Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2365
Peer-review started: February 12, 2019
First decision: March 20, 2019
Revised: April 12, 2019
Accepted: April 29, 2019
Article in press: April 29, 2019
Published online: May 21, 2019
Processing time: 101 Days and 19.8 Hours
Lenvatinib is one of the first-line tyrosine kinase inhibitors used for unresectable hepatocellular carcinoma (HCC). In the present study, we evaluated the potential of early changes in the time-intensity curve (TIC) of arterial phase on contrast-enhanced ultrasound (CEUS) as early imaging biomarkers of lenvatinib efficacy.
To evaluate the potential of the early changes in the TIC of CEUS as early imaging biomarkers of lenvatinib efficacy in patients with unresectable HCC.
We analyzed 20 consecutive patients with unresectable HCC treated with lenvatinib from March to November 2018. Tumor response at 8 wk was assessed by computed tomography using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). CEUS was performed at baseline before treatment (Day 0) and on day 7 (Day 7), and the images were analyzed in the arterial phase for 20 seconds after the contrast agent arrived at the target tumor. Three perfusion parameters were extracted from the TICs: the slope of wash-in (Slope), time to peak (TTP) intensity, and the total area under the curve (AUC) during wash-in. The rate of change in the TIC parameters between Day 0 and Day 7 was compared between treatment responders and non-responders based on mRECIST.
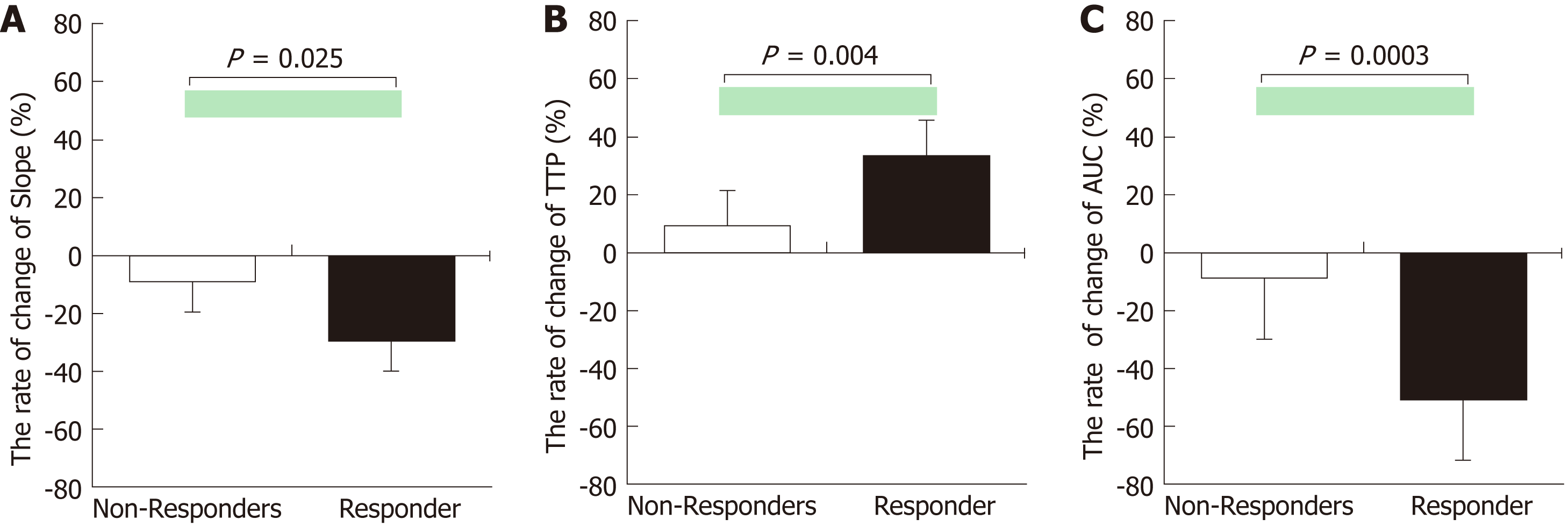
The rate of change for all TIC parameters showed significant differences between the responders (n = 9) and non-responders (n = 11) (Slope, P = 0.025; TTP, P = 0.004; and AUC, P = 0.0003). The area under the receiver operating curve values for slope, TTP, and AUC for the prediction of responders were 0.805, 0.869, and 0.939, respectively.
CEUS may be useful for the early prediction of tumor response to lenvatinib therapy in patients with unresectable HCC.
Core tip: Lenvatinib is one of the first-line tyrosine kinase inhibitors used for unresectable hepatocellular carcinoma (HCC). In the present study, we evaluated the potential of early changes in the time-intensity curve (TIC) of arterial phase on contrast-enhanced ultrasound (CEUS) as early imaging biomarkers of lenvatinib efficacy. The rate of change for TIC parameters showed precisely reflect the therapeutic effects. CEUS may be useful for the early prediction of tumor response to lenvatinib therapy in patients with unresectable HCC.
- Citation: Kuorda H, Abe T, Fujiwara Y, Okamoto T, Yonezawa M, Sato H, Endo K, Oikawa T, Sawara K, Takikawa Y. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol 2019; 25(19): 2365-2372
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2365
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver [1]. Unfortunately, the overall prognosis for patients with HCC is poor, and more than half of the patients are diagnosed at a stage when the tumor is unresectable. The treatment options for unresectable HCC are limited, and oral administration of sorafenib, a receptor tyrosine kinase inhibitor, has been the only treatment that substantially prolongs survival[2,3]. In the SHARP study, compared to the placebo group, the sorafenib group had an improved overall survival (OS) (median OS, 7.9 mo vs 10.7 mo)[4]. However, the clinical benefits of sorafenib are modest, and the survival rates in patients with unresectable HCC remain low. Lenvatinib is an oral multikinase inhibitor targeting the vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor (PDGF) receptor α, RET, and KIT[5]. The phase III REFLECT trial including 954 patients with previously untreated unresectable HCC demonstrated that lenvatinib had a treatment effect on OS by statistical confirmation of non-inferiority when compared to sorafenib, the standard of care[6]. Furthermore, lenvatinib also demonstrated a significantly higher overall response rate (ORR) compared to sorafenib [24.1% vs 9.2%; odds ratio, 3.13; 95% confidence interval (CI): 2.15-4.56; P < 0.0001]. In recent years, lenvatinib has become available as a single agent for the first-line treatment of patients with advanced or unresectable HCC[7].
There is a critical need for effective early methods for evaluating targeted therapies to enable individualized medicine in a clinical setting. Contrast-enhanced ultrasound (CEUS) is considered to be a useful technique for evaluating microvascularization, which is essential for tumorigenesis since angiogenesis is the basis for neoplastic growth. Lassau et al. have reported that the time-intensity curve (TIC) parameters obtained from CEUS of tumors correlated well with prognosis[8]. Furthermore, Frampas et al. showed that CEUS may be a potential surrogate marker of tumor response during targeted therapy, and the area under the curve (AUC), one of the TIC parameters, was useful for assessing blood flow[9]. However, there have been no reports designed to assess the usefulness of CEUS for early prediction of the efficacy of lenvatinib therapy.
This study investigated whether early changes in the TIC parameters of CEUS are useful indicators of the therapeutic effects of lenvatinib therapy.
HCC was diagnosed on the basis of an increasing course of α-fetoprotein, dynamic computed tomography (CT), contrast-enhanced magnetic resonance imaging (MRI), and pathological findings. Between March and November 2018, 22 consecutive HCC patients with (1) an Eastern Cooperative Oncology Group (ECOG) performance status score of 2 or less, (2) Child-Pugh liver function class A, and (3) Barcelona Clinic Liver Cancer stage B or C were enrolled in this prospective study to assess the potential of CEUS findings as early imaging biomarkers of lenvatinib efficacy. Two patients were excluded from the analysis owing to data corruption, and so a total of 20 patients were finally included in this study. One target tumor per patient was studied. The baseline characteristics of the patients are summarized in Table 1.
| Variables | All (n = 20) |
| Age, yr | 68.6 ± 8.4 |
| Gender, male : female | 19:1 |
| BMI, kg/m2 | 22.2 ± 4.2 |
| ECOG PS, 0:1: | 18:2 |
| Etiology, HBV:HCV:alcohol:others | 7:7:3:3 |
| AST, IU/L | 52.2 ± 38.1 |
| ALT, IU/L | 45.1 ± 26.3 |
| T.Bil, mg/dL | 0.7 ± 0.4 |
| Alb, g/dL | 3.4 ± 0.5 |
| PT, % | 82.2 ± 15.2 |
| Plt, × 104/μL | 19.7 ± 6.1 |
| Child–Pugh score, 5:6 points | 8:12 |
| Median AFP, ng/mL (range) | 268 (4.5-53000) |
| Intrahepatic tumor size, cm | 6.6 ± 6.3 |
| Number of intrahepatic tumors, single : multiple | 3:17 |
| Portal vein invasion, n (%) | 9 (45.0) |
| Extrahepatic metastasis, n (%) | 15 (75.0) |
| Previous treatment, n (%) | 19 (95.0) |
| Initial dose of lenvatinib, 8:12 mg/d | 16:4 |
The study was approved by the local Ethics Committee of the Iwate Medical University (MH2018-533). The patients provided written informed consent prior to the study, in accordance with the principles of the Declaration of Helsinki (revision of Fortaleza, 2013).
Lenvatinib (Eisai, Tokyo, Japan) was administered at an initial dose of 8 or 12 mg/d based on the patient's body weight. If grade 3 or 4 adverse events judged to be clinically significant were observed, either the dose was adjusted, or treatment was interrupted according to the guidelines for the administration of lenvatinib. Baseline dynamic CT or MRI was performed within a week before treatment initiation. The target tumor was evaluated using dynamic CT at 8 wk after administering lenvatinib, based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST)[10]. Treatment responders were defined as patients who showed a complete response (CR) or partial response (PR). Non-responders were defined as patients who had stable disease (SD) or progressive disease (PD).
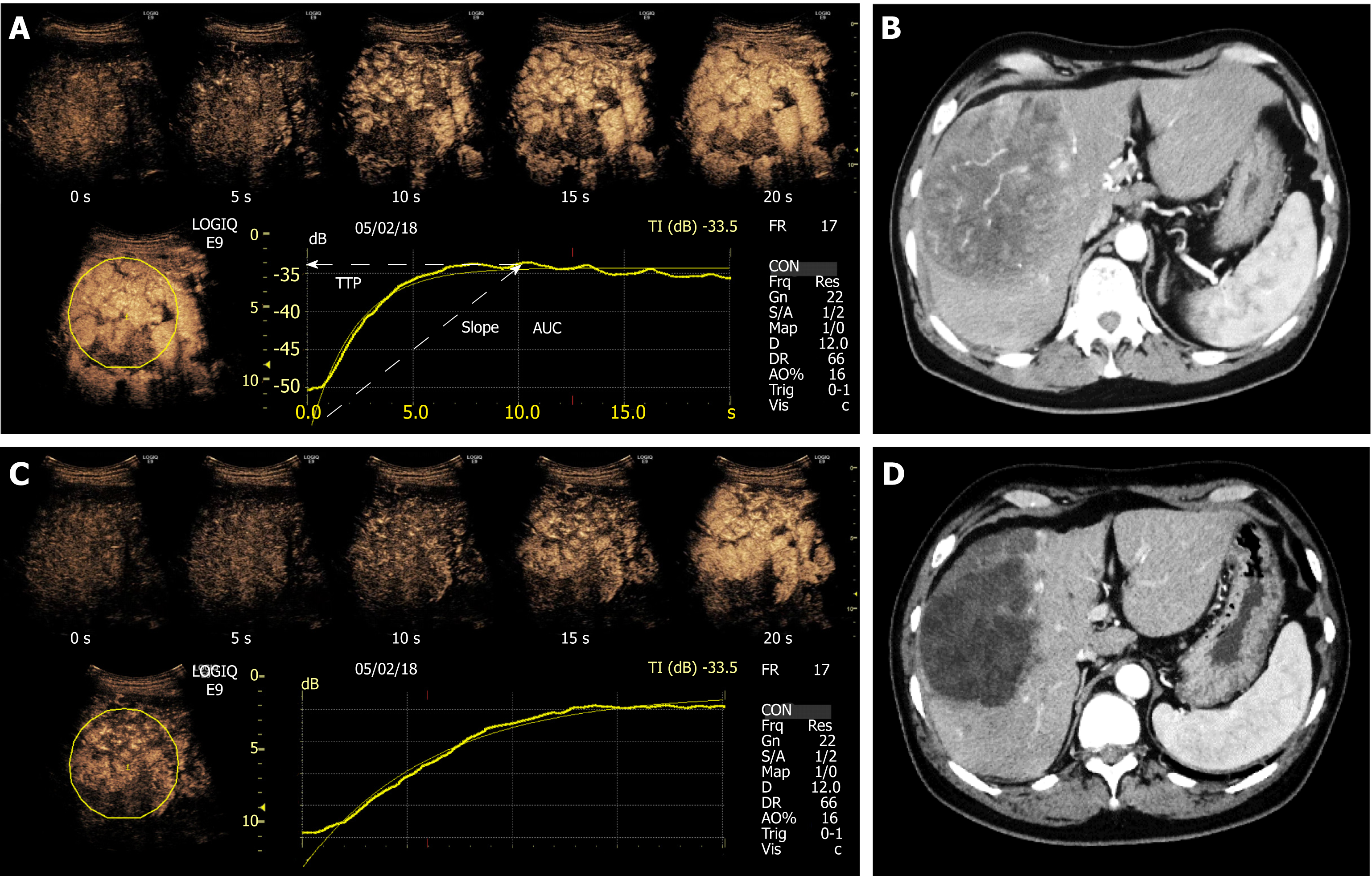
CEUS was performed at baseline before the initiation of treatment, and then on Day 7, for evaluation. We selected Day 7 for reference in previous studies on sorafenib therapy[11-15]. Ultrasonography was performed using a LOGIQ E9 XDclear 2.0 ultrasound scanner (GE Healthcare, Wauwatosa, WI, United States) and C1-6-D convex array probe (frequency, 4 MHz). Prior to CEUS, B-mode ultrasonography was performed to examine the slices of target images, and the slice with the largest diameter was selected. All ultrasound images were analyzed by one radiologist who was blinded from the treatment information. CEUS imaging was recorded for 2 min immediately after injection of a bolus (0.0075 mL/kg) of Sonazoid (Daiichi Sankyo, Tokyo, Japan) using the amplitude modulation mode. The acoustic power of the contrast harmonic sonography was set at the default setting with a mechanical index of 0.2-0.3, a rate of 17 frames per second, and a dynamic range of 66 dB. The gain, image depth and transmit focus were optimized for each patient at baseline examination, and the same settings were used at follow up. The cine sequences were saved in the DICOM file format for subsequent analyses.
A specific calibration file provided by the vendor for the GE Logiq E9 was used in the analysis software to convert ultrasound images to linearized data for TIC analysis. A circular region of interest was established within the demarcated margins of the target tumor as illustrated in Figure 1, which was automatically positioned by the software to adjust for respiratory motion on the following images of the sequence. We analyzed the CEUS images in the arterial phase for 20 s after the contrast agent arrived at the target tumor. Three perfusion parameters were extracted from the TICs: the slope of wash-in (Slope), time to peak (TTP) intensity, and the total area under the TIC (AUC) during wash-in. For each parameter, the rate of change was calculated as follows: {[values after administration of lenvatinib (Day 7) minus baseline values (Day 0)]/baseline values (Day 0) × 100(%)}[16]. The resultant values were compared between the responders and non-responders based on mRECIST.
Statistical analyses were performed using the SPSS software program (version 23, IBM, Armonk, NY, United States). The values are shown as the means ± standard deviation or as the medians (range) according to the distribution of the values. The Mann-Whitney U test was used to compare differences between responders and non-responders. Receiver operating characteristic (ROC) curves were constructed, and area under the ROC curve (AUROC) was calculated using the trapezoidal rule. Optimal cut-off values for prediction of responders were identified from the highest Youden index. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated using cut-offs obtained from the ROC curves. P-values < 0.05 were considered to be statistically significant.
All 20 patients were evaluated based on the imaging findings obtained by enhanced CT at 8 wk after starting lenvatinib therapy. On the basis of mRECIST, 0, 9, 8, and 3 patients were found to have CR, PR, SD, and PD, respectively [ORR, 45.0%; disease control rate (DCR), 85.0%]. Thus, 9 and 11 patients were classified as responders and non-responders, respectively, after 8 wk of lenvatinib therapy. There were no statistically significant differences in the ORR and DCR from those in patients receiving the initial dose of lenvatinib.
In responders, the TIC parameters were as follows: median slope Day 0/Day 7: 1.51 dB/s/1.09 dB/s, P = 0.018; median TTP Day 0/Day 7: 10.56 s/12.43 s, P = 0.003; median AUC Day 0/Day 7: 266.51/156.44, P = 0.001. In contrast, the TIC parameters in non-responders were as follows: median slope Day 0/Day 7: 1.52 dB/s/1.33 dB/s, P = 0.511; median TTP Day 0/Day 7: 11.02 s/11.84 s, P = 0.247; median AUC Day 0/Day 7: 258.14/229.65, P = 0.322 (Table 2). There were no significant differences in any TIC parameters between patients with SD and PD.
| Responders (n = 9) | Non-responders (n = 11) | |||
| Day 0 | Day 7 | Day 0 | Day 7 | |
| Slope | 1.51 [1.31, 1.68] | 1.09 [0.84, 1.23] | 1.52 [1.22, 1.62] | 1.33 [0.86, 1.71] |
| P value | 0.018 | 0.511 | ||
| TTP | 10.56 [9.33, 12.13] | 12.43 [11.94, 13.94] | 11.02 [8.53, 12.51] | 11.84 [10.15, 13.82] |
| P value | 0.003 | 0.247 | ||
| AUC | 266.51 [225.38, 296.67] | 156.44 [123.05, 178.91] | 258.14 [191.61, 299.51] | 229.65 [176.10, 269.50] |
| P value | 0.001 | 0.322 | ||
The rate of change for all TIC parameters showed significant differences between the responders and non-responders (Slope, P = 0.022; TTP, P = 0.019; AUC, P = 0.003) (Figure 2). The AUROC values for the rate of change of slope, TTP, and AUC for prediction of responders were 0.818, 0.869, and 0.939, respectively (Table 3).
| Slope (95%CI) | TTP (95%CI) | AUC (95%CI) | |
| AUROC | 0.818 (0.602-0.944) | 0.869 (0.728-0.975) | 0.939 (0.812-0995) |
| Cut-off value (%) | -11.765 | +9.495 | -25.714 |
| Sensitivity | 0.889 (0.540-0.978) | 0.889 (0.540-0.978) | 0.896 (0.616-0.989) |
| Specificity | 0.545 (0.304-0.786) | 0.818 (0.510-0.917) | 0.909 (0.648-0.995) |
| PPV | 0.615 (0.583-0.793) | 0.800 (0.611-0.907) | 0.833 (0.727-0.918) |
| NPV | 0.857 (0.731-0.922) | 0.878 (0.741-0.962) | 0.900 (0.747-0.992) |
In this study, we enrolled 20 patients with unresectable HCC and used CEUS to make an early prediction of the efficacy of lenvatinib therapy. The results of the study confirm that real-time observation of the perfusion within HCC is possible by CEUS, and responders to lenvatinib show a change in perfusion in the arterial phase on CEUS within a few days of starting the therapy. In our study, the rate of change in the slope, TTP, and AUC on Day 7 was significantly different in the responders and non-responders. The findings of CEUS performed at the earliest stage of therapy reflected the results of the CT evaluation performed 8 weeks after starting lenvatinib therapy, thereby suggesting that CEUS can predict the clinical outcomes at an early stage of therapy. To the best of our knowledge, this is the first prospective study to assess the potential of CEUS for making early predictions of clinical outcomes following lenvatinib therapy.
Besides its antitumor effects, lenvatinib is also known to be antiangiogenic based on its interaction with VEGFR2[5]. Since angiogenesis is necessary for tumor growth, the changes in tumor perfusion seem to reflect changes in tumor vitality. Therefore, CEUS is relevant as a modality for monitoring biologically essential changes in response to lenvatinib therapy. Several studies have reported that the changes in perfusion measured by CEUS may predict treatment responses in patients with HCC receiving sorafenib[11-15]. Sugimoto et al[14] showed that the AUC during wash-in on day 14 of sorafenib therapy was useful for the early prediction of tumor response. We observed a similar trend; the rate of change of the AUC during wash-in on day 7 of lenvatinib therapy was significantly different between the responders and non-responders. In the REFLECT trial, lenvatinib demonstrated a significantly higher ORR than sorafenib[6], which was potentially due to its stronger effect on tumor perfusion.
The evaluation of tumor perfusion by CEUS is a simple, non-invasive test that can be done in real time. Such a prediction tool is also cost-effective since it helps avoid adverse events and may enable individualized treatment for unresectable HCC in the future.
This study has some limitations. First, the sample size was small. Larger-scale prospective clinical studies will be needed to confirm these findings. Second, unlike dynamic CT and MRI, which are commonly used to assess tumor angiogenesis, CEUS is an operator-dependent examination.
In conclusion, this study demonstrates that with contrast enhancement in the arterial phase, the differences in the TIC before and after lenvatinib therapy may serve as useful indicators of therapeutic outcomes for patients with unresectable HCC. In particular, the rate of change for AUC appear to precisely reflect the therapeutic effects. Therefore, TIC analysis could help in the early prediction of the clinical outcomes of lenvatinib therapy.
Lenvatinib is one of the first-line tyrosine kinase inhibitors used for unresectable hepatocellular carcinoma (HCC).
The overall prognosis for patients with HCC is poor, and more than half of the patients are diagnosed at a stage when the tumor is unresectable. The treatment options for unresectable HCC are limited, and oral administration of sorafenib, a receptor tyrosine kinase inhibitor, has been the only treatment that substantially prolongs survival.
To evaluate the potential of the early changes in the time-intensity curve (TIC) of contrast-enhanced ultrasound (CEUS) as early imaging biomarkers of lenvatinib efficacy in patients with unresectable HCC.
We analyzed 20 consecutive patients with unresectable HCC treated with lenvatinib from March to November 2018. Tumor response at 8 wk was assessed by computed tomography using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). CEUS was performed at baseline before treatment (Day 0) and on day 7 (Day 7), and the images were analyzed in the arterial phase for 20 seconds after the contrast agent arrived at the target tumor. Three perfusion parameters were extracted from the TICs: the slope of wash-in (Slope), time to peak (TTP) intensity, and the total area under the curve (AUC) during wash-in. The rate of change in the TIC parameters between Day 0 and Day 7 was compared between treatment responders and non-responders based on mRECIST.
The rate of change for all TIC parameters showed significant differences between the responders (n = 9) and non-responders (n = 11) (Slope, P = 0.025; TTP, P = 0.004; and AUC, P = 0.0003). The area under the receiver operating curve values for slope, TTP, and AUC for the prediction of responders were 0.805, 0.869, and 0.939, respectively.
To the best of our knowledge, this is the first prospective study to assess the potential of CEUS for making early predictions of outcomes following lenvatinib therapy, which makes it a significant contribution to the literature.
Further, we believe that this paper will be of interest to the readership especially hepatologists and oncologists because we demonstrate that CEUS may be useful for the early prediction of tumor response in patients with unresectable HCC treated with lenvatinib.
The authors thank Ms. Yuriko Mikami and Ms. Kouko Motodate for their excellent technical assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ho HK, Vradelis S, Yarmohammadi H S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3249] [Cited by in F6Publishing: 3511] [Article Influence: 292.6] [Reference Citation Analysis (3)] |
| 2. | Gauthier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol Res. 2013;43:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4438] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9747] [Article Influence: 609.2] [Reference Citation Analysis (1)] |
| 5. | Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, Minoshima Y, Iwata M, Funahashi Y. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3128] [Cited by in F6Publishing: 3293] [Article Influence: 548.8] [Reference Citation Analysis (0)] |
| 7. | Kudo M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers (Basel). 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, Taieb S, Aziza R, Sarran A, Labbe-Devilliers C, Gallix B, Lucidarme O, Ptak Y, Rocher L, Caquot LM, Chagnon S, Marion D, Luciani A, Feutray S, Uzan-Augui J, Coiffier B, Benastou B, Koscielny S. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Frampas E, Lassau N, Zappa M, Vullierme MP, Koscielny S, Vilgrain V. Advanced Hepatocellular Carcinoma: early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary results. Eur J Radiol. 2013;82:e205-e211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2583] [Cited by in F6Publishing: 3001] [Article Influence: 214.4] [Reference Citation Analysis (36)] |
| 11. | Shiozawa K, Watanabe M, Ikehara T, Shimizu R, Shinohara M, Igarashi Y, Sumino Y. Evaluation of sorafenib for advanced hepatocellular carcinoma with low α-fetoprotein in arrival time parametric imaging using contrast-enhanced ultrasonography. J Med Ultrason (2001). 2017;44:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Knieling F, Waldner MJ, Goertz RS, Strobel D. Quantification of dynamic contrast-enhanced ultrasound in HCC: prediction of response to a new combination therapy of sorafenib and panobinostat in advanced hepatocellular carcinoma. BMJ Case Rep. 2012;2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Shiozawa K, Watanabe M, Ikehara T, Kogame M, Kikuchi Y, Igarashi Y, Sumino Y. Therapeutic evaluation of sorafenib for hepatocellular carcinoma using contrast-enhanced ultrasonography: Preliminary result. Oncol Lett. 2016;12:579-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, Imai Y. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Lamuraglia M, Escudier B, Chami L, Schwartz B, Leclère J, Roche A, Lassau N. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006;42:2472-2479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Ueda N, Nagira H, Sannomiya N, Ikunishi S, Hattori Y, Kamida A, Koyanagi Y, Shimabayashi K, Sato K, Saito H, Hirooka Y. Contrast-Enhanced Ultrasonography in Evaluation of the Therapeutic Effect of Chemotherapy for Patients with Liver Metastases. Yonago Acta Med. 2016;59:255-261. [PubMed] [Cited in This Article: ] |