Published online Jun 21, 2019. doi: 10.3748/wjg.v25.i23.2935
Peer-review started: March 25, 2019
First decision: April 11, 2019
Revised: April 18, 2019
Accepted: April 29, 2019
Article in press: April 29, 2019
Published online: June 21, 2019
Processing time: 90 Days and 8.2 Hours
Clinically significant portal hypertension (CSPH) and severe portal hypertension (SPH) increase the risk for decompensation and life-threatening complications in liver cirrhosis. Pathologic angiogenesis might contribute to the formation of these conditions. Placental growth factor (PlGF) and Nogo-A protein are biomarkers of pathological angiogenesis, but data on their role in liver cirrhosis and portal hypertension is scarce.
To determine plasma levels of PlGF and Nogo-A in patients with liver cirrhosis, CSPH, SPH and potential to predict portal hypertension.
A cohort of 122 patients with hepatitis C virus and/or alcohol-induced liver cirrhosis with characterized hepatic venous pressure gradient (HVPG) were included in the study. Demographic data, medical history, Child-Turcotte-Pugh and Model of End Stage liver disease score, clinical chemistry, liver stiffness values were recorded on the day of the procedure prior HVPG measurement. The degree of portal hypertension was determined by the invasive HVPG measurement. Nogo-A and PlGF plasma levels were evaluated using enzyme linked immunosorbent assay. The control group consisted of 30 healthy age- and sex- matched individuals.
Peripheral PlGF levels were higher and Nogo-A levels were lower in patients with liver cirrhosis (23.20 vs 9.85; P < 0.0001 and 2.19 vs 3.12; P = 0.004 respectively). There was a positive linear correlation between peripheral levels of PlGF and HVPG (r = 0.338, P = 0.001) and negative linear correlation between the peripheral Nogo-A levels and HVPG (r = -0.267, P = 0.007). PlGF levels were higher in CSPH and SPH (P = 0.006; P < 0.0001) whereas Nogo-A levels were lower (P = 0.01; P < 0.033). Area under the curve for the diagnosis of CSPH for PlGF was 0.68 (P = 0.003) and for Nogo-A - 0.67 (P = 0.01); for SPH 0.714 (P < 0.0001) and 0.65 (P = 0.014) respectively. PlGF levels were higher and Nogo-A levels were lower in patients with esophageal varices (P < 0.05). PlGF cut-off value of 25 pg/mL distinguished patients with CSPH at 55.7% sensitivity and 76.7% specificity; whereas Nogo-A cut-off value of 1.12 ng/mL was highly specific (93.1%) for the diagnosis of CSPH.
Plasma PlGF levels were higher while Nogo-A levels were lower in patients with liver cirrhosis and portal hypertension. Biomarkers showed moderate predictive value in determining CSPH and SPH.
Core tip: In this study, we aimed to evaluate plasma levels of angiogenesis mediators placental growth factor and Nogo-A protein in patients with liver cirrhosis, clinically significant portal hypertension, severe portal hypertension as well as biomarker potential to predict clinically significant and severe portal hypertension. Higher levels of placental growth factor have previously been associated with portal hypertension in animal models; however, data in patients with liver cirrhosis are scarce. To date this is the first study to evaluate Nogo-A protein levels in patients with liver cirrhosis and portal hypertension. Furthermore, to our best knowledge this is the first study to evaluate prognostic potential of these biomarkers to detect clinically significant and severe portal hypertension. We believe that this study adds additional knowledge on the complex pathogenesis of portal hypertension and might provide new insights for future research of new diagnostic approaches and treatment targets in the field.
- Citation: Gelman S, Salteniene V, Pranculis A, Skieceviciene J, Zykus R, Petrauskas D, Kupcinskas L, Canbay A, Link A, Kupcinskas J. Plasma Nogo-A and placental growth factor levels are associated with portal hypertension in patients with liver cirrhosis. World J Gastroenterol 2019; 25(23): 2935-2946
- URL: https://www.wjgnet.com/1007-9327/full/v25/i23/2935.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i23.2935
Portal hypertension (PH) is a consequence of liver cirrhosis and can cause serious life-threatening complications. The degree of portal hypertension is one of the most important prognostic factors for complications and decompensation of liver cirrhosis and is defined by the hepatic venous pressure gradient (HVPG)[1]. The presence of clinically significant portal hypertension (CSPH; HVPG ≥ 10 mmHg) increases the risk for the formation of varicose veins and decompensation whereas severe portal hypertension (SPH; HVPG ≥ 12 mmHg) increases the risk for variceal bleeding and death[1-3]. In order to optimize the care of patients with liver cirrhosis, it is essential to detect early PH and prevent the development of CSPH or treat already present CSPH to avoid decompensation[4]. As current options for the prevention and treatment of CSPH are limited to attenuating splanchnic vasodilatation, new insights and deeper knowledge about pathogenetic me-chanisms of PH and molecules involved, would aid in search for newer and more effective treatment strategies[5].
The main pathophysiological mechanism of PH in cirrhosis is an increase in intrahepatic vascular resistance (IHVR), mostly caused by structural changes in liver tissue, but functional disorders of liver circulation are also important pathogenetic factors[1]. Recent studies suggest that pathologic angiogenesis could also contribute to an increase in IHVR, causing splanchnic hyperemia, portosystemic collateralization and pathologic angiogenesis inside the liver, worsening an already existing PH[5-7].
Pathologic angiogenesis has been reported to result from the upregulation of proangiogenic factors and simultaneous downregulation of angiogenesis inhibitors, thus both stimuli might be important and contribute to the pathophysiological mechanisms[8].
Vascular endothelial growth factor (VEGF) family has been recognized as one of the major proangiogenic mediators associated with PH[9] in number of animal studies[10-12] and only two small human studies[13,14] demonstrating beneficial effects on PH when blocking VEGF signaling. However this could cause deleterious side effects, as angiogenesis is essential for tissue healing and regeneration[5]. Placental growth factor (PlGF), a member of the VEGF family, on the other hand could be a more promising target as it has been reported only to enhance angiogenesis in pathological conditions, not affecting normal tissues[15]. As a porangiogenic factor PlGF escalates the proliferation, migration and survival of the endothelial cells, intensifies the proliferation of the mesenchymal cells and regulates the contraction of the mural cells[16]. The expression and function of PlGF has been addressed in several animal studies[17,18], however data on plasma levels of PlGF in patients with liver cirrhosis, portal hypertension and it’s complications is scarce.
Angiogenesis is a two way process including activation and inhibition of an-giogenesis stimuli and a number of endogenous angiogenesis inhibitors have been identified, some of them being associated with PH[19,20]. Nogo-A and Nogo-B proteins are novel recently discovered angiogenesis mediators that belong to the reticulon 4 protein family. Nogo-A protein, most widely examined as a potent neurite growth inhibitor, has never been studied in patients with liver cirrhosis or PH. Studies have examined the expression of Nogo-A in cardiomyocytes[21], enteric nervous system[22], in ocular diseases[23] and hepatocellular carcinoma[24]. Nogo-A has also been reported to be a negative regulator of retinal and CNS[25,26] angiogenesis, but the effects on an-giogenesis in liver diseases remains unknown. As Nogo-B protein, a splice variant of Nogo-A, is associated with liver fibrogenesis and liver cirrhosis[27,28], angiogenesis[29,30] and endogenous tissue repair[31], we hypothesized that Nogo-A, similarly to Nogo-B, might be associated with liver cirrhosis and the pathogenesis of PH.
In this study we aimed to evaluate plasma levels of PlGF and Nogo-A in liver cirrhosis patients with normal portal pressure and portal hypertension as well as in controls. We also aimed to evaluate the potential of plasma PlGF and Nogo-A levels as biomarkers to predict CSPH and SPH as well as complications of portal hy-pertension.
The study included patients with hepatitis C virus and/or alcohol induced liver cirrhosis who underwent a scheduled HVPG measurement or transjugular liver biopsy in the Department of Gastroenterology, Lithuanian University of Health Sciences from September 2015 to December 2017. A total of 290 patients were examined, out of which 122 were included in the study. The main criteria for the exclusion from the study were: Pre- or posthepatic causes of portal hypertension, cardiovascular, kidney disease, diabetes, neurodegenerative diseases, active infection, hepatocellular carcinoma or cancer of other location. We also excluded the patients with the history of current use of beta-blockers or other vasoactive drugs. De-mographic data, medical history (presence of ascites, esophageal varices), Child-Turcotte-Pugh and Model of End Stage liver disease (MELD) score, clinical chemistry, liver stiffness (Fibroscan, Echosens, France), plasma levels of Nogo-A and PlGF, HVPG values were recorded on the day of the procedure prior HVPG measurement.
Liver cirrhosis was diagnosed according to clinical, laboratory and radiologic data and/or histology; the presence of portal hypertension was diagnosed by hepatic vein catheterization.
Thirty healthy volunteers, matched for age and sex, with normal liver enzymes and normal liver stiffness measurements were included as controls for peripheral levels of Nogo-A and PlGF.
The study was approved by Kaunas Region Biomedical Research Ethics Committee (2015-08-24, No. BE-2-28, Kaunas, Lithuania). Every participant provided a written informed consent to participate in the study and study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Peripheral blood samples were obtained on the day of the procedure. Blood samples from the hepatic vein were obtained during the HVPG measurement in EDTA tubes. Samples were centrifuged at room temperature in 3500 g for 10 min, plasma was separated and stored in -80 °C for further analysis. Sandwich enzyme-linked immunosorbent assay (ELISA) kits were used to determine Nogo-A (Elabscience, United States) and PlGF (Abbexa LTD, United Kingdom) plasma levels, according to manufacturers’ protocols using SunriseTM (Tecan Trading AG, Switzerland) mi-croplate reader with 450 nm wavelength filter and MagellanTM (Tecan Trading AG, Switzerland) software. In each ELISA kit both controls and patients were included.
The degree of portal hypertension was determined by the invasive HVPG mea-surement. The procedure was performed by the same experienced radiologist according to the standard as described by Groszmann et al[32]. At least three repeated measurements were performed to determine free and wedged hepatic vein pressure for calculation of HVPG. HVPG values of 1-5 mmHg were considered to represent normal portal pressure, whereas portal hypertension was diagnosed at a HVPG ≥ 6 mmHg. HVPG ≥ 10 mmHg was considered to be CSPH and ≥ 12 mmHg-SPH.
Statistical analysis was performed using SPSS 25.0 software. Descriptive statistics are provided as mean and standard deviation (SD), or as median and range for non-parametric data. Differences between the groups were assessed with the Student’s t test or Mann-Whitney’s test as appropriate. Differences between three groups were assessed by one-way ANOVA test or the Wilcoxon test, when appropriate. Correlations were performed by means of Spearman’s correlation for PlGF and Pearson’s correlation for Nogo-A and expressed by Spearman’s or Pearson’s co-efficient. Univariate regression analysis was performed to identify the relationship of PlGF and Nogo-A with PH and its complications. Receiver operating characteristic (ROC) curves were created to assess the predictive values of PlGF and Nogo-A for CSPH, SPH and complications with area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). The value with the best sensitivity and specificity in AUC analysis (Youden’s Index) was chosen for further analysis. Statistical significance was established at P < 0.05 and expressed as aP < 0.05.
One hundred and twenty-two patients with liver cirrhosis and 30 controls were included in the study. Sixty-seven patients (54.9%) were male and mean age was 50.45 (± 11.13). Sixty-one (50%) patient had alcohol induced liver cirrhosis and sixty-one (50%)-hepatitis C virus cirrhosis. Seventy-five (61.5%) patients were classified as having Child-Pugh A, 30 (24.6%) as Child-Pugh B and 17 (13.9%) as Child-Pugh C cirrhosis. Fourteen patients (11.5%) had normal HVPG, eighty-six (70.5 %) had HVPG ≥ 10 mmHg, of which 70 (57.4%) had HVPG ≥ 12 mmHg. Demographic, clinical and endoscopic characteristics are displayed in Table 1. Peripheral Nogo-A and PlGF levels were examined in 100 patients each and hepatic Nogo-A and PlGF levels were examined in 30 patients each.
| Variable | Patients (n = 122) | Controls (n = 30) |
| Sex (male/female; %) | 55/45 | 50/50 |
| Age (yr; SD) | 51.14 (± 9.73) | 48.92 (± 15.46) |
| BMI (kg/m2; SD) | 26.04 (± 4.63) | 25.30 (± 3.51) |
| Etiology (N (%) of patients) | ||
| Alcohol induced cirrhosis | 61 (50) | |
| HCV cirrhosis | 61 (50) | |
| Child-Pugh class (A/B/C; %) | 61.5/24.6/13.9 | |
| MELD score (SD) | 11.15 (± 4.32) | |
| TE (kPa; SD) | 31.37 (± 19.79) | |
| Ascites (% of patients) | 38.5 | |
| Varices (% of patients) | ||
| Absent | 45.1 | |
| F1 | 27 | |
| F2 | 18.9 | |
| F3 | 9 | |
| Risk signs of bleeding (N of patients) | 24 | |
| HVPG (mmHg; SD) | 13.70 (± 6.52) | |
| HVPG 1-5 mmHg (% of patients) | 11.5 | |
| HVPG 5-9 mmHg (% of patients) | 19.7 | |
| CSPH; HVPG ≥ 10 mmHg (% of patients) | 70.5 | |
| SPH; HVPG ≥ 12 mmHg (% of patients) | 57.4 |
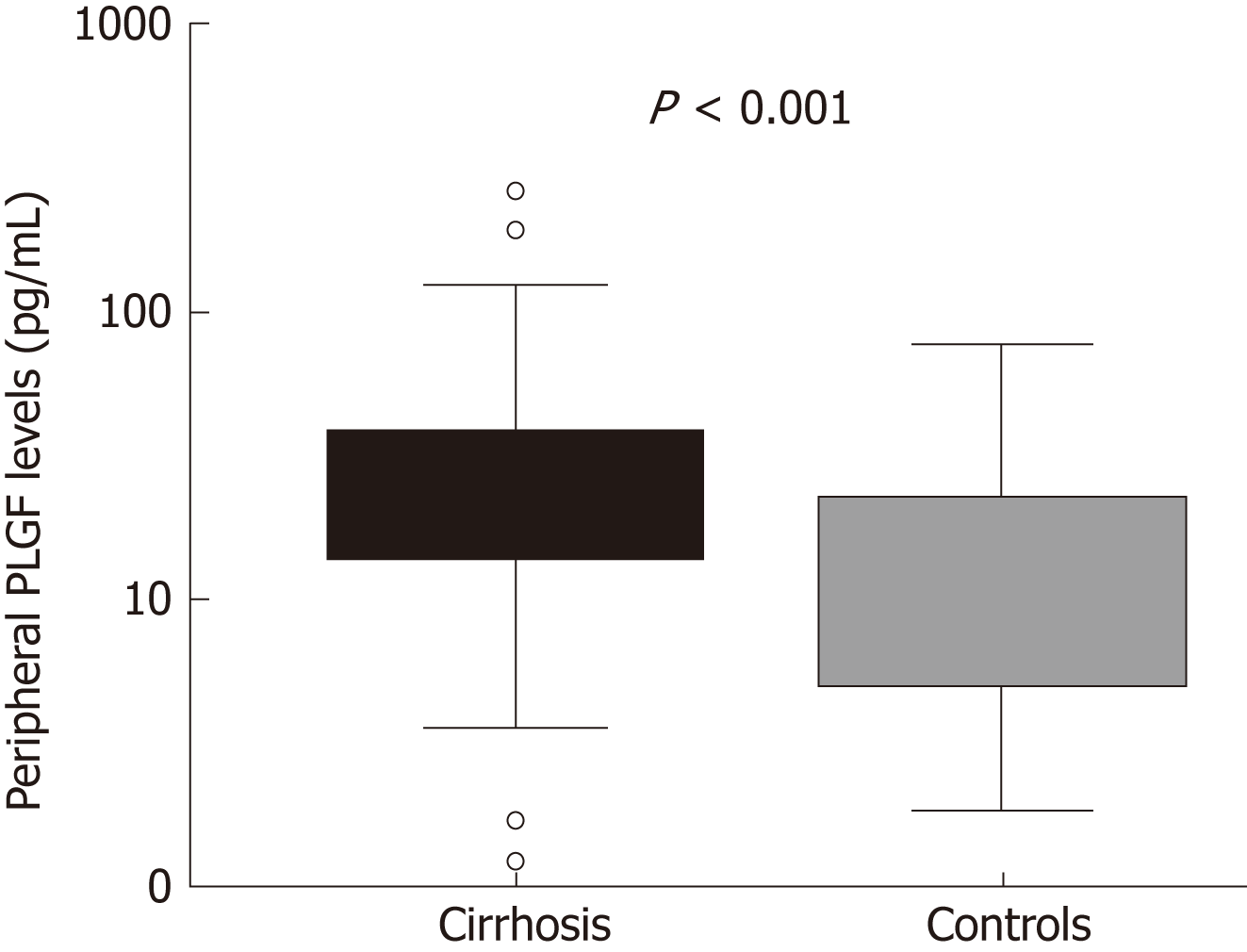
Median peripheral PlGF levels were higher in patients with liver cirrhosis when compared to controls (23.20; range 14.08-37.70 pg/mL vs 9.85; range 4.88-22.72 pg/mL; P < 0.0001; Figure 1). Peripheral PlGF levels were higher in patients with alcohol-induced cirrhosis when compared to patients with hepatitis C virus cirrhosis (29.10; range 16.52-41.71 pg/mL vs 20.92; range 13.72-34.42 pg/mL; P = 0.049) and both were significantly higher than in controls (9.85; range 4.88-22.72 pg/mL). Levels of PlGF at the hepatic vein did not differ significantly from those at the peripheral vein (25.22 pg/mL, range 10.51-34.73 vs 21.22 pg/mL, range 9.48-44.87; P = 0.289). There was a positive linear correlation between the peripheral PlGF levels and Child-Pugh score (r = 0.424; P < 0.0001). Peripheral PlGF levels were increasing with the Child-Pugh stage: in patients with Child-Pugh class A PlGF was 19.64 pg/mL (IQR: 13.38-31.42 pg/mL), in Child-Pugh class B 29.65 pg/mL (IQR: 18.95-46.29 pg/mL) and in Child-Pugh class C 32.80 pg/mL (IQR: 28.00-49.93 pg/mL). PlGF levels were significantly higher in Child-Pugh class B (P < 0.042) and class C (P < 0.002) when compared to Child-Pugh class A. Positive linear correlation was also observed with MELD score (r = 0.283; P < 0.006). PlGF levels at the hepatic vein correlated with Child-Pugh score (r = 0.384; P = 0.036) and were significantly higher in Child-Pugh C stage (P = 0.007), when compared to Child-Pugh A and B stage.
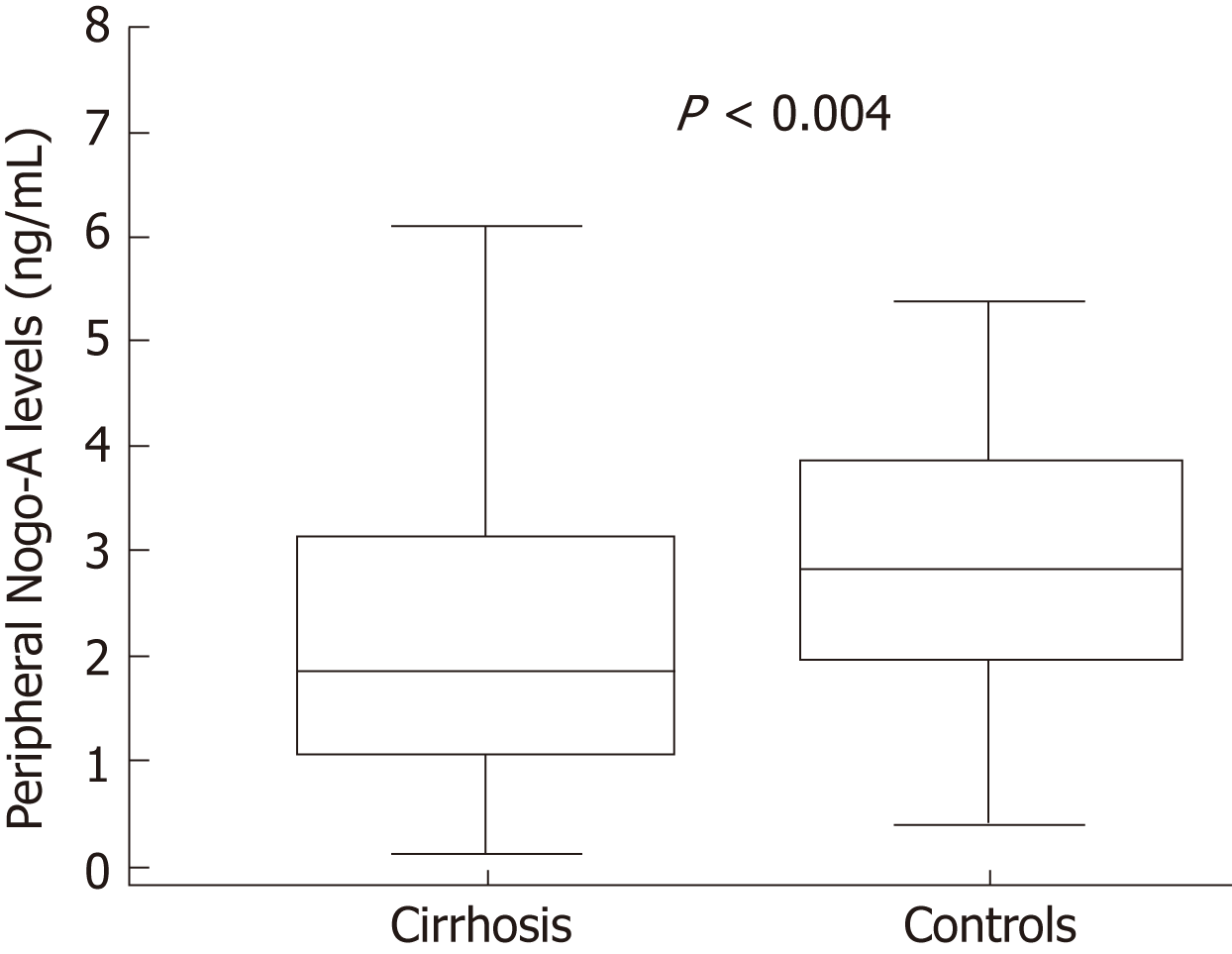
Mean peripheral mean levels of Nogo-A protein were lower in patients with liver cirrhosis (2.19 ± 1.47 ng/mL) when compared to controls (3.12 ± 1.54 ng/mL; P = 0.004; Figure 2). Peripheral Nogo-A protein levels did not differ between the alcohol-induced (2.22 ± 1.59 ng/mL) and hepatitis C virus cirrhosis (2.02 ± 1.33 ng/mL; P = 0.785), however both were significantly lower than in controls (3.15 ± 1.48 ng/mL). Peripheral levels of Nogo-A were significantly lower when compared to the levels at the hepatic vein (1.70 ± 1.19 ng/mL vs 3.48 ± 2.27 ng/mL respect. P < 0.0001). No significant correlation was observed between peripheral and hepatic Nogo-A levels and Child-Pugh score as well as MELD score. Peripheral Nogo-A levels did not differ between the Child-Pugh stage groups (Child-Pugh A 2.19 ± 1.37 ng/mL; Child-Pugh B 1.9 ± 1.49 ng/mL and Child-Pugh C 2.17 ± 1.84 ng/mL).
To evaluate the relationship of plasma PlGF and Nogo-A levels with liver stiffness we calculated the correlation of biomarkers with values of transient elastography (TE; Fibroscan). Transient elastography measurements were available for 74 patients. There was a positive linear correlation between peripheral PlGF values and TE (r = 0.364; P < 0.001), but hepatic levels of PlGF did not correlate with liver stiffness. Peripheral Nogo-A levels did not correlate with liver stiffness.
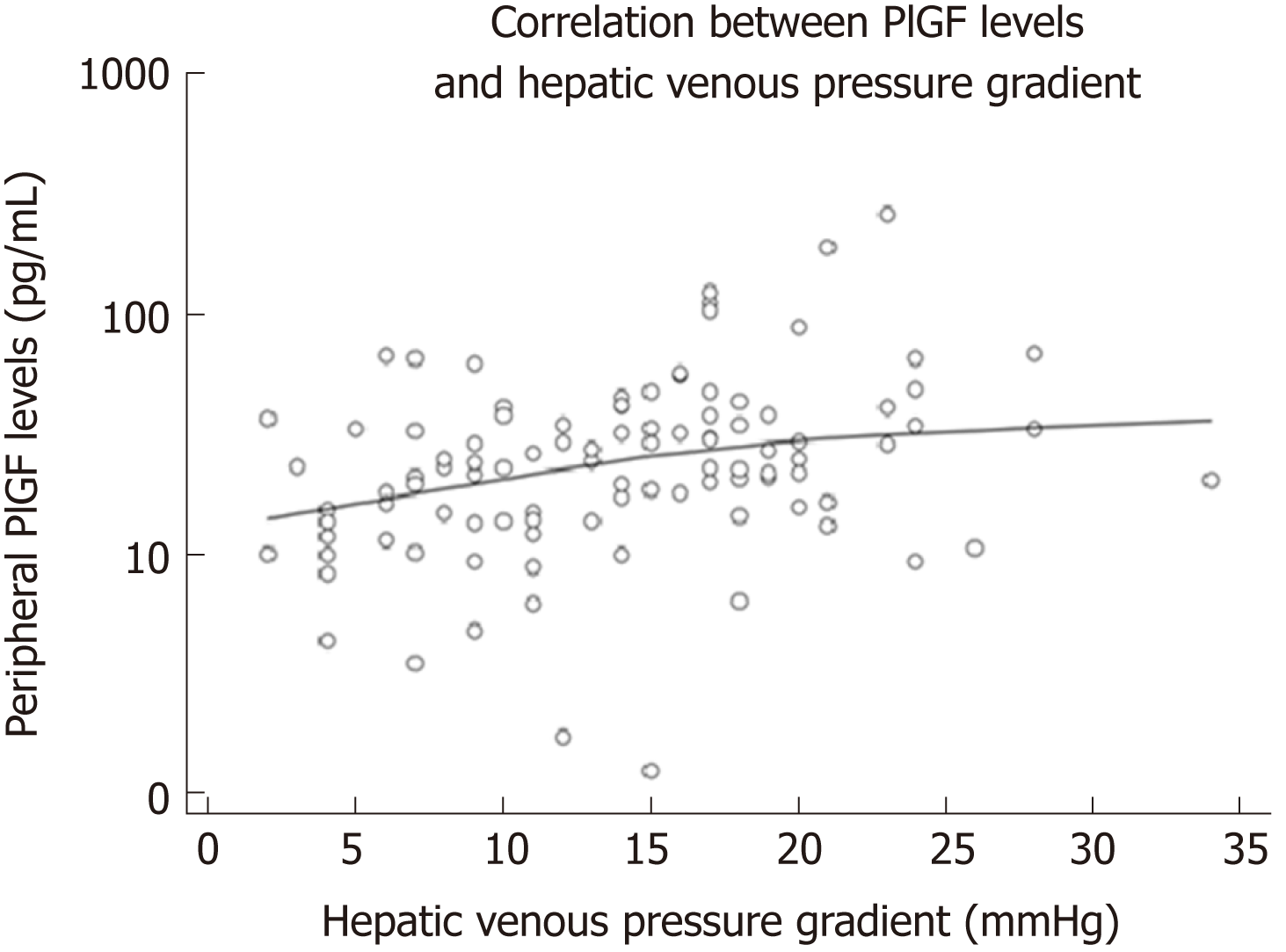
There was a positive linear correlation between peripheral levels of PlGF and HVPG (r = 0.338, P = 0.001, Figure 3). Hepatic PlGF levels did not correlate with HVPG. Higher peripheral PlGF values were significantly associated with ascites (OR = 5.59; P = 0.018). Linear regression revealed that an increase of PlGF by 10 points increased the HVPG by 0.5 mmHg (P = 0.003).
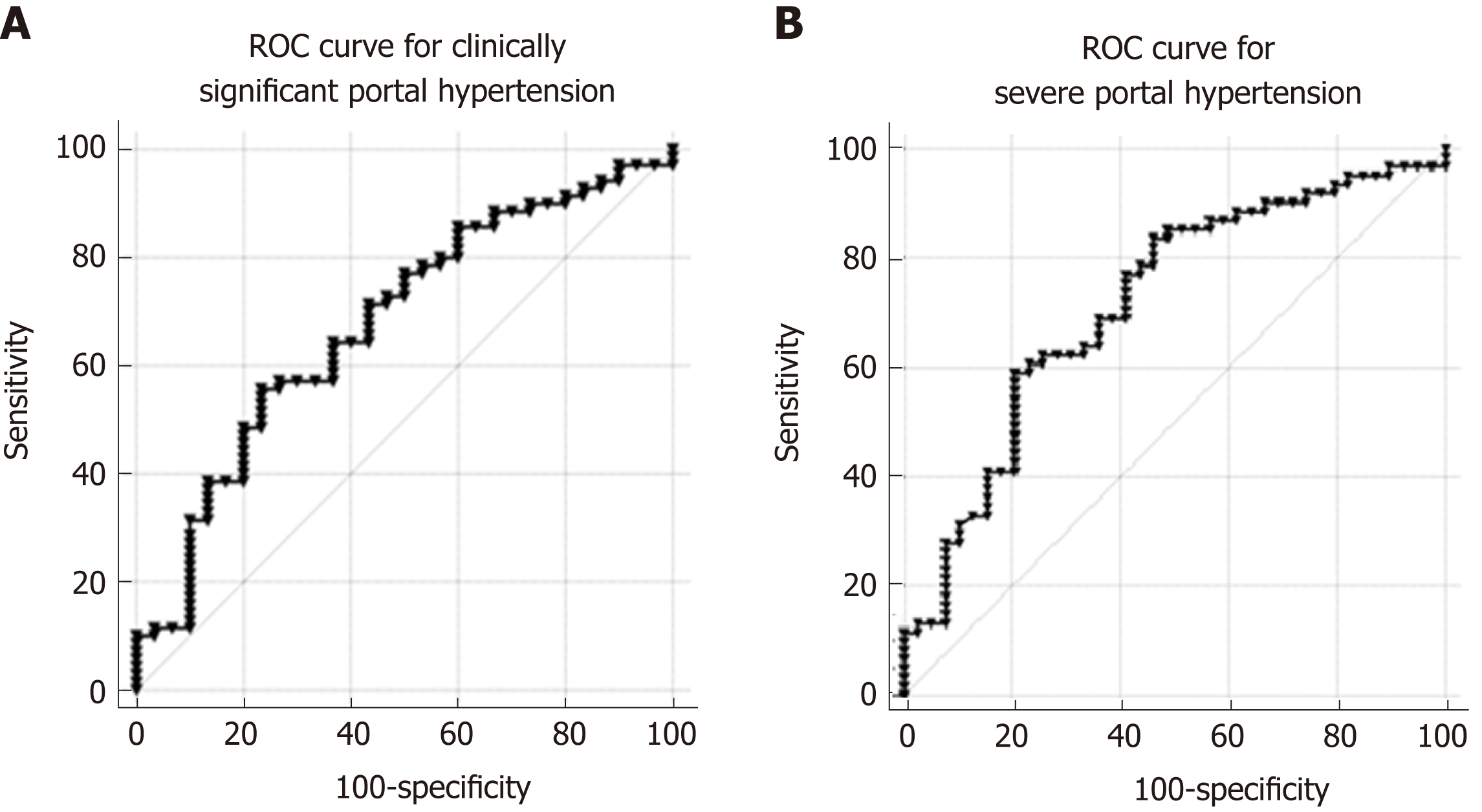
Peripheral levels of PlGF were significantly higher in patients with CSPH (28.20 pg/mL; range 17.09-41.17 pg/mL) than in patients without CSPH (17.20 pg/mL; range 10.12-26.00 pg/mL; P = 0.006), as well as in patients with SPH (29.27 pg/mL; range 19.83-42.68 pg/mL) when compared to patients without SPH (15.39 pg/mL; range 10.15-24.99 pg/mL; P < 0.0001). AUC for the diagnosis of CSPH was 0.68 (CI: 0.56-0.79; P = 0.003 Figure 4A) and for the diagnosis of SPH 0.714 (CI: 0.61-0.82; P < 0.0001 Figure 4B). A cut-off value of 25 pg/mL provided the most accurate sensitivity and specificity to discriminate between patients with and without CSPH, whereas a cut-off value of 26.8 pg/mL helped to differentiate between the patients with and without SPH (Table 2). When classifying patients according to the cut-off values, OR for the presence of CSPH was 4.13 (P = 0.004) and for the presence of SPH 5.58 (P < 0.0001).
| Cut-off value1 | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P value | |
| CSPH | ||||||
| PLGF | 25 pg/mL | 55.7 | 76.7 | 84.8 | 42.6 | 0.003 |
| Nogo-A | 1.12 ng/mL | 36.6 | 93.1 | 92.9 | 37.5 | 0.01 |
| SPH | ||||||
| PlGF | 26.8 pg/mL | 59.0 | 79.5 | 81.8 | 55.4 | 0.0001 |
| Nogo-A | 1 ng/mL | 33.3 | 92.5 | 87 | 48 | 0.014 |
| High-risk varicose veins | ||||||
| PlGF | 19.83 pg/mL | 90.5 | 44.2 | 30.6 | 94.4 | 0.034 |
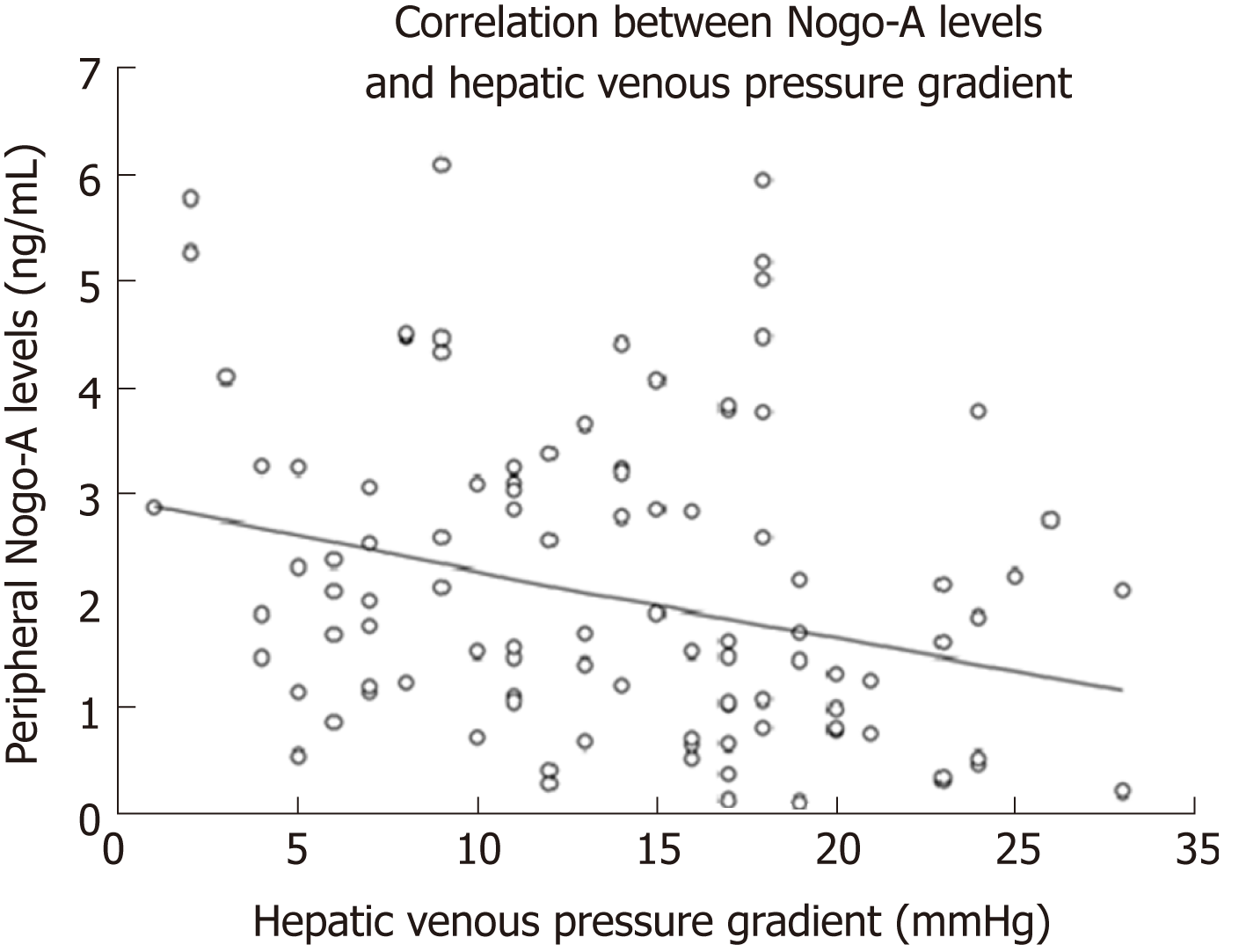
There was a negative linear correlation between the peripheral Nogo-A levels and HVPG (r = -0.267, P = 0.007, Figure 5). Hepatic Nogo-A levels did not correlate with HVPG. Linear regression showed an increase of HVPG by 1.2 mmHg per decrease of Nogo-A levels by 1 point (P = 0.007).
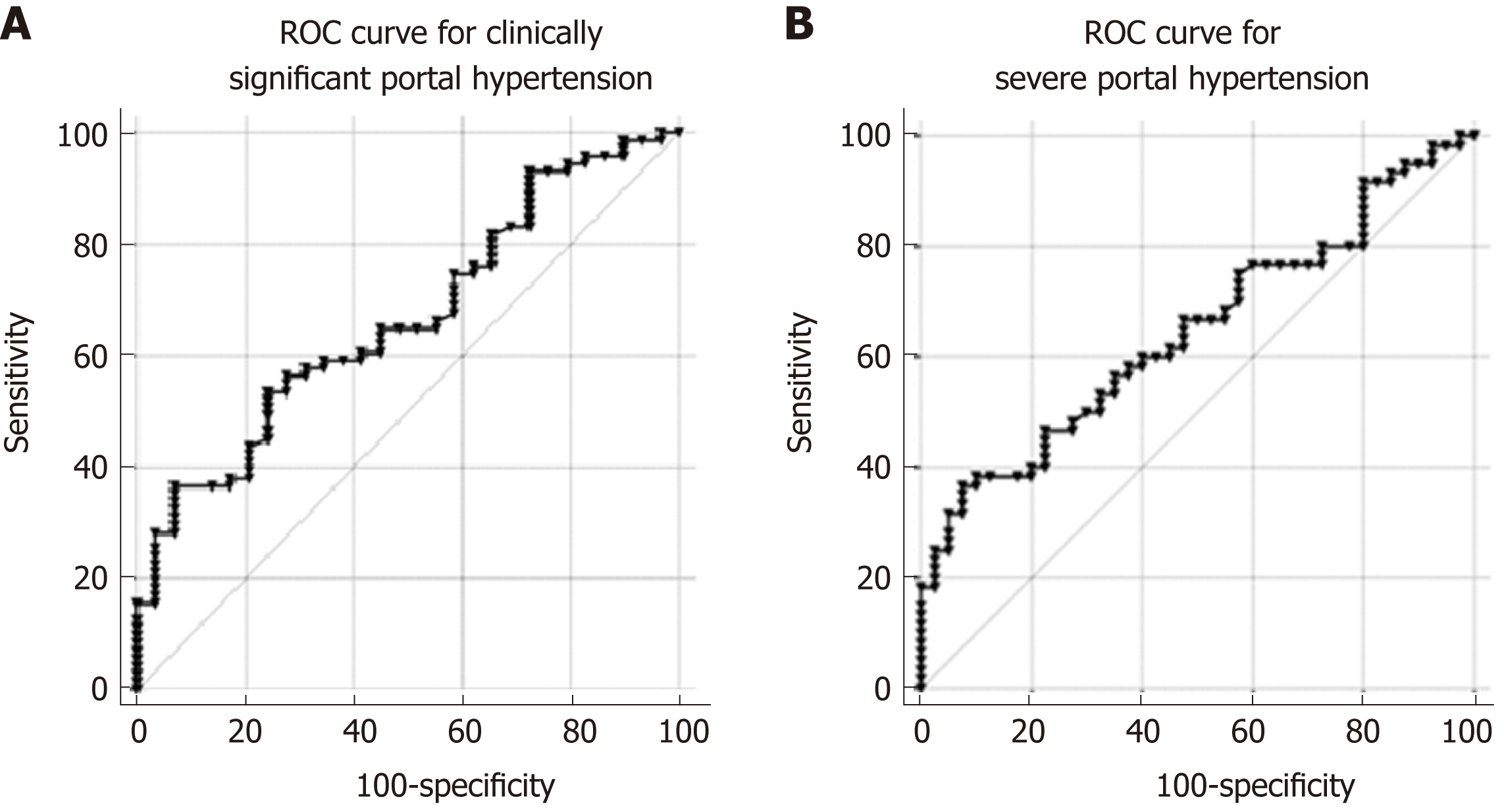
Peripheral levels of Nogo-A were significantly lower in patients with CSPH (1.96 ± 1.39 ng/mL) than in patients without CSPH (2.77 ± 1.53 ng/mL; P = 0.011), as well as in patients with SPH (1.94 ± 1.46 ng/mL) when compared to patients without SPH (2.57 ± 1.42 ng/mL; P < 0.033). AUC for the diagnosis of CSPH was 0.67 (CI: 0.55-0.78; P = 0.01 Figure 6A) and for the diagnosis of SPH 0.65 (CI: 0.54-0.75; P = 0.014 Figure 6B). A cut-off value of 1.12 ng/mL yielded the most accurate sensitivity and specificity to differentiate between patients with and without CSPH whereas a cut-off value of 1 ng/mL helped to differentiate between the patients with and without SPH (Table 2). When classifying patients according to the cut-off values, OR for the presence of CSPH was 7.8 (P = 0.008) and for the presence of SPH 6.12 (P = 0.006).
Both peripheral PlGF and Nogo-A were significantly associated with esophageal varices. Peripheral PlGF values were higher and Nogo-A levels were lower in patients with esophageal varices when compared to patients without esophageal varices (PlGF 29.96 pg/mL; range 20.34-44.03 vs 17.73 pg/mL; range 11.76-27.97; P = 0.001; and Nogo-A 1.94 ± 1.48 ng/mL vs 2.52 ± 1.41 ng/mL; P < 0.05, respectively). The PlGF cut-off value of 19.83 pg/mL and Nogo-A cut-off value of 2.3 ng/mL were the most sensitive and specific in order to discriminate patients having esophageal varices. When categorizing patients according to peripheral PlGF levels above 19.83 pg/mL, the OR for the presence of esophageal varices was 5.25 (P = 0.0001). With peripheral Nogo-A levels below 2.3 ng/mL, the OR for the presence of esophageal varices was 3.2 (P = 0.007). However, the levels of biomarkers did not differ between different grades of esophageal varices (Figures 1-3).
To evaluate the esophageal varices with high risk of bleeding we selected a separate group of patients with large (Figure 3) esophageal varices and with varices containing red wale marks or cherry red spots on endoscopy (n = 21). Higher peripheral PlGF levels were associated with high risk esophageal varices (OR = 7.512; P = 0.01). AUC for the presence of high-risk esophageal varices was 0.62 (CI: 0.54-0.77) (Table 2).
With growing burden on chronic liver diseases worldwide[33], the optimization of care provided to the patients with liver cirrhosis has become a priority. According to the Baveno VI consensus meeting the detection and treatment of early PH as well as the prevention and timely treatment of CSPH, in order to avoid complications and decompensation, are of utmost importance[4]. The consensus has encouraged the search for new treatment strategies to target different pathogenetic components of PH[4].
This prospective study was aimed to gain deeper knowledge on the molecules involved in the abnormal angiogenesis-one of the pathogenetic components of PH. We have demonstrated differences of proangiogenic mediator PlGF and angiogenesis inhibitor Nogo-A plasma levels in patients with liver cirrhosis, when compared to controls. We have also determined a clear correlation of these mediators to PH and complications of PH. Furthermore, we have for the first time demonstrated that plasma concentration of Nogo-A is lower in liver cirrhosis and PH, as well as the predictive values of plasma PlGF and Nogo-A levels in diagnosing CSPH, SPH and PH complications. This information provides further insights in the pathogenesis of PH in liver cirrhosis and might be useful in creating new noninvasive diagnostic models for CSPH, as it is one of the most important prognostic factors for patients with liver cirrhosis.
Data of recent research suggests that angiogenesis plays an important role in the development of PH by contributing to all pathogenetic processes involved in the formation of PH and its complications: An increased intra-hepatic resistance due to the establishment of abnormal liver angioarchitecture, portosystemic collateralization and possibly development and maintenance of splanchnic hyperemia as well as hy-perdynamic circulation[7,15,20]. This evidence supporting the role of angiogenesis in the pathogenesis of PH arises from the animal and human studies involving a potent proangiogenic factor-VEGF. VEGF has been shown to be overexpressed in the splanchnic circulation in animal models of PH[11,12], an increased VEGF mediated angiogenesis was observed in animal models as well as cirrhotic patients with PH[34,35].
PlGF is a member of VEGF family, which is upregulated in pathological tissues and almost undetectable in healthy tissues[16], making it a perspective candidate for the therapeutic strategies. PlGF has been identified by multiple studies as a disease modifying agent in various organs and organ systems, including heart, muscles, skin, solid tumors, nervous system, colon, lungs[16]. In liver PlGF has been found to be associated with inflammation, fibrosis, angiogenesis[17,18] and portal hypertension[36-38]. The role of PlGF in portal hypertension is believed to be associated with pathological angiogenesis and vascular maturing through the action on VEGFR-1 receptor[5,20]. Thus PlGF antibodies or PlGF knockout decreased neo-vascularization, splanchnic blood flow and portal pressure in animal models[9,17,39]. To our knowledge only one previous study evaluated the association of PlGF to liver cirrhosis and HVPG in humans. Van Steenkiste et al[17] reported the increase of PlGF expression in cirrhotic liver, increase in plasma PlGF levels in patients with alcoholic hepatitis and a linear correlation between plasma PlGF levels and HVPG. Our findings support this evidence as we have also observed increased plasma PlGF levels in patients with hepatitis C virus and alcohol-induced liver cirrhosis as well as linear correlation between plasma PlGF levels and HVPG. PlGF levels at the hepatic vein did not differ from the levels at the peripheral vein, suggesting that PlGF levels remain stable after passing to the systemic circulation. The reason why in our study hepatic PlGF levels did not correlate to HVPG needs further research and we are planning to explore this phenomenon with a higher study sample. Furthermore, in our study we have observed that plasma PlGF levels were predictors of CSPH (sensitivity 55.7%, specificity 76.7%) and SPH (sensitivity 59%, specificity 79%) in patients with cirrhosis. Another important finding of our study was the association of plasma PlGF levels with high-risk esophageal varices. The cut-off value of 19.83 pg/mL predicted high-risk esophageal varices with high sensitivity 90% and high negative predictive value 94.4%. Hence plasma PlGF levels could be promising candidates for noninvasive diagnosis of PH and help distinguish the patients with high risk for variceal bleeding.
Nogo-A is a member of reticulon 4 (RTN4) family of membrane-associated multifunctional proteins[40]. There are three isoforms of RTN4 protein: Nogo-A, Nogo-B and Nogo C. Nogo-A was initially most widely studied as a neurite growth inhibitor and was believed to be mainly expressed in the nervous system[36,41]. Apart from the nervous system, Nogo-A was shown to be expressed in the heart tissue, testis and liver, although in the latter the signal was considered unspecific[42]. Furthermore, a recent study by Ramo et al[43] has reported that Nogo-A and Nogo-B are simultaneously expressed in human hepatoma, fibroblast and neuronal cells, concluding than none of the isoforms should be considered a cell type specific isoform and revealed a wider range of functions for Nogo-A outside the nervous system. Another study addressing the expression of Nogo-A in the liver was performed by Hao et al[24], where the authors reported that Nogo-A was highly expressed in four liver cancer cell lines in vitro and the depletion of Nogo-A protein suppressed cancer cell proliferation. To our best knowledge, this study is the first to demonstrate the relationship of Nogo-A with liver cirrhosis and PH in humans. Furthermore, the cut-off value of 1.12 ng/mL helped to differentiate CSPH with high specificity (93.1%) and positive predictive value (92.9%), showing that Nogo-A might be used as a non-invasive marker for the diagnosis of CSPH. Nogo-A levels at the hepatic vein were significantly higher than in the peripheral vein, suggesting that the protein undergoes some metabolism processes in the systemic circulation. However to explain this phenomenon further mechanistic studies are required.
In conclusion, plasma PlGF levels were higher and Nogo-A plasma levels were lower in patients with liver cirrhosis and PH. Both biomarkers showed correlation with HVPG and only moderate predictive value in determining clinically significant, severe portal hypertension and high-risk esophageal varices.
The main limitation of our study is the lack of mechanistic studies concerning Nogo-A protein in healthy liver tissue and cirrhotic tissue, which could better explain the expression of the protein in healthy and diseased liver.
Portal hypertension (PH) is a consequence of liver cirrhosis and can cause serious life-threatening complications. The degree of portal hypertension is one of the most important prognostic factors and is defined by the hepatic venous pressure gradient (HVPG). In order to optimize the care of patients with liver cirrhosis, it is essential to detect early PH and prevent the development of clinically significant PH (CSPH) to avoid decompensation. As current options for prevention and treatment of CSPH are limited to attenuating splanchnic vasodilatation, new insights and deeper knowledge about pathogenetic mechanisms of PH and molecules involved, would aid in search for newer and more effective treatment strategies.
With growing burden on chronic liver diseases worldwide, the optimization of care provided to the patients with liver cirrhosis has become a priority. According to the Baveno VI consensus meeting the detection and treatment of early PH as well as the prevention and timely treatment of CSPH, in order to avoid complications and decompensation, are of utmost importance. The consensus has encouraged the search for new treatment strategies to target different patho-genetic components of PH. Recent studies suggest that one of these alternative pathogenetic components is pathologic angiogenesis. Pathologic angiogenesis has been reported to result from the upregulation of proangiogenic factors and simultaneous downregulation of angiogenesis inhibitors, thus both stimuli might be important and contribute to the pa-thophysiological mechanisms. This prospective study was aimed to gain deeper knowledge on the molecules involved in abnormal angiogenesis-one of the pa-thogenetic components of PH. PlGF has previously been associated with portal hypertension in animal models; however, data in patients with liver cirrhosis are scarce. Nogo-A protein has not been previously evaluated in patients with liver cirrhosis and PH.
In this study we aimed to evaluate plasma levels of PlGF and Nogo-A in liver cirrhosis patients with normal portal pressure and portal hypertension as well as in controls. We also aimed to evaluate the potential of plasma PlGF and Nogo-A levels as biomarkers to predict CSPH and SPH as well as complications of portal hypertension.
A cohort of 122 patients with hepatitis C virus and/or alcohol-induced liver cirrhosis with characterized HVPG were included in the study. Demographic data, medical history, Child-Turcotte-Pugh and Model of End Stage liver disease score, clinical chemistry, liver stiffness values were recorded on the day of the procedure prior HVPG measurement. The degree of portal hypertension was determined by the invasive HVPG measurement. Nogo-A and placental growth factor levels in plasma were evaluated using enzyme liked im40munosorbent assay. The control group consisted of 30 healthy age- and sex- matched individuals
We have demonstrated differences of proangiogenic mediator PlGF and angiogenesis inhibitor Nogo-A plasma levels in patients with liver cirrhosis, when compared to controls. We have also determined a clear correlation of these mediators to PH and complications of PH. Furthermore, we have for the first time demonstrated that plasma concentration of Nogo-A is lower in liver cirrhosis and PH, as well as the predictive values of plasma PlGF and Nogo-A levels in diagnosing CSPH, SPH and PH complications. Futher research will be addressed to evaluate Nogo-A expression in healthy and cirrhotic liver.
We have for the first time demonstrated that plasma concentration of Nogo-A is lower in liver cirrhosis and PH, as well as the predictive values of plasma PlGF and Nogo-A levels in diagnosing CSPH, SPH and PH complications. We believe that our study expands the knowledge on pathologic angiogenesis and its role in the pathogenesis of PH as well as molecules involved. We have demonstrated that indeed PH is a complicated pathology with multiple pathogenetic pathways, which are important in optimizing the care of patients with portal hypertension. This information provides further insights in the pathogenesis of PH in liver cirrhosis and might be useful in creating new noninvasive diagnostic models for CSPH, as it is one of the most important prognostic factors for patients with liver cirrhosis. We have proposed the new hypothesis that Nogo-A protein is associated with portal hypertension and pathologic angiogenesis. This study examined two novel non-invasive markers of portal hypertension, which could be useful in creatinf noninvasive diagnostic models for PH, or new treatment targets. We have demonstrated that two biomarkers of pathologic angiogenesis have moderate predictive value in diagnosing CSPH and SPH as well as high risk esophageal varices. Our study demonstrated that biomarkers of pathologic angiogenesis are associated with liver cirrhosis and PH and have moderate ability to predict CSPH and SPH. These findings might be useful in creating new noninvasive diagnostic models for PH as well as new treatment targets of PH.
Future research will be directed towards gaining more detailed information about Nogo-A protein expression in healthy and cirrhotic liver as well as further understanding of Nogo-A protein roles outside the central nervous system. We plan to conduct mechanistic studies, using liver cell models as well as liver tissue biopsy specimens.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lithuania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garbuzenko DV, Gencdal G, Swierczynski JT, Yoshioka K S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Snowdon VK, Guha N, Fallowfield JA. Noninvasive evaluation of portal hypertension: emerging tools and techniques. Int J Hepatol. 2012;2012:691089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811-16819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2290] [Article Influence: 229.0] [Reference Citation Analysis (3)] |
| 5. | Schwabl P, Laleman W. Novel treatment options for portal hypertension. Gastroenterol Rep (Oxf). 2017;5:90-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Garbuzenko DV, Arefyev NO, Kazachkov EL. Antiangiogenic therapy for portal hypertension in liver cirrhosis: Current progress and perspectives. World J Gastroenterol. 2018;24:3738-3748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 7. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and Fibrogenesis in Chronic Liver Diseases. Cell Mol Gastroenterol Hepatol. 2015;1:477-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Geerts AM, De Vriese AS, Vanheule E, Van Vlierberghe H, Mortier S, Cheung KJ, Demetter P, Lameire N, De Vos M, Colle I. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int. 2006;26:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 11. | Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodés J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | State-of-the-art conference on azidothymidine therapy for early HIV infection. Am J Med. 1990;89:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Coriat R, Gouya H, Mir O, Ropert S, Vignaux O, Chaussade S, Sogni P, Pol S, Blanchet B, Legmann P, Goldwasser F. Reversible decrease of portal venous flow in cirrhotic patients: a positive side effect of sorafenib. PLoS One. 2011;6:e16978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 16. | Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med. 2012;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Van Steenkiste C, Ribera J, Geerts A, Pauta M, Tugues S, Casteleyn C, Libbrecht L, Olievier K, Schroyen B, Reynaert H, van Grunsven LA, Blomme B, Coulon S, Heindryckx F, De Vos M, Stassen JM, Vinckier S, Altamirano J, Bataller R, Carmeliet P, Van Vlierberghe H, Colle I, Morales-Ruiz M. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology. 2011;53:1629-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Li X, Yao QY, Liu HC, Jin QW, Xu BL, Zhang SC, Tu CT. Placental growth factor silencing ameliorates liver fibrosis and angiogenesis and inhibits activation of hepatic stellate cells in a murine model of chronic liver disease. J Cell Mol Med. 2017;21:2370-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967-3979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Gana JC, Serrano CA, Ling SC. Angiogenesis and portal-systemic collaterals in portal hypertension. Ann Hepatol. 2016;15:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Sarkey JP, Chu M, McShane M, Bovo E, Ait Mou Y, Zima AV, de Tombe PP, Kartje GL, Martin JL. Nogo-A knockdown inhibits hypoxia/reoxygenation-induced activation of mitochondrial-dependent apoptosis in cardiomyocytes. J Mol Cell Cardiol. 2011;50:1044-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Osborne SL, Corcoran SL, Prinjha RK, Moore SE. Nogo A expression in the adult enteric nervous system. Neurogastroenterol Motil. 2004;16:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Pernet V. Nogo-A in the visual system development and in ocular diseases. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1300-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Hao CQ, Zhou Y, Wang JP, Peng MJ, Xie YM, Kang WZ, Sun L, Wang PZ, Wan CL, He L, Cai L, Jia ZS. Role of NogoA in the regulation of hepatocellular carcinoma SMMC7721 cell apoptosis. Mol Med Rep. 2014;9:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Tata M, Ruhrberg C, Fantin A. Vascularisation of the central nervous system. Mech Dev. 2015;138 Pt 1:26-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Wälchli T, Pernet V, Weinmann O, Shiu JY, Guzik-Kornacka A, Decrey G, Yüksel D, Schneider H, Vogel J, Ingber DE, Vogel V, Frei K, Schwab ME. Nogo-A is a negative regulator of CNS angiogenesis. Proc Natl Acad Sci USA. 2013;110:E1943-E1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Zhang D, Utsumi T, Huang HC, Gao L, Sangwung P, Chung C, Shibao K, Okamoto K, Yamaguchi K, Groszmann RJ, Jozsef L, Hao Z, Sessa WC, Iwakiri Y. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 648] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 28. | Men R, Wen M, Dan X, Zhu Y, Wang W, Li J, Wu W, Liu X, Yang L. Nogo-B: A potential indicator for hepatic cirrhosis and regulator in hepatic stellate cell activation. Hepatol Res. 2015;45:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Zhao B, Chun C, Liu Z, Horswill MA, Pramanik K, Wilkinson GA, Ramchandran R, Miao RQ. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116:5423-5433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Teng RJ, Rana U, Afolayan AJ, Zhao B, Miao QR, Konduri GG. Nogo-B receptor modulates angiogenesis response of pulmonary artery endothelial cells through eNOS coupling. Am J Respir Cell Mol Biol. 2014;51:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Yu J, Fernández-Hernando C, Suarez Y, Schleicher M, Hao Z, Wright PL, DiLorenzo A, Kyriakides TR, Sessa WC. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc Natl Acad Sci USA. 2009;106:17511-17516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 33. | Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38 Suppl 1:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 34. | Sieber CC, Sumanovski LT, Stumm M, van der Kooij M, Battegay E. In vivo angiogenesis in normal and portal hypertensive rats: role of basic fibroblast growth factor and nitric oxide. J Hepatol. 2001;34:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Cejudo-Martín P, Ros J, Navasa M, Fernández J, Fernández-Varo G, Ruiz-del-Arbol L, Rivera F, Arroyo V, Rodés J, Jiménez W. Increased production of vascular endothelial growth factor in peritoneal macrophages of cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2001;34:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Salcedo Mora X, Sanz-Cameno P, Medina J, Martín-Vílchez S, García-Buey L, Borque MJ, Moreno-Otero R. Association between angiogenesis soluble factors and disease progression markers in chronic hepatitis C patients. Rev Esp Enferm Dig. 2005;97:699-706. [PubMed] |
| 37. | Krawczyk M, Zimmermann S, Hess G, Holz R, Dauer M, Raedle J, Lammert F, Grünhage F. Panel of three novel serum markers predicts liver stiffness and fibrosis stages in patients with chronic liver disease. PLoS One. 2017;12:e0173506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Li X, Jin Q, Yao Q, Zhou Y, Zou Y, Li Z, Zhang S, Tu C. Placental Growth Factor Contributes to Liver Inflammation, Angiogenesis, Fibrosis in Mice by Promoting Hepatic Macrophage Recruitment and Activation. Front Immunol. 2017;8:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Van Steenkiste C, Geerts A, Vanheule E, Van Vlierberghe H, De Vos F, Olievier K, Casteleyn C, Laukens D, De Vos M, Stassen JM, Carmeliet P, Colle I. Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology. 2009;137:2112-24.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Yang YS, Strittmatter SM. The reticulons: a family of proteins with diverse functions. Genome Biol. 2007;8:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Sui YP, Zhang XX, Lu JL, Sui F. New Insights into the Roles of Nogo-A in CNS Biology and Diseases. Neurochem Res. 2015;40:1767-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553-3567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 345] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 43. | Rämö O, Kumar D, Gucciardo E, Joensuu M, Saarekas M, Vihinen H, Belevich I, Smolander OP, Qian K, Auvinen P, Jokitalo E. NOGO-A/RTN4A and NOGO-B/RTN4B are simultaneously expressed in epithelial, fibroblast and neuronal cells and maintain ER morphology. Sci Rep. 2016;6:35969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |