Published online May 7, 2019. doi: 10.3748/wjg.v25.i17.2133
Peer-review started: November 26, 2018
First decision: December 28, 2018
Revised: March 27, 2019
Accepted: March 29, 2019
Article in press: March 30, 2019
Published online: May 7, 2019
Processing time: 165 Days and 17.8 Hours
Patients with neuroendocrine tumors (NETs) of the gastrointestinal tract suffer frequently from chronic diarrhea. A well characterized medical advice containing zeolite (Detoxsan® powder) was applied to patients suffered from therapy-refractory diarrhea either by its frequency or by watery stool, despite receiving standard pharmacotherapy according to the guidelines for carcinoid syndrome and comorbidities. Detoxsan® powder acts as an adsorbent and might reduce significantly symptoms of diarrhea in patients suffering from NETs.
To overcome the therapy-refractory diarrhea of patients with NETs by the zeolite containing medical advice Detoxsan® powder.
A total of 20 patients (12 female and 8 male) suffering from diarrhea either by its frequency or from watery stool caused by NETs were included. In each patient, the diagnosis had been confirmed by histology and somatostatin receptors expression proven by positron emission tomography/computed tomography using Ga-68-labeled somatostatin analogs. All patients received standard-of-care pharmacotherapy and were additionally given Detoxsan® powder as an extemporaneous drug containing 90% natural Cuban zeolite and 10% magnesium aspartate. Recommended daily dosage ranges between 3 g once to three times per day. Each day dose and bowel movements were documented by the patients themselves in a pre-defined table. Additionally to the bowel movements quantitative determinations of serotonin, urea, creatinine and single ions were performed within the serum of the patients by commercially available equipment used as a matter of routine in the clinic.
All patients enrolled in this pilot study did not only suffer from NETs, but also from comorbidities and treatment-resistant diarrhea. There was insufficient control of diarrhea, most probably due to the secretion of hormones like serotonin produced by the slowly growing and highly differentiated NETs. All patients only took Detoxsan® powder as an antidiarrheal drug. In general, response effects need several days to become perceptible and require an intake of Detoxsan® powder for an extended time period or intermittently, if persisting stabilization of bowel movements could not be achieved. A correlation between NET grade, part and size of bowel resection and functionality of the tumor could not be demonstrated. Therefore, diarrhea seemed to be based on the metabolic activity of the well-differentiated NETs, which eventually led to treatment resistance. In summary, 14 out of the 20 patients (70%) declared to be very content with using Detoxsan® powder and observed a significant reduction of diarrhea, while the effective dose and intake period that resulted in a symptom relief varied individually.
Detoxsan® powder is able to reduce significantly symptoms of NET-related diarrhea in the majority of patients. The duration of taking Detoxsan® powder and its dosage vary individually.
Core tip: A well characterized zeolite (Detoxsan® powder) was applied to patients with neuroendocrine tumors (NETs) of the gastrointestinal tract suffered from therapy-refractory diarrhea either by its frequency or by watery stool, despite receiving standard pharmacotherapy according to the guidelines for carcinoid syndrome and comorbidities. In 14 of 20 patients (70%) bowel movement rate could be normalized. Thus, Detoxsan® powder acts as an adsorbent and is able to reduce significantly symptoms of diarrhea in patients suffering from NETs. However, dose and period of intake have to be individually adjusted.
- Citation: Langbein T, Dathe W, Deuerling A, Baum RP. Efficacy of Detoxsan® powder on diarrhea caused by gastrointestinal neuroendocrine tumors. World J Gastroenterol 2019; 25(17): 2133-2143
- URL: https://www.wjgnet.com/1007-9327/full/v25/i17/2133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i17.2133
There are diverse etiologies of diarrhea due to different or tissue-dependent mechanisms[1]. Chronic diarrhea can be caused by neuroendocrine tumors (NETs) of the gastrointestinal tract (previously called “carcinoids”), which lead to significantly reduced quality of life. Application of synthetic analogues of somatostatin[2] is one of the established treatment options to control characteristic symptoms like flushing and diarrhea as well as to inhibit tumor growth. A significant proportion of patients might stop responding to somatostatin analogue therapy or this treatment alone might not achieve adequate symptom relief. Therefore, new and active substances are certainly required to alleviate relevant symptom burden like diarrhea. A prospective phase III clinical study using Telotristat Etiprate (XermeloTM), a novel serotonin synthesis inhibitor, reported a significant decrease in daily bowel movements frequency, decrease in serotonin production and increase in quality of life[3,4]. Telotristat Etiprate is a tryptophan hydroxylase inhibitor, and thereby reduces the production of serotonin and diminishes daily bowel movements. Since 2017, XermeloTM is approved in by the FDA as well as by EMA for the treatment of carcinoid syndrome-related diarrhea in combination with somatostatin analog therapy[5]. It implies that the most recently approved drug is based on the inhibition of the production of serotonin.
Serotonin or 5-hydroxytryptamine (5-HT) is well known as the cerebral ‘hormone of happiness’, while the enteric 5-HT is a polyfunctional signaling molecule, acting as a paracrine factor, an endocrine hormone or a growth factor, which is important in gastrointestinal motility, enteric neurogenesis and intestinal inflammation[6]. Approximately 95% of 5-HT is synthesized in the gastrointestinal tract, stored in mucosal enterochromaffin cells and released by mechanical and chemical stimulations[7]. Spohn and Mawe[8] could show that bacteria within the lumen of the bowel influence serotonin synthesis and release by enterochromaffin cells. Furthermore, colon epithelial cells directly exposed to serotonin are primed for inflammatory reactions. Thus, the elevated serotonin level seems to be an important innate immune response, induces inflammatory genes in the gut and may be responsible at least partially for 5-HT-mediated pathogenesis in patients with inflammatory bowel disease[9]. On the other hand, the amino acid tryptophan acting as the precursor of both serotonin and niacin (vitamin B3), which may induce niacin deficiency by uncontrolled serotonin production as reviewed[10]. The clinical complex appearance of NETs – the so called “carcinoid syndrome” – is most frequently based on serotonin overproduction and involves severe diarrhea and flushing as well as bronchial obstruction, wheezing muscle wasting as well as proximal myopathy and may lead to carcinoid heart disease consisting of a secondary tricuspid valve insufficiency due to endocardial fibrosis[10]. Thus, serotonin downregulation within the gastrointestinal tract might cause reduction of bowel movements.
Natural zeolites are characterized by attractive properties such as adsorption, ion-exchange and molecular sieving. Due to the lattice structure of the aluminosilicates with channels and cavities, they possess an excellent binding capacity for ions, toxins and other harmful substances, which privilege them for medical and biomedical applications. Therefore, they are widely used in dietary supplements, as active ingredients in drugs or carriers for drugs, adjuvants in anticancer therapy and several other applications[11-15]. In particular, the natural zeolite clinoptilolite has been traditionally used in a large number of biomedical applications, due to its physico-chemical stability and biological compatibility. It has proven to be an effective anti-diarrheic drug[16]. Furthermore, a majority of the patients suffering from irritable bowel syndrome with diarrhea responded effectively to artificially enhanced clinoptilolite[17]. The natural Cuban zeolite used in this study is already available in Germany as an extemporaneous mixture (Detoxsan® Pulver) and is composed of two types of zeolite structures having different pore sizes: clinoptilolite, a medium-pore 10-membered ring zeolite and mordenite, a large-pore 12-membered ring zeolite[18]. Moreover, this Cuban zeolite is able to adsorb remarkable amounts of the biogenic amine histamine and water[18-20]. It has been applied for the first time to patients suffering from severe diarrhea caused by NETs. In diarrhea related to medullary thyroid cancer, montmorillonite clay had been applied successfully in 10 patients as a pilot study[21]. Montmorillonite clay belongs to the layered aluminium silicates while zeolites used in this study are characterized by 3-dimensional crystal lattices with different characteristics[22,23].
In the present study, a total of 20 patients (12 female and 8 male) suffering from diarrhea either by its frequency or from watery stool caused by NET were included, age ranged from 39 and 83 years (mean age of 64.1 years) (Table 1). In each patient, the diagnosis had been confirmed by histology and somatostatin receptors expression proven by positron emission tomography/computed tomography using Ga-68-labeled somatostatin analogs[24]. All patients received standard-of-care phar-macotherapy (Table 2) and were additionally given Detoxsan® powder (Detoxsan® Puder) as an extemporaneous drug containing 90% natural Cuban zeolite (clinoptilolite and mordenite) and 10% magnesium aspartate. Intake of Detoxsan® powder was commenced with low doses of 3 × 1 g/d or 2 × 2 g/d and increased up to 3 × 3 g/d or 3 to 5 g every 4 h if necessary, as recommended in the literature about common non-infectious diarrhea for few days only[16]. Individually tailored dose of Detoxsan® powder will be mentioned in detail in the ‘Results’ section. Each day dose and bowel movements were documented by the patients themselves in a pre-defined table.
| Patient | Female | Male | Age (yr) | Grading | Functionality | Resection (cm) | HBP | Others |
| 1 | X | 55 | G2 | yes, WD | IL, 62 + CC, 4 | X | ||
| 2 | X | 68 | G2 | yes, WD | IL, 40 | X | X | |
| 3 | X | 72 | G2 | yes, WD | IL, 56 + CO, 11 | X | X | |
| 4 | X | 79 | G1 | yes, WD | ileocecal | X | ||
| IL + jejunum, 150 | ||||||||
| 5 | X | 60 | G1 | yes, WD | hemicolectomy | X | X | |
| 6 | X | 65 | G2 | yes, WD | hemicolectomy | X | ||
| 7 | X | 47 | G2 | yes, WD | hemicolectomy, 220 | X | ||
| 8 | X | 77 | G1 | yes, WD | hemicolectomy, 120 | X | ||
| 9 | X | 43 | G2 | no, WD | no | X | ||
| 10 | X | 61 | G2 | MD, Serotonin | ileocecal, 40 | X | ||
| 11 | X | 73 | G1 | yes, WD | hemicolectomy, IL | X | ||
| 12 | X | 57 | G1 | yes, WD | mid IL | X | ||
| 13 | X | 63 | G2 | yes, WD | IL segments | X | X | |
| 14 | X | 48 | G2 | yes, WD | IL, 23 + CO, 31 | X | X | |
| 15 | X | 64 | G2 | yes, WD | IL, 70 | X | ||
| 16 | X | 77 | unknown | WD, Chromogranin | IL, 64 | X | ||
| 17 | X | 74 | G2 | yes, WD | IL, 18 | X | ||
| 18 | X | 78 | unknown | yes, WD | no | X | X | |
| 19 | X | 83 | G1 | Chromogranin | no | X | ||
| 20 | X | 39 | G1 | no, well diff. | no | X | X | |
| Total | 12 | 8 | ||||||
| Average age | 61.1 | 68.7 | 64.1 |
| Patient | Nutricion | Somatostatin | Dosage | Interval | Loperamide1 | Disease | NET type | Primary | Metastases | in | PRRT | |||
| consultation | analog | stage | Liver | Lymph | Bone | Lung | Peritoneum | |||||||
| 1 | yes | OCT | 30 mg | 4 wk | if required | IV | functional | midgut | x | x | x | 2nd | ||
| 2 | yes | OCT | 20 mg | 4 wk | if required | IIIb | functional | midgut | x | x | x | 3rd | ||
| 3 | yes | OCT | 30 mg | 4 wk | no | IV | functional | midgut | x | x | x | x | 2nd | |
| 4 | yes | LAN | 120 mg | 6 wk | no | IV | functional | midgut | x | x | x | x | 2nd | |
| 5 | yes | LAN | 120 mg | 3 wk | no | IV | functional | midgut | x | x | 2nd | |||
| 6 | yes | OCT | 30 mg | 4 wk | no | IIIb | functional | midgut | x | x | 4th | |||
| 7 | yes | OCT | 30 mg | 4 wk | no | IIIb | functional | midgut | x | x | 3rd | |||
| 8 | yes | LAN | 120 mg | 4 wk | if required | IV | functional | midgut | x | x | 2nd | |||
| 9 | yes | no | no | IV | nonfunctional | pancreas | x | x | x | 1st | ||||
| 10 | yes | OCT | 30 mg | 4 wk | no | IV | functional | midgut | x | x | x | 3rd | ||
| OCT s.c. | + 100 µg | if required | ||||||||||||
| 11 | yes | OCT | 30 mg | 4 wk | no | IIIb | functional | midgut | x | x | 1st | |||
| 12 | yes | LAN | 120 mg | 4 wk | if required | IV | functional | midgut | x | 2nd | ||||
| 13 | yes | OCT | 30 mg | 4 wk | if required | IV | functional | midgut | x | 3rd | ||||
| 14 | yes | LAN | 120 mg | 4 wk | no | IV | functional | appendix | x | 2nd | ||||
| 15 | yes | OCT | 30 mg | 4 wk | no | IV | functional | midgut | x | x | x | 3rd | ||
| 16 | yes | OCT | 20 mg | 4 wk | if required | IV | functional | midgut | x | x | 2nd | |||
| 17 | yes | LAN | 120 mg | 4 wk | if required | IIIb | functional | midgut | x | x | x | 2nd | ||
| 18 | yes | OCT | 30 mg | 3 wk | if required | IV | functional | CUP | x | x | 3rd | |||
| 19 | yes | OCT | 30 mg | 4 wk | no | IV | nonfunctional | pancreas | x | x | x | 1st | ||
| 20 | yes | OCT | 30 mg | 4 wk | no | IV | functional | CUP | x | x | 3rd | |||
Serum serotonin levels were measured by a commercially available ELISA kit (Serotonin FAST ELISA, DRG Instruments GmbH, Marburg, Germany) according to instructions given by the company. Incubation was performed at room temperature for 15 min and the antigen antibody complex containing serotonin was determined at 450 nm. Urea had been quantified by a kinetic assay using urease and glutamate dehydrogenase. In the first step urea is split by urease into ammonia and in the second enzymatic reaction the released ammonia forms L-glutamate from 2-oxoglutate using NADH. The rate of decrease of NADH measured at 340 nm is directly proportional to the concentration of urea in the assay[25]. Determination of single ions was performed by ion-selective electrode (ISE) using commercially available equipment (Tecan’s Sunrise absorbance microplate reader). The electrochemical sensors are helpful tools for qualitative and quantitative ion measurements[26]. Therefore, ISE serves as the standard method in our laboratory.
All patients enrolled in this pilot study did not only suffer from NETs, but also from comorbidities and treatment-resistant diarrhea (Table 1). They took Detoxsan® powder as an antidiarrheal drug, moreover, the intake of loperamide, which is a commercially available antidiarrheal drug, was recommended as well (See Table 2). Nevertheless, in the predefined table documented by the patients themselves, we could not found the intake of loperamide by the patients.
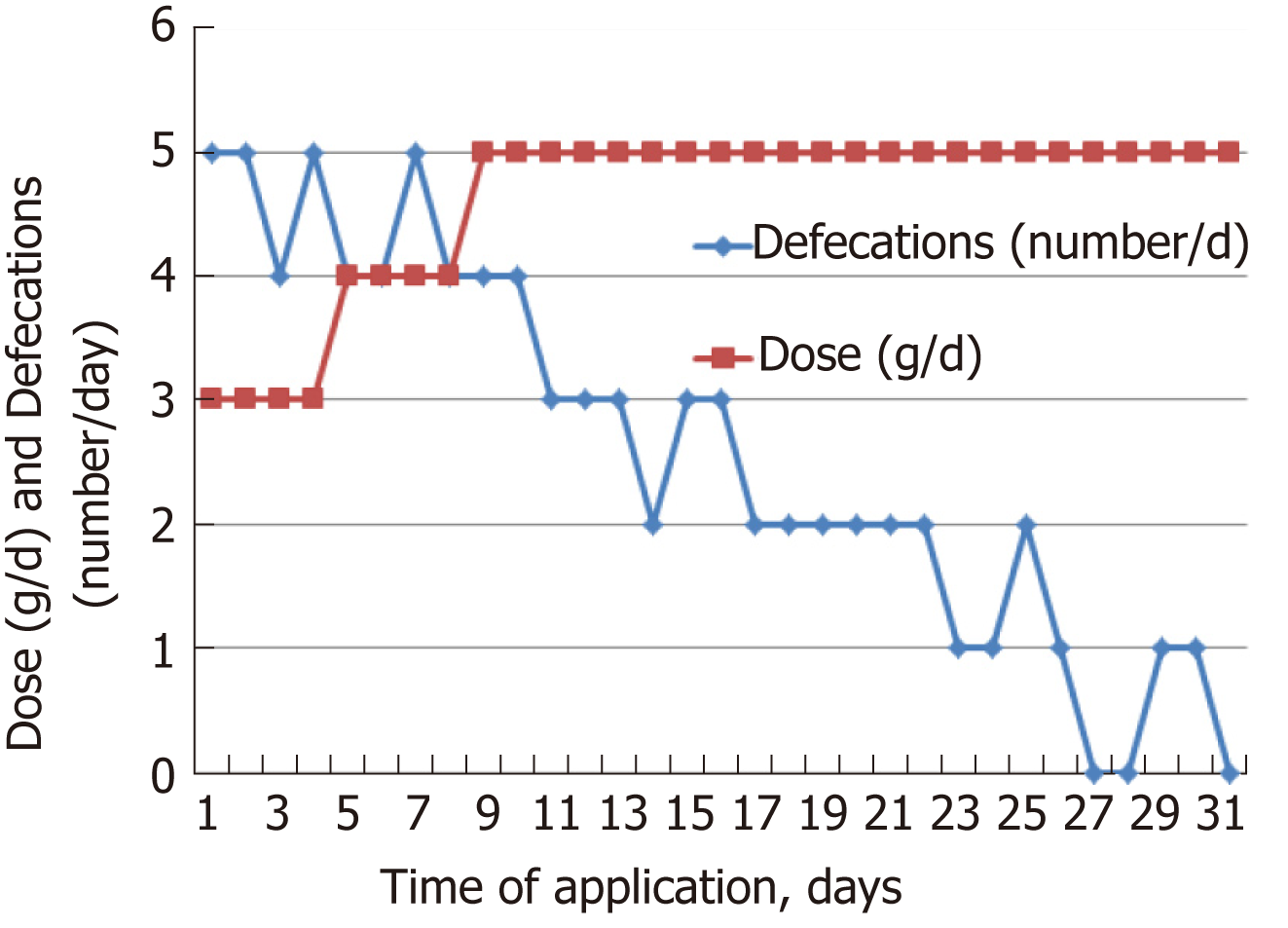
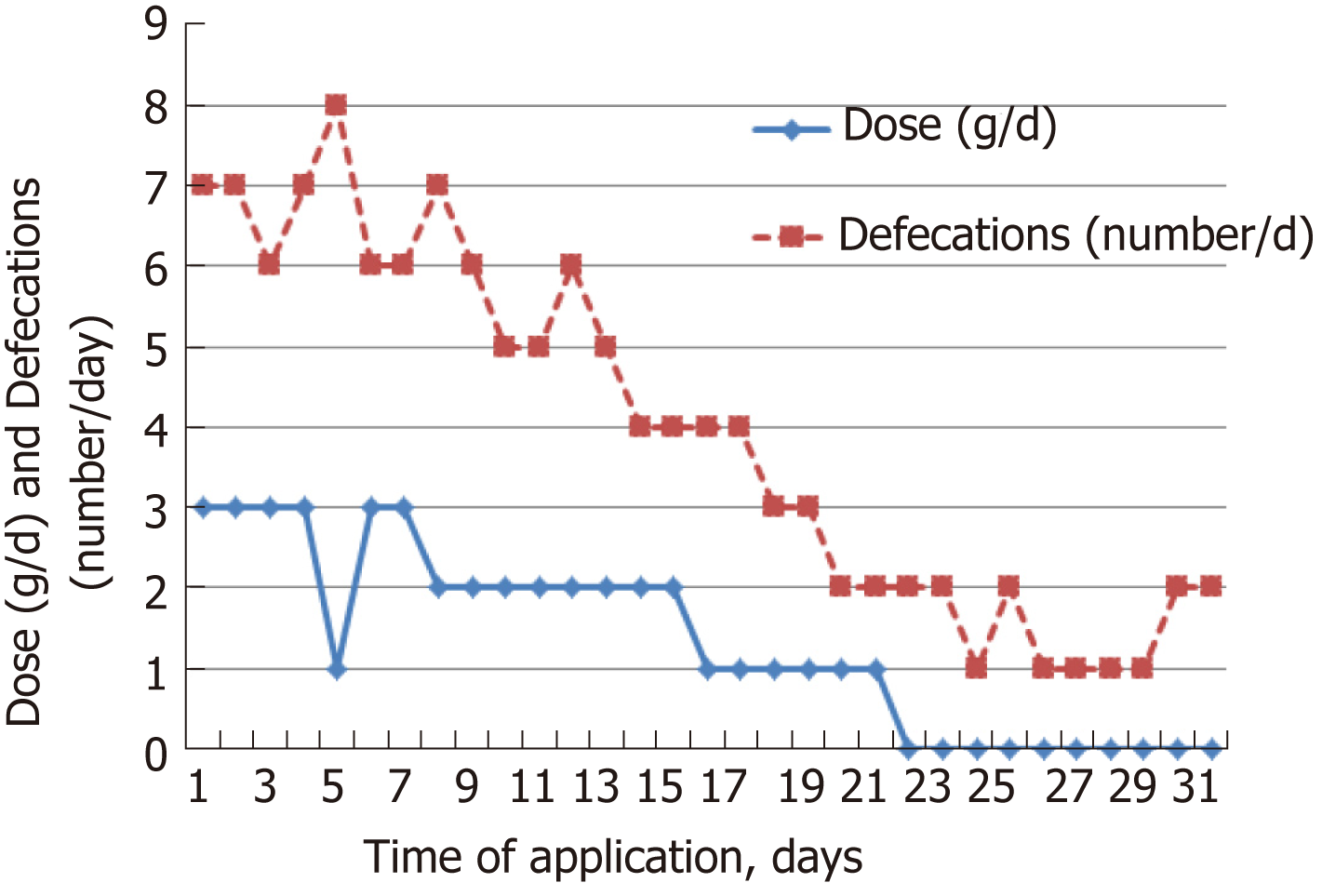
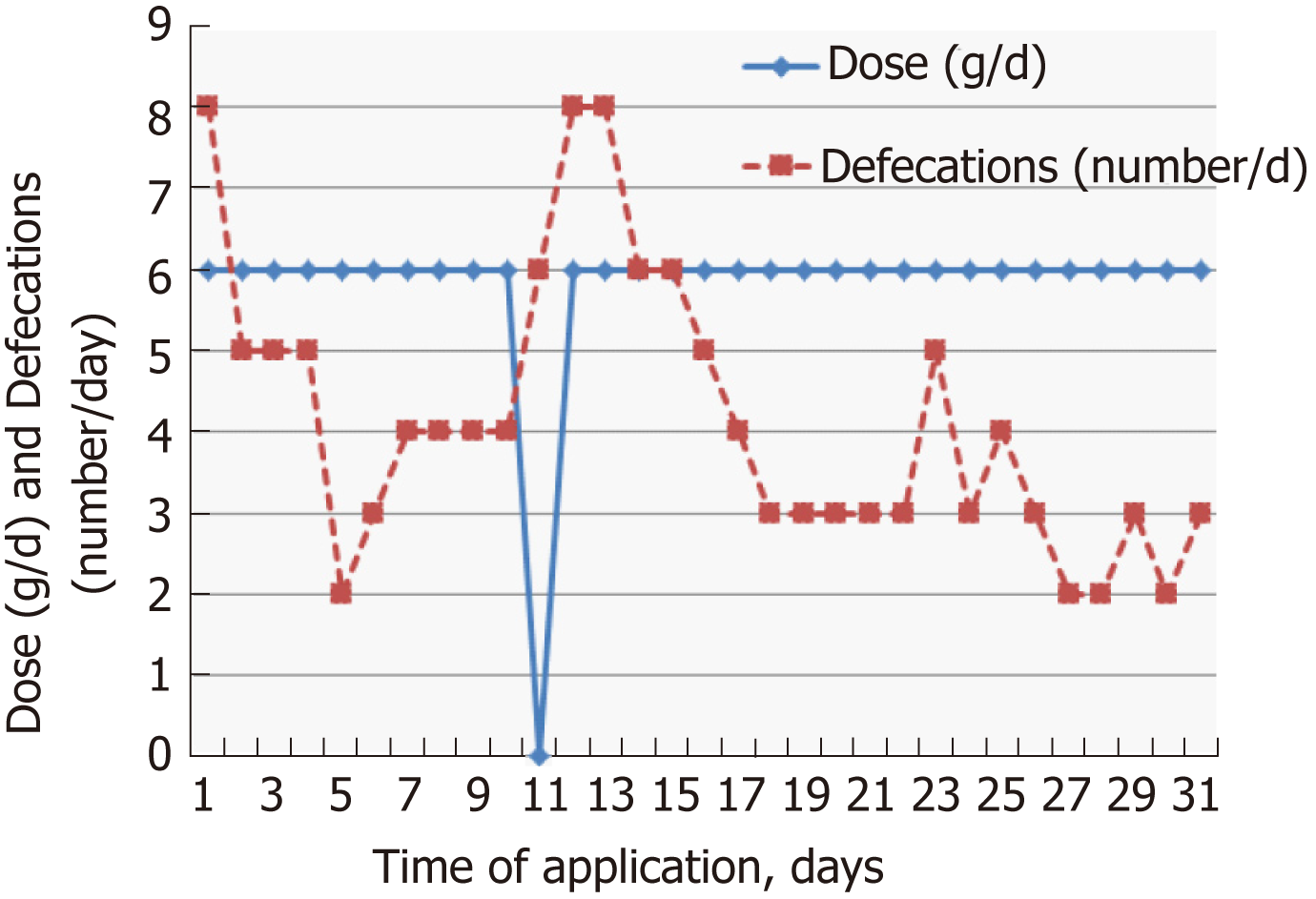
The individual dose of Detoxsan® powder was adapted by patients themselves with an increase until a significant reduction of bowel movements was reached (Figure 1) or a decrease if bowel movements frequency declined (Figure 2). In general, response effects need several days to become perceptible and require an intake of Detoxsan® powder for an extended time period or intermittently, if persisting stabilization of bowel movements could not be achieved (Figure 3). However, the use of Detoxsan® powder did not satisfy every patient or could reduce bowel movements (Tables 3 and 4). Three patients could not benefit even at a higher concentration and stopped daily intake ahead of schedule. Three patients reached only partial reduction of diarrhea. In general, response rates of Detoxsan® powder appears to correlate with patient’s nutrition, e.g. raw salad, fatty food and sauerkraut were reported to have negative effects on diarrhea despite intake of the powder.
| Patient | Empirical evaluation of the patients | Intake time (d) | Dose (g/d) | Bowel movement | ||
| Satisfied | Uncertain | Non-satisfied | (frequency per day) | |||
| 1 | X | 41 | 4 x 5 | 9 to 12 | ||
| 2 | X | 10 | 6 x 3 | 8 to 11 | ||
| 3 | X | 160 | 1 x 3 | 1 to 3 | ||
| 4 | X | 118 | 3 x 3 | 2 to 3 | ||
| 5 | X | 201 | 3 x 3 | 3 to 5 | ||
| 6 | X | 106 | 3 x 3 | 1 to 3 | ||
| 7 | X | 215 | 2 x 2 | 3 | ||
| 8 | X | 605 | 2 x 2 | 1 to 3 | ||
| 9 | X | 21 | 3 x 1 | 1 to 2 | ||
| 10 | X | 134 | 2 x 2 | 3 to 5 | ||
| 11 | X | 220 | 3 x 1 | 2 to 3 | ||
| 12 | X | 427 | 2 x 3 | 2 to 3 | ||
| 13 | X | 138 | 2 x 2 | 1 to 2 | ||
| 14 | X | 31 | 2 x 3 | 2 to 4 | ||
| 15 | X | 21 | 1 x 3 | 1 to 2 | ||
| 16 | X | 31 | 1 x 5 | 1 | ||
| 17 | X | 20 | 2 x 3 | 6 to 7 | ||
| 18 | X | 62 | 2 x 3 | 3 to 4 | ||
| 19 | X | 31 | 1 x 3 | 1 to 2 | ||
| 20 | X | 31 | 2 x 3 | 1 to 3 | ||
| Total | 15 | 3 | 3 | |||
| Percentage | 70.0 | 15.0 | 15.0 | |||
| Detoxsan® powder intake (d) | days | Dose (g/d) | Bowel movements (frequency per day) | Empirical evaluation, daily | |
| Satisfied | Non-satisfied | ||||
| 1 to 2 | 2 x 2 | 5 to 8 | 2 | ||
| 3 to 8 | 2 x 2 | 1 to 4 | 6 | ||
| 9 to 11 | 2 x 2 | 5 to 8 | 3 | ||
| 12 to 13 | 2 x 3 | 5 to 8 | 2 | ||
| 14 to 26 | 2 x 2 | 1 to 4 | 13 | ||
| 27 to 28 | 2 x 2 | 5 to 8 | 2 | ||
| 29 to 31 | 2 x 2 | 1 to 4 | 3 | ||
| 32 to 43 | 2 x 3 | 1 to 4 | 12 | ||
| 44 to 49 | 2 x 3 | 5 to 8 | 6 | ||
| 50 to 75 | 2 x 3 | 1 to 4 | 26 | ||
| 76 to 79 | 2 x 3 | 5 to 8 | 4 | ||
| 80 to 87 | 2 x 3 | 1 to 4 | 8 | ||
| 88 to 91 | 2 x 3 | 5 to 8 | 4 | ||
| 92 to 120 | 2 x 3 | 1 to 4 | 28 | ||
| Sum of days | 96 | 23 | |||
In summary, 14 out of the 20 patients (70%) declared to be very content with using Detoxsan® powder and observed a significant reduction of diarrhea, while the effective dose and intake period that resulted in a symptom relief varied individually (Table 3). Moreover, some patients stopped oral application when bowel movement became regular, whereas other patients extended intake in order to keep bowel movements at a low tolerable level. Apparently, that individual decision seemed to be dependent on the tolerance of patients with the number of bowel movements and stool consistency, in which a variance could be observed. A correlation between NET grade, part and size of bowel resection and functionality of the tumor could not be demonstrated (Tables 1-3). Therefore, diarrhea seemed to be based on the metabolic activity of the well-differentiated NETs, which eventually led to treatment resistance.
The serum serotonin level appears to be one of the major factors responsible for diarrhea in NETs[3-5], which is why blood serotonin level was determined and recorded on follow-up (Table 5). Only in 6 patients (patients 4, 5, 10, 14, 19, 20), serotonin level was measured in a short time interval before and after intake of Detoxsan® powder. Independent of the potential wide range of level of this biogenic amine, there was a significant decrease in the serotonin level during the period of intake in all that cases. To evaluate if other commonly detected laboratory parameters are affected by Detoxsan® powder intake, we determined exemplarily the levels of creatinine and urea as well as the cations sodium (Na), potassium (K) and calcium (Ca) in the blood (Table 5). In the creatinine and urea levels only a slight increase was measured during the use of Detoxsan® powder while the investigated electrolytes did not exhibit any significant changes of their level.
| Patient | Serotonin (µg/L) | Creatinine (µmol/L) | Urea (mmol/L) | Sodium (mmol/L) | Potassium (mmol/L) | Calcium (mmol/L) |
| 1 | (1.554) 2.442 | (58.0) 62.4 | (3.1) 2.7 | (139) 134 | (3.7) 5.2 | (2.3) 2.2 |
| 2 | (ND) 940 | (126.7) 142.3 | (5.5) 7.3 | (139) 142 | (4.0) 4.2 | (2.4) 2.4 |
| 3 | (289) 530 | (59.0) 69.8 | (4.1) 5.2 | (138) 133 | (4.9) 4.4 | (2.3) 2.2 |
| 4 | (919) 718 | (75.0) 92.1 | (4.0) 4.8 | (134) 140 | (3.9) 4.0 | (2.2) 2.3 |
| 5 | (1.834) 1.330 | (57.9) 91.9 | (4.4) 5.4 | (144) 145 | (4.2) 3.9 | (2.3) 2.3 |
| 6 | (398) 540 | (72.8) 91.8 | (7.8) 10.4 | (141) 137 | (4.3) 4.1 | (2.0) 2.1 |
| 7 | (ND) 318 | (53.0) 46.0 | (3.1) 3.1 | (139) 139 | (3.9) 3.4 | (2.3) 2.1 |
| 8 | (946) 399 | (167.5) 204.7 | (4.7) 9.9 | (142) 145 | (4.2) 5.0 | (2.3) 2.1 |
| 9 | (394) 575 | (73.2) 66.9 | (3,7) 5.1 | (136) 138 | (4.3) 4.4 | (2.3) 2.3 |
| 10 | (2.500) 688 | (60.5) 83.7 | (3.6) 3.8 | (146) 142 | (3.8) 4.5 | (2.3) 2.3 |
| 11 | (263) 1.939 | (59.3) 93.8 | (6.8) 6.5 | (144) 142 | (4.3) 4.2 | (2.1) 2.0 |
| 12 | (1.102) 1.025 | (85.6) 70,6 | (4.1) 3.4 | (143) 142 | (3.9) 3.2 | (2.2) 2.2 |
| 13 | (1.230) 708 | (70.0) 69.5 | (5.4) 3.5 | (144) 145 | (4.0) 4.3 | (2.4) 2.5 |
| 14 | (1.967) 775 | (46,9) 54,9 | (2.6) 3.2 | (142) 143 | (4.4) 4.3 | (2.3) 2.3 |
| 15 | (2.052) 2.500 | (75.1) 110.7 | (4.6) 6.3 | (137) 141 | (3.9) 3.8 | (2.3) 2.3 |
| 16 | (477) 353 | (58.6) 55.9 | (5.8) 7.0 | (144) 146 | (4.2) 4.8 | (2.2) 2.4 |
| 17 | (1.224) 1.446 | (50.3) 48.3 | (3.5) 3.9 | (143) 142 | (4.0) 4.0 | (2.3) 2.3 |
| 18 | (1.185) 2.500 | (52.7) 61.6 | (3.5) 3.4 | (143) 140 | (4.2) 4.3 | (2.3) 2.3 |
| 19 | 95 (63) | (155.0) 171.5 | (8.2) 13.9 | (133) 137 | (4.1) 4.6 | (2.3) 2.3 |
| 20 | 68 (64) | (75.5) 74.3 | (5.3) 5.8 | (144) 142 | (4.5) 4.1 | (2.4) 2.4 |
All the patients enrolled in this pilot study did not only suffer from NETs, but also from comorbidities and treatment-resistant diarrhea due to reduced resorption capacity of the bowl with a consequent intestinal failure (Table 1)[27,28]. Grading and differentiation of NETs were categorized according to the recent WHO classification[29]. Length of bowel resection was included in the characterization of patient status. Principally, patients were treated according to the Theranostic concept for NETs based on national and international guidelines[24]. However, there was insufficient control of diarrhea, most probably due to the secretion of hormones like serotonin produced by the slowly growing and highly differentiated NETs[2].
Whether the serotonin level decrease in 6 patients is based on the adsorption of serotonin by the zeolite – similar to the histamine uptake – or the decline of the chemically-labile serotonin is caused by other mechanisms is still the subject of ongoing investigations[18,19]. Nevertheless, these 6 patients were part of the satisfied ones with respect to the bowel movements (Table 2) corresponding to correlation of serotonin level and diarrhea[3-5]. For further investigations it is worth to determine the level of 5-hydroxyindoleacetic acid in a 24-h urine collection. It is a stable metabolite of serotonin and by this way its level can be determined indirectly[30]. It was not done in our present research.
Despite the known facts that enteric serotonin is a polyfunctional signaling molecule, an essential component of the gastrointestinal inflammatory response and a bioactive component in developing and mature animals, the effect of this amine on diarrhea is still not comprehensively understood[6]. In weaning mice e.g., stress-induced diarrhea is considered to be caused by deregulation of the mucosal immune system (among others). Interestingly, mucosal immunity was decreased in the duodenum and jejunum without being affected in the ileum and colon[31,32]. Given the fact that the intestinal tract is the largest immune organ of the human body, serotonin might be therefore considered to be a link between the gut and the immune regulation[33,34]. Moreover, histamine is one of the most important biogenic amines and strongly involved in immunological reactions. It has been described as ‘an undercover agent in multiple rare diseases’, because many pathological inflammatory processes are involved with histamine as well[35].
The positive effect of zeolite on treatment-resistant diarrhea caused by NETs might be related to several origins. High adsorption capacity for histamine and possibly other biogenic amines like serotonin, the antiphlogistic effect of this mineral as well as its high water uptake capacity are potentially responsible for reducing diarrhea complaints[18-20]. This kind of treatment differs significantly from all other drugs in the field because Detoxsan® powder acts only via its inherent adsorption properties within the gastrointestinal tract; neither does it penetrate into the blood circulation nor directly influence regulation processes. Furthermore, it is noteworthy that the histamine uptake of the Cuban zeolite (and possibly of other biogenic amines) is significantly higher compared to other zeolites containing only clinoptilolite and no additional mordenite[19]. The clinical treatment of patients with NETs with regard to their functional complaints is recently focused to the application of somatostatin receptor inhibitors[36]. Thus, the aim of both methods appears similar while the pharmacological approach is quite different. The slight increase of the creatinine and urea levels during the use of Detoxsan® powder might be interpreted as a mild decrease in the kidney filtration process while the metabolic liver function seems to be unaffected.
In conclusion, the contemporaneous mixture Detoxsan® powder is able to reduce significantly symptoms of NET-related diarrhea in the majority of patients. The effect of this zeolite seems to be due to its high capacity to bind water, histamine and possibly serotonin, too, within the gastrointestinal tract and to removal of those compounds via stool. The duration and dose of Detoxsan® powder intake varies individually. In some patients the normalization of bowel movement could be observed within a few weeks while some patients need to use it permanently in order to maintain acceptable quality of life without diarrhea. Recommended daily dosage requires an individual adaptation and ranges between 3 g once to three times per day. The clinical reduction of the diarrhea symptoms by Detoxsan® powder comes without a relevant negative influence on other biochemical parameters.
Therapy-refractory diarrhea in neuroendocrine tumor (NET) patients reduces quality of life, strongly restricts their daily routine and is therefore a highly clinical unmet need.
Motivation of this investigation was reduce bowel movements in chronic diarrhea patients and by this to achieve a significant improvement in their quality of life.
To overcome the therapy-refractory diarrhea of patients with NETs by the zeolite containing medical advice Detoxsan® powder.
For this purpose, patients were offered a well characterized zeolite product which is known to adsorb biogenic amines and water in large extent and it does not enter into the blood stream. The patients have been informed in detail about the product, the individual adaptation of the dosage and the documentation in a predefined table. Due to the fact that diarrhea syndrome is a disturbance of the daily routine the patients enrolled in this clinical trial respected these recommendations and showed an excellent compliance. In addition to the clinical trial, we determined some biochemical parameters in order to monitor if some undesired changes occur.
It is the first time that a well characterized zeolite product is able to reduce bowel movements in patients suffered by therapy-refractory diarrhea caused by NETs over a long time. In 14 of 20 patients (70%) enrolled in the trial bowel movement rate could be normalized. The application time to reach an acceptable bowel movement oscillated between few weeks up to a permanent or intermittent use. Also the dosage oscillated between 3 g per day up to three times 3 g per day. All of the 14 responder patients appreciated the normalization of the bowel movements in spite of the individual adaptation of dosage and time. There were no side effects. However, it is not clear which factors influence the reduction of diarrhea. At least one component namely serotonin seems to be involved in this physiological process. Therefore, the adsorption of this biogenic amine by this type of zeolite is under investigation. Furthermore, the serotonin metabolite 5-hydroxyindoleacetic acid should be determined in a 24-h urine collection. Using this method the natural serotonin secretion can be determined indirectly.
The new finding of this study is the effective application of a well characterized zeolite product in patients suffered by therapy-refractory diarrhea caused by NETs. The attractive properties of the lattice structure of this mineral for excellent binding capacity for water, amines and harmful substances seem to possess a key function in overcome diarrhea symptoms in both temporary application and long term use. The individual dosage and period of application in order to receive the best reduction of diarrhea indicate that the physiological process of these symptoms is not fully understood and requires further investigations. For the clinical practice it is important to accept the individuality of this treatment to overcome patient’s diarrhea and improve their daily routine.
We observed that a well characterized natural zeolite is able to overcome therapy-refractory diarrhea caused by NETs via passing the gastrointestinal tract only. However not all effects could be answered satisfactorily. Therefore, future research should be focused on the one hand to the adsorption of serotonin and other trigger substances for diarrhea by this zeolite. On the other hand the clinical treatment of NETs patients requires the determination of the biochemical factors in both blood and faeces in correlation the resected bowel part and the effect of this zeolite. The aim of these investigations should be the selective dosage of the mineral product on the basis of biochemical values and/or surgical data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Capurso G, Cidon EU, Krishnan T S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Camilleri M, Sellin JH, Barrett KE. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology. 2017;152:515-532.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31:169-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Lamarca A, Barriuso J, McNamara MG, Hubner RA, Valle JW. Telotristat ethyl: a new option for the management of carcinoid syndrome. Expert Opin Pharmacother. 2016;17:2487-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Masab M, Saif MW. Telotristat ethyl: Proof of principle and the first oral agent in the management of well-differentiated metastatic neuroendocrine tumor and carcinoid syndrome diarrhea. Cancer Chemother Pharmacol. 2017;80:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Markham A. Telotristat Ethyl: First Global Approval. Drugs. 2017;77:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 454] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 7. | Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol. 2001;17:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling - the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Regmi SC, Park SY, Ku SK, Kim JA. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic Biol Med. 2014;69:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Mota JM, Sousa LG, Riechelmann RP. Complications from carcinoid syndrome: Review of the current evidence. Ecancermedicalscience. 2016;10:662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Pavelić K, Hadzija, M. Medical applications of zeolites In: Auerbach SM, Cerrado KA, Dutta PK. Handbook of Zeolite Science and Technology. CRC Press. 2003;1143-1174. [DOI] [Full Text] |
| 12. | Andronikashvili T, Pagava K, Kurashvili T, Eprikashvili L. Possibility of application of natural zeolites for medical purposes. Bull Georg Nat Acad Sci. 2009;3:158-167. |
| 13. | Colella C. A critical reconsideration of biomedical and veterinary applications of natural zeolites. Clay Mineral. 2011;46:295-309. [DOI] [Full Text] |
| 14. | Milić J, Daković A, Krajišnik D, Rottinghaus GE. Modified natural zeolites–functional characterization and biomedical application. In: Tiwari A. Advanced healthcare materials. Scrivener Publishing: Beverly MA. 2014;361-403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Laurino C, Palmieri B. Zeolite: "The Magic Stone"; Main Nutritional, Environmental, Experimental And Clinical Fields Of Application. Nutr Hosp. 2015;32:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Rodríguez-Fuentes G, Barrios MA, Iraizoz A, Perdomo I, Cedré B. Enterex: Antidiarrheic drug based on purified natural clinoptilolite. Zeolites. 1997;19:441-448. [RCA] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Lamprecht JC, Ellis S, Snyman JR, Laurens I. The effects of an artificially enhanced clinoptilolite in patients with irritable bowel syndrome. S Afr Fam Pract. 2017;59:18-22. |
| 18. | Selvam T, Schwieger W, Dathe W. Natural Cuban zeolites for medical use and their histamine binding capacity. Clay Mineral. 2014;49:501–512. [DOI] [Full Text] |
| 19. | Selvam T, Schwieger W, Dathe W. Histamine-binding capacities of different natural zeolites: A comparative study. Environ Geochem Health. 2018;40:2657-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Cervini-Silva J, Nieto-Camacho A, Kaufhold S, Ufer K, Palacios E, Montoya A, Dathe W. Antiphlogistic effect by zeolite as determined by a murine inflammation model. Micropor Mesopor Mat. 2016;228:207-214. [DOI] [Full Text] |
| 21. | Dadu R, Hu MI, Cleeland C, Busaidy NL, Habra M, Waguespack SG, Sherman SI, Ying A, Fox P, Cabanillas ME. Efficacy of the Natural Clay, Calcium Aluminosilicate Anti-Diarrheal, in Reducing Medullary Thyroid Cancer-Related Diarrhea and Its Effects on Quality of Life: A Pilot Study. Thyroid. 2015;25:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Cadars S, Guégan R, Garaga MN, Bourrat X, Le Forestier L, Fayon F, Vu Huynh T, Allier T, Nour Z, Massiot D. New insights into the molecular structures, compositions, and cation distributions in synthetic and natural montmorillonite clays. Chem Mat 2012, 24: 4376-4389. . [DOI] [Full Text] |
| 23. | Ambruster T, Gunter ME. Crystal structures of natural zeolites. Rev Mineral Geochem. 2001;45:1-67. [DOI] [Full Text] |
| 24. | Lee ST, Kulkarni HR, Singh A, Baum RP. Theranostics of Neuroendocrine Tumors. Visc Med. 2017;33:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Rick W. Klinische Chemie und Mikroskopie. Springer-Verlag Berlin Heidelberg: New York 1990; 245-246. [DOI] [Full Text] |
| 26. | Bakker E, Qin Y. Electrochemical sensors. Anal Chem. 2006;78:3965-3984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 27. | Lal S, Teubner A, Shaffer JL. Review article: Intestinal failure. Aliment Pharmacol Ther. 2006;24:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Lamprecht G, Pape UF, Witte M, Pascher A. und DGEM Steering Committee. S3-Leitlinie der Deutschen Gesellschaft für Ernährungsmedizin e.V. in Zusammenarbeit mit der AKE, der GESKES und der DGVS Klinische Ernährung in der Gastroenterologie (Teil 3) – Chronisches Darmversagen. Aktuelle Ernährungsmedizin. 2014;39:e57–e71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Wood EK, Kruger R, Bennion A, Cooke BM, Lindell S, Schwandt M, Goldman D, Barr CS, Suomi SJ, Higley JD. Low Inherent Sensitivity to the Intoxicating Effects of Ethanol in Rhesus Monkeys with Low CSF Concentrations of the Serotonin Metabolite 5-Hydroxyindoleacetic Acid. Alcohol Clin Exp Res. 2018;42:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Dong Y, Han Y, Wang Z, Qin Z, Yang C, Cao J, Chen Y. Role of serotonin on the intestinal mucosal immune response to stress-induced diarrhea in weaning mice. BMC Gastroenterol. 2017;17:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Dong Y, Wang Z, Qin Z, Cao J, Chen Y. Role of serotonin in the intestinal mucosal epithelium barrier in weaning mice undergoing stress-induced diarrhea. J Mol Histol. 2018;49:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M. Mammalian gut immunity. Biomed J. 2014;37:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Guseva D, Holst K, Kaune B, Meier M, Keubler L, Glage S, Buettner M, Bleich A, Pabst O, Bachmann O, Ponimaskin EG. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20:1516-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Pino-Ángeles A, Reyes-Palomares A, Melgarejo E, Sánchez-Jiménez F. Histamine: An undercover agent in multiple rare diseases? J Cell Mol Med. 2012;16:1947-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Gut P, Waligórska-Stachura J, Czarnywojtek A, Sawicka-Gutaj N, Bączyk M, Ziemnicka K, Fischbach J, Woliński K, Kaznowski J, Wrotkowska E, Ruchała M. Management of the hormonal syndrome of neuroendocrine tumors. Arch Med Sci. 2017;13:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |