, albumin, AST, ALT, γ-GT, prothrombin time, HBeAg status, HBV-DNA levels, inflammatory grade, and liver fibrosis stage. Except for liver fibrosis stage, the multivariate linear regression analysis revealed no associations between those factors and liver stiffness values of MRE. However, BMI and liver fibrosis stage were independent factors affecting liver stiffness values of 2D-SWE, and these data suggested that BMI might be a confounder that decreases liver stiffness values of 2D-SWE, potentially causing underestimation of the real liver fibrosis stage. The reason why BMI affect liver stiffness measurements of 2D-SWE is not clear. A possible explanation is that high BMI is the most common condition associated with hepatic steatosis, and several studies have shown that the liver stiffness value of US-based elastography is fundamentally influenced by hepatic liver fat content[33,34]. On the other hand, a few clinical studies revealed that hepatic steatosis did not affect liver stiffness values of MRE[35,36].
There are some limitations to the present study. First, the use of liver biopsy as the reference standard for assessing liver fibrosis has limitations associated with sampling errors, as well as intra- and interobserver variability, which are at least partly linked to the size of the biopsy. Second, despite MRE has the best effectiveness, it is much more expensive than 2D-SWE and is available only in tertiary centers. Third, as the sample size of this study is relatively small, the present results need to be validated independently in further studies.
In conclusion, MRE might be a non-invasive and more precise measurement for the assessment of significant fibrosis compared to 2D-SWE as well as serum-based indices in treatment-naive CHB patients with high viral loads but with normal or mildly elevated ALT levels who should be considered for initiation of antiviral therapy depending on the presence of significant fibrosis.
Accurate detection of significant fibrosis (fibrosis stage 2 or higher on the METAVIR scale) is important especially for chronic hepatitis B (CHB) patients with high viral loads but with normal or mildly elevated alanine aminotransferase (ALT) levels because the presence of significant fibrosis is accepted as the indication for antiviral treatment. Liver biopsy is the reference standard for diagnosing significant fibrosis, but it is an invasive procedure. Consequently, non-invasive imaging-based measurements, such as magnetic resonance elastography (MRE) or two-dimensional shear-wave elastography (2D-SWE), have been proposed for the quantitative assessment of liver fibrosis.
Liver biopsy is still considered the “gold standard” for the evaluation of significant fibrosis in CHB patients. However, its utilization is often restricted because its invasiveness can cause life‐threatening complications. Moreover, tissue obtained via biopsy represents approximately only 1/50000 of the liver volume, which may result in a sampling error and is associated with considerable interobserver variability in the microscopic evaluation. Furthermore, repeating the liver biopsy to monitor changes in liver fibrotic burden is generally not feasible in clinical practice.
The objective of this study was to evaluate the liver stiffness values of MRE and two-dimensional SWE (2D-SWE) to assess liver fibrosis and to compare their diagnostic performances with those of FIB-4 and APRI for the prediction of significant fibrosis, which is an indicator for initiating antiviral therapy in treatment-naïve CHB patients with high viral loads but with borderline-normal or mildly elevated ALT levels.
The study enrolled 63 treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels who underwent liver biopsy before a decision was made to initiate antiviral therapy. MRE and 2D-SWE were performed, and serum-based indices, such as FIB-4 and APRI, were calculated. The diagnostic performances of MRE, 2D-SWE, FIB-4, and APRI for assessing significant fibrosis (≥ F2) and cirrhosis (F4) were evaluated with liver histology as the reference standard, using receiver operating characteristic analyses.
The liver fibrosis stage was F0/F1 in 19, F2 in 14, F3 in 14, and F4 in 16 patients, respectively. MRE significantly discriminated F2 from F0/1 (P = 0.022), whereas 2D-SWE showed a broad overlap in distinguishing those stages. MRE showed a higher correlation coefficient value with fibrosis stage than 2D-SWE with fibrosis stage (0.859 vs 0.647, Spearman test; P < 0.001). Multiple-regression analyses showed that fibrosis stage was the only factor affecting the values of MRE (P < 0.001), whereas body mass index (P = 0.042) and fibrosis stage (P < 0.001) were independent factors affecting 2D-SWE values. The MRE performance for diagnosing significant fibrosis was better than FIB-4 (P = 0.002) and APRI (P = 0.010), whereas the performance of 2D-SWE was not significantly different from that of FIB-4 or APRI.
MR elastography might be a non-invasive and more precise measurement for the assessment of significant fibrosis compared to 2D-SWE as well as serum-based indices in treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels who should be considered for initiation of antiviral therapy depending on the presence of significant fibrosis.
There are some limitations to the present study. First, the use of liver biopsy as the reference standard for assessing liver fibrosis has limitations associated with sampling errors, as well as intra and interobserver variability, which are at least partly linked to the size of the biopsy. Second, despite MRE has the best effectiveness, it is much more expensive than 2D-SWE and is available only in tertiary centers. Third, as the sample size of this study is relatively small, the present results need to be validated independently in further studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheungpasitporn W, Ierardi E, Mihaila RG, Tamori A, Yao D S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 318] [Article Influence: 53.0] [Reference Citation Analysis (2)] |
| 2. | Cho EJ, Kim SE, Suk KT, An J, Jeong SW, Chung WJ, Kim YJ. Current status and strategies for hepatitis B control in Korea. Clin Mol Hepatol. 2017;23:205-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 3. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1532] [Cited by in F6Publishing: 1500] [Article Influence: 187.5] [Reference Citation Analysis (2)] |
| 4. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3323] [Article Influence: 474.7] [Reference Citation Analysis (0)] |
| 5. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1652] [Cited by in F6Publishing: 1754] [Article Influence: 219.3] [Reference Citation Analysis (0)] |
| 6. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1678] [Cited by in F6Publishing: 1683] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 7. | Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 230] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 8. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in F6Publishing: 1446] [Article Influence: 96.4] [Reference Citation Analysis (1)] |
| 9. | Srinivasa Babu A, Wells ML, Teytelboym OM, Mackey JE, Miller FH, Yeh BM, Ehman RL, Venkatesh SK. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. Radiographics. 2016;36:1987-2006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Martínez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Zeng DW, Dong J, Liu YR, Jiang JJ, Zhu YY. Noninvasive models for assessment of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2016;22:6663-6672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Wu Z, Dong X, Wang G, Zhao H; China HepB-Related Fibrosis Assessment Research Group. Clinical noninvasive markers for antiviral therapy decision in chronic hepatitis B with alanine aminotransferase less than two times upper limit of normal. J Viral Hepat. 2019;26:287-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 14. | Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, Levine D. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276:845-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 15. | Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, Taouli B. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology. 2018;286:738-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 16. | Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, Lee KB, Han JK, Choi BI. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 17. | Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two-dimensional Shear-Wave Elastography Performance in the Noninvasive Evaluation of Liver Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum Fibrosis Indexes. Radiology. 2017;283:873-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 19. | Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014;24:70-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Lee JE, Lee JM, Lee KB, Yoon JH, Shin CI, Han JK, Choi BI. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography. Korean J Radiol. 2014;15:210-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22:433-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: Review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45:1276-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Liu J, Zhao J, Zhang Y, Ji Y, Lin S, Dun G, Guo S. Noninvasive Assessment of Liver Fibrosis Stage Using Ultrasound-Based Shear Wave Velocity Measurements and Serum Algorithms in Patients With Viral Hepatitis B: A Retrospective Cohort Study. J Ultrasound Med. 2017;36:285-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 3157] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 25. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 3062] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 26. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2969] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 27. | Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M, Chen J, Keaveny AP, Bridges M, Bohte A, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440-451.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 28. | Xiao H, Shi M, Xie Y, Chi X. Comparison of diagnostic accuracy of magnetic resonance elastography and Fibroscan for detecting liver fibrosis in chronic hepatitis B patients: A systematic review and meta-analysis. PLoS One. 2017;12:e0186660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Lee Yj. , Lee JM, Lee JE, Lee KB, Lee ES, Yoon JH, Yu MH, Baek JH, Shin CI, Han JK, Choi BI. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2014;39:326-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 514] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 31. | Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, Aryafar H, Sirlin CB, Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 147] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 32. | Ichikawa S, Motosugi U, Morisaka H, Sano K, Ichikawa T, Tatsumi A, Enomoto N, Matsuda M, Fujii H, Onishi H. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn Reson Imaging. 2015;33:26-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, Marzano A, Rizzetto M. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 35. | Lee DH, Lee JM, Han JK, Choi BI. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013;38:1215-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Venkatesh SK, Wang G, Teo LL, Ang BW. Magnetic resonance elastography of liver in healthy Asians: normal liver stiffness quantification and reproducibility assessment. J Magn Reson Imaging. 2014;39:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |