Published online Apr 21, 2019. doi: 10.3748/wjg.v25.i15.1899
Peer-review started: January 11, 2019
First decision: February 13, 2019
Revised: March 3, 2019
Accepted: March 15, 2019
Article in press: March 16, 2019
Published online: April 21, 2019
Cytomegalovirus (CMV) remains a critical complication after solid-organ transplantation. The CMV antigenemia (AG) test is useful for monitoring CMV infection. Although the AG-positivity rate in CMV gastroenteritis is known to be low at onset, almost all cases become positive during the disease course. We treated a patient with transverse colon perforation due to AG-negative CMV gastroenteritis, following a living donor liver transplantation (LDLT).
The patient was a 52-year-old woman with decompensated liver cirrhosis as a result of autoimmune hepatitis who underwent a blood-type compatible LDLT with her second son as the donor. On day 20 after surgery, upper and lower gastrointestinal endoscopy (GE) revealed multiple gastric ulcers and transverse colon ulcers. The biopsy tissue immunostaining confirmed a diagnosis of CMV gastroenteritis. On day 28 after surgery, an abdominal computed tomography revealed transverse colon perforation, and simple lavage and drainage were performed along with an urgent ileostomy. Although the repeated remission and aggravation of CMV gastroenteritis and acute cellular rejection made the control of immunosuppression difficult, the upper GE eventually revealed an improvement in the gastric ulcers, and the biopsy samples were negative for CMV. The CMV-AG test remained negative, therefore, we had to evaluate the status of the CMV infection on the basis of the clinical symptoms and GE.
This case report suggests a monitoring method that could be useful for AG-negative CMV gastroenteritis after a solid-organ transplantation.
Core tip: The cytomegalovirus (CMV) antigenemia (AG) test is useful for monitoring recipients for posttransplantation CMV infection. Although the AG-positivity rate in CMV gastroenteritis is known to be low at onset, most cases become positive during the disease course. We managed a patient with a complicated condition with a transverse colon perforation caused by AG-negative CMV gastroenteritis, after a living donor liver transplantation. This case report presents a method that could be important monitoring for AG-negative CMV gastroenteritis after solid-organ transplantation.
- Citation: Yokose T, Obara H, Shinoda M, Nakano Y, Kitago M, Yagi H, Abe Y, Yamada Y, Matsubara K, Oshima G, Hori S, Ibuki S, Higashi H, Masuda Y, Hayashi M, Mori T, Kawaida M, Fujimura T, Hoshino K, Kameyama K, Kuroda T, Kitagawa Y. Colon perforation due to antigenemia-negative cytomegalovirus gastroenteritis after liver transplantation: A case report and review of literature. World J Gastroenterol 2019; 25(15): 1899-1906
- URL: https://www.wjgnet.com/1007-9327/full/v25/i15/1899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i15.1899
Although cytomegalovirus (CMV) infection can remain latent since childhood, it can be reactivated due to immunosuppression. While CMV gastroenteritis presents with clinical symptoms, such as abdominal pain, nausea, vomiting and melena, a definitive diagnosis is made based on endoscopic findings and the histopathological examination of biopsy tissues. The CMV-antigenemia (AG) positivity rate at the onset of gastroenteritis has been reported to be approximately 20%-30%[1]. Although gastrointestinal perforation due to CMV gastroenteritis is not uncommon[2], such an occurrence has rarely been reported after organ transplantation[3]. Autoimmune hepatitis is an autoimmune disease that commonly develops in middle-aged or older woman and usually causes chronic and progressive liver damage. In regard to treatment, immunosuppressants, especially prednisolone, are commonly used. Liver transplantation is the final therapeutic option for patients, such as in a recently reported case on a patient with autoimmune hepatitis who developed decompensated cirrhosis due to an insufficient response to medical treatment.
We managed a patient with a complicated condition, with transverse colon perforation that was caused by AG-negative CMV gastroenteritis, after a living donor liver transplantation (LDLT). Here, we report on this case, which was difficult to diagnose and treat.
Abdominal pain and fullness.
The patient was a 52-year-old Asian woman, who was diagnosed with liver dysfunction during a medical examination in her twenties. A diagnosis of autoimmune hepatitis was made at 40 years of age. When the patient was 46 years old, the patient developed ascites, which improved with oral steroids. However, with disease progression, she developed decompensated cirrhosis at 51 years old that was resistant to medical management. She was then referred to our department.
There was no other significant medical history.
The patient was a nonsmoker and had stopped drinking socially 5 years prior. Her occupation was a housewife. There was no relevant family history.
According to the Eastern Cooperative Oncology Group Performance Status, her performance status was 2. At the physical examination, the patient’s height was 155 cm, her weight was 47 kg, and her vitals were stable; yellowish bulbar conjunctivae, ascites, and bilateral pedal edema were observed.
The Child-Pugh score was 11 points in class C, and the Model for end stage liver disease score was 11 points. The serologic tests for CMV showed that the patient was IgG positive (+), IgM negative (-), and AG negative, which is indicative of past CMV infection. A PCR test for CMV was not performed routinely before transplantation at our facility and was not performed in this case.
Abdominal computed tomography (CT) revealed liver cirrhosis with ascites before LDLT.
A blood-type compatible LDLT was performed using a left lobe graft, with the patient’s second son as the donor (20 years old, CMV IgG+/IgM-, which is indicative of past CMV infection). The graft-to-recipient weight ratio was 0.73, the operation duration was 849 min, and the bleeding volume was 822 mL. At our facility, in accordance with the protocol of CMV monitoring and treatment after a liver transplantation, CMV-AG is tested twice a week, but a CMV-PCR test is not performed routinely. In addition, prophylactic ganciclovir (GCV) is not administered, but GCV is initiated when the patient becomes CMV-AG positive or in the case of a seropositive donor.
Initially, cyclophosphamide (CyA), prednisolone (PSL) and mizoribine (MIZ) were used as the postoperative immunosuppressants, in accordance with the protocol of our facility[4,5], however, for this patient, MIZ was replaced by mycophenolate mofetil (MMF) due to pancytopenia, and CyA was replaced by tacrolimus (FK) due to renal failure. As the patient had jaundice and persistently elevated aspartate transaminase and alanine transaminase levels, a liver biopsy was performed on the 10th day after transplantation. The histopathological examination was negative for both acute cellular rejection (ACR) and CMV hepatitis, so her condition was suspected to be drug-induced or caused by cholestasis.
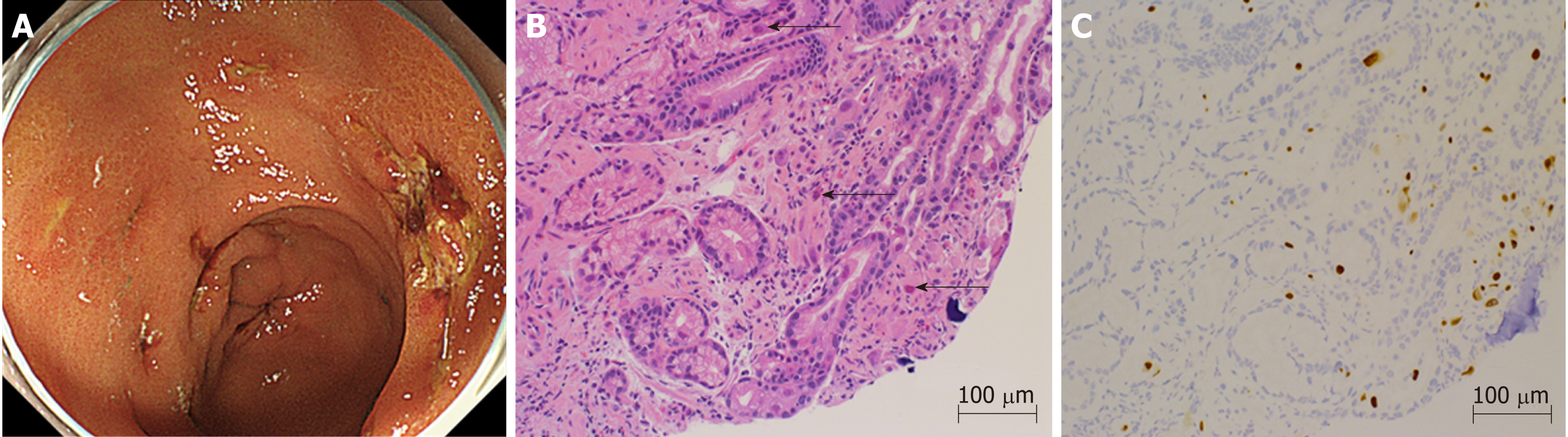
Starting on the 12th day after transplantation, the patient’s anemia worsened, and she required frequent packed red blood cell transfusions. Further investigations confirmed thrombocytopenia, jaundice, and renal failure. We suspected thrombotic microangiopathy (TMA), even though the peripheral smear was negative for fragmented red blood cells, and a fresh frozen plasma transfusion and FK dose reduction were carried out. The CMV-AG remained negative, and there were no clinical findings that were characteristic of a CMV infection, but prophylactic GCV administration was initiated. Thereafter, the thrombocytopenia gradually improved. On the 20th day after transplantation, the patient reported abdominal pain and black-colored stools, so upper gastrointestinal endoscopy was performed, which showed multiple gastric ulcers (Figure 1A). A biopsy tissue sample, taken from an ulcer, showed large cells with intranuclear inclusions with hematoxylin and eosin (HE) staining (Figure 1B), and CMV-positive cells were observed through immunostaining (Figure 1C). Once the diagnosis of CMV gastroenteritis was confirmed, GCV, which had already been initiated, was continued, and the dosage of all 3 immunosuppressants, FK, PSL, and MMF, was reduced.
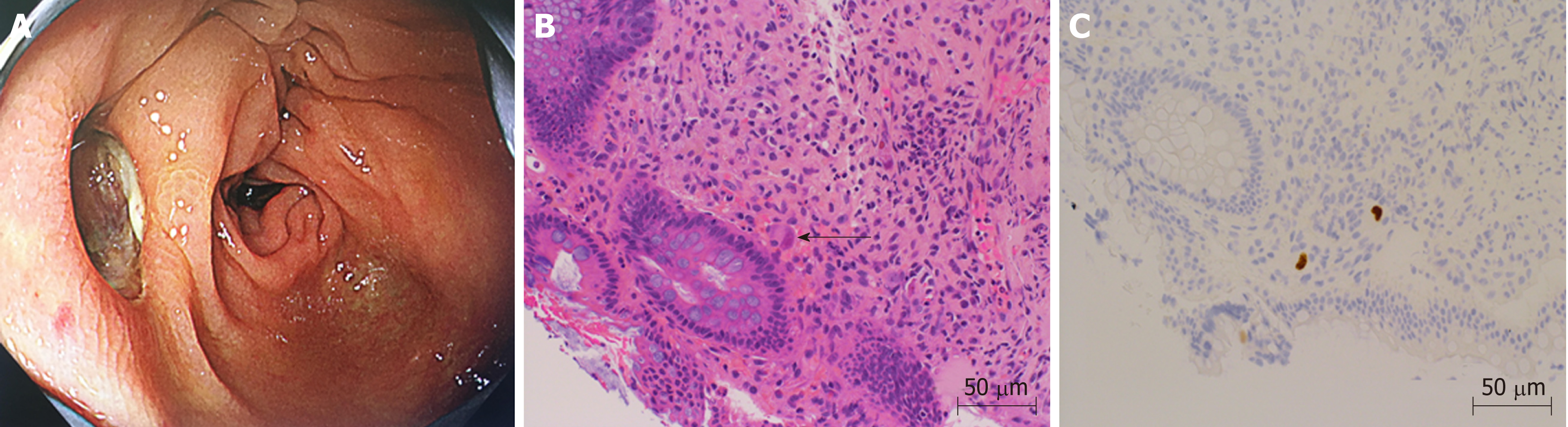
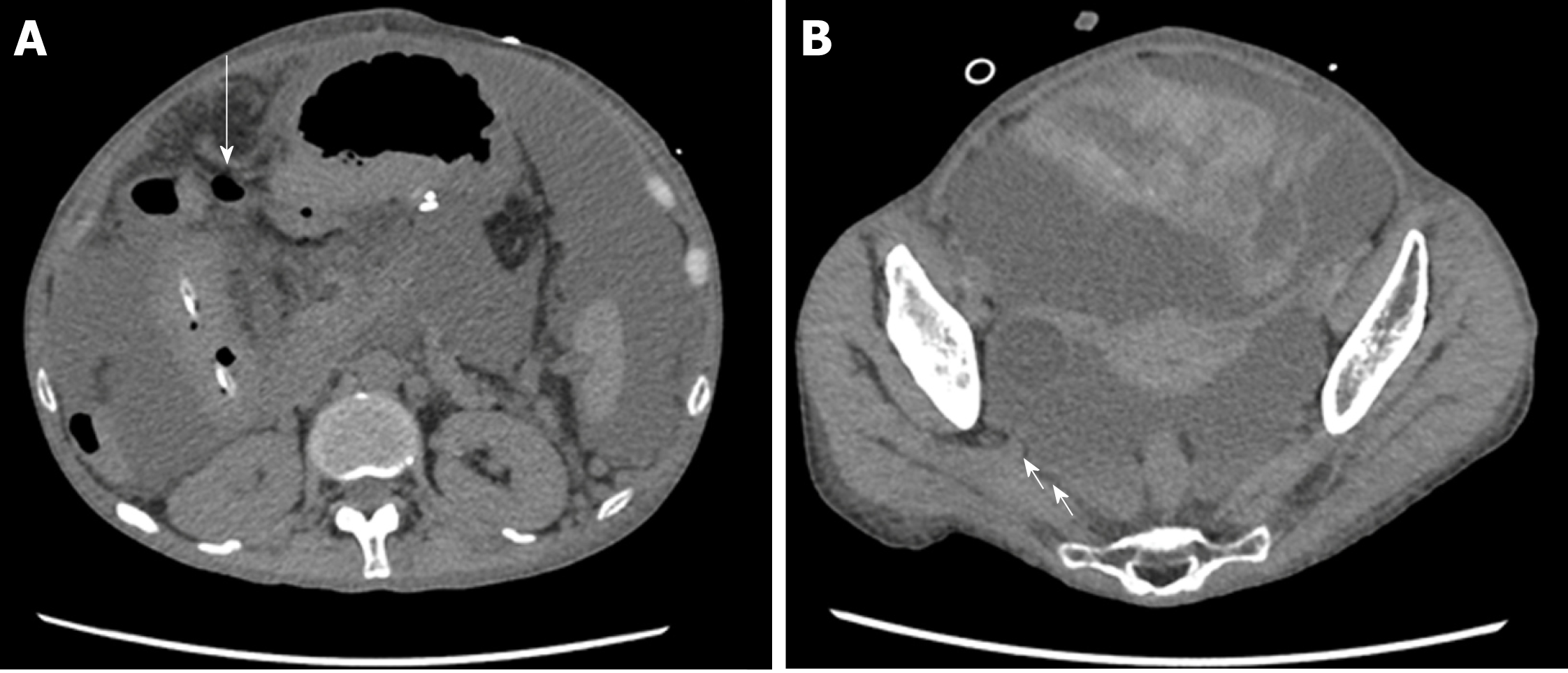
On the 26th day after transplantation, the patient had frequent, watery diarrhea, for which a lower gastrointestinal endoscopy was performed, and a deep ulcer was observed in the transverse colon (Figure 2A). The biopsy tissue diagnosis of the ulcerative lesion revealed large cells with intranuclear inclusions with HE-staining (Figure 2B) and CMV-positive cells with immunostaining (Figure 2C). On the 28th day after transplantation, we noted the findings of abdominal pain, fever and an increased inflammatory response. A plain abdominal CT scan revealed intraperitoneal free air adjacent to the transverse colon (Figure 3A) and hemorrhagic ascites in the pelvis (Figure 3B).
The patient was diagnosed with gastrointestinal perforation, and an emergency surgery was performed. When the abdomen was incised, contaminated ascites were not observed. In the transverse colon, an impending perforation with a thinned serous membrane was confirmed.
The rejection activity index score of the liver tissues collected during the surgery was found to be P2, B1, and V1 during the histopathologic examination, indicating ACR.
The final diagnosis of the presented case was transverse colon perforation due to CMV gastroenteritis.
GCV was administered for CMV gastroenteritis.
During the emergency surgery, as it was difficult to remove the adhesions with the surrounding areas, thus, the site of perforation was left as it was. Simple lavage with drainage was performed, and ileostomy was performed for acute pan-peritonitis due to transverse colon perforation.
The repeated remission and aggravation of CMV gastroenteritis, ACR and TMA made the control of immunosuppression extremely difficult. CMV-AG remained negative throughout the disease course, but the CMV-PCR test result was 220 copies/mL. On the 46th day after transplantation, a follow-up upper gastrointestinal endoscopy showed that the gastric ulcer was finally resolving, and the biopsies were also negative for CMV. The patient was ambulatory at discharge on the 86th day after transplantation.
Although CMV infection is often contracted in childhood, it usually remains latent. It is often reactivated due to immunosuppression after an organ transplantation[6]. Most Japanese individuals are infected with CMV in early childhoods, and it usually remains latent. Therefore, even if a donor candidate had a past CMV infection, he or she is not excluded as a donor. When the recipient is seronegative while the donor is seropositive for the CMV antibody, the risk of onset of CMV infection after organ transplantation is high, and appropriate monitoring and prophylactic measures are necessary[7]. However, when the recipient is seropositive, whether the recipient is at risk of infection when the donor is seropositive or seronegative is controversial.
The diagnostic methods for CMV include (1) the isolation and identification of CMV from blood, urine and pharyngeal secretions; (2) the CMV-AG test, which involves the detection of CMV-antigen-positive polymorphonuclear leukocytes in peripheral blood using a monoclonal antibody; (3) the CMV-PCR method, which involves amplifying CMV-DNA from blood and the bronchoalveolar lavage fluid for the identification; and (4) cytopathological and histopathological examinations, which involve the evaluation of the target organs of CMV infection with endoscopy and biopsy to detect the intranuclear inclusion bodies of giant cells through HE staining and CMV antigens through immunostaining using an anti-CMV monoclonal antibody[6,7]. Because both the sensitivity and specificity of the CMV-AG test are reported to be 70%-90%[8,9] and the levels are indicative of disease severity and treatment response, the test is said to be effective for infection monitoring. The CMV-AG test is therefore used as an indicator for the initiation and completion of treatment. The sensitivity and specificity of the CMV-PCR method are reported to be superior to the CMV-AG test[10-14], and while a quantitative estimation of the number of copies is possible with the former, the positive cut-off value varies among different reports[9-11,13,15]. The CMV-PCR test is not covered by health insurance providers in Japan.
CMV gastroenteritis exhibits clinical symptoms such as nausea, vomiting, abdominal pain and melena, and it is characteristic to find multiple gastrointestinal ulcers during endoscopy[6,10,15]. The diagnosis is based on endoscopic findings and biopsy tissue examination and analysis. The diagnosis is confirmed when the intranuclear inclusion bodies of giant cells are observed using HE stains and CMV antigens are identified through immunostaining[6,10,14,16]. When an ulcerative lesion is observed in the gastrointestinal tract of an immunosuppressed patient, the site should be biopsied and investigated, keeping CMV infection in mind. Although it has been reported that in CMV gastroenteritis, the CMV-AG positive rate is approximately 20% at onset and that false-negatives results occur frequently[1], the CMV-AG status becomes positive in most cases during the disease course, thus making this test a useful parameter for monitoring the response to treatment[1,8,14,17]. While there have been reports on rare cases in which patients who had CMV gastroenteritis had CMV-AG results that remained negative throughout the disease course after bone marrow transplantation[13,18,19], there have been no such reports associated with solid-organ transplantation; however, there have been some reports in which the CMV-AG test was positive when an intestinal perforation was caused by CMV gastroenteritis[20,21], including a report in which intestinal perforation were caused by CMV gastroenteritis in patients taking immunosuppressants to treat rheumatoid arthritis[2]. Although it has been reported that routine upper gastrointestinal endoscopy during the follow-up for CMV gastroenteritis does not lead to differences in recurrence rates and treatment effects[17], there are also cases such as ours, where the patient suffers from localized CMV infection but does not achieve viremia and remains CMV-AG negative throughout the disease course. An examination of symptomatic patients through regular upper gastrointestinal endoscopy and biopsy may be an important monitoring method, and it is necessary to tailor the medical management on a case-to-case basis.
Although there have been reports on cases with gastrointestinal perforation due to CMV gastroenteritis, this complication is extremely rare after a solid organ transplantation[2,3]. Recently, there was a report showing that CMV infects vascular endothelial cells and causes ulcers and perforation locally in the intestinal mucosa after solid-organ transplantation[21].
In our case, in addition to the long-term history of oral PSL therapy for autoimmune hepatitis before transplantation, posttransplantation immunosuppression was essential. This may have caused CMV gastroenteritis with strong, localized inflammation that led to intestinal perforation without viremia.
Although CMV infection tends to occur at least three week after transplantation[3], patients taking immunosuppressive drugs before transplantation may be affected earlier[6].
Atsumi et al[22] and Kemeny et al[23] have reported on patients presenting with a combination of polyoma virus infection and acute graft rejection after kidney transplantation. However, the coexistence of an infection and rejection represents a combination of contradictory illnesses and constitutes a rare presentation. In our case, the patient was first diagnosed with ACR through an intraoperative liver biopsy sample taken during the emergency procedure for gastrointestinal perforation in an advanced stage of CMV infection. It was a combination of an infection and a rejection, which again is an extremely rare presentation, which made the management difficult.
TMA is a condition in which polymers of von Willebrand factor are secreted due to vascular endothelial cell dysfunction that is caused by various factors, for e.g., graft-versus-host disease (GVHD), ABO-incompatible transplantation, calcineurin inhibitor (CNI) administration, and fungal and viral (e.g., human immunodeficiency virus, CMV and adenovirus) infections[24,25]. This secretion promotes platelet thrombus formation, leading to multiorgan failure due to microangiopathy. Although the recommended criteria for diagnosing TMA have been previously reported, there are no set diagnostic criteria. Our hospital, as reported previously[26], follows the protocol of a diagnosis based on clinical examination findings and the appearance of fragmented red blood cells in the peripheral smear, combined with tests showing hemolytic anemia, thrombocytopenia and increased bilirubin and lactate dehydrogenase levels.
CMV has been reported to exhibit a TMA-like disease state by infecting vascular endothelial cells and making them dysfunctional[27,28]. Java et al[29], Waiser et al[30] reported a patient with newly developed TMA due to CMV infection in a kidney transplant recipient. In our case, treatment with GCV was initiated as the patient presented with symptoms of TMA prior to the detection of CMV infection. Therefore, a CMV infection could be suspected in a TMA-like disease presentation.
In patients who are chronically treated with immunosuppressants before organ transplantation, endoscopy should be performed according to symptoms at an earlier stage after transplantation. As a reliable test, the CMV-PCR test is reported to be superior to the CMV-AG test, but it is not covered by health insurance providers in Japan.
After solid-organ transplantation, it is necessary to monitor symptomatic patients for CMV gastroenteritis, even when the CMV-AG assay remains negative throughout the course of the illness. Additionally, a CMV infection should be suspected when a TMA-like disease presentation is observed, and the patient should be investigated accordingly, to rule out this diagnosis.
We acknowledge Mr. Gotou Masaki (Department, LSI Medience Corporation) for advice on the antigenemia assay evaluation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF, Boin IFSF, Huerta-Franco MR, Jeong KY S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Mori T, Mori S, Kanda Y, Yakushiji K, Mineishi S, Takaue Y, Gondo H, Harada M, Sakamaki H, Yajima T, Iwao Y, Hibi T, Okamoto S. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:431-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ozaki T, Yamashita H, Kaneko S, Yorifuji H, Takahashi H, Ueda Y, Takahashi Y, Kaneko H, Kano T, Mimori A. Cytomegalovirus disease of the upper gastrointestinal tract in patients with rheumatic diseases: a case series and literature review. Clin Rheumatol. 2013;32:1683-1690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Vincenzi R, Fonseca EA, Chapchap P, Machado MCC, Roda KMO, Candido HL, Benavides MR, D'Assuncao MA, Afonso RC, Turine P, Marson FP, Neto JS. Pancreas-preserving duodenectomy after living donor liver transplantation for invasive cytomegalovirus disease. Pediatr Transplant. 2017;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Hibi T, Tanabe M, Hoshino K, Fuchimoto Y, Kawachi S, Itano O, Obara H, Shinoda M, Shimojima N, Matsubara K, Morikawa Y, Kitagawa Y. Cyclosporine A-based immunotherapy in adult living donor liver transplantation: accurate and improved therapeutic drug monitoring by 4-hr intravenous infusion. Transplantation. 2011;92:100-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Hibi T, Shinoda M, Itano O, Obara H, Kitago M, Abe Y, Yagi H, Tanaka M, Hoshino K, Fujino A, Kuroda T, Kawachi S, Tanabe M, Shimazu M, Kitagawa Y. Steroid minimization immunosuppression protocol using basiliximab in adult living donor liver transplantation for hepatitis C virus-related cirrhosis. Hepatol Res. 2015;45:1178-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Azevedo LS, Pierrotti LC, Abdala E, Costa SF, Strabelli TM, Campos SV, Ramos JF, Latif AZ, Litvinov N, Maluf NZ, Caiaffa Filho HH, Pannuti CS, Lopes MH, Santos VA, Linardi Cda C, Yasuda MA, Marques HH. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70:515-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A; Transplantation Society International CMV Consensus Group. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 472] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 8. | Boeckh M, Bowden RA, Goodrich JM, Pettinger M, Meyers JD. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood. 1992;80:1358-1364. [PubMed] [Cited in This Article: ] |
| 9. | David-Neto E, Triboni AH, Paula FJ, Vilas Boas LS, Machado CM, Agena F, Latif AZ, Alencar CS, Pierrotti LC, Nahas WC, Caiaffa-Filho HH, Pannuti CS. A double-blinded, prospective study to define antigenemia and quantitative real-time polymerase chain reaction cutoffs to start preemptive therapy in low-risk, seropositive, renal transplanted recipients. Transplantation. 2014;98:1077-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Nagata N, Kobayakawa M, Shimbo T, Hoshimoto K, Yada T, Gotoda T, Akiyama J, Oka S, Uemura N. Diagnostic value of antigenemia assay for cytomegalovirus gastrointestinal disease in immunocompromised patients. World J Gastroenterol. 2011;17:1185-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Boaretti M, Sorrentino A, Zantedeschi C, Forni A, Boschiero L, Fontana R. Quantification of cytomegalovirus DNA by a fully automated real-time PCR for early diagnosis and monitoring of active viral infection in solid organ transplant recipients. J Clin Virol. 2013;56:124-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kwon S, Jung BK, Ko SY, Lee CK, Cho Y. Comparison of quantitation of cytomegalovirus DNA by real-time PCR in whole blood with the cytomegalovirus antigenemia assay. Ann Lab Med. 2015;35:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Dahi PB, Perales MA, Devlin SM, Olson A, Lubin M, Gonzales AM, Scaradavou A, Kernan NA, O'Reilly RJ, Giralt S, Jakubowski A, Koehne G, Papadopoulos EB, Ponce DM, Sauter C, Papanicolaou G, Barker JN. Incidence, nature and mortality of cytomegalovirus infection after double-unit cord blood transplant. Leuk Lymphoma. 2015;56:1799-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Mori T, Okamoto S, Matsuoka S, Yajima T, Wakui M, Watanabe R, Ishida A, Iwao Y, Mukai M, Hibi T, Ikeda Y. Risk-adapted pre-emptive therapy for cytomegalovirus disease in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;25:765-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Durand CM, Marr KA, Arnold CA, Tang L, Durand DJ, Avery RK, Valsamakis A, Neofytos D. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis. 2013;57:1550-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711-5719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 408] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 17. | Seo M, Kim do H, Gong EJ, Ahn JY, Lee JH, Jung KW, Choi KD, Song HJ, Lee GH, Jung HY, Kim JH, Lee SO, Choi SH, Kim YS, Woo JH, Kim SH. Is Follow-Up Endoscopy Necessary in Upper Gastrointestinal Cytomegalovirus Disease? Medicine (Baltimore). 2016;95:e3389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Yanada M, Yamamoto K, Emi N, Naoe T, Suzuki R, Taji H, Iida H, Shimokawa T, Kohno A, Mizuta S, Maruyama F, Wakita A, Kitaori K, Yano K, Hamaguchi M, Hamajima N, Morishima Y, Kodera Y, Sao H, Morishita Y. Cytomegalovirus antigenemia and outcome of patients treated with pre-emptive ganciclovir: retrospective analysis of 241 consecutive patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:801-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Mori T, Okamoto S, Watanabe R, Yamazaki R, Tsukada Y, Nagayama H, Ishida A, Ikeda Y. Incidence of cytomegalovirus (CMV) infection in allogeneic hematopoietic stem cell recipients at low risk of CMV infection. Bone Marrow Transplant. 2002;29:1005-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | De Bartolomeis C, Collini A, Barni R, Ruggieri G, Bernini M, Carmellini M. Cytomegalovirus infection with multiple colonic perforations in a renal transplant recipient. Transplant Proc. 2005;37:2504-2506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Zandberg M, de Maar EF, Hofker HS, Homan van der Heide JJ, Rosati S, van Son WJ. Initial cytomegalovirus prophylaxis with ganciclovir: no guarantee for prevention of late serious manifestations of CMV after solid organ transplantation. Neth J Med. 2005;63:408-412. [PubMed] [Cited in This Article: ] |
| 22. | Atsumi H, Asaka M, Kimura S, Imura J, Fujimoto K, Chikazawa Y, Nakagawa M, Okuyama H, Yamaya H, Moriyama M, Tanaka T, Suzuki K, Yokoyama H. A case of second renal transplantation with acute antibody-mediated rejection complicated with BK virus nephropathy. Clin Transplant. 2010;24 Suppl 22:35-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Kemény E, Hirsch HH, Eller J, Dürmüller U, Hopfer H, Mihatsch MJ. Plasma cell infiltrates in polyomavirus nephropathy. Transpl Int. 2010;23:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Nakazawa Y, Hashikura Y, Urata K, Ikegami T, Terada M, Yagi H, Ishizashi H, Matsumoto M, Fujimura Y, Miyagawa S. Von Willebrand factor--cleaving protease activity in thrombotic microangiopathy after living donor liver transplantation: a case report. Liver Transpl. 2003;9:1328-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Kobayashi T, Wada H, Usui M, Sakurai H, Matsumoto T, Nobori T, Katayama N, Uemoto S, Ishizashi H, Matsumoto M, Fujimura Y, Isaji S. Decreased ADAMTS13 levels in patients after living donor liver transplantation. Thromb Res. 2009;124:541-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Kishida N, Shinoda M, Itano O, Obara H, Kitago M, Hibi T, Yagi H, Abe Y, Matsubara K, Odaira M, Tanabe M, Shimazu M, Kitagawa Y. Increased Incidence of Thrombotic Microangiopathy After ABO-Incompatible Living Donor Liver Transplantation. Ann Transplant. 2016;21:755-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Narimatsu H, Kami M, Hara S, Matsumura T, Miyakoshi S, Kusumi E, Kakugawa Y, Kishi Y, Murashige N, Yuji K, Masuoka K, Yoneyama A, Wake A, Morinaga S, Kanda Y, Taniguchi S. Intestinal thrombotic microangiopathy following reduced-intensity umbilical cord blood transplantation. Bone Marrow Transplant. 2005;36:517-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Rahbar A, Söderberg-Nauclér C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79:2211-2220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Java A, Edwards A, Rossi A, Pandey R, Gaut J, Delos Santos R, Miller B, Klein C, Brennan D. Cytomegalovirus-induced thrombotic microangiopathy after renal transplant successfully treated with eculizumab: case report and review of the literature. Transpl Int. 2015;28:1121-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Waiser J, Budde K, Rudolph B, Ortner MA, Neumayer HH. De novo hemolytic uremic syndrome postrenal transplant after cytomegalovirus infection. Am J Kidney Dis. 1999;34:556-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |