Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2402
Peer-review started: February 6, 2019
First decision: March 27, 2019
Revised: April 4, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 28, 2019
Processing time: 111 Days and 6.2 Hours
The purpose of this review is to provide a definitive account of small intestinal mucosal structure and interpretation. The coeliac lesion has been well known, but not well described to date and this review aims to identify the interpretative difficulties which have arisen over time with the histological assessment of coeliac disease. In early coeliac interpretation, there were significant inaccuracies, particularly surrounding intraepithelial lymphocyte counts and the degree of villous flattening which occurred in the tissue. Many of these interpretive pitfalls are still encountered today, increasing the potential for diagnostic errors. These difficulties are mostly due to the fact that stained 2-dimensional sections can never truly represent the 3-dimensional framework of the intestinal tissue under investigation. Therefore, this review offers a critical account occasioned by these 2-dimensional interpretative errors and which, in our opinion, should in general be jettisoned. As a result, we leave a framework regarding the true 3-dimensional knowledge of mucosal structure accrued over the 70-year period of study, and one which is available for future reference.
Core tip: The purpose of this review is to provide a definitive account of small intestinal mucosal structure and interpretation. We offer a critical account and give opinion that current testing protocols need to be altered. We then leave a framework regarding the true 3-dimensional knowledge of mucosal structure accrued over the 70-year period of study, and one which is available for future reference.
- Citation: Charlesworth RP, Marsh MN. From 2-dimensional to 3-dimensional: Overcoming dilemmas in intestinal mucosal interpretation. World J Gastroenterol 2019; 25(20): 2402-2415
- URL: https://www.wjgnet.com/1007-9327/full/v25/i20/2402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i20.2402
The clinical features of “idiopathic steatorrhoea” characterising a fatty diarrhoea (and later renamed “coeliac disease”), was well-recognised throughout the 19th century. This was considered a disease of adults and distinguishable from (Indian) Tropical Sprue. A similar malabsorptive disease (“coeliac disease”) was familiar to paediatricians, but its identity with the adult condition was realised only following introduction of intestinal (capsule) biopsy techniques.
The ‘coeliac lesion’ was, hitherto, unknown since the earliest attempts at histology were greatly frustrated from the use of autolysed post-mortem material. With the use of fresh capsule biopsies (about 1950s onwards), it became clear that the typical “flat” lesion is worse in the upper jejunum but far less marked around the mid-jejunum. This suggested that a ‘gluten peptidase’ deficiency could be aetiological, despite knowledge that intestinal peptidases are not protein specific. This incorrect view was overtaken (1970’s onwards) towards immune mechanisms.
Interest in intestinal immunological function was formally initiated at the Sir William Dunn School of Pathology in Oxford by (Sir) James Gowans and his doctoral student Julie Knight. Using the newly-produced 3H-labelled thymidine, Gowans first demonstrated the re-circulatory potential of lymphocytes within thoracic duct lymph (TDL)[1], thereby establishing one of the important physiological properties of the mesenteric immune system. Knight’s interest, however, was in TDL “blasts” which she demonstrated to home specifically to intestinal tissues as (IgA-secreting[2,3]) plasma cells, an exclusive re-circulatory pathway effected via the β7-MADCAM-1 receptor on post-capillary venules[4].
The physiological role of this pathway was functionally proved with mice orally primed with ferritin, leading to an accumulation of IgA-anti-ferritin plasma cells throughout the small intestine and colon[5]. This study prompted later work on rectal gluten challenge, which was of high diagnostic specificity and easy to perform[6-8]. The technique never caught on, probably resulting from a lack of understanding about the functions of the mesenteric immune system and because of ingrained views that coeliac disease only affects (or “is a disease of”) the upper jejunum. Nevertheless, it should be remembered that the re-circulation of mesenteric blasts, once antigen- primed, emigrate to all mucosal surfaces, thus providing an important defence mechanism especially protective for lactating humans and animals[9,10]. Far greater current interest involves the rise in circulating CD4+ lymphocytes identified with the “tetramer test” following oral priming of known coeliacs with gluten; this, or other, approaches may soon supplant the diagnostic relevance of intestinal biopsies in the near future[11-13].
The purpose of this paper, therefore, is to provide a definitive account of small intestinal mucosal structure and interpretation. In moving from the 2-dimensional into the 3-dimensional reality, we wish to identify the interpretative difficulties that have arisen over time across this divide, and are still relevant to current-day biopsy evaluation. This essay offers a critical account occasioned by these 2-dimensional interpretative errors and which, in our opinion, should in general be jettisoned. As a result, we leave a framework regarding the true 3-dimensional knowledge of mucosal structure accrued over the 70-year period of study, and one which is available for future reference.
For several decades, it was assumed that coeliac disease implied a severe intestinal lesion lacking demonstrable villi. Then, as attention was drawn to the lymphocytic packing of the epithelium, intraepithelial lymphocytes (IEL) began to be quantitated with reference to epithelial cells. Initially, counts > 40/100 enterocytes were regarded as abnormal, but this was a high figure probably due to the use of thick hematoxylin and eosin (H&E) sections. Nevertheless, it is curious that definitive attempts which define “normality” have only recently been made, with estimated means of 23-27[14-16]. Importantly, it should be understood that there can be no specific “upper limit” to these counts because the IEL response is graded and cumulative across control and diseased mucosae. An overlap between “normality” and “disease” is thus an inevitable feature of any count. In other words, the response is not biphasic, and is thus analogous to other biological characteristics such as blood pressure, height, or acid secretion. Despite some enthusiasm for immunohistochemical dissection of the IEL population, data reveal that H&E counts are the most reliable in routine practice[15].
When based on theoretical criteria the IEL count, in fact, is inaccurate and overestimates IEL populations upwards by a factor of × 2[17]. There are two reasons for this anomaly. First, in flat (Marsh III) lesions the IEL are increased in size[18], while epithelial cells are markedly smaller[19]: comparisons become invalid in these circumstances since, in the 2-dimensional tissue section, larger objects produce more sectioned profiles thus leading to higher counts: (the corollary is that small objects provide very few sectioned profiles). Here, it should be remembered that only sectioned profiles of real, 3-dimensional lymphocytes are being enumerated: that’s the difference.
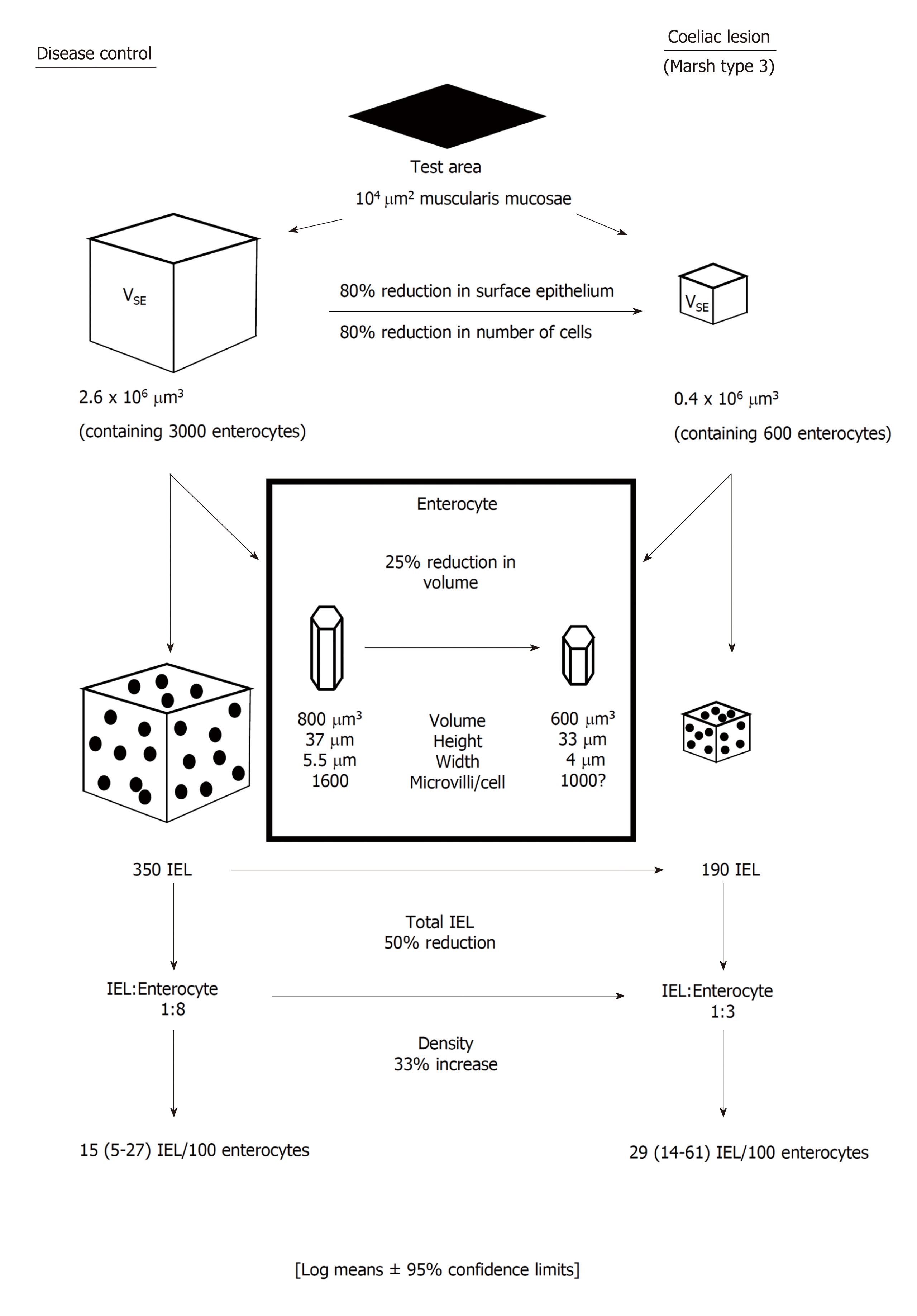
Second, IEL counts can only be performed relative to epithelial cell nuclei, since adjacent cell boundaries cannot be effectively defined. It is also the case, contrary to general assumptions, that enterocytes are not arranged on the basement membrane in regular, parallel lines: indeed, both scanning and transmission EM reveal their hexagonal packing. As a result of that form of surface packing, any random section unavoidably sustains about 50% losses of epithelial cell nuclei[17], which is the factor principally contributing to over-estimation of IEL populations. In passing, we should observe that this impasse can neither be side-stepped with the use of basement membrane as control. The difficulty here is due to the different sizes (widths) of control and coeliac enterocytes (Figure 1). In order to obtain correct results, only about 50-60 μm basement membrane from a coeliac lesion would be needed for accurate, comparative counts relative to 100 μm from a control specimen[17].
Such drawbacks with the original counting method have been revealed with computerised image analysis. Here, ‘absolute’ values for epithelial, crypt and lamina propria volumes and their contained cellular components across the mucosal spectrum, are generated with the use of a reference 100 μm × 100 μm test area of muscularis mucosae. This methodology[15,17,19,20] clearly provides far more dynamic and extensive information (Figure 1) beyond Taavela’s recent discovery and, apparently, much-needed “validation” of crypt/villus ratios[20]. But such ratios (for those more conversant with the literature) have been in use for nearly 50 years[21]: indeed, parenthetically, one might ask why this old-fashioned methodology still requires a local, departmentally-based “validation” at this late stage?
It seems not to be appreciated generally that crypt/villous ratios are quite inaccurate, despite a growing tendency for their use in mucosal measurement, and in absence of solid data for “normal” crypts and villi. Interpreting the crypt-villus border is entirely subjective and thus operator-dependent. Moreover, ratios arbitrarily set at ≥ 2 need radical revision, since if crypt depth is assumed at about 80 μm, then the corresponding “villi” would be < 200 μm, which is impossible since, below a height of about 350 μm, villi are already amalgamated into convolutions[22]. On those grounds, one would need ratios of at least 4-6 to be in the correct “normal” range, and one not always possibly obtained: below this “normal” range, no proper villi exist for measurement.
The use of computer-assisted image analysis has clearly demonstrated that as the mucosa flattens, (Marsh III stage) the total number of enterocytes is far more reduced when compared with absolute IEL numbers. It is that empirical fact that highlights the erroneous, subjective view that IEL still appear to be infiltrating the epithelium. In absolute terms, however, IEL are markedly diminished in the flat lesion, this loss now falling numerically within the normal IEL range. The increased mitotic activity[23] and higher proportion of “blasts”[18] among Marsh III IEL may represent a proliferative response to those incurred losses. That possibility would require further analysis, even involving a subset of IEL that might be sensitive to these overall losses.
It parallel with the reservations noted above, readers should be aware that lamina propria volumes increase twofold with progressive flattening[24,25], indicating that the use of high-power fields for cell counts within this mucosal compartment are similarly invalid, when compared with normal values. Counting cells relative to some independent variable or designated test area obviates this kind of technical error[24-27].
These problems generally arise because stained (2-dimensional) sections never truly represent the 3-dimensional framework of the tissue sample under scrutiny. For any cell count to be accurate, and to avoid counting the same cells in consecutive sections (as may well have occurred in many publications), the “effective section thickness” (EST), according to Weibel[28], must be determined.
This is easily done. EST is the sum of actual section thickness (perhaps 4μm) plus mean diameter of the cell of interest (usually taken as 5 μm). More preferably, these should be nuclear diameters since their overall size distributions are much tighter. Once EST has been determined, it is essential that successive sections should be separated by at least a gap equivalent to EST. Use of immunohistochemical markers may seem more accurate a means of counting cell types. Nevertheless, it is always difficult relating a decorated cell membrane to its nucleus, leading to over-estimated cell counts, and thus greater inaccuracies, as the ROC curves clearly indicate in respect of previous immunohistological studies[15].
Cumulative computerised image analysis data (Figure 1) reveal the extensive tissue and cellular changes which occur as the mucosa flattens. These have been used in the laboratory for decades in studies of intestinal responses to gluten, which include changes in surface epithelium, crypts, and the lamina propria. In one of the latest papers[15] such a panoramic view of these vast collective changes is illustrated but which could never be illustrated purely with micrographs alone. Neither could they be conceptualised just with use of “moving” crypt/villus ratios.
Next, a word about how much material needs sampling to produce accurate, and reproducible, measurements. Some workers obviously rely on “numerical digit preference”: this simply means the adoption of an arbitrary number of observations, for example 20 crypts or 50 cell types, or some other number which looks or feels “right”. For optimal results, it is useful to know how many individual measurements should be undertaken in order to provide reasonably precise, and reproducible, results. This is formally known as the “Quotient of accumulative addition of values”[29]. In practice, the approach is far easier than the title suggests. All that is necessary is to calculate and to update the means of each successive measurement, until that mean value becomes constant: that is the correct number of observations to make: it could then, perhaps, be scaled up to a convenient value. In other words, if the mean becomes constant after 17 observations have been made, it might be useful to scale up to 20. Use of this simple methodology indicates the appropriate number of observations or counts that are precisely needed for each item under scrutiny. If fewer observations were to be used than those determined by the cumulative means method, the resulting data are very likely to be non-reproducible, non-comparable, and thus erroneous. On the contrary, doing too many counts would not improve performance, but merely incur a considerable waste of time! Few papers seem to recognise the relevance of these simple measures, as well as the importance of their outcomes.
With these precautionary methods understood, the image-analysis computer (Kontron) is calibrated with a grid in the eyepiece, or stage of the microscope. The electronic pencil is used to make outlines of the particular aspect of mucosa under investigation, such as a volume compartment, or the total numbers of a specific cell-type contained within one of those mucosal compartments. The independent test measure is a nominal square of muscularis mucosae, 100 μm × 100 μm. Measuring is done along 100 μm lengths of the muscularis mucosae (“Y” axis), while for “Z” axis measurements, the section thickness has to be taken into account. If volume measurements are being made with 4 μm sections, then only 100/4, or 25 μm × 100 μm observations along the muscularis would be necessary. For cell counts, EST must be calculated as explained above, in order to avoid counting the same cell in successive sections. Thus, bearing in mind the example given above, 100/9 along the “Z” axis, or 11 multiples of 100 μm (along the “Y” axis) would deliver the absolute cell count overlying the test area.
The computer is programmed to calculate final volumes, or counts per mucosal volume. Details of the procedure are fully outlined elsewhere[26], and in our individual papers: there is insufficient space here to go into more specific operational details. The entire methodology is very simple (say, in comparison with the grid counting technique of Guix[25]), and does not require a great deal of observer input once the computer is programmed for the particular need: this obviously avoids the important factor of operator fatigue and is thus an additional benefit.
It is also our view that this methodology overcomes, to a large extent, the problem of subjective interpretations, especially regarding cell counts. It is our view that our individual cell counts, expressed as “absolute” values, are probably as accurate as any could be. However, it must be stressed that the use of optimal technical methodology helps considerably in getting the right answers. Although this is possible with thin H&E sections, the preferred approach was to use Epon embedded material, sectioned at 1μm with very sharp glass knives on ultramicrotomes, stained with toluidine blue and examined (and counted) under oil-immersion optics using a × 100 plan-apochromat objective lens. Given those conditions, there is only one plane of focus which greatly facilitates the procedure. Moreover. this approach confers the advantage of driving a costly research microscope in “top gear”. Toluidine blue gives a distinctive hue to each cell type within the lamina, so making recognition less difficult. It also makes IEL counting far easier to perform and provides an accurate answer, rather than the relative values with their inherent errors obtained when related to 100 enterocytes (see above).
Understandings about mucosal histology and its derangements were unknown when Shiner proposed that the coeliac lesion reflects “villous atrophy”. Doubtless she was influenced by Wood’s recent description of the true, irreversible gastric atrophy of pernicious anaemia, resulting in life-long failure of intrinsic factor production. Following Castle’s striking experimental discoveries, together with the synthesis of cyanocobalamin (B12) and folic acid, the post-war 1950’s spawned great interest in megaloblastic anaemias: and with coeliac patients presenting with folate deficiency it is not surprising that acid output was closely investigated. Indeed, and most surprisingly, about 50% patients with coeliac disease, dermatitis herpetiformis and tropical sprue (from the mid-1920’s to mid-1980’s) were found to be either totally or partially achlorhydric. For full data, see Table IV in Marsh 1992[30]. But Anderson’s demonstration of villous regeneration following gluten restriction should have indicated that the coeliac lesion results from a different process, which was not atrophy[31]. Despite that, and 70 years on, we have laboratories around the world still asserting that the gold standard for diagnosis is a flat, “atrophic” mucosa. Unfortunately, the severe coeliac lesion is neither a gold standard (Table 1), nor the result of an atrophic mode of pathology.
| Factors causing mucosal change | ||
| Mucosal remodelling | Food antigens | Gluten |
| Milk | ||
| Soya | ||
| Egg | ||
| Fish | ||
| Drugs | Olmartesan | |
| Cytotoxic drugs | ||
| Colchicine | ||
| Vinca | ||
| Neomycin | ||
| Ipilimumab | ||
| Autoimmune associated | MVID | |
| Autoimmune enteropathy | ||
| Collagenous sprue | ||
| IDDM | ||
| Cerebral ataxia | ||
| DH | ||
| Sarcoidosis | ||
| Miscellaneous | Malnutrition | |
| Crohn’s disease | ||
| Intestinal lymphoma | ||
| Z-E syndrome | ||
| Eosinophilic enteritis | ||
| Antigen-based | Tropical sprue | |
| GVHD | ||
| Parasitic | ||
| Giardiasis | ||
| Cryptosporidiosis | ||
| Enterocytozoon bieneusi | ||
| Infection | HIV | |
| TB | ||
| Whipple’s disease | ||
| Bacterial overgrowth | ||
| Immunodeficiencies | ||
| Helicobacter pylori | ||
| Post-infectious diarrhoea | ||
| Altered villous heights | Preparative artefacts | Forceps trauma |
| Fixation contraction | ||
| Embedding contraction | ||
| Imperfect sectioning | ||
| Social factors | Age | |
| Ethnicity Gender | ||
| Genetic | ||
| background | ||
| Geo-cultural environment | ||
| Diet | ||
| Microbiome | Intestinal microenvironment | |
| Maternal | ||
| Infections | ||
| Miscellaneous | Diurnal variation | |
| Parasites | ||
| Allergy | ||
| Antigenic challenge | ||
| Various drugs | ||
| Variations in cell shape | Morphogenic | Cell-cell contact |
| Physiologic | Ion-channel fluxes | |
| Mechano-sensors | ||
| Hydrostatic | ||
| Pathological | Various enteropathies | |
| Drug-induced | Colchicine, etc | |
| True mucosal atrophy | Chronic radiation damage | |
| Chronic ischaemia | ||
| Long-term disuse | ||
The other curious outcome was that over the years, it was never queried how the mucosa becomes flat, even though there was no surprise at its regenerative potential in the wake of a gluten-free diet. In fact in those earlier times, following Anderson[31], mucosal recovery became an additional and essential diagnostic requirement. But there were hints of the manner of flattening.
First, many case reports did indicate that the mucosal lesion evolves over time, at different rates, and with differing functional outcomes, and that a flat mucosa is not a given. Yet the progression implied by these reports seemed not to be apparent to the authors[32-37].
Second, continuing dose/time gluten-response studies in Manchester showed initially a progressive dose-responsive IEL infiltrate, followed by crypt hypertrophy, and then flattening, in that changing sequence[38]. Moreover, that crypt hypertrophy and IEL infiltrates also occurred in graft-versus-host experiments strongly suggested that the coeliac challenge data, likewise, are a T-cell dependent phenomena[39].
Third, examination of many biopsies from coeliac families revealed the same range of mucosal alterations, although many individuals were asymptomatic irrespective of mucosal morphology[40].
Fourth, a similar spectrum of mucosal changes was found in cases of Indian tropical sprue which, analogous to the host response to gluten in coeliac disease, can only reflect a host-produced response to high levels of intestinal bacterial colonisation[41].
Although it took nearly 40 years for this classification to become formalised, it has since served for almost 30 years. Most importantly, in addition to the insights given to the immunohistologic basis of host responses to environmental antigen challenge this classification, in widening the goalposts, has over the years brought many more subjects to diagnosis than would have obtained with the older criterion. The classification although revolutionary for its time should have been suspected. Despite that, many workers, even in recent years, have called these early Marsh 0, I and II lesions “non-specific”. But a moment of careful thought should make it clear that no tissue could ever be deemed “non-specific” since it must reflect the physiological or pathological state of its place of origin within the patient’s body. Within context, however, infiltrated villi indicate gluten sensitisation when seen in a family setting with appropriate genetic backgrounds, and the ingestion of gluten-containing products. In previous work, we included these early lesions under the umbrella of microscopic enteritis: for the faint-hearted, a list of relevant differential diagnoses is given in that paper[42].
Furthermore, these early stages do have diagnostic value, and have become useful criteria for introducing gluten-restriction. That cannot be said of the Oberhuber manipulation of Marsh III lesions, which merely reproduces Shiner’s older stages of differential grades of villous shortening: unfortunately, at this stage of mucosal remodelling, there are no villi to comment on. These so-called progressive grades of mucosal change were never defined structurally in quantitative terms, then or now, so it is anyone’s guess how they are gauged by those who still cling to this useless, poorly-based modification. What we need are numbers, and not alphabetical fragments[43]. Oberhuber’s sub-classification of Marsh III has neither diagnostic value, implications for treatment, nor prognostic significance. We know of no paper in which these gradings have then been found valuable either for informing research or clinical purposes. That might be some food for thought by clinical gastroenterologists. On the other hand, pathologists might be well advised to discontinue using this impoverished sub-classification and think about employing their valuable time along more fruitful avenues: it seems as though that may be some time in coming.
We have already asserted that the reshaping of the mucosa through its varied grades is not caused by atrophy of the villi: that no longer makes any sense, irrespective of the dogged adherence to this terminology. Despite its continued use, even by centres of “coeliac excellence”, the atrophic bits still await identification. Furthermore, do these atrophic bits recover totally, or in part, with a gluten-free diet? From the manner in which some papers are written, it is clear that many investigators are convinced that flattening means progressive loss of each individual villus down to the level of the crypt openings. That is also an erroneous view: but that reflects Shiner’s original misconception when creating her “partial”, “subtotal” and “total villous atrophy” categories, and now continued under Oberhuber’s guise of Marsh 3a, b and c.
These sentiments are entirely inconsistent with the processes through which the mucosa is re-organised. It is to that subject to which we now turn.
Despite the extensive literature on the developing foetal intestinal mucosa, its 3-dimensional features are less well portrayed, so there is little knowledge about how villi are formed. Although villi in adult mucosae may be free-standing structures, they also most definitely arise from low ridges, visible by scanning EM[44,45]. These ridges therefore seem to be of importance in delineating the villous superstructure of the mucosa. They were first portrayed by Creamer in postmortem material from which the surface cells had been shed[46,47]. Nowadays, their occurrence and likely roles in mucosal remodelling are largely unknown and unappreciated. These short-range, curvi-linear ridges form a quasi-orthogonal network across the mucosal surface, lying between the openings of the “wells” into which about 10-20 individual crypt tubes open[48].
Creamer also showed that this ridged network undergoes considerable hypertrophy as the mucosa begins to flatten. The ridges grow up vertically between adjacent villi, thus progressively amalgamating them into broad convolutions, a process which, as viewed histologically, invariably leads to an erroneous designation of “broad, flat-topped, stunted villi”. Additionally, enlargement of the transverse ridges would complete the upward growth into the formation of mosaic plateaux. The latter rise to some 200 μm above the basal mucosal surface, thus comprising refashioned parts of villous territory[49,50]. The mucosa is “flat” - yet that is not due to attrition of every villus down to the crypt interface. “Flatness” is not quite what it seems, despite opinions to the contrary. Another difficulty concerns how and where cells are shed from these mosaic outgrowths. Despite this continuing uncertainty about cell loss from deformed mucosal surfaces, these losses are counteracted by four regenerative factors: an increase in cell migration rates from the crypts[51], and within the crypts by an increased growth fraction, together with reductions both in duration of the mitotic process itself and of the inter-mitotic interval[52].
Together with the enormous increases in volume of the lamina propria, this protective response is vast and requires considerable remodelling of the connective tissue framework of the mucosa - including all basement membranes, and especially a drastic re-construction of the entire mucosal micro-vasculature. This is an immense procedure as revealed by dynamic computer-assisted computerised morphometry of the changing mucosal landscape[15]. Yes, there is some flattening of the villi, but this is by no means an atrophic process. At Marsh stage III, the mucosa may look flat, but that appearance is an outcome of this vast reorganising hypertrophic process that results in the production of high mosaic plateaux, indicative, overall, of a fairly healthy reorganisational process and which, notably, is capable of complete recovery.
These structural mucosal reorganisations provide a framework upon which to investigate gene switching and their products which are co-ordinated in effecting these progressive changes. Some work in this direction, although very sporadic[53,54], is indicative of the task ahead, if we are fully to understand this process entirely. Some very interesting approaches towards this end have already been published by Lundin’s group in Oslo and Wijmenga’s in The Netherlands[55,56], but far more needs to be done.
The development of completely novel tests (serological or immunological) for coeliac disease is a welcome[12,13] sign of progress in comparison with histology which has tended to be static or even backward looking[57,58], other than from one or two novel developments using computer-aided morphometric analyses[26] or logistic regression analyses[8]. As we have noted above, such tests may come to supplant biopsy usage in the near future for purely diagnostic purposes. These would also reduce the burden, clinically as a procedure, and relieve patients of some discomfort.
In addition to the development of novel diagnostics, research has also been carried out to improve diagnostic pathology in the newer application of statistical analysis of tissue measurements. One such technique which shows promise is linear discriminant analysis (LDA). This technique, when applied to biological data, aims to assign patients to one or more groups on the basis of a series of measurements from which a linear function may be derived[59]. Discriminant analysis has been already shown to predict patient groupings in rheumatoid arthritis, Parkinson’s disease, diabetes, Alzheimer’s disease and coronary artery disease.
With our last studies, the goal was to move away from analysis solely reliant on the subjective abilities of the observer: the endeavour was to gain more objective diagnostic end-points. In the first of these studies[59], it was shown that by developing algorithmic metrics, it was possible to tighten histological diagnosis by reference to specifically chosen biopsy features, referable to mucosal dimensions, and populations of goblet cells, monocytes and lymphocytes within the epithelium and lamina propria. The algorithms distinguished healthy biopsies from coeliac biopsies in about 80% of cases. Despite this success, further understanding of the mechanisms behind coeliac mucosal remodelling by simultaneously examining various gene expressions was sought.
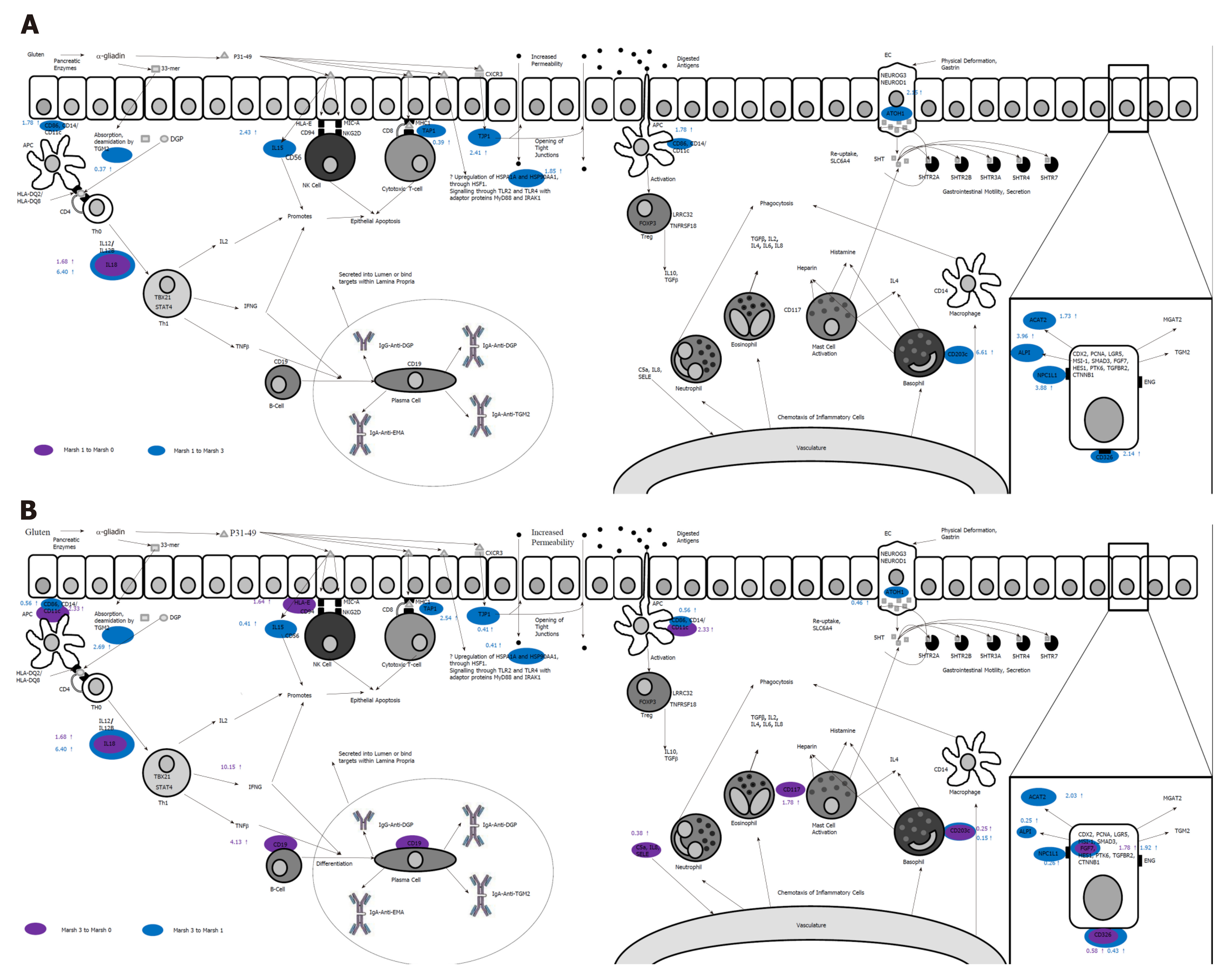
In a subsequent study[60], we showed that by using a novel panel of 26 (coeliac) candidate genes, each Marsh category revealed its own unique mRNA-expressed signature. Moreover, it became clear that the expression profile of the genes studied changed from an “innate” pattern of expression to that of an “adaptive” response which was accompanied by an observed decrease in structural (intestinal mucosal) gene expression. These changes are explored in Figure 2. From these newer observations, a further set of linear discriminant algorithms was defined capable of successfully accounting for 99% of the observed experimental variation in discriminating coeliac patients into each stage of Marsh’s classification. This study therefore showed that the analysis of gene expression in coeliac disease is a reliable measure of disease severity since it is not subject to the same observer biases always frustrating histological assessments.
Finally in the most recent study[61], a combination of genetic and histological data produced the most accurate discriminant algorithms, capable of separating patients into each Marsh category. Their use also highlighted the power of the discriminant algorithms, in that very subtle changes in both gene expression and tissue morphology were able to be detected and used to separate patients.
While discriminant analysis has been demonstrated by these and other studies to be a useful tool in classifying disease severity in coeliac disease, most have only used it to separate coeliac disease patients from healthy controls. More research is required to further explore whether or not the Marsh score groupings can be discriminated from each other and whether patients can be accurately assigned to a particular Marsh score using selected, but discriminatory tissue features. It is also worth noting that for histological studies using this technique, only a small number of variables have been employed in the building these functions. It is likely that the addition of more variables into the building process would produce more accurate equations, such as cell heights, crypt height and IEL infiltration, and neutrophil emigration into the lamina propria, or changes in crypt cell mitotic activity: such possibilities have still to be explored. Other provisos arising from this work concerns the very small number of patient biopsies available from local hospital sources. Furthermore, the reading by commercial histopathologists did not provide criteria for individual mucosal classifications. Nevertheless, with future tightening of these weaknesses, such enhanced diagnostic power will be an aid to parallel tests, like the “tetramer” (Oslo) or “triple score” (Tampere) tests on offer, or at least provide a firmer basis for the evaluation of immuno-serological diagnostic testing.
This has been a critical review of the past 60-70 years of intestinal mucosal interpretation. Overall, the results have not been electrifying. Much of the measurement has used ad hoc procedures without much reference to, or indeed interest in, a proper morphometric basis[26,28]. For various reasons, as specified above, IEL counts, comparisons between lamina propria cellularity, and villus/crypt ratios are flawed procedures because of no defined or agreed numerical foundation, relative to an invariant index within the mucosa. Over the last few years, there has been a strong tendency to disregard mucosal biopsies as important in diagnosis, since interest has increasingly turned towards genetic or serologic testing as a preferred means of diagnosing coeliac disease. This downgrading of histology has not helped to foster a greater awareness and precision in mucosal measurement.
We hardly know the criteria by which a “normal” mucosa should be identified[49], yet many papers frequently refer to “normal” mucosae without any reference whatsoever to precise measurement, as might be agreed by most laboratories. Likewise, many authors seem to be happy in accepting a villous/crypt ratio ≥ 2, which is impossible because there are no villous projections below about 50 μm as a result of mucosal remodelling. Villi are very pliant structures and soon become modified in shape if conditions favour mucosal flattening. Without properly conducted surface microscopy with a dissecting microscope, these changes no longer are fully appreciated or understood: moreover, 2-dimensional histology continues to assert the presence of “flat-topped” villi, which in reality are misinterpretations of either convolutions or mosaic plateaux. It seems to be hardly recognised that the 2-dimensional histological section does not immediately map onto the 3-dimensional reality of the whole tissue block from which it was taken.
From all this, we need to jettison into historical oblivion such outworn, inappropriate terms like “atrophy”, or words such as “partial”, “subtotal” or “total” atrophy when applied to mucosal structure, especially when at that stage of mucosal remodelling, no villi are actually present. And for similar reasoning, similar sentiments apply to the misguided subdivision of Marsh III. Nevertheless, these subdivisions continue to appear in most papers on coeliac mucosa without any thought of precise (measured) definition. Moreover, we know of no paper in which these subdivisions were ever employed to sharpen diagnosis, inform treatment, or anticipate future prognostic effects of dietary control.
From that perspective, we need to start again.
The small intestinal mucosa should be conceived in terms of upper and lower compartments. The upper “villous” compartment comprises villi and inter-villous ridges, in a dynamic reciprocating relationship. Good shaped “normal” villi, about 350-650 μm in height, arise from very slender ridges whose contours define the basal origins of villi. And, because of their quasi-orthogonal arrangement across the mucosal surface, the burgeoning ridges account for the linear arrangement of convolutions, as evident from dissecting microscopy. As we have indicated above, with further remodelling, these ridges ultimately coalesce villous material into flat-topped mosaic plateaux.
The lower compartment comprises crypt tubes and their communicating “basins”, which empty into a smaller number of “wells”[48]. A single well may accommodate up to 20 crypt tubes, via the basins, which means there can be no such entity as a “crypt-villus unit”: that is a misguided interpretation of the 2-dimensional section. Furthermore, this micro-geometry makes the vertical measurement of crypt height somewhat more difficult than is pre-supposed in a large number of papers. The upper reaches of many crypts are curved inwards in order to be able to enter their respective basins. This arrangement affects not only measures of crypt height, but also impacts the determination of villus/crypt ratios. The upper horizontally-disposed circumferences of the wells define the crypt/villous border. This border of wells and basins is most difficult to define histologically, simply because they comprise 3-dimensional depressions or voids into the mucosal surface. That is, in section, there is nothing to see, thus making interpretation difficult. Hence the need for a careful assessment of surface morphology before the specimen is prepared for sectioning.
We need additional information for this task, such as mRNA locations of the origin of villous cell membrane hydrolases (esterase, alkaline phosphatase) thus to define where villi begin. That information we do not have. There may be other genes or their products which might help in this task, for example, in defining the upper limits of the crypts for use in the 2-D world of tissue sections. We also need far more genetic data upon which to define the earlier stages of the Marsh classification of mucosal remodelling. Some approaches to this end have begun[53-56], although much more needs to be done, such that a confident identification of each stage can be defined from gene activations, their periods of activity, or the products elaborated. It is now apparent that the subepithelial stroma has major influences on the differentiation and maintenance of epithelial structure[61-63]. The conversation between mesenchyme and epithelium needs to be learned and its influences determined. Such approaches must, surely, be the required technologies upon which the histologic assessment of other forms of coeliac treatments must depend, rather than the stultifying boring and retrograde procedures recommended recently by two ad hoc expert “committees”[57,58].
Finally, in our attempt to clarify the distance between 3-dimensional reality and the non-matching appearance of 2-dimensional tissue sections, arises the need to model the mucosa with use of computerised technology[49]. Such approaches would serve to make clearer the complex reality of the 3-dimensional world of the tissue biopsy and its relationship to the 2-dimensional world of the tissue section. In addition, these models could be very usefully employed in defining how the microvasculature is re-modelled as the mucosa itself undergoes progressive re-modelling into convolutions and mosaic plateaux. Furthermore, it would be of great interest to define the points at which the microvasculature begins to produce new capillaries in order to facilitate mucosal regeneration (with gluten restriction) leading to the ultimate re-formation of villous processes as the inter-villous ridges slowly recede.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gabriel S, Ierardi E S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in the rat. Proc R Soc Lond B Biol Sci. 1964;159:257-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 729] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Crabbe PA, Carbonara AO, Heremans JF. The normal human intestinal mucosa as a major source of plasma cells containing gamma-a-immunoglobulin. Lab Invest. 1965;14:235-248. [PubMed] |
| 3. | Tomasi TB, Tan EM, Solomon A, Prendergast RA. Characteristics of an immune system common to certain external secretions. J Exp Med. 1965;121:101-124. [PubMed] |
| 4. | Kantele JM, Arvilommi H, Kontiainen S, Salmi M, Jalkanen S, Savilahti E, Westerholm M, Kantele A. Mucosally activated circulating human B cells in diarrhea express homing receptors directing them back to the gut. Gastroenterology. 1996;110:1061-1067. [PubMed] |
| 5. | Crabbé PA, Nash DR, Bazin H, Eyssen DV, Heremans JF. Antibodies of the IgA type in intestinal plasma cells of germfree mice after oral or parenteral immunization with ferritin. J Exp Med. 1969;130:723-744. [PubMed] |
| 6. | Loft DE, Marsh MN, Sandle GI, Crowe PT, Garner V, Gordon D, Baker R. Studies of intestinal lymphoid tissue. XII. Epithelial lymphocyte and mucosal responses to rectal gluten challenge in celiac sprue. Gastroenterology. 1989;97:29-37. [PubMed] |
| 7. | Loft DE, Marsh MN, Crowe PT. Rectal gluten challenge and diagnosis of coeliac disease. Lancet. 1990;336:953. [PubMed] |
| 8. | Ensari A, Marsh MN, Morgan S, Lobley R, Unsworth DJ, Kounali D, Crowe PT, Paisley J, Moriarty KJ, Lowry J. Diagnosing coeliac disease by rectal gluten challenge: a prospective study based on immunopathology, computerized image analysis and logistic regression analysis. Clin Sci (Lond). 2001;101:199-207. [PubMed] |
| 9. | Quiding-Järbrink M, Lakew M, Nordström I, Banchereau J, Butcher E, Holmgren J, Czerkinsky C. Human circulating specific antibody-forming cells after systemic and mucosal immunizations: differential homing commitments and cell surface differentiation markers. Eur J Immunol. 1995;25:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Barratt M, Powell J, Allen W, Porter P, Marsh MN. Immunopathology of intestinal disorders in farm animals. In: March MN. Immunopathology of the Small Intestine. Marsh MN. Chichester: John Wiley 1987; 253-281. |
| 11. | Ráki M, Fallang LE, Brottveit M, Bergseng E, Quarsten H, Lundin KE, Sollid LM. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc Natl Acad Sci USA. 2007;104:2831-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Brottveit M, Ráki M, Bergseng E, Fallang LE, Simonsen B, Løvik A, Larsen S, Løberg EM, Jahnsen FL, Sollid LM, Lundin KE. Assessing possible celiac disease by an HLA-DQ2-gliadin Tetramer Test. Am J Gastroenterol. 2011;106:1318-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Sarna VK, Skodje GI, Reims HM, Risnes LF, Dahal-Koirala S, Sollid LM, Lundin KEA. HLA-DQ:gluten tetramer test in blood gives better detection of coeliac patients than biopsy after 14-day gluten challenge. Gut. 2018;67:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Cabanne A, Vázquez H, Argonz J, Moreno ML, Nachman F, Niveloni S, Mazure R, Kogan Z, Gómez JC, Mauriño E, Bai JC. Clinical utility of counting intraepithelial lymphocytes in celiac disease intestinal mucosa. Acta Gastroenterol Latinoam. 2007;37:20-28. [PubMed] |
| 15. | Marsh MN, Heal CJ. Evolutionary Developments in Interpreting the Gluten-Induced Mucosal Celiac Lesion: An Archimedian Heuristic. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Rostami K, Marsh MN, Johnson MW, Mohaghegh H, Heal C, Holmes G, Ensari A, Aldulaimi D, Bancel B, Bassotti G, Bateman A, Becheanu G, Bozzola A, Carroccio A, Catassi C, Ciacci C, Ciobanu A, Danciu M, Derakhshan MH, Elli L, Ferrero S, Fiorentino M, Fiorino M, Ganji A, Ghaffarzadehgan K, Going JJ, Ishaq S, Mandolesi A, Mathews S, Maxim R, Mulder CJ, Neefjes-Borst A, Robert M, Russo I, Rostami-Nejad M, Sidoni A, Sotoudeh M, Villanacci V, Volta U, Zali MR, Srivastava A. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66:2080-2086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Crowe PT, Marsh MN. Morphometric analysis of intestinal mucosa. VI--Principles in enumerating intra-epithelial lymphocytes. Virchows Arch. 1994;424:301-306. [PubMed] |
| 18. | Marsh MN. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology. 1980;79:481-492. [PubMed] |
| 19. | Crowe PT, Marsh MN. Morphometric analysis of small intestinal mucosa. IV. Determining cell volumes. Virchows Arch A Pathol Anat Histopathol. 1993;422:459-466. [PubMed] |
| 20. | Taavela J, Koskinen O, Huhtala H, Lähdeaho ML, Popp A, Laurila K, Collin P, Kaukinen K, Kurppa K, Mäki M. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One. 2013;8:e76163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Chapman BL, Henry K, Paice F, Coghill NF, Stewart JS. Measuring the response of the jejunal mucosa in adult coeliac disease to treatment with a gluten-free diet. Gut. 1974;15:870-874. [PubMed] |
| 22. | Ensari A, Marsh MN. Coeliac disease: critical evaluations in the interpretation of mucosal histology. Turk J Gastro. 2019;30:389-397. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Marsh MN. Studies of intestinal lymphoid tissue. IV--The predictive value of raised mitotic indices among jejunal epithelial lymphocytes in the diagnosis of gluten-sensitive enteropathy. J Clin Pathol. 1982;35:517-525. [PubMed] |
| 24. | Dhesi I, Marsh MN, Kelly C, Crowe P. Morphometric analysis of small intestinal mucosa. II. Determination of lamina propria volumes; plasma cell and neutrophil populations within control and coeliac disease mucosae. Virchows Arch A Pathol Anat Histopathol. 1984;403:173-180. [PubMed] |
| 25. | Guix M, Skinner JM, Whitehead R. Measuring intraepithelial lymphocytes, surface area, and volume of lamina propria in the jejunal mucosa of coeliac patients. Gut. 1979;20:275-278. [PubMed] |
| 26. | Marsh MN, Crowe P, Moriarty K, Ensari A. Morphometric analysis of intestinal mucosa: the measurement of volume compartments and cell volumes in intestinal mucosa. In: March MN.. Coeliac Disease: Methods and Protocols, Methods in Molecular Medicine #41. Totowa Humana Press 2002; . |
| 27. | Marsh MN, Hinde J. Inflammatory component of celiac sprue mucosa. I. Mast cells, basophils, and eosinophils. Gastroenterology. 1985;89:92-101. [PubMed] |
| 28. | Weibel ER. Stereological Methods, Vol 1. New York: Academic Press 1979; . |
| 29. | Dunnill M. Quantitative methods in histology. In: Dyke SC. Recent Advances in Clinical Pathology. London: Churchill, 1968; 401-416. |
| 30. | Marsh MN. The mucosal pathology of gluten sensitivity. In: Marsh MN. Coeliac Disease. Oxford: Blackwell Scientific Publications, 1992; 136-191. |
| 31. | Anderson CM. Histological changes in the duodenal mucosa in coeliac disease. Reversibility during treatment with a wheat gluten free diet. Arch Dis Child. 1960;35:419-427. [PubMed] |
| 32. | Fry L, Seah PP, McMinn RM, Hoffbrand AV. Lymphocytic infiltration of epithelium in diagnosis of gluten-sensitive enteropathy. Br Med J. 1972;3:371-374. [PubMed] |
| 33. | McConnell RB, Whitwell F. Letter: Small-intestinal histology in coeliac disease. Lancet. 1975;2:418. [PubMed] |
| 34. | Scott BB, Losowsky MS. Coeliac disease with mild mucosal abnormalities: a report of four patients. Postgrad Med J. 1977;53:134-138. [PubMed] |
| 35. | Egan-Mitchell B, Fottrell PF, McNicholl B. Early or pre-coeliac mucosa: development of gluten enteropathy. Gut. 1981;22:65-69. [PubMed] |
| 36. | Marsh MN. Studies of intestinal lymphoid tissue. XIII. Immunopathology of the evolving celiac sprue lesion. Pathol Res Pract. 1989;185:774-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Mäki M, Holm K, Koskimies S, Hällström O, Visakorpi JK. Normal small bowel biopsy followed by coeliac disease. Arch Dis Child. 1990;65:1137-1141. [PubMed] |
| 38. | Marsh MN, Loft D, Garner V, Gordon D. Time/dose responses of coeliac mucosae to graded oral challenges with Frazer’s fraction III of gliadin.. Eur J Gastroenterol Hepatol. 1992;4:667-674. |
| 39. | Mowat AM, Ferguson A. Intraepithelial lymphocyte count and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982;83:417-423. [PubMed] |
| 40. | Marsh MN, Bjarnason I, Shaw J, Ellis A, Baker R, Peters TJ. Studies of intestinal lymphoid tissue. XIV--HLA status, mucosal morphology, permeability and epithelial lymphocyte populations in first degree relatives of patients with coeliac disease. Gut. 1990;31:32-36. [PubMed] |
| 41. | Marsh MN. Celiac and allied sprue syndromes. In: Sleisenger M, Fordtran J. Seminars in Gastrointestinal Disease 1992, 3: 214-223. |
| 42. | Rostami K, Aldulaimi D, Holmes G, Johnson MW, Robert M, Srivastava A, Fléjou JF, Sanders DS, Volta U, Derakhshan MH, Going JJ, Becheanu G, Catassi C, Danciu M, Materacki L, Ghafarzadegan K, Ishaq S, Rostami-Nejad M, Peña AS, Bassotti G, Marsh MN, Villanacci V. Microscopic enteritis: Bucharest consensus. World J Gastroenterol. 2015;21:2593-2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Rostami K, Ensari A, Ciacci C, Srivastava A, Volta U, Villanacci V, Marsh MN. Coeliac biopsies: numbers are valid, alphabets not. Gut. 2018;67:2069-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Marsh MN. The scanning electron microscope and its application to the investigation of intestinal structure. In: Badenoch J, Brooke BN. Recent Advances in Gastroenterology, 2nd Edition. London: Churchill Livingstone 1972; 81-135. |
| 45. | Toner PG, Carr KE, Ferguson A, Mackay C. Scanning and transmission electron microscopic studies of human intestinal mucosa. Gut. 1970;11:471-481. [PubMed] |
| 46. | Creamer B, Leppard P. Post-mortem examination of a small intestine in the coeliac syndrome. Gut. 1965;6:466-471. [PubMed] |
| 47. | Loehry CA, Creamer B. Three-dimensional structure of the human small intestinal mucosa in health and disease. Gut. 1969;10:6-12. [PubMed] |
| 48. | Cocco AE, Dohrmann MJ, Hendrix TR. Reconstruction of normal jejunal biopsies: three-dimensional histology. Gastroenterology. 1966;51:24-31. [PubMed] |
| 49. | Marsh MN, Rostami K. What Is A Normal Intestinal Mucosa? Gastroenterology. 2016;151:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Padykula HA, Strauss EW, Ladman AJ, Gardner FH. A morphologic and histochemical analysis of the human jejunal epithelium in nontropical sprue. Gastroenterology. 1961;40:735-765. [PubMed] |
| 51. | Trier JS, Browning TH. Epithelial-cell renewal in cultured duodenal biopsies in celiac sprue. N Engl J Med. 1970;283:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Watson AJ, Wright NA. Coeliac disease. Morphology and cell kinetics of the jejunal mucosa in untreated patients. Clin Gastroenterol. 1974;3:11-31. [PubMed] |
| 53. | Daum S, Bauer U, Foss HD, Schuppan D, Stein H, Riecken EO, Ullrich R. Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy specimens from patients with coeliac disease. Gut. 1999;44:17-25. [PubMed] |
| 54. | Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni L, MacDonald TT, Pallone F. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Diosdado B, van Bakel H, Strengman E, Franke L, van Oort E, Mulder CJ, Wijmenga C, Wapenaar MC. Neutrophil recruitment and barrier impairment in celiac disease: a genomic study. Clin Gastroenterol Hepatol. 2007;5:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Brottveit M, Beitnes AC, Tollefsen S, Bratlie JE, Jahnsen FL, Johansen FE, Sollid LM, Lundin KE. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 57. | Hindryckx P, Levesque BG, Holvoet T, Durand S, Tang CM, Parker C, Khanna R, Shackelton LM, D'Haens G, Sandborn WJ, Feagan BG, Lebwohl B, Leffler DA, Jairath V. Disease activity indices in coeliac disease: systematic review and recommendations for clinical trials. Gut. 2018;67:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Ludvigsson JF, Ciacci C, Green PH, Kaukinen K, Korponay-Szabo IR, Kurppa K, Murray JA, Lundin KEA, Maki MJ, Popp A, Reilly NR, Rodriguez-Herrera A, Sanders DS, Schuppan D, Sleet S, Taavela J, Voorhees K, Walker MM, Leffler DA. Outcome measures in coeliac disease trials: the Tampere recommendations. Gut. 2018;67:1410-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 59. | Charlesworth RPG, Andronicos NM, Scott DR, McFarlane JR, Agnew LL. Can the sensitivity of the histopathological diagnosis of coeliac disease be increased and can treatment progression be monitored using mathematical modelling of histological sections? - A pilot study. Adv Med Sci. 2017;62:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Charlesworth RPG, Agnew LL, Scott DR, Andronicos NM. Celiac disease gene expression data can be used to classify biopsies along the Marsh score severity scale. J Gastroenterol Hepatol. 2019;34:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Charlesworth RPG, Agnew LL, Scott DR, Andronicos NM. Equations defined using gene expression and histological data resolve coeliac disease biopsies within the Marsh score continuum. Comput Biol Med. 2019;104:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Ensari A, Marsh MN. Exploring the villus. Gastroenterol Hepatol Bed Bench. 2018;11:181-190. [PubMed] |
| 63. | De Gregorio V, Imparato G, Urciuolo F, Netti PA. Micro-patterned endogenous stroma equivalent induces polarized crypt-villus architecture of human small intestinal epithelium. Acta Biomater. 2018;81:43-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |