Published online Jun 7, 2019. doi: 10.3748/wjg.v25.i21.2549
Peer-review started: February 17, 2019
First decision: April 16, 2019
Revised: April 19, 2019
Accepted: April 29, 2019
Article in press: April 29, 2019
Published online: June 7, 2019
Processing time: 111 Days and 7.7 Hours
Gastrointestinal angiodysplasias (GIADs), also called angioectasias, are the most frequent vascular lesions. Its precise prevalence is unknown since most of them are asymptomatic. However, the incidence may be increasing since GIADs affect individuals aged more than 60 years and population life expectancy is globally increasing worldwide. They are responsible of about 5% to 10% of all gastrointestinal bleeding (GIB) cases. Most GIADs are placed in small bowel, where are the cause of 50 to 60% of obscure GIB diagnosed with video capsule endoscopy. They may be the cause of fatal severe bleeding episodes; nevertheless, recurrent overt or occult bleeding episodes requiring repeated expensive treatments and disturbing patient’s quality-of-life are more frequently observed. Diagnosis and treatment of GIADs (particularly those placed in small bowel) are a great challenge due to insidious disease behavior, inaccessibility to affected sites and limitations of available diagnostic procedures. Hemorrhagic causality out of the actively bleeding lesions detected by diagnostic procedures may be difficult to establish. No treatment guidelines are currently available, so there is a high variability in the management of these patients. In this review, the epidemiology and pathophysiology of GIADs and the status in the diagnosis and treatment, with special emphasis on small bowel angiodysplasias based on multiple publications, are critically discussed. In addition, a classification of GIADs based on their endoscopic characteristics is proposed. Finally, some aspects that need to be clarified in future research studies are highlighted.
Core tip: Gastrointestinal angiodysplasias (GIADs) are the most frequent vascular malformations. They cause 10% of all gastrointestinal bleeding and 50% of small bowel bleeding cases. In most patients, they manifest with frequent bleeding episodes, requiring expensive treatments and disturbing patient’s quality of life. In this review, we summarize the main aspects related to epidemiology, risk factors and pathophysiology of GIADs. The diagnostic challenge of causality and the efficiency of different treatment modalities available are critically discussed. A novel classification of GIADs based on endoscopic characteristics is proposed and future research directions that may improve diagnostic and therapeutic results will be highlighted.
- Citation: García-Compeán D, Del Cueto-Aguilera ÁN, Jiménez-Rodríguez AR, González-González JA, Maldonado-Garza HJ. Diagnostic and therapeutic challenges of gastrointestinal angiodysplasias: A critical review and view points. World J Gastroenterol 2019; 25(21): 2549-2564
- URL: https://www.wjgnet.com/1007-9327/full/v25/i21/2549.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i21.2549
Gastrointestinal angiodysplasias (GIADs), also called angioectasias, are vascular malformations composed of dilated and tortuous arterial or venous capillaries, which are usually smaller than 5 mm in diameter and located in the mucosal and submucosal layers of the gastrointestinal tract. They are part of a group of vascular aberrations, some of which are hereditary, such as hereditary hemorrhagic telangiectasia and others are acquired, such as gastric antral vascular ectasia, radiation-induced vascular ectasia, Dieulafoy’s lesion and GIADs[1]. GIADs are the most frequent vascular lesions. They are responsible for approximately 10% of gastrointestinal bleeding (GIB) cases and are difficult to diagnose, particularly those placed in the small bowel. Current treatment is very diverse (since therapeutic guidelines have not been established) and bleeding recurrences remain frequent in most cases[2].
In this review, we will address the epidemiology and pathophysiology of the lesions and critically discuss the status in the diagnosis and treatment of GIADs with special emphasis on small bowel angiodysplasias (SBADs). In addition, we will propose a simple classification of angiodysplasias based on their endoscopic characteristics and will discuss the clinical manifestations and bleeding recurrence frequency probably associated to different types of angiodysplasias. Finally, we will point out some aspects that need to be clarified in future research studies.
The prevalence of GIADs is difficult to determine because most of them are asymptomatic. They are incidentally found in 1% to 5% of patients who underwent endoscopic studies and remain asymptomatic in a period of 3 years of follow-up[3,4]. These vascular lesions are mostly detected in patients older than 60 years, so it is thought that they have a degenerative component[4,5]. This association may indicate that its globally incidence is increasing, as the population is becoming older, particularly in developed countries.
GIADs can be found in any segment of the digestive tract. Before the introduction of the video capsule endoscopy (VCE) and the device-assisted enteroscopy (DAE), it was thought that GIADs occurred more frequently in the caecum and ascending colon. In recent studies, in which the entire digestive tract is reviewed, it has been found that these lesions are more frequent in the small bowel (57%-80%), particularly in the proximal segment, followed by the colon (44%) and the stomach (32%)[6,7]. These lesions are located in more than one segment of the digestive tract in 60% of cases[8]. It is reported that they can be responsible of 4% to 7% of nonvariceal upper GIB cases[8,9]. Moreover, 77% of patients with GIADs experience at least one episode of visible bleeding (hematemesis or melena) with or without hemodynamic instability, while the remaining ones have transfusion-dependent or intravenous iron infusion-dependent chronic anemia due to occult bleeding. In the colon, 3% to 40% of the bleeding cases are caused by GIADs[10,11]. In the 2% of such cases, bleeding may be a cause of death, particularly in elderly patients presenting cardiovascular disease or chronic renal failure (CRF)[12].
Several morbid conditions associated with the presence of bleeding GIADs have been identified. The causal relationship between such morbid conditions and the GIADs is difficult to elucidate, since they could be confounding factors because angiodysplasias are more frequent in elderly individuals. In addition, the use of anticoagulant, antithrombotic and platelet antiaggregant drugs are only involved in the induction of bleeding from preexistent vascular lesions.
The association between bleeding GIADs and aortic stenosis has been extensively described. This condition is known as Heyde's disease[13]. Similar association has not been found in other valvular heart diseases[14]. The most accepted explanation of this association is that aortic stenosis could cause the destruction of high-molecular-weight von Willebrand factor (vWF) multimers, leading to acquired von Willebrand disease with a tendency to bleed[15]. Improvement of anemia and reduction or disappearance of bleeding recurrences from GIADs have been described in many patients who underwent aortic valve replacement[16,17]. However, this issue has not been conclusively resolved and currently, the indication of this surgical treatment for GIADs is controversial[18]. The association of bleeding SBADs and low-flow left ventricular assist devices implantation in patients with advanced heart failure has been recently reported. A similar pathogenic mechanism that was described for aortic stenosis, may be involved in this association[19].
On the other hand, GIADs are very common in patients with CRF, and their incidence is further increased by the duration and severity of the renal disease[20]. GIADs are responsible for 19% to 32% of episodes of lower GIB in patients with CRF compared to 5% to 6%, observed in the general population. The most constant factor in the induction of bleeding in CRF patients is platelet dysfunction, which include impaired platelet adhesion and aggregation, produced by extrinsic and intrinsic factors[21,22]. It is not clear if the CRF induces generation of vascular lesions, or if only induces the bleeding due to the hemorrhagic diathesis or both. The risk of bleeding is even greater in patients undergoing hemodialysis where the use of anticoagulants is frequent and uremia is higher[23]. Recently, a significant association between SBADs and other comorbidities such as chronic obstructive pulmonary disease, venous thromboembolism, cardiovascular disease, and liver cirrhosis has been found[24,25] (Table 1).
| Risk factors for gastrointestinal angiodysplasias |
| 1 Age > 60 yr |
| 2 Chronic obstructive pulmonary disease |
| 3 Aortic stenosis (Heyde´s disease) |
| 4 Low-flow left ventricular assist devices |
| 5 Von Willebrand disease |
| 6 Venous thromboembolism |
| 7 Ischemic heart disease |
| 8 Liver cirrhosis |
| 9 Drugs1: Anti-platelet |
| Anti-coagulant |
| Anti-thrombotic |
The pathophysiology of GIADs is not precisely known. Currently, several theories have been proposed, which have different points of contact among them:
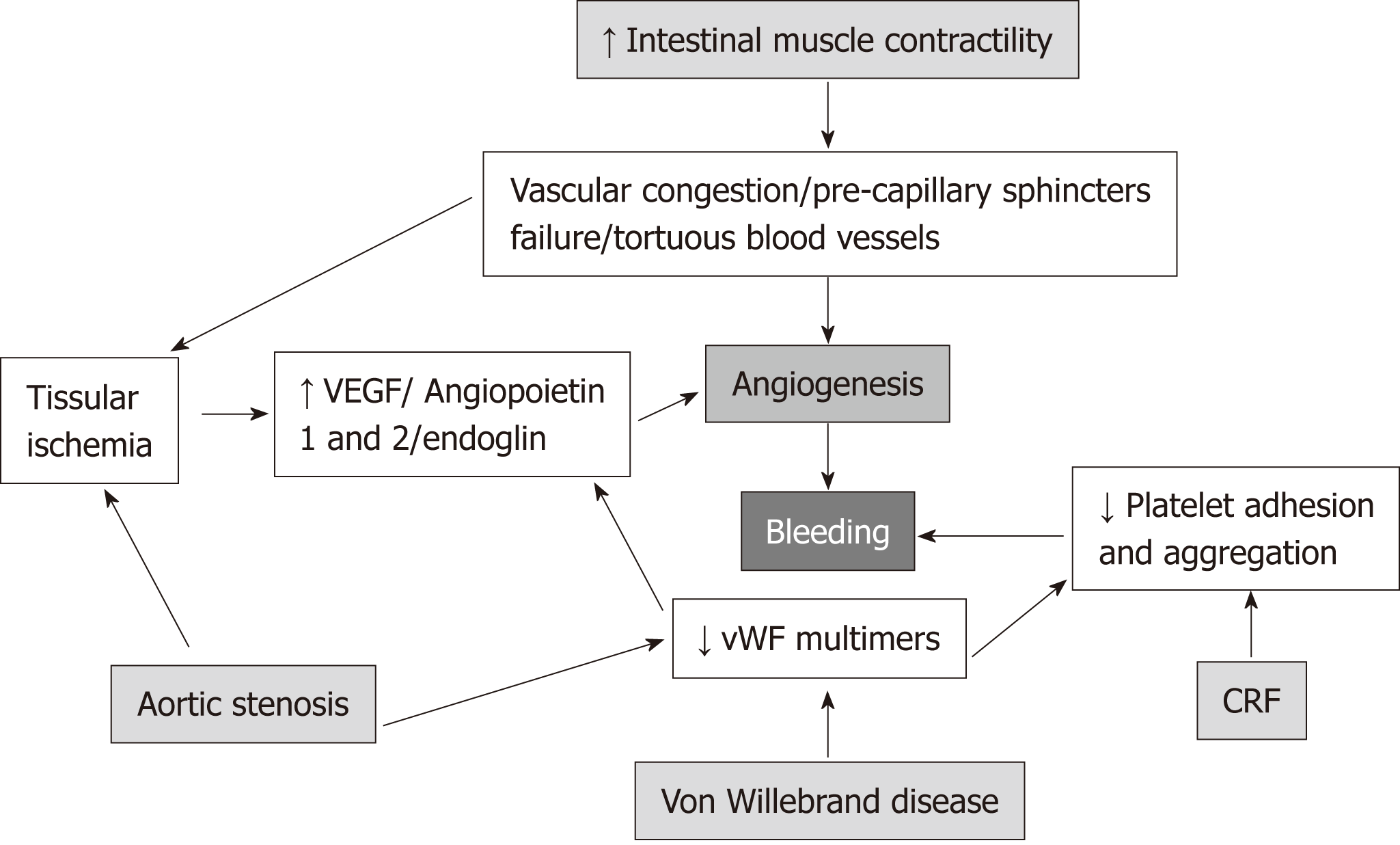
it has been suggested that a repeated and sustained increase in smooth muscle contractions of the muscularis propria would cause intermittent obstruction of the sub mucosal veins of the intestinal wall, which would lead to capillary congestion and insufficiency of the precapillary sphincters with the formation of arteriovenous collaterals[26]. This theory might explain the presence of these lesions in the cecum and the ascending colon, where the intraluminal pressure is higher and the walls are thinner than in other segments of the digestive tract. However, it does not explain the presence of such lesions in other segments thereof such as the stomach.
It has been proposed that muscular contractions of the muscularis propria would trigger a chronic hypoxemia, which would lead to angiogenesis mediated by chemical agents, of which the most important is the vascular endothelial growth factor (VEGF)[27]. This chemical mediator is released in response to tissue hypoxia and is a central active agent in the early stages of angiogenesis[28]. A similar mechanism could also explain the association between GIADs and aortic stenosis[29].
The proliferation of blood vessels (a process known as angiogenesis) could be induced by the primary or secondary reduction of high-molecular-weight vWF multimers, which increases VEGF-dependent angiogenesis and reduces platelet adhesion and aggregation. Finally, a decrease in platelet adhesion and aggregation by drug-induced inhibition of erythropoietin has been observed in CRF[30] (Figure 1).
In support of these angiogenesis theories, elevated tissue expression and serum VEGF levels have been found in patients with GIADs compared to normal subjects[30,31]. Another recent study has found a significant association between GIADs and abnormality of the metabolic pathway of angiopoietins suggesting the potential role of angiopoietin-1 (Ang-1) and Ang-2 in the development of vascular lesions[32].
The finding of non-bleeding GIADs by endoscopy in patients with GIB confronts the clinician with the dilemma of the bleeding responsibility of such lesions. Most authors suggest that in the absence of other evident actively bleeding source, the responsibility of GIAD may be accepted[33]. However, this assumption may be reasonably questioned. On the other hand, clinical or endoscopic systems for estimating probability of bleeding causality of GIADs have not been assessed. This issue is complicated by the fact that a significant number of GIADs are asymptomatic and most of them have a very low probability of bleeding in a period of 3 years after detection[4,34].
GIADs are more common in the small bowel than in other segments of the digestive tract (57%-80%), where they are the cause of 5% of all GIB cases. Before the 2000s, the detection of SBADs was very difficult, since the available diagnostic procedures [scintigraphy, simple angiography, computed tomography (CT), magnetic resonance imaging (MRI)] had low sensitivity and specificity[35,36], therefore multiple diagnostic procedures and hospitalizations were usually performed to these patients before diagnosis, thus increasing the costs of medical care[37]. CT or MRI angiography moderately improved these numbers[38]. Push enteroscopy, which was the only available deep endoscopic method, allowed the revision of only the proximal third of the small bowel[39]. In the last decade, with the introduction of VCE and DAE, the detection of SBADs as the cause of GIB has increased[40-42]. There are three types of DAE: Double-balloon, single-balloon and spiral enteroscopy, which allow the complete or partial revision of SB through retrograde and /or anterograde access[43-45]. These methods are as efficacious as VCE for SBADs detection[46,47] and have the advantage that allow the application of endoscopic therapies to vascular lesions. However, they are invasive and expensive procedures, requiring sedation administration and expertise operating personal; therefore, they are reserved exclusively for endoscopic treatment of lesions, leaving the VCE as first-line diagnostic procedure in obscure GIB (OGIB) patients.
Multiple studies have reported that OGIB is the most frequent indication of VCE, representing from 70% to 75% of cases[48,49]. The presence of GIADs has been demonstrated in about 50% to 60% of such cases[50-52]. In 40% to 60% of cases, SBADs are multiple and 20% coexists with lesions located outside the small bowel, particularly in the colon[53]. From 50% to 80% of the lesions is located in the jejunum and duodenum, 5% to 20% in the ileum and 60% of patients is over 60 years of age[54]. Despite the achievement of recent advances in the diagnosis of SBADs, some important points must be taken into account for interpretation of procedure results. (1) Limitations of the VCE in detection of lesions due to accelerated peristalsis, presence of intestinal detritus or the concealment of lesions by the mucosal folds; (2) The incomplete revision of small bowel with VCE and DAE, which is observed in 10%-16% and 40%-50% of cases respectively[55]; (3) Observer experience in the recognition of lesions. Detection of GIADs is usually easy when they present as typical bright red spots; however, mucosal erosions or erythema may be confounded with GIADs and conversely, pale red angiodysplasias may be unnoticed[56,57].
The bleeding recurrence of SBADs is greater than that of the colon and stomach[58]. In a retrospective study that involved 56 patients with SBADs, the bleeding recurrence occurred in 80% of the cases, the first bleeding episode was observed in an average of 10.7 mo of follow-up, 70% of the cases required transfusions, 67% hospital readmissions and 50% endoscopic, pharmacological or surgical therapy. Deaths related to bleeding occurred in 3.5% of cases. Multiple lesions and cardiovascular diseases were independent predictors of bleeding[59].
Another study showed that SBADs, in presence of simultaneous angiodysplasias in other segments of the digestive tract (colon or stomach), are 4 times more likely to have a bleeding recurrence in one year after diagnosis[60]. SBADs were the cause of severe visible bleeding in 35% of cases[58].
Based on the endoscopic characteristics determined with VCE or DAE, SBADs may be classified in several types, possibly related with different extent of bleeding causality and bleeding recurrence (Table 2).
| Type | Endoscopic characteristics | Bleeding causality | Clinical manifestations | Bleeding recurrence probability |
| Type 1 | Punctuated or patchy lesions with non-pulsatile active bleeding | Certain | Overt bleeding; high frequency of hemodynamic instability | Very high, without hemostatic treatment |
| Type 2 | Non-actively bleeding lesion; stigmata of hemorrhage (ulcer, adherent clot, digested blood debris) | High | Frequently overt bleeding; lower frequency of hemodynamic instability than Type 1 lesions | Highly likely |
| Type 3 | Bright red spots; typical images | Moderate or mild | Overt or occult bleeding; low or null frequency of hemodynamic instability Iron deficiency anemia | Moderate rate; frequently IDA is dependent on iron supplements or blood transfusion |
| Type 4 | Pale red spots | Low or null | Generally occult bleeding Chronic IDA; extra digestive cause of bleeding should be ruled out | When other sources of bleeding have been excluded, re-bleeding is low |
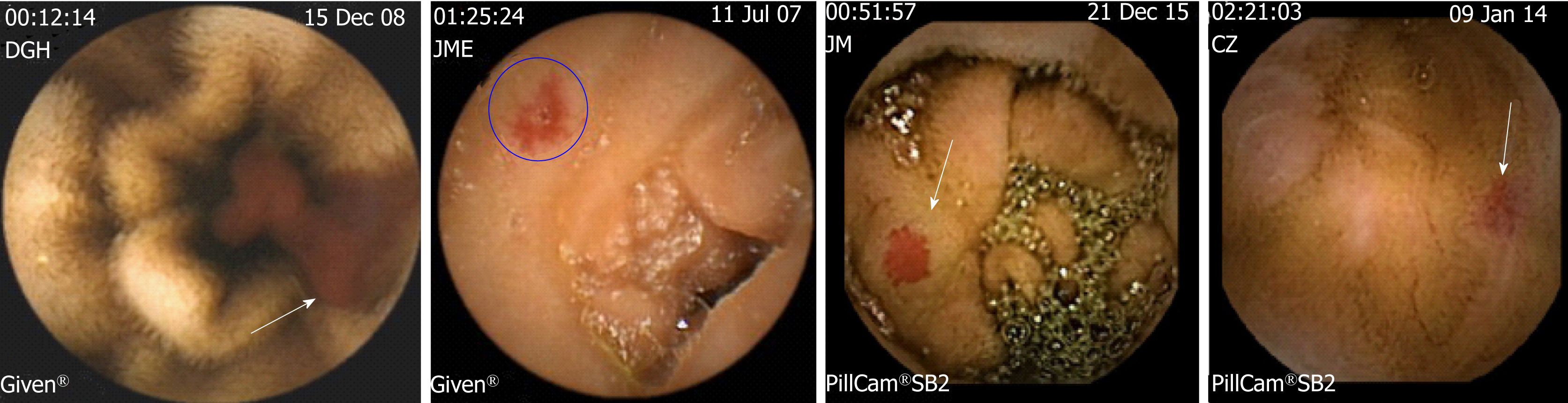
Punctuate or patchy lesions with non-pulsatile active bleeding (which correspond to type 1a and 1b lesions of Yano-Yamamoto classification)[61] (Figure 2A). Patients may have hemodynamic instability and overt GIB. The bleeding recurrence may be very high in the absence of a hemostatic treatment.
Non-actively bleeding lesions showing bleeding stigmata (adherent clot, ulceration or digested blood debris)[62] (Figure 2B). Patients may be hemodynamically stable and may present overt bleeding. Bleeding causality is highly probable and the bleeding recurrence may be high.
Bright-red color patchy spots due to intense vascular congestion (Figure 2C). In the absence of other sources of bleeding, it is moderately likely that such lesions may be the cause of bleeding. Patients with this type of lesions may have occult or overt bleeding in similar proportions and moderate frequency of bleeding recurrence leading to blood transfusion or intravenous iron infusions-dependent anemia.
Pale-red color patchy spots. These lesions are sometimes difficult to discern from erythema or artefacts in VCE studies (Figure 2D). Patients have iron deficiency anemia due to occult bleeding more frequently than overt bleeding. However, in some cases, anemia may be dependent on intravenous iron infusions or blood transfusions. These lesions have a low probability of being responsible of GIB. Therefore, any other source of digestive or extradigestive bleeding should be ruled out by measuring serum iron levels, screening fecal occult blood by the immunological test or a hematologic evaluation. The frequency of bleeding recurrence may be the lowest compared to the other type of lesions.
In non-actively bleeding lesions (types 2 to 4), the influence of the number and extent of lesions on bleeding causality and bleeding recurrence rate is unknown. The stratification of GIADs in combination with the presence or absence of comorbidities and with the concomitant use of pharmacologic anticoagulant agents may be helpful for selecting patients in whom, aggressive hemostatic invasive procedures (radiologic, endoscopic or surgical treatment), long-term prophylactic pharmacological therapy or only clinical observation must be prescribed.
Bleeding SBADs are more difficult to treat than gastric or colonic angiodysplasias due to their inaccessibility. GIB due to angiodysplasias is a frequent therapy challenge, no treatment guidelines are currently available, and so there is a high variability in the management of these patients[63,64].
There are three treatment modalities: (1) Hemostatic; (2) Prophylactic; and (3) Rescue therapy. The former is applied to patients in whom continuous or intermittent persistent-active bleeding has been demonstrated and who frequently have hemodynamic instability, such condition is observed in around 10% of cases[65]. Prophylactic treatment aims to prevent bleeding recurrence in a long-term and rescue treatment is used when other modalities have failed. Whichever the treatment used, it is mandatory to stop the use of antithrombotic, antiaggregant or anticoagulant drugs.
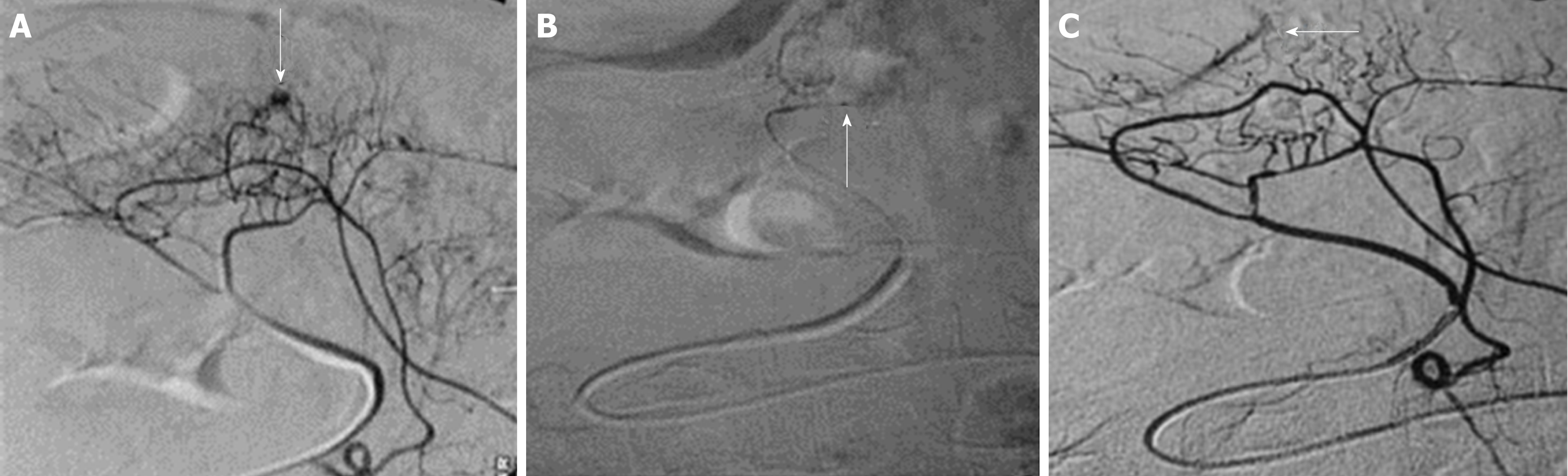
Selective embolization by angiography: This procedure has a high hemostatic effectiveness from 80% to 90%[66-68]. It is performed by selective catheterization of the vessel nourishing the bleeding lesion and subsequent injection of embolizing agents[69] (Figure 3). The most commonly used agents are biodegradable sponges and microcoils. This procedure is associated with complications in 5% to 9% of cases, of which 2% are severe[70,71] and include hematomas, bowel infarction, arterial dissection, thrombosis, and pseudoaneurysms. Although this procedure has more complications than endoscopic therapy, it is more frequently used in patients with actively bleeding SBADs due to their inaccessibility. However, it is a complex procedure, which requires special technical equipment and highly trained operators in endovascular radiology.
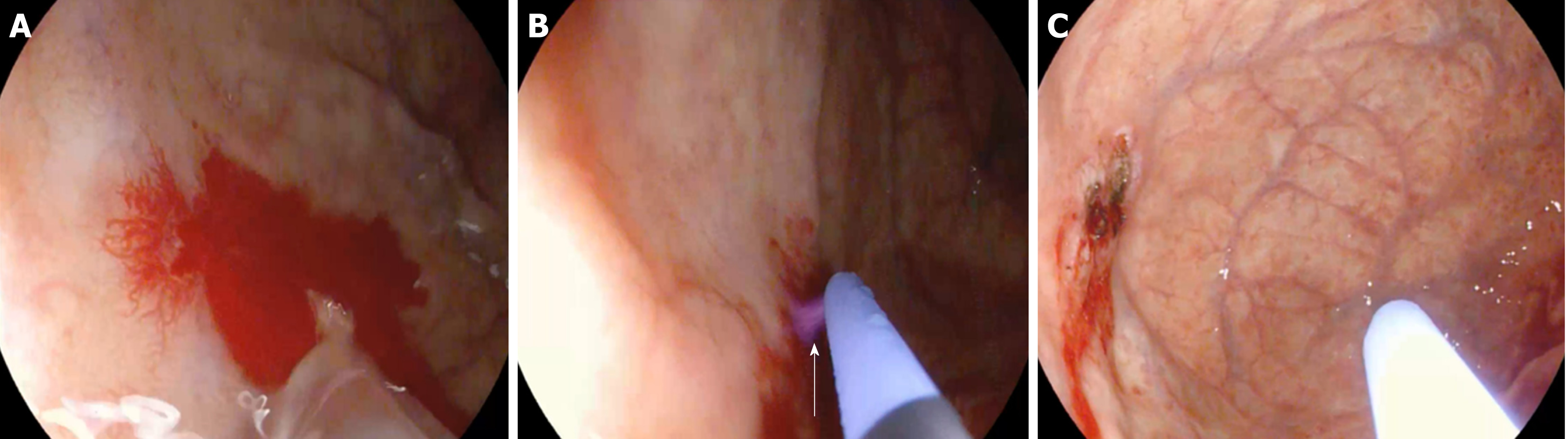
Endoscopic treatment: It consists of the direct coagulation of the vascular lesions with several methods of which, the most used and clinically evaluated, is the argon plasma coagulation (APC)[72-75]. This procedure uses a mixture of electric current and argon gas that is applied through a non-contact endoscopic probe to the vascular lesion (Figure 4). This method has low penetration in the mucosa thus, the risks of perforation are quite low[75]. Other endoscopic contact-probe methods such as monopolar electrocoagulation (Heat Probe, Olympus, Japan) and bipolar electrocoagulation (Gold Probe, Boston Scientific) as well as photo coagulation with Nd:YAG (Neodymium: Yttrium- Aluminium-Garnet) and Argon Laser have been used for long time[76-79]. The endoscopic contact-probe methods such as electrocoagulation and laser photocoagulation techniques have similar hemostatic effectiveness compared to APC for bleeding GIADs[80], conversely, they have higher incidence of perforation particularly when they are used in the colon, where intestinal thin walls are present. Laser photocoagulation is a high cost technique that requires specialist expertise to deliver the treatment[81].
Hemoclipping[82-83] and multiple endoscopic band ligation[84,85] have been reported to be effective as hemostatic therapy of bleeding vascular lesions. Nevertheless, reports are anecdotal with low number of patients; therefore, these techniques may be only used in selected cases, probably as rescue therapy. In conclusion, the first line recommended endoscopic treatment for bleeding GIADs is APC[86].
Endoscopic therapy is indicated for patients with few vascular lesions confined to one digestive tract segment and accessible to the endoscope. It has similar hemostatic effectiveness to that observed in selective angiographic embolization; however, bleeding recurrences are very frequent[33]. In a study of 183 with GIADs patients who underwent endoscopic treatment, there was a one-year bleeding recurrence rate of 34.5%, while recurrence rate at 2 years or more was 42% to 60%[74,87,88]. In a systematic review of 24 articles involving 490 patients with GIADs treated with endoscopic therapy, the bleeding recurrence rate was similar to that observed in patients without treatment (42.7% vs 49.2%)[89].
The complication rate of endoscopic therapy is lower than that observed in selective embolization by angiography (1.7%), with intestinal perforation being the most frequent one. Sub mucosal saline injection (with or without adrenaline) to form a fluid cushion in the colon before APC therapy, has been demonstrated to be effective for reducing this complication[90,91].
Explosion of colonic gas is a rare complication that has been observed with the use of APC and electrocoagulation techniques. It is thought to be secondary to accumulation of combustible gases in the colon due to poor bowel preparation. So, a full colonic cleansing with oral purgatives is highly recommended before performing these techniques[92,93]. The results in the treatment of the SBADs are poorer than those from the colon, with a higher bleeding recurrence rate being observed (78%)[59].
The high recurrence of bleeding in GIADs patients after endoscopic treatment could be due to the presence of untreated lesions, not endoscopically detected (particularly in small bowel) or the neoformation of lesions. Due to the long-term ineffectiveness of endoscopic therapy, we think that it should be followed with a long-term prophylactic pharmacological treatment or it can be performed “on demand”, when bleeding recurrences occur[94]. In this context, combination therapy (endoscopic or radiological as hemostatic methods followed by pharmacological treatment as prophylactic therapy) has not been systematically evaluated. In a study with 52 patients with bleeding GIADs, treatment with APC followed by monthly ad-ministration of lanreotide during one year, proved to be more effective than isolated endoscopic treatment since it significantly reduced the recurrence of bleeding and the long-term requirements for blood transfusions[95].
Currently, highly effective prophylactic treatment for GIADs is not available for clinical use. Nevertheless, several pharmacological agents have been evaluated recently; some of them have shown promising results (Table 3).
| Author (yr) | Design | n | Treatment | Follow–up (mo) | Results |
| Hormonal therapy | |||||
| Van Cutsem et al[98], 1990 | DB, Cr Ov, Rx vs P, | 10 | Oral Ethinyloestradiol and norethisterone for 6 mo | 6 | Significant reduction of transfusion requirement in Rx patients vs controls (P < 0.003). Non-significant side effects in treated patients |
| Barkin and Ross[99],1998 | Cohort | 25 | Oral Mestranol and norethynodrel or norethinidrone | 18 (1-52) | None patients rebled during Rx. Side effects in 44% of patients |
| Junquera et al[100], 2001 | R, DB, Rx vs P | Rx = 33 P = 35 | Oral Norethisterone and ethinylstradiol for 1-2 yr | 12 (12-36) | Bleeding recurrence: Rx = 39% vs P = 46%; P = NS |
| Somatostatin analogues | |||||
| Nardone et al[106], 1999 | Cohort | 17 | SC Octreotide 100 µg/8 h for 6 mo | 12 | Rates of complete, partial and non-response were 59%, 23% and 18% respectively; non-significant side effects |
| Junquera et al[107], 2007 | Cohort, Rx vs P | Rx = 32 P = 38 | SC Octreotide 50 µg/12 h for 12-24 mo | 13 (12-36) | Bleeding recurrence: Rx = 23% vs P = 48% (P = 0.04); major adverse effects: Rx = 3.1% vs P = 2.6% |
| Scaglione et al[111], 2007 | Cohort | 13 | IM Lanreotide 10 mg/mo for 12 mo | 33 (12-60) | Rates of complete, partial and non-response were 70% and 23% respectively; non-significant side effects were observed |
| Molina et al[111], 2009 | Cohort | 11 | IM Lanreotide 20 mg/mo | 15 (5-48) | Patients with serious comorbidities; treatment reduced transfusion requirements and bleeding related hospitalizations |
| Bon et al[113], 2012 | Cohort | 15 | IM Lanreotide 20 mg/mo for 12 mo | 14 (10-36) | Rx significantly reduced bleeding recurrences, transfusion requirements and increased serum Hb levels; side effect were rare |
| Holleran et al[108], 2016 | Cohort | 24 | IM Lanreotide 20 mg/mo for 3 mo | 8 (3-17) | Rates of complete, partial and non-response were 70%, 20% and 10% respectively; adverse events: 30% |
| Thalidomide | |||||
| Ge et al[121], 2011 | Cohorts Rx vs C | R = 28 C: 27 | Oral thalidomide 100 mg/day for 4 mo; oral iron 400 mg/day for 4 mo | 39 (8-52) | Rate of response; Rx = 71.4 vs 3.7%; non-significant side effects |
| Garrido et al[119], 2012 | Cohort | 12 | Oral 200 mg/day for 4 mo | 4 | Increase of serum Hb levels; severe side effects requiring Rx discontinuation in 17% |
Hormonal drugs: This therapy (a mixture of estrogens and progestogens) began to be used in the 80s. Initial studies reported a favorable effect on the recurrence of bleeding[96-99]. However, they were retrospective and non-controlled studies with few patients. A good quality, multicenter, prospective, controlled, double-blind, placebo-compared study involving 72 patients with acute and chronic bleeding treated with hormonal drugs for 1 to 3 years showed no therapeutic benefit[100]. This study was key to abandon the use of these drugs for GIADs therapy.
Somatostatin analogues: In the 90s, octreotide and lanreotide began to be used in the treatment of bleeding GIADs. These drugs are somatostatin analogues and their mechanism of action on GIADs is probably multiple. It is suggested that they have a hemodynamic effect on the splanchnic circulation; they inhibit angiogenesis-promoter chemical-factors such as VEGF, b-FGF and IGF-1 k. In addition, these drugs may stimulate relaxation of intestinal smooth muscle thus reducing the chronic obstruction of sub mucosal veins[101,102].
Initially, a beneficial effect of subcutaneous octreotide administration on bleeding recurrence was reported in isolated cases or short series of patients with bleeding GIADs refractory to other treatments[103-105]. A study involving 17 patients, who received 300 µg/day for 6 mo, showed a reduced requirement for treatment of anemia in 82.3% of the patients[106]. Another long-term placebo-compared study involving 70 patients treated with 100 µg daily for 1 to 2 years demonstrated a 1- and 2-year effectiveness of 77 and 68% vs 55 and 36% of the control group, respectively (P = 0.03). In this study, octreotide therapy and the number of previous episodes of bleeding were independent predictors of efficacy[107].
In the 2000s, the first studies with lanreotide (which is a drug used in the treatment of acromegaly and the symptoms caused by carcinoid syndrome) were published. The monthly parenteral administration of this drug facilitates the patient's treatment compliance. In three publications, monthly administration of 10 to 20-mg to patients with bleeding GIADs, showed a beneficial effect of 75% to 90% of cases. This effectiveness was measured by reduction of blood transfusion and iron infusions requirement as well as bleeding episodes and bleeding-related hospitalizations reduction. However, almost all the studies published to date are retrospective, with a small number of patients, and non-comparative[108-113]. A meta-analysis and systematic review of 24 studies involving 831 individuals with GIADs showed that octreotide and lanreotide treatment is more effective than endoscopic therapy in reducing recurrence of bleeding in the short and long term[72].
Finally, treatment with somatostatin analogues is associated with a low frequency of adverse effects. Most of them are digestive (diarrhea, abdominal pain) and are tolerable, rarely necessitating interruption of treatment. However, these drugs should be cautiously used in patients with asymptomatic cholecystolithiasis or difficult-to-treat diabetes mellitus[114].
Thalidomide: A series of isolated reports on the beneficial effect of thalidomide in the treatment and prevention of bleeding recurrence of GIADs[115-119] has been recently published. It is thought that thalidomide acts by inhibiting angiogenesis by suppressing VEGF[120]. In a randomized controlled trial involving 52 patients who received orally 100 mg daily of thalidomide vs 400 mg of iron for 4 mo, it was observed that the therapeutic response, which was measured by a 50% reduction in bleeding episodes during one year, was significantly higher in the patients who received the drug (71% vs 4%). Thalidomide reduced the number of transfusion-dependent patients (11 vs 48%) and re-hospitalizations due to bleeding (39% vs 100%). However, patients treated with thalidomide showed more adverse reactions than controls (71.4% vs 33.3%, respectively) of which fatigue, constipation, dizziness and peripheral edema were the most frequent[121].
In conclusion, prophylactic therapy with somatostatin analogues or thalidomide seems to be an effective alternative of GIADs. However, prospective, controlled, randomized studies with a sufficient number of patients are required in order to confirm this issue. A cost-benefit ratio assessment should be included in these studies, since these are high-cost drugs at present, especially somatostatin analogues.
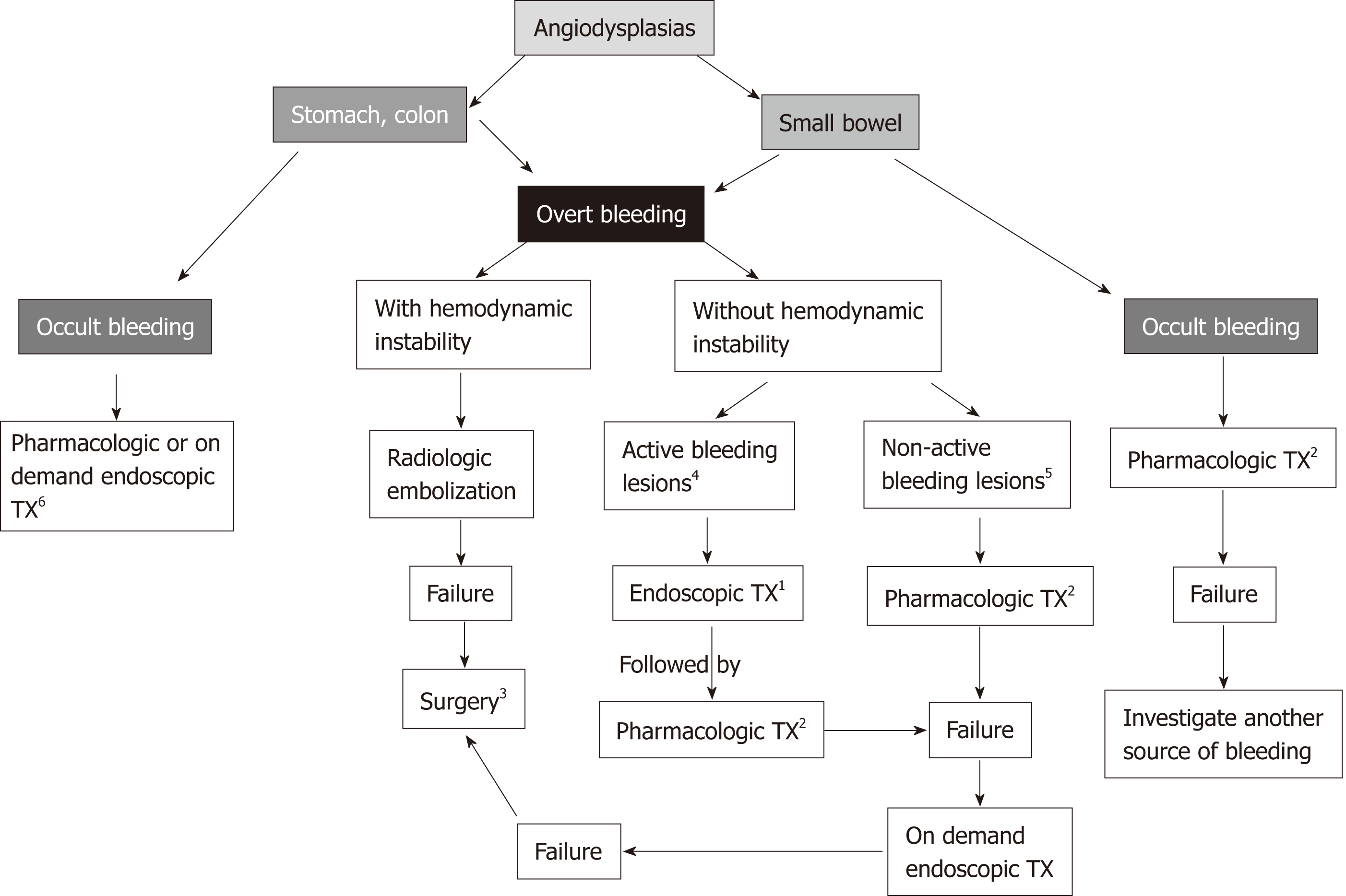
This therapy is used in patients with acute bleeding or recurrent chronic bleeding refractory to initially administered measures. There is no defined rescue treatment strategy, so radiological, endoscopic and pharmacological treatments have been sequentially or combinedly used with this aim[75,122,123]. Notwithstanding, the most effective rescue treatment is surgical resection[124]. However, it is associated with high perioperative morbidity and mortality rates of 23% and 12%, respectively[125], since most patients with GIADs are of advanced age and frequently suffer diverse comorbidities. After the introduction of technically advanced non-surgical methods for GIADs, as those previously described in this review, the number of patients undergoing surgery has significantly reduced. Nonetheless, surgery remains a valid therapeutic alternative, which must be considered when the other therapeutic modalities have failed, in patients with acceptable operative risk, who have focalized lesions in any segment of the digestive tract and bleeding causality of vascular lesions is confirmed[126]. The Figure 5 describe a practical guide for the treatment of GIADs based on the place where they are located, the clinical manifestations and the endoscopic type of lesions.
The genesis of GIADs is closely linked to angiogenesis. This is a complex process, in which multiple chemical mediators participate [VEGF, endoglin, Ang-1, Ang-2, platelet derived growth factor, and tumor necrosis factor alpha (TNFα)]. An increase in VEGF has been found in response to tissue hypoxemia[30]. Endoglin is a trans-membranal glycoprotein that is strongly expressed in endothelial cells during the process of angiogenesis[127]. Ang-2, (which is a member of the angiopoietin family), rises rapidly in response to angiogenic stimuli and it is thought to induce the formation of immature and unstable blood vessels[128]. vWF stores in endothelial cells and regulates the release of Ang-2 and integrin by a complex interaction with the VEGF receptor[32].
A recently published study with GIAD patients showed a significantly lower serum of Ang-1/Ang-2 ratio compared to subjects without GIADs. In angiodysplastic tissue, significant differences in the messenger RNA expression of Ang-1 and Ang-2 and its Tyrosine kinase receptor 2 were found[32]. Another recent prospective study showed high Ang-2 levels and low tendency of Ang-1 and TNF-α levels in patients with SBADs compared to individuals who had bleeding from other causes[129].
Despite the progress achieved in this field, the clinical use of chemical markers of angiogenesis as diagnostic tools is still in early stage. However, it is a promising area of research. Biological markers of angiogenesis may be useful tools for evaluation of severity of lesions, prediction of bleeding recurrence, and therapeutic response. It must be remembered that the somatostatin analogues and thalidomide have an anti-angiogenic effect. The deeper knowledge of the pathogeneses of GIADs, particularly in relation to angiogenesis, will possibly contribute to the identification of more highly specific angiogenesis mediators and stimulate the development of highly selective antagonist agents directed against specific targets in order to improve therapeutic results.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Singh S, Sitkin S S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Gordon FH, Watkinson A, Hodgson H. Vascular malformations of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2001;15:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Jackson CS, Strong R. Gastrointestinal Angiodysplasia: Diagnosis and Management. Gastrointest Endosc Clin N Am. 2017;27:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Ueno S, Nakase H, Kasahara K, Uza N, Kitamura H, Inoue S, Mikami S, Matsuura M, Chiba T. Clinical features of Japanese patients with colonic angiodysplasia. J Gastroenterol Hepatol. 2008;23:e363-e366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564-567. [PubMed] |
| 5. | Dodda G, Trotman BW. Gastrointestinal angiodysplasia. J Assoc Acad Minor Phys. 1997;8:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Bollinger E, Raines D, Saitta P. Distribution of bleeding gastrointestinal angioectasias in a Western population. World J Gastroenterol. 2012;18:6235-6239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | DeBenedet AT, Saini SD, Takami M, Fisher LR. Do clinical characteristics predict the presence of small bowel angioectasias on capsule endoscopy? Dig Dis Sci. 2011;56:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Clouse RE, Costigan DJ, Mills BA, Zuckerman GR. Angiodysplasia as a cause of upper gastrointestinal bleeding. Arch Intern Med. 1985;145:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 59] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Moretó M, Figa M, Ojembarrena E, Zaballa M. Vascular malformations of the stomach and duodenum: An endoscopic classification. Endoscopy. 1986;18:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 10. | Farrell JJ, Friedman LS. Review article: The management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2005;21:1281-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, Jowell P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: A randomized controlled trial. Am J Gastroenterol. 2005;100:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Cappell MS, Gupta A. Changing epidemiology of gastrointestinal angiodysplasia with increasing recognition of clinically milder cases: Angiodysplasia tend to produce mild chronic gastrointestinal bleeding in a study of 47 consecutive patients admitted from 1980-1989. Am J Gastroenterol. 1992;87:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Heyde EC. Gastrointestinal Bleeding in Aortic Stenosis. N Engl J Med. 1958;259:196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 14. | Shoenfeld Y, Eldar M, Bedazovsky B, Levy MJ, Pinkhas J. Aortic stenosis associated with gastrointestinal bleeding. A survey of 612 patients. Am Heart J. 1980;100:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: Is acquired von Willebrand's disease the link? Lancet. 1992;340:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Scheffer SM, Leatherman LL. Resolution of Heyde's syndrome of aortic stenosis and gastrointestinal bleeding after aortic valve replacement. Ann Thorac Surg. 1986;42:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Cappell MS, Lebwohl O. Cessation of recurrent bleeding from gastrointestinal angiodysplasias after aortic valve replacement. Ann Intern Med. 1986;105:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Mehta PM, Heinsimer JA, Bryg RJ, Jaszewski R, Wynne J. Reassessment of the association between gastrointestinal arteriovenous malformations and aortic stenosis. Am J Med. 1989;86:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Tabibian JH, Rhoades DP, Forde KA, McLean RC, Chandrasekhara V. Timing of Gastrointestinal Bleeding After Implantation of Left Ventricular Assist Devices Associates With Anatomic Location, Presentation, and Management. Clin Gastroenterol Hepatol. 2019;17:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: Role of vascular ectasia. Am J Gastroenterol. 1996;91:2329-2332. [PubMed] |
| 21. | Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Kringen MK, Narum S, Lygren I, Seljeflot I, Sandset PM, Trøseid AM, Johansen PW, Brørs O, Holthe MR. Reduced platelet function and role of drugs in acute gastrointestinal bleeding. Basic Clin Pharmacol Toxicol. 2011;108:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Weigert AL, Schafer AI. Uremic bleeding: Pathogenesis and therapy. Am J Med Sci. 1998;316:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Holleran G, Hall B, Hussey M, McNamara D. Small bowel angiodysplasia and novel disease associations: A cohort study. Scand J Gastroenterol. 2013;48:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Igawa A, Oka S, Tanaka S, Kunihara S, Nakano M, Aoyama T, Chayama K. Major predictors and management of small-bowel angioectasia. BMC Gastroenterol. 2015;15:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Boley SJ, Sammartano R, Adams A, DiBiase A, Kleinhaus S, Sprayregen S. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72:650-660. [PubMed] |
| 27. | Baum S, Athanasoulis CA, Waltman AC, Galdabini J, Schapiro RH, Warshaw AL, Ottinger LW. Angiodysplasia of the right colon: A cause of gastrointestinal bleeding. AJR Am J Roentgenol. 1977;129:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 109] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6954] [Article Influence: 316.1] [Reference Citation Analysis (0)] |
| 29. | Heer M, Sulser H, Hany A. Angiodysplasia of the colon: An expression of occlusive vascular disease. Hepatogastroenterology. 1987;34:127-131. [PubMed] |
| 30. | Junquera F, Saperas E, de Torres I, Vidal MT, Malagelada JR. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Fujita H, Momoi M, Chuganji Y, Tomiyama J. Increased plasma vascular endothelial growth factor levels in patients with angiodysplasia. J Intern Med. 2000;248:268-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Holleran G, Hall B, O'Regan M, Smith S, McNamara D. Expression of Angiogenic Factors in Patients With Sporadic Small Bowel Angiodysplasia. J Clin Gastroenterol. 2015;49:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Fan GW, Chen TH, Lin WP, Su MY, Sung CM, Hsu CM, Chi CT. Angiodysplasia and bleeding in the small intestine treated by balloon-assisted enteroscopy. J Dig Dis. 2013;14:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | Pennazio M, Eisen G, Goldfarb N; ICCE. ICCE consensus for obscure gastrointestinal bleeding. Endoscopy. 2005;37:1046-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, Gisbert JP, Bujanda L, Castro M, Muñoz M, Del-Pino MD, Garcia S, Calvet X. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Junquera F, Quiroga S, Saperas E, Pérez-Lafuente M, Videla S, Alvarez-Castells A, Miró JR, Malagelada JR. Accuracy of helical computed tomographic angiography for the diagnosis of colonic angiodysplasia. Gastroenterology. 2000;119:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Foutch PG, Sawyer R, Sanowski RA. Push-enteroscopy for diagnosis of patients with gastrointestinal bleeding of obscure origin. Gastrointest Endosc. 1990;36:337-341. [RCA] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 100] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Gerson LB. Double-balloon enteroscopy: The new gold standard for small-bowel imaging? Gastrointest Endosc. 2005;62:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P, Barbaro F, Cellier C, Charton JP, Delvaux M, Despott EJ, Domagk D, Klein A, McAlindon M, Rosa B, Rowse G, Sanders DS, Saurin JC, Sidhu R, Dumonceau JM, Hassan C, Gralnek IM. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 42. | Shim KN, Moon JS, Chang DK, Do JH, Kim JH, Min BH, Jeon SR, Kim JO, Choi MG; Korean Gut Image Study Group. Guideline for capsule endoscopy: Obscure gastrointestinal bleeding. Clin Endosc. 2013;46:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Saygili F, Saygili SM, Oztas E. Examining the whole bowel, double balloon enteroscopy: Indications, diagnostic yield and complications. World J Gastrointest Endosc. 2015;7:247-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Manno M, Barbera C, Bertani H, Manta R, Mirante VG, Dabizzi E, Caruso A, Pigo F, Olivetti G, Conigliaro R. Single balloon enteroscopy: Technical aspects and clinical applications. World J Gastrointest Endosc. 2012;4:28-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Morgan D, Upchurch B, Draganov P, Binmoeller KF, Haluszka O, Jonnalagadda S, Okolo P, Grimm I, Judah J, Tokar J, Chiorean M. Spiral enteroscopy: prospective U.S. multicenter study in patients with small-bowel disorders. Gastrointest Endosc. 2010;72:992-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Shishido T, Oka S, Tanaka S, Aoyama T, Watari I, Imagawa H, Yoshida S, Chayama K. Diagnostic yield of capsule endoscopy vs double-balloon endoscopy for patients who have undergone total enteroscopy with obscure gastrointestinal bleeding. Hepatogastroenterology. 2012;59:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 47. | Brito HP, Ribeiro IB, de Moura DTH, Bernardo WM, Chaves DM, Kuga R, Maahs ED, Ishida RK, de Moura ETH, de Moura EGH. Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: A systematic review and meta-analysis. World J Gastrointest Endosc. 2018;10:400-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G; ESGE Clinical Guidelines Committee. European Society of Gastrointestinal Endoscopy (ESGE): Recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 49. | Arakawa D, Ohmiya N, Nakamura M, Honda W, Shirai O, Itoh A, Hirooka Y, Niwa Y, Maeda O, Ando T, Goto H. Outcome after enteroscopy for patients with obscure GI bleeding: Diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc. 2009;69:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 51. | Rastogi A, Schoen RE, Slivka A. Diagnostic yield and clinical outcomes of capsule endoscopy. Gastrointest Endosc. 2004;60:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: Validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Cappell MS. Spatial clustering of simultaneous nonhereditary gastrointestinal angiodysplasia. Small but significant correlation between nonhereditary colonic and upper gastrointestinal angiodysplasia. Dig Dis Sci. 1992;37:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Singh A, Baptista V, Stoicov C, Cave DR. Evaluation of small bowel bleeding. Curr Opin Gastroenterol. 2013;29:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | ASGE Technology Committee. Chauhan SS, Manfredi MA, Abu Dayyeh BK, Enestvedt BK, Fujii-Lau LL, Komanduri S, Konda V, Maple JT, Murad FM, Pannala R, Thosani NC, Banerjee S. Enteroscopy. Gastrointest Endosc. 2015;82:975-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Inter-observer variations on interpretation of capsule endoscopies. Eur J Gastroenterol Hepatol. 2006;18:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Pezzoli A, Cannizzaro R, Pennazio M, Rondonotti E, Zancanella L, Fusetti N, Simoni M, Cantoni F, Melina R, Alberani A, Caravelli G, Villa F, Chilovi F, Casetti T, Iaquinto G, D'imperio N, Gullini S. Interobserver agreement in describing video capsule endoscopy findings: A multicentre prospective study. Dig Liver Dis. 2011;43:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Lecleire S, Iwanicki-Caron I, Di-Fiore A, Elie C, Alhameedi R, Ramirez S, Hervé S, Ben-Soussan E, Ducrotté P, Antonietti M. Yield and impact of emergency capsule enteroscopy in severe obscure-overt gastrointestinal bleeding. Endoscopy. 2012;44:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Holleran G, Hall B, Zgaga L, Breslin N, McNamara D. The natural history of small bowel angiodysplasia. Scand J Gastroenterol. 2016;51:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Mai SH, Chao DC, Liao SY, Jackson CS. Nonisolated Small Bowel Gastrointestinal Angiodysplasias are Associated With Higher Rebleeding Rates When Compared With Isolated Small Bowel Gastrointestinal Angiodysplasia on Video Capsule Endoscopy. J Clin Gastroenterol. 2018;52:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Yano T, Yamamoto H, Sunada K, Miyata T, Iwamoto M, Hayashi Y, Arashiro M, Sugano K. Endoscopic classification of vascular lesions of the small intestine (with videos). Gastrointest Endosc. 2008;67:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 62. | Steger AC, Galland RB, Hemingway A, Wood CB, Spencer J. Gastrointestinal haemorrhage from a second source in patients with colonic angiodysplasia. Br J Surg. 1987;74:726-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Grooteman KV, van Geenen EJ, Drenth JP. High variation in treatment strategies for gastrointestinal angiodysplasias. Eur J Gastroenterol Hepatol. 2016;28:1082-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Chetcuti Zammit S, Koulaouzidis A, Sanders DS, McAlindon ME, Rondonotti E, Yung DE, Sidhu R. Overview of small bowel angioectasias: Clinical presentation and treatment options. Expert Rev Gastroenterol Hepatol. 2018;12:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Beg S, Ragunath K. Review on gastrointestinal angiodysplasia throughout the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2017;31:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Darcy M. Treatment of lower gastrointestinal bleeding: Vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Kuo WT, Lee DE, Saad WE, Patel N, Sahler LG, Waldman DL. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 68. | Patel TH, Cordts PR, Abcarian P, Sawyer MA. Will transcatheter embolotherapy replace surgery in the treatment of gastrointestinal bleeding?(2)(2). Curr Surg. 2001;58:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Othman MHM, Radwan MEM, Korany M, Maghraby H, Abdel-Rahem EM. Gastrointestinal bleeding caused by angiodysplasia; one stop angiographic diagnosis and endovascular treatment by super selective embolization with polyvinyl alcohol particles. Egyptian J Radiol Nucl Med. 2010;41:491-496. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 70. | Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol. 2009;12:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Mirsadraee S, Tirukonda P, Nicholson A, Everett SM, McPherson SJ. Embolization for non-variceal upper gastrointestinal tract haemorrhage: A systematic review. Clin Radiol. 2011;66:500-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Jackson CS, Gerson LB. Management of gastrointestinal angiodysplastic lesions (GIADs): A systematic review and meta-analysis. Am J Gastroenterol. 2014;109:474-83; quiz 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Kushnir VM, Tang M, Goodwin J, Hollander TG, Hovis CE, Murad FM, Mullady DK, Azar RR, Jonnalagadda SS, Early DS, Edmundowiz SA, Chen CH. Long-term outcomes after single-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Dig Dis Sci. 2013;58:2572-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | May A, Friesing-Sosnik T, Manner H, Pohl J, Ell C. Long-term outcome after argon plasma coagulation of small-bowel lesions using double-balloon enteroscopy in patients with mid-gastrointestinal bleeding. Endoscopy. 2011;43:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 75. | Olmos JA, Marcolongo M, Pogorelsky V, Varela E, Dávolos JR. Argon plasma coagulation for prevention of recurrent bleeding from GI angiodysplasias. Gastrointest Endosc. 2004;60:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Rogers BH. Endoscopic diagnosis and therapy of mucosal vascular abnormalities of the gastrointestinal tract occurring in elderly patients and associated with cardiac, vascular, and pulmonary disease. Gastrointest Endosc. 1980;26:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Laine L. Therapeutic endoscopy and bleeding ulcers. Bipolar/multipolar electrocoagulation. Gastrointest Endosc. 1990;36:S38-S41. [PubMed] |
| 78. | Cello JP, Grendell JH. Endoscopic laser treatment for gastrointestinal vascular ectasias. Ann Intern Med. 1986;104:352-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Naveau S, Aubert A, Poynard T, Chaput JC. Long-term results of treatment of vascular malformations of the gastrointestinal tract by neodymium YAG laser photocoagulation. Dig Dis Sci. 1990;35:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | ASGE Standards of Practice Committee. Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (2)] |
| 81. | Rutgeerts P, Van Gompel F, Geboes K, Vantrappen G, Broeckaert L, Coremans G. Long term results of treatment of vascular malformations of the gastrointestinal tract by neodymium Yag laser photocoagulation. Gut. 1985;26:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 97] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Moparty B, Raju GS. Role of hemoclips in a patient with cecal angiodysplasia at high risk of recurrent bleeding from antithrombotic therapy to maintain coronary stent patency: A case report. Gastrointest Endosc. 2005;62:468-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Pishvaian AC, Lewis JH. Use of endoclips to obliterate a colonic arteriovenous malformation before cauterization. Gastrointest Endosc. 2006;63:865-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Matsui S, Kamisako T, Kudo M, Inoue R. Endoscopic band ligation for control of nonvariceal upper GI hemorrhage: Comparison with bipolar electrocoagulation. Gastrointest Endosc. 2002;55:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Junquera F, Brullet E, Campo R, Calvet X, Puig-Diví V, Vergara M. Usefulness of endoscopic band ligation for bleeding small bowel vascular lesions. Gastrointest Endosc. 2003;58:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Vargo JJ. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Rahmi G, Samaha E, Vahedi K, Delvaux M, Gay G, Lamouliatte H, Filoche B, Saurin JC, Ponchon T, Rhun ML, Coumaros D, Bichard P, Manière T, Lenain E, Chatellier G, Cellier C. Long-term follow-up of patients undergoing capsule and double-balloon enteroscopy for identification and treatment of small-bowel vascular lesions: A prospective, multicenter study. Endoscopy. 2014;46:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Landi B, Cellier C, Gaudric M, Demont H, Guimbaud R, Cuillerier E, Couturier D, Barbier JP, Marteau P. Long-term outcome of patients with gastrointestinal bleeding of obscure origin explored by push enteroscopy. Endoscopy. 2002;34:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Romagnuolo J, Brock AS, Ranney N. Is Endoscopic Therapy Effective for Angioectasia in Obscure Gastrointestinal Bleeding?: A Systematic Review of the Literature. J Clin Gastroenterol. 2015;49:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Norton ID, Wang L, Levine SA, Burgart LJ, Hofmeister EK, Rumalla A, Gostout CJ, Petersen BT. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc. 2002;56:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Suzuki N, Arebi N, Saunders BP. A novel method of treating colonic angiodysplasia. Gastrointest Endosc. 2006;64:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Josemanders DF, Spillenaar Bilgen EJ, van Sorge AA, Wahab PJ, de Vries RA. Colonic explosion during endoscopic polypectomy: Avoidable complication or bad luck? Endoscopy. 2006;38:943-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Zinsser E, Will U, Gottschalk P, Bosseckert H. Bowel gas explosion during argon plasma coagulation. Endoscopy. 1999;31:S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Hayat M, Axon AT, O'Mahony S. Diagnostic yield and effect on clinical outcomes of push enteroscopy in suspected small-bowel bleeding. Endoscopy. 2000;32:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Chetcuti Zammit S, Sidhu R, Sanders D. Refractory Anaemia Secondary to Small Bowel Angioectasias - Comparison between Endotherapy Alone versus Combination with Somatostatin Analogues. J Gastrointestin Liver Dis. 2017;26:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Junquera F, Santos J, Saperas E, Armengol JR, Malagelada JR. [Estrogen and progestagen treatment in digestive hemorrhage caused by vascular malformations]. Gastroenterol Hepatol. 1995;18:61-65. [PubMed] |
| 97. | Lewis BS, Salomon P, Rivera-MacMurray S, Kornbluth AA, Wenger J, Waye JD. Does hormonal therapy have any benefit for bleeding angiodysplasia? J Clin Gastroenterol. 1992;15:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet. 1990;335:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Barkin JS, Ross BS. Medical therapy for chronic gastrointestinal bleeding of obscure origin. Am J Gastroenterol. 1998;93:1250-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (3)] |
| 100. | Junquera F, Feu F, Papo M, Videla S, Armengol JR, Bordas JM, Saperas E, Piqué JM, Malagelada JR. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 101. | Sami SS, Al-Araji SA, Ragunath K. Review article: Gastrointestinal angiodysplasia - pathogenesis, diagnosis and management. Aliment Pharmacol Ther. 2014;39:15-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 102. | Holleran G, McNamara D. An overview of angiodysplasia: Management and patient prospects. Expert Rev Gastroenterol Hepatol. 2018;12:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Bowers M, McNulty O, Mayne E. Octreotide in the treatment of gastrointestinal bleeding caused by angiodysplasia in two patients with von Willebrand's disease. Br J Haematol. 2000;108:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Rossini FP, Arrigoni A, Pennazio M. Octreotide in the treatment of bleeding due to angiodysplasia of the small intestine. Am J Gastroenterol. 1993;88:1424-1427. [PubMed] |
| 105. | Andersen MR, Aaseby J. Somatostatin in the treatment of gastrointestinal bleeding caused by angiodysplasia. Scand J Gastroenterol. 1996;31:1037-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Nardone G, Rocco A, Balzano T, Budillon G. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Junquera F, Saperas E, Videla S, Feu F, Vilaseca J, Armengol JR, Bordas JM, Piqué JM, Malagelada JR. Long-term efficacy of octreotide in the prevention of recurrent bleeding from gastrointestinal angiodysplasia. Am J Gastroenterol. 2007;102:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 108. | Holleran G, Hall B, Breslin N, McNamara D. Long-acting somatostatin analogues provide significant beneficial effect in patients with refractory small bowel angiodysplasia: Results from a proof of concept open label mono-centre trial. United European Gastroenterol J. 2016;4:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 109. | Molina Infante J, Pérez Gallardo B, Hernández Alonso M, Mateos Rodríguez JM, Dueñas Sadornil C, Fernández Bermejo M. [Octreotide long acting release for severe obscure gastrointestinal haemorrhage in elderly patients with serious comorbidities]. Med Clin (Barc). 2009;133:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Nardone G, Compare D, Scarpignato C, Rocco A. Long acting release-octreotide as "rescue" therapy to control angiodysplasia bleeding: A retrospective study of 98 cases. Dig Liver Dis. 2014;46:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Scaglione G, Pietrini L, Russo F, Franco MR, Sorrentini I. Long-acting octreotide as rescue therapy in chronic bleeding from gastrointestinal angiodysplasia. Aliment Pharmacol Ther. 2007;26:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 112. | Orsi P, Guatti-Zuliani C, Okolicsanyi L. Long-acting octreotide is effective in controlling rebleeding angiodysplasia of the gastrointestinal tract. Dig Liver Dis. 2001;33:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 113. | Bon C, Aparicio T, Vincent M, Mavros M, Bejou B, Raynaud JJ, Zampeli E, Airinei G, Sautereau D, Benamouzig R, Michopoulos S. Long-acting somatostatin analogues decrease blood transfusion requirements in patients with refractory gastrointestinal bleeding associated with angiodysplasia. Aliment Pharmacol Ther. 2012;36:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 114. | Szilagyi A, Ghali MP. Pharmacological therapy of vascular malformations of the gastrointestinal tract. Can J Gastroenterol. 2006;20:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Shurafa M, Kamboj G. Thalidomide for the treatment of bleeding angiodysplasias. Am J Gastroenterol. 2003;98:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Heidt J, Langers AM, van der Meer FJ, Brouwer RE. Thalidomide as treatment for digestive tract angiodysplasias. Neth J Med. 2006;64:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Bauditz J, Lochs H, Voderholzer W. Macroscopic appearance of intestinal angiodysplasias under antiangiogenic treatment with thalidomide. Endoscopy. 2006;38:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Dabak V, Kuriakose P, Kamboj G, Shurafa M. A pilot study of thalidomide in recurrent GI bleeding due to angiodysplasias. Dig Dis Sci. 2008;53:1632-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | Garrido A, Sayago M, López J, León R, Bellido F, Márquez JL. Thalidomide in refractory bleeding due to gastrointestinal angiodysplasias. Rev Esp Enferm Dig. 2012;104:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 120. | Kamalaporn P, Saravanan R, Cirocco M, May G, Kortan P, Kandel G, Marcon N. Thalidomide for the treatment of chronic gastrointestinal bleeding from angiodysplasias: A case series. Eur J Gastroenterol Hepatol. 2009;21:1347-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Ge ZZ, Chen HM, Gao YJ, Liu WZ, Xu CH, Tan HH, Chen HY, Wei W, Fang JY, Xiao SD. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology. 2011;141:1629-37.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 122. | Iannone A, Principi M, Barone M, Losurdo G, Ierardi E, Di Leo A. Gastrointestinal bleeding from vascular malformations: Is octreotide effective to rescue difficult-to-treat patients? Clin Res Hepatol Gastroenterol. 2016;40:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Nardone G, Compare D, Martino A, Rocco A. Pharmacological treatment of gastrointestinal bleeding due to angiodysplasias: A position paper of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2018;50:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 124. | Gifford SM, Peck MA, Reyes AM, Lundy JB. Methylene blue enteric mapping for intraoperative localization in obscure small bowel hemorrhage: Report of a new technique and literature review: Combined intraoperative methylene blue mapping and enterectomy. J Gastrointest Surg. 2012;16:2177-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 125. | Czymek R, Kempf A, Roblick UJ, Bader FG, Habermann J, Kujath P, Bruch HP, Fischer F. Surgical treatment concepts for acute lower gastrointestinal bleeding. J Gastrointest Surg. 2008;12:2212-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 126. | Tonea A, Andrei S, Andronesi D, Ionescu M, Gheorghe C, Herlea V, Hortopan M, Andrei A, Andronesi A, Popa C, Popescu I. [Difficulties in diagnosis and surgical treatment of the angiodysplasia of the gastrointestinal tract]. Chirurgia (Bucur). 2008;103:513-528. [PubMed] |
| 127. | Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 512] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 128. | Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150-4156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 565] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 129. | Holleran G, Hussey M, Smith S, McNamara D. Assessment of serum angiogenic factors as a diagnostic aid for small bowel angiodysplasia in patients with obscure gastrointestinal bleeding and anaemia. World J Gastrointest Pathophysiol. 2017;8:127-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |