Published online Mar 14, 2019. doi: 10.3748/wjg.v25.i10.1266
Peer-review started: December 6, 2018
First decision: January 6, 2019
Revised: January 16, 2019
Accepted: January 18, 2019
Article in press: January 18, 2019
Published online: March 14, 2019
Processing time: 99 Days and 17.1 Hours
Asymptomatic children with Crohn’s disease (CD) require ongoing monitoring to ensure early recognition of a disease exacerbation.
In a cohort of pediatric CD patients, we aimed to assess the utility of serial fecal calprotectin measurements to detect intestinal inflammatory activity and predict disease relapse.
In this prospective longitudinal cohort study, children with CD on infliximab therapy in clinical remission were included. Fecal calprotectin levels were assessed at baseline and at subsequent 2-5 visits. Clinical and biochemical disease activity were assessed using the Pediatric Crohn’s Disease Activity Index, C-reactive protein and erythrocyte sedimentation rate at baseline and at visits over the following 18 mo.
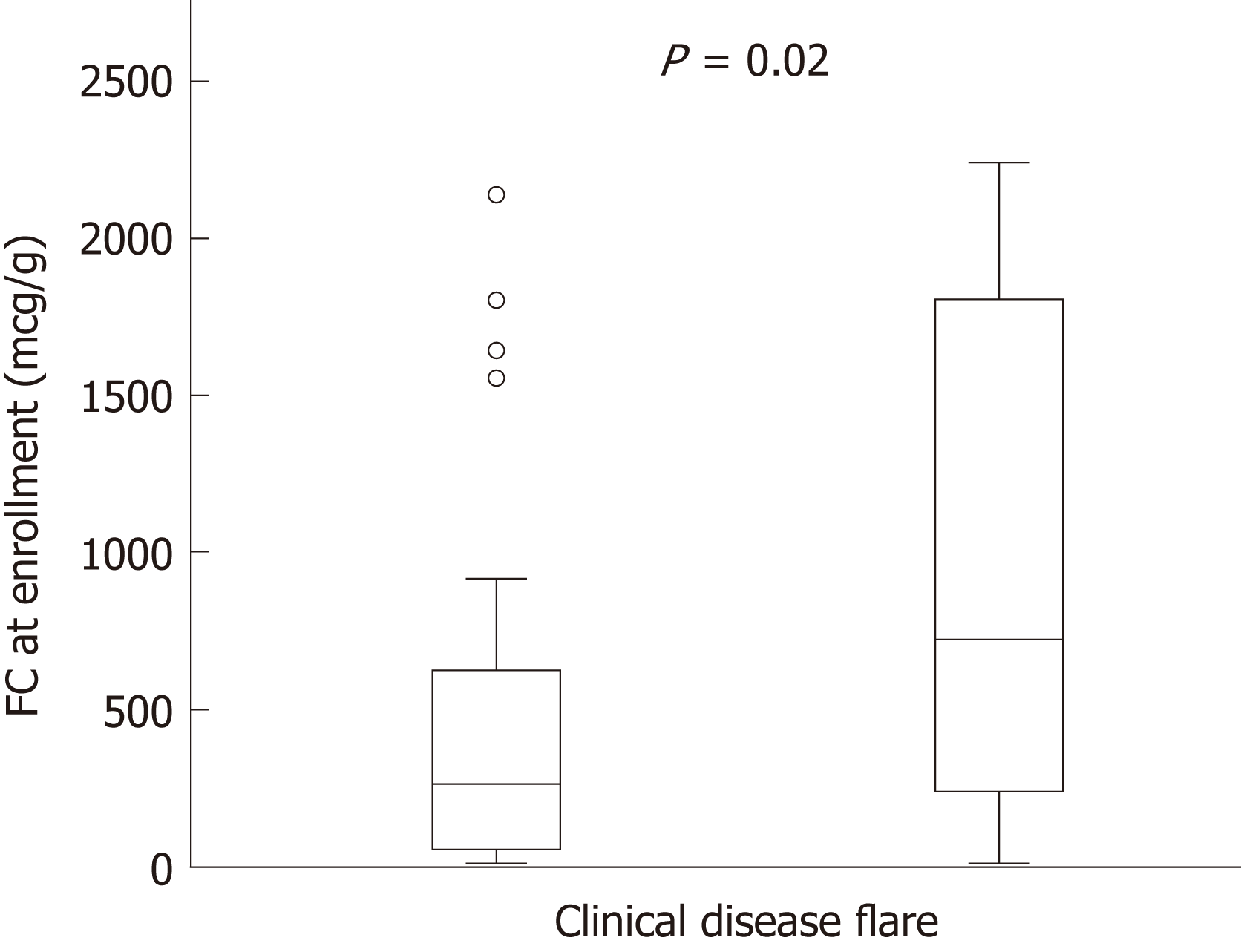
53 children were included and eighteen patients (34%) had a clinical disease relapse during the study. Baseline fecal calprotectin levels were higher in patients that developed symptomatic relapse [median (interquartile range), relapse 723 μg/g (283-1758) vs 244 μg/g (61-627), P = 0.02]. Fecal calprotectin levels > 250 μg/g demonstrated good predictive accuracy of a clinical flare within 3 mo (area under the receiver operator curve was 0.86, 95% confidence limits 0.781 to 0.937).
Routine fecal calprotectin testing in children with CD in clinical remission is useful to predict relapse. Levels > 250 μg/g are a good predictor of relapse in the following 3 mo. This information is important to guide monitoring standards used in this population.
Core tip: It has becoming increasingly evident that many children with Crohn’s disease (CD) who are in clinical remission continue to have ongoing active intestinal inflammation. This prospective paediatric study demonstrates the utility of fecal calprotectin monitoring in asymptomatic children with CD. In this study, a significant number of children were found to have elevated levels despite clinical remission, and levels of > 250 μg/g were found to be a good predictor of clinical relapse in the subsequent 3 mo.
- Citation: Foster AJ, Smyth M, Lakhani A, Jung B, Brant RF, Jacobson K. Consecutive fecal calprotectin measurements for predicting relapse in pediatric Crohn’s disease patients. World J Gastroenterol 2019; 25(10): 1266-1277
- URL: https://www.wjgnet.com/1007-9327/full/v25/i10/1266.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i10.1266
Crohn’s disease (CD) is a type of inflammatory bowel disease (IBD) with clinical course characterized by periods of disease remission and exacerbation. In children, worsening disease activity can be complicated by poor growth and development, hospitalizations, surgeries, and school absences. In addition, quality of life is correlated with disease activity, further supporting the importance of maintaining disease remission[1]. IBD patients have regular follow-up appointments with their gastroenterologist to closely monitor the inflammatory state of the disease with the aim of implementing timely adjustments to medical therapy to prevent worsening of disease activity and development of complicated disease. Unfortunately, clinical symptoms typically lag behind the early intestinal inflammatory changes. Consequently treatment changes are often initiated only after deterioration in clinical status has occurred reinforcing the need for closer monitoring and better evaluation of intestinal inflammatory activity.
Clinicians utilize different tests to monitor inflammatory burden and predict relapse in children with IBD. The most accurate methods include endoscopic evaluation, capsule endoscopy and magnetic resonance enterography; though, these options are invasive, costly, time consuming and can have associated complications[2-4]. Alternatively, less expensive and less invasive serum biomarkers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are also used to monitor inflammatory activity[5]. However, up to one third of children with IBD were found to have normal ESR and CRP levels at diagnosis despite endoscopic evidence of active inflammation, thus demonstrating the lack of sensitivity of these markers to reliably detect active intestinal inflammation[5].
More recently research efforts have been directed towards the use of fecal calprotectin (FC), a stool test that is non-invasive, sensitive and less expensive than imaging or endoscopic procedures[6,7]. Calprotectin is a calcium and zinc binding cytosolic protein found in neutrophilic granulocytes released upon their activation[8]. This protein is stable in feces, and can be measured as a marker of intestinal inflammation[9]. Indeed, FC levels have been reported to correlate with endoscopic evidence of mucosal healing a clinical measure that has been associated with a lower two-year probability for a clinical disease flare[9,10]. In particular, FC levels < 250 μg/g have been found to be a reliable marker of endoscopic mucosal healing in IBD patients[11].
While FC has been studied extensively in disease diagnosis, responses to therapy and post-operative monitoring[12-16], there is a paucity of robust data evaluating the predictive role of FC for disease relapse in pediatric IBD patients in clinical disease remission. Studies in adults have shown that FC is an accurate marker of intestinal inflammation and provides a useful tool for predicting disease relapse in IBD patients in clinical remission[7,17,18]. Most recently, a prospective study in adults evaluated the accuracy of routine FC measurements to predict flares in IBD patients on maintenance infliximab therapy, showing that FC was a good predictor of relapse and remission over the subsequent 4 mo[19]. However, knowledge in pediatric patients is limited, as prior studies lack long-term, prospective data using validated instruments to define remission[20-23]. The aim of this study was to evaluate the accuracy of serial FC measurements to predict clinical flares in pediatric CD patients on maintenance anti-tumor necrosis factor (anti-TNF) therapy and in clinical remission.
In this blinded prospective longitudinal cohort study, pediatric patients with CD receiving infliximab, and in clinical remission were consecutively recruited from BC Children’s Hospital Division of Gastroenterology, Hepatology and Nutrition in Vancouver, British Columbia, Canada between June 2013 and May 2015. Ethical approval was obtained from the University of British Columbia Clinical Research Ethics Board and the British Columbia Children’s and Women’s Research Review Committee. Written informed consent was obtained prior to inclusion in the study.
Children ages 6-18 years old with an established diagnosis of moderate to severe CD based on the revised Porto criteria were included[24]. Disease was characterized using the Paris classification based on age at diagnosis, disease location and behaviour[25]. All participants were on maintenance infliximab infusions (i.e., > 14 wk from initiation of therapy) at dosing intervals between 4 and 8 wk, and in clinical remission at commencement of the study as defined by a Pediatric CD Activity Index (PCDAI) score of < 10[26]. Potential subjects were excluded if they had at any time during the study known infectious enteritis or colitis, regular use of non-steroidal anti-inflammatory drugs (> 2 tablets/wk), or a diagnosis of celiac disease. All subjects were required to have a minimum of 3 stool samples and on infliximab throughout the study period.
Sample size was determined pragmatically based on the number of patients available at our center and fulfilling inclusion criteria. Review of our clinical IBD database showed 73 patients with CD receiving infliximab infusions. Based on our departmental data over the preceding 4 years, we estimated an additional 10-12 new patients per year eligible for the study. With 70%-80% enrolment, we estimated a potential sample size of 58 to 66 patients. All consecutive cases during the study period fulfilling inclusion criteria were invited to participate.
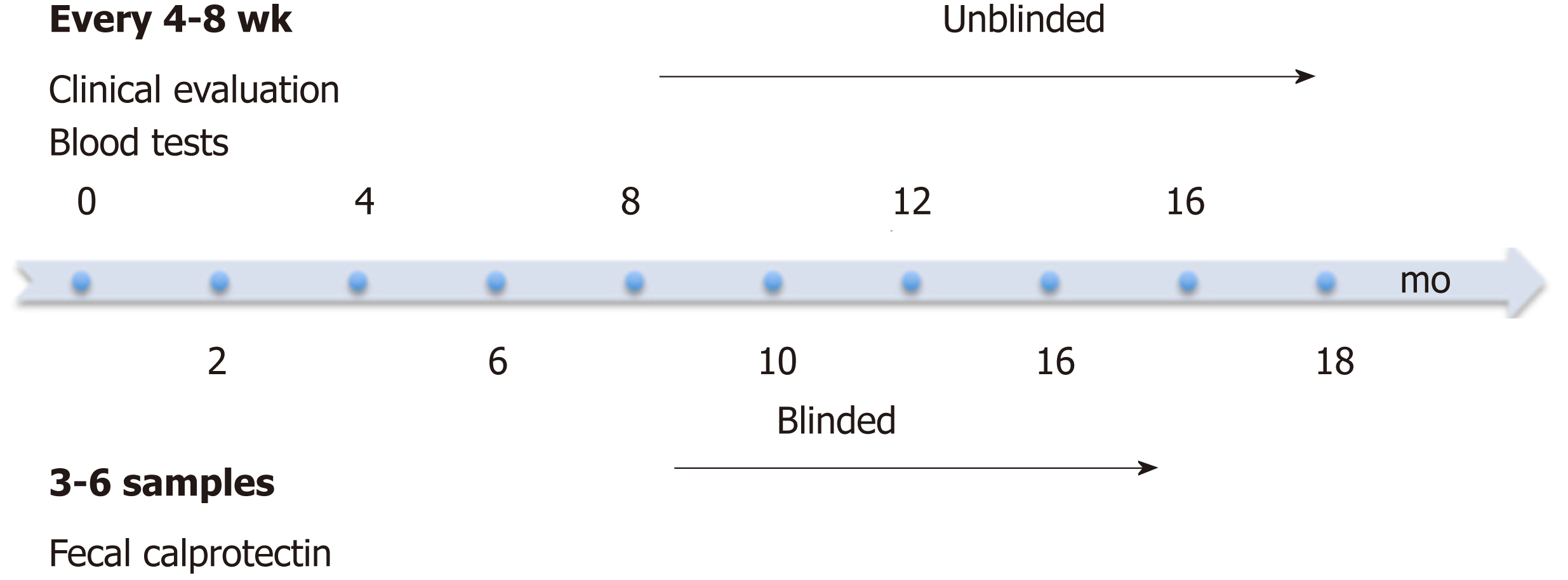
At enrolment patients provided a baseline stool sample, for baseline FC level, followed by collections at subsequent 2-5 infliximab infusion visits (Figure 1). Treating physicians, patients and families were blinded to FC results and no management decisions during the study were based on FC results. Stool samples were stored at 4 °C and processed within 7 d of collection to ensure stability of FC; 100 mg of stool was extracted using the Buhlmann Smart Prep kit following vendor’s instructions, and frozen at - 80°C until the day of the assay. Manual ELISA was performed within 4 mo of sample extraction using the Buhlmann fCAL® ELISA kit (Buhlmann EK-CAL, rev 2012-11-20) following vendor’s instructions for the lower range procedure (working 10-600 μg/g). Samples with levels > 600 μg/g were diluted according to vendor protocol and re-assayed. A FC level < 50 μg/g stool was considered to be within the normal range. Imprecision studies and comparability of patient results with other laboratories using the same assay were performed as part of the method validation (See Supplementary data, Supplemental Figures 1 and 2).
In addition, stool samples from 25 healthy children between ages 6 to 18 years with no inflammatory conditions were tested. These subjects were recruited from the general population and were screened for gastrointestinal symptoms and diseases, associated inflammatory conditions, and nonsteroidal anti-inflammatory drugs use.
The treating physician evaluated clinical disease activity at study enrolment and each subsequent visit (4-8 weekly) using a PCDAI score (Figure 1). Standard laboratory investigations including a complete blood count, CRP, ESR and albumin were measured at each infusion visit and available to the physician. Appropriate results were used to calculate the PCDAI including hematocrit, ESR, albumin, height, weight and disease symptoms. Patients were followed for 18 mo to provide long-term data on routine clinical evaluation and to monitor for clinical disease relapse. The primary endpoint was symptomatic disease relapse defined as a PCDAI score of ≥ 10 with a change of at least 10 points from the prior visit.
Patient characteristics included age, sex, age of diagnosis, disease location and behaviour, other medications (both IBD and non-IBD related), IBD-related surgeries, time from diagnosis to infliximab start, and infliximab infusion dosing and interval were collected. Any medication changes including the addition of new medications or changes in the infliximab infusions (dosing or time interval) were recorded.
Continuous variables following a skewed distribution are presented using median values and interquartile range (IQR) and Wilcoxon signed-rank test was used to compare differences between patients that relapsed and those that remained in clinical remission. Categorical data are presented as percentages. Time-to-event data are presented using Kaplan-Meier survival curves for FC, ESR, CRP and albumin levels. One predictor logistic regression and receiver operating characteristic (ROC) curve analyses for FC, ESR, CRP and albumin were used to evaluate the cumulative incidence of flares in a 2-wk time window around (before or after) 3 and 6 mo after the FC and a 4-wk window around 12 mo after the FC. Specificity, sensitivity and optimal threshold values of FC were determined from ROC curve analysis. The area under the curve (AUC) of the ROC was used to determine accuracy of FC, which was defined as poor (0.6-0.7), fair (0.7-0.8), good (0.8-0.9) and excellent (0.9-1.0)[27].
Variance components analysis and Bland-Altman plots were used to evaluate the correlation between FC levels completed at our laboratory, the comparison laboratory, and the Buhlmann Diagnostics comparison samples. A significance level of P < 0.05 was used in our analysis. Statistical analysis was performed using R (version 3.3.3)[28] and cp-R Clinical Chemistry Software V 0[29].
Of the 25 healthy children evaluated, 17 had FC levels < 25 μg/g, 7 had levels between 50 μg/g and 200 μg/g, and 1 had a level > 200 μg/g. Additional stool samples were evaluated from 3 subjects with FC levels > 50 μg/g. The subject with the highest level (247.9 μg/g) had a normal repeat FC (12.4 μg/g) and the other 2 subjects had levels of 47.6 μg/g (original 114.1 μg/g) and 88.5 μg/g (90.1 μg/g). The median (IQR) of the group of health controls (following normalization of FC levels in the two individuals) was 35 µg/g (IQR 20-50 μg/g).
Sixty-two patients were prospectively enrolled. Three subjects withdrew because of difficulty completing stool samples, 3 were transferred to adult care, 1 had a change in diagnosis to ulcerative colitis, and 2 patients stopped infliximab treatment during the study. Consequently, 53 children were analyzed. Thirty-six patients (68%) were male and the median follow-up time was 1.5 years. A total of 205 stool samples were collected during the study with a mean of 4 stool samples per patient. In addition to infliximab, 46 (87%) of patients received co-immunosuppressive therapy, with azathioprine prescribed most commonly (51% of patients). Patients’ clinical characteristics are shown in Table 1.
| Clinical characteristics of the pediatric Crohn’s disease | |
| Males | 36 (68) |
| Age years (median, IQR) | 14.9 (11.9-16.0) |
| Age at diagnosis years (median, IQR) | 10.9 (8.3-13.1) |
| Duration of disease years (median, IQR) | 3.0 (1.3-5.1) |
| Concomitant immunosuppressant | |
| Azathioprine | 27 (51) |
| Methotrexate | 19 (36) |
| None | 7 (13) |
| Previous IBD related surgery | |
| Perianal surgery | 1 (2) |
| Resectional surgery | 6 (11) |
| Crohn’s disease: Age at diagnosis (Paris classification) | |
| A1a: 0-< 10 yr | 20 (38) |
| A1b: 10-< 17 yr | 33 (62) |
| Crohn’s disease: Location (Paris classification) | |
| L1 | 5 (9) |
| L2 | 22 (42) |
| L3 | 26 (49) |
| L4 | 35 (66) |
| Crohn’s disease: Behaviour (Paris classification) | |
| B1: non-stricturing, non-penetrating | 31 (59) |
| B2: stricturing | 12 (23) |
| B3: penetrating | 7 (13) |
| B2B3: penetrating and structuring | 3 (5) |
| P: Perianal disease | 17 (32) |
| Crohn’s disease: Growth impairment (Paris classification) | |
| Evidence of growth delay | 0 (0) |
| Infliximab dosing | |
| Interval between infliximab doses (wk), mean (range) | 7 (4-8) |
| Mean dose (mg/kg) | 6.8 |
At enrolment when all patients were in clinical remission (PCDAI < 10), serum inflammatory markers and FC were initially evaluated (baseline data). The cumulative number of patients with symptomatic relapse was 18 (34%), and the mean time from enrolment to clinical flare was 7.1 mo (range, 1.5-15 mo). Baseline FC level in the group with symptomatic relapse was higher than those remaining in clinical remission [median 723 μg/g (IQR 283-1758) vs 244 μg/g (IQR 61-627), P = 0.02] (Figure 2). The median FC level at the visit prior to relapse was 765 μg/g (IQR 504-1800). Four outliers in the non-relapsing group had elevated FC at enrolment; 1 was 6 mo post diagnosis and FC levels gradually decreased to the normal range with no intervention, 2 had a decrease in FC levels after antibiotic administration for concurrent infections (urinary tract infection, pharyngitis) but FC levels remained elevated (> 400 μg/g) and the final outlier had a persistent FC levels > 1800 μg/g at study completion but remained in clinical remission. Both ESR and CRP levels were elevated in this individual triggering infliximab dose escalation; however this did not impact the FC level.
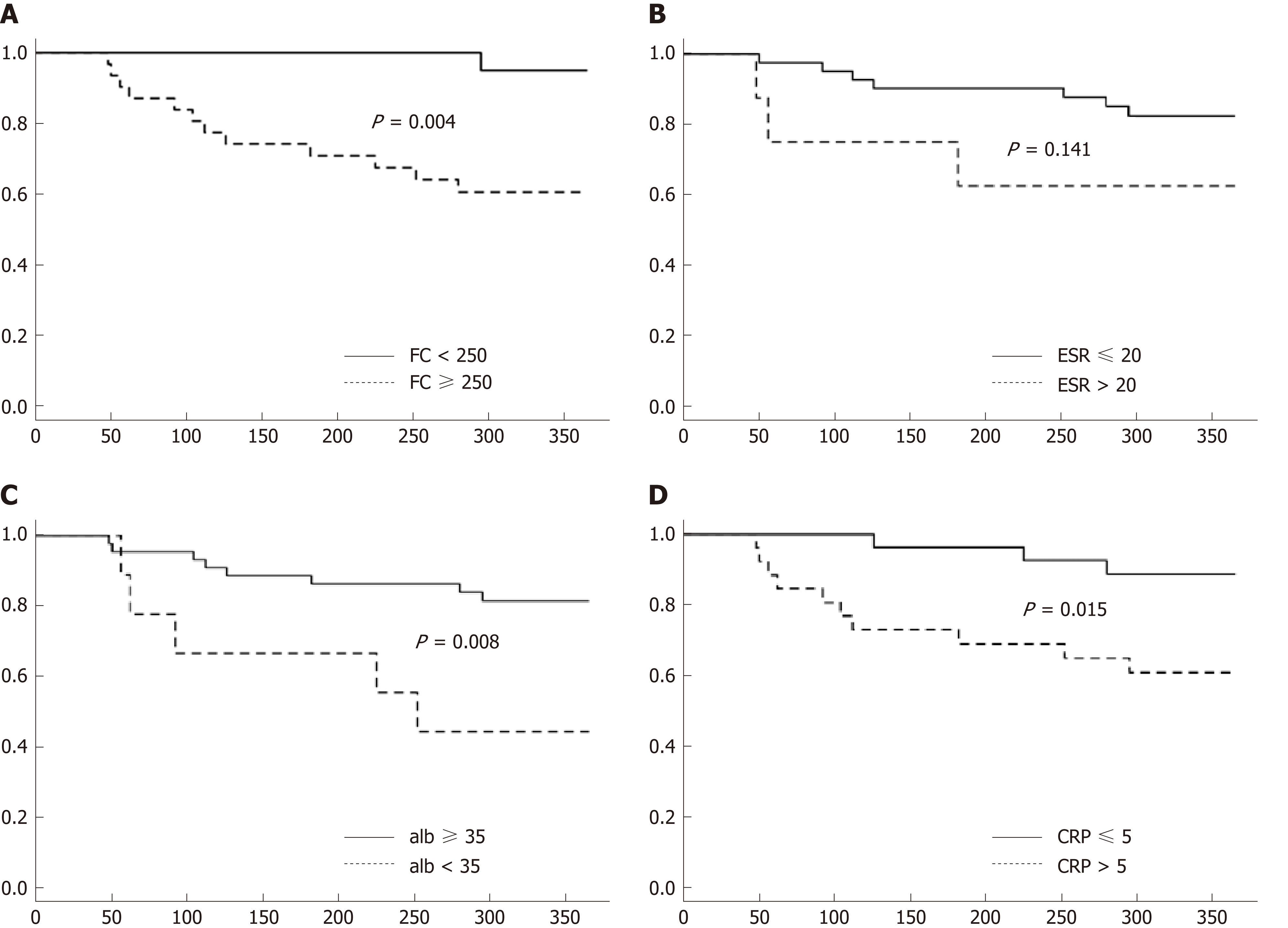
To further assess risk of clinical disease relapse in relationship to baseline FC levels, time to clinical relapse curves were calculated for the first year of the study using baselines FC levels < or ≥ 250 μg/g. Significant differences in relapse rates were noted in patients with FC < 250 μg/g compared to those with levels ≥ 250 μg/g (Figure 3A, P = 0.004). Significant differences were also noted for baseline CRP levels ≤ 5 and > 5 mg/L (Figure 3C, P = 0.015) and albumin < 35 and ≥ 35 g/L (Figure 3D, P = 0.008). However, baseline ESR levels < 20 and ≥ 20 mm/h were not significantly different in predicting clinical relapse over time (Figure 3B, P = 0.141).
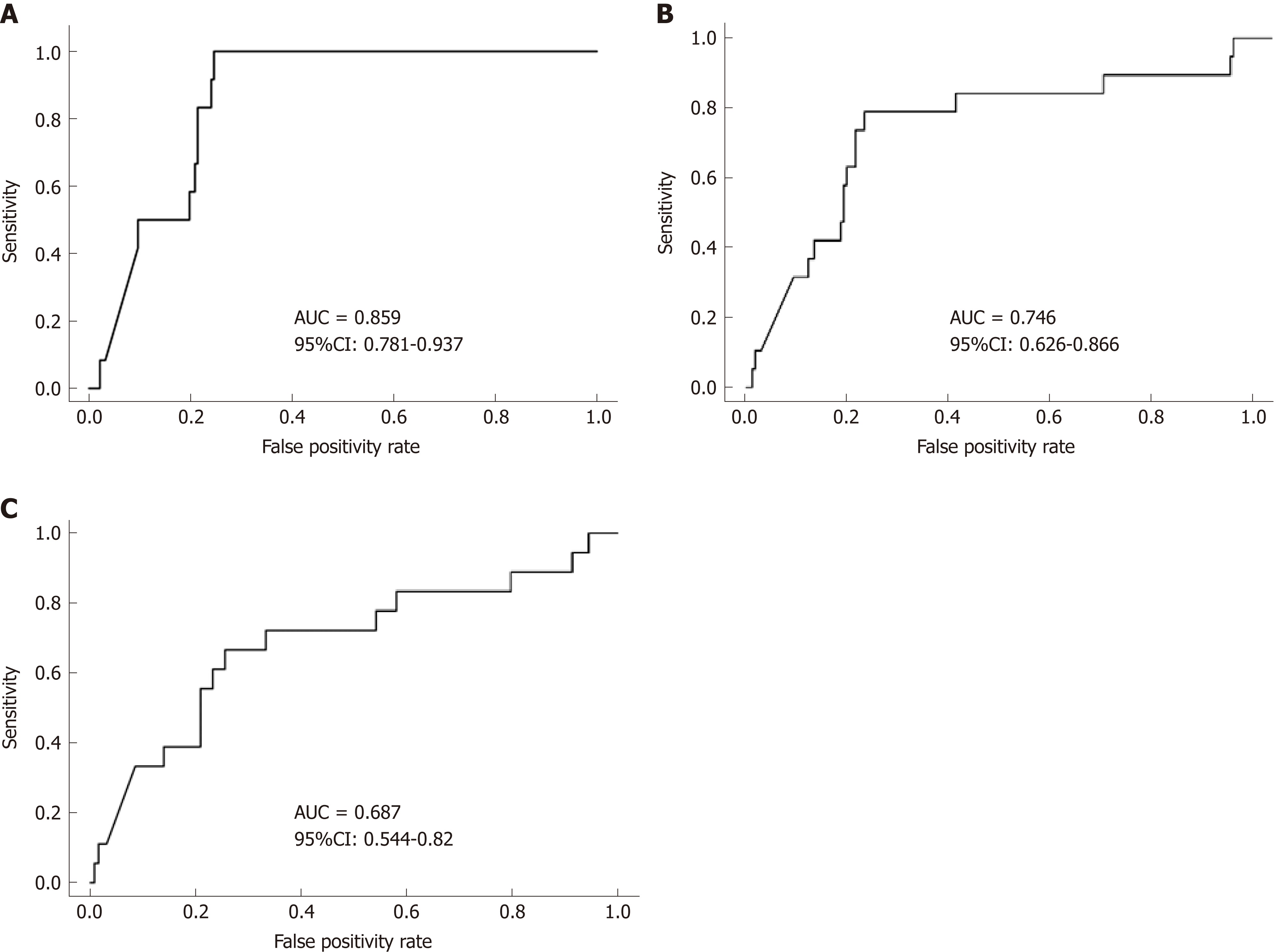
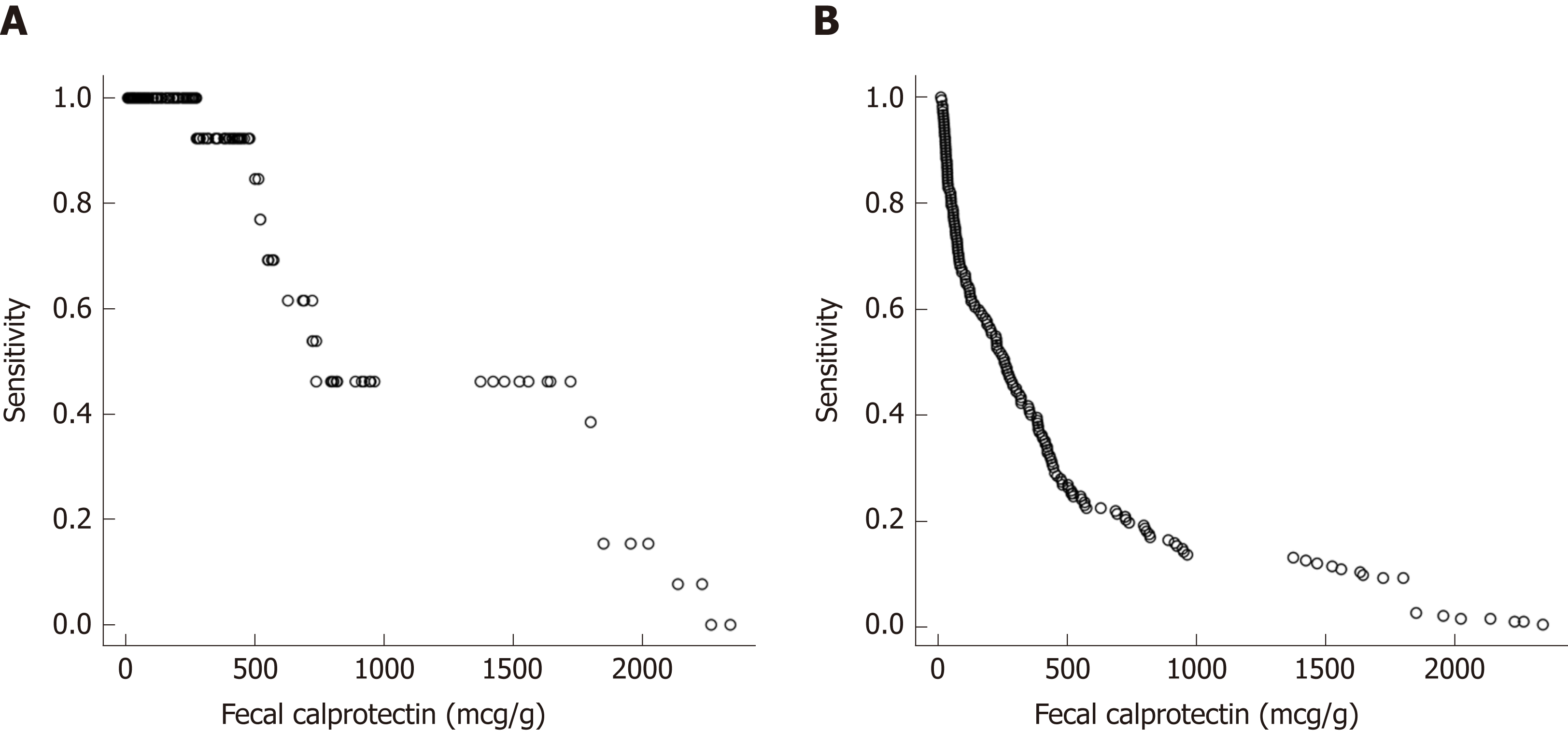
Repeated measures analysis was used to determine if FC, ESR, CRP and albumin at an individual visit were predictive of a clinical disease relapse at subsequent visits. Visits with clinical relapse carried over from a previous visit were excluded. FC had good accuracy in predicting clinical disease relapse within 3 mo of collection with a ROC value of 0.86 [95% confidence interval (CI) 0.781 to 0.937], (Figure 4A). In contrast, the ROC values for ESR, CRP and albumin were lower at 0.651 (95% CI 0.481-0.821), 0.697(95%CI 0.527-0.866) and 0.773 (95%CI 0.662-0.883) respectively. The ability of FC to predict clinical disease relapse decreased significantly at 6 and 12-mo time intervals from stool collection with ROC values of 0.746 (95%CI 0.626-0.866) and 0.687 (95%CI 0.554-0.82) respectively (Figure 4B and C). ESR, CRP and albumin were also unreliable predictors at 6 and 12-mo time points (Supplemental Table 1). Repeated measures analysis showed that a FC value of approximately 500 μg/g provided a sensitivity of 85% and specificity of 73% for predicting clinical relapse at the next visit (Figure 5A and B).
Management decisions were based on PCDAI score, clinical assessment and serum laboratory values. We expected that high FC would be associated with a high PCDAI and vice versa, however this relationship was only seen in 24 children (45%) (Supplemental Figures 3 and 4). In the remaining 29 children the PCDAI and FC level did not correlate. Despite normal FC levels (< 250 μg/g) throughout the study in 14 patients, 4 (29%) had a medication escalation (Supplemental Figure 3). FC levels > 250 μg/g were reported in 39 (74%) patients at one or more time points, however 25 (64%) did not have a clinical flare, and in 4 of these patients (16%) therapy was actually de-escalated (Supplemental Figure 4). In the 14 patients with a clinical flare and raised FC, six (43%) had escalation of therapy, while eight (57%) had no medication change (Supplemental Figure 4).
Several studies in adult IBD patients have reported a strong association between elevated FC levels and clinically active inflammatory disease as well as the utility of FC to predict disease relapse[17-19,30]. However, in children the data is more limited and mostly confined to retrospective data collection or prospective studies with single FC measurements and short study follow-up times[20-23]. This prospective study is the first to evaluate serial blinded FC measurements to assess their value in predicting clinical disease relapse in children with IBD. Our study showed that serial FC levels could predict clinical disease relapse in patients with quiescent CD on infliximab during an 18-mo follow up period. Moreover, FC demonstrated good predictive accuracy of clinical disease relapse within 3 mo and was superior, but not significantly so, to other currently available serum biomarkers at predicting clinical relapse.
Consistent with the adult and pediatric studies that reported higher baseline FC levels in patients who developed symptomatic relapse, our patients who developed symptomatic relapse had significantly higher baseline median FC levels than those patients who maintained clinical remission (723 μg/g vs 244 μg/g). Reported cut-off of FC to predict risk of relapse in adults and children with IBD vary widely ranging from 120-340 μg/g in adults[18,19,31] and 108-500 μg/g in paediatrics[20,21,23]. Dierderen et al[20] in their recent retrospective study in pediatric IBD patients noted that a baseline FC of 250 µg/g was predictive of symptomatic relapse within 6-mo, with a hazard ratio of 1.46 (95%CI: 1.21-1.77) with a good predictive accuracy (AUC: 0.82). Similarly, in our study, Kaplan-Meier time-to-relapse plots using FC level ≥ 250 μg/g determined that patients followed prospectively over 18-mo had a significant 7.9-fold (95%CI 1.1-56.1) increased risk of developing a clinical relapse than patients with lower values (P = 0.004). Moreover, FC had good accuracy in predicting clinical disease relapse within 3 mo of collection with an ROC value of 0.86 (95%CI 0.781-0.937).
Dierderen et al[20] reported an optimal FC cut off in pediatric IBD patients (n = 114) of 350 μg/g with a sensitivity and specificity of 82% and 79% and a positive and negative predictive value of 41% and 96% respectively. Conversely, in a short term cross-sectional, observational, prospective cohort study in adult patients with IBD in clinical remission on maintenance Infliximab a FC concentration > 160 μg/g was reported to have a sensitivity and specificity for predicting relapse of 91.7% and 82.9% respectively[32]. In contrast, we determined that FC values > 500 μg/g provided the best sensitivity and specificity to predict a clinical disease relapse at the next visit.
A comparable study to ours but in adult IBD patients in clinical remission on anti-TNF therapy followed prospectively until relapse or for 16-mo reported that FC levels ≥ 300 μg/g predicted relapse over the following 4 mo with a probability of 78.3%[19]. Moreover, two consecutive FC values ≥ 300 μg/g increased the probability of predicting clinical relapse to 85.7%. However, the study population included both CD (n = 71) and ulcerative colitis (n = 24) patients. In the CD cohort a FC value > 290 μg/g predicted relapse at any time over the following 4 mo with a probability of 96%. The variability in FC cut offs is multifactorial and likely related to differences in study design, populations, duration of follow-up, use of clinical indicators of symptomatic relapse, differences in methodology for FC quantification and fewer FC measurements. Notably, our study prospectively assessed serial FC only in CD patients on infliximab in clinical remission and the methodology for FC quantification was validated in our laboratory prior to measuring levels. Indeed, FC levels < 500 μg/g correlated more closely than those in the upper range and given that our study found optimal cut-offs for predicting relapse to be < 600 μg/g, using the lower range procedure is valid.
Notably, in keeping with adult IBD practice, our study shows that children should also have FC levels checked every 3 mo to ensure adequate monitoring. Indeed, earlier detection of intestinal inflammation, with more timely management changes could result in fewer disease flares, improvement in patient quality of life and ultimately a decreased burden on the health care system.
A major strength of our study was the blinded and prospective longitudinal design with repeated measurements of FC, ESR, CRP and PCDAI scores. The blinding of FC results ensured that therapeutic changes were based upon our current disease monitoring practices and were not made based upon the FC result. The repeated measures allowed us to evaluate the temporal relationship between test results and clinical relapse providing new information that prior studies are lacking[20]. Furthermore, evaluation of management decisions following study completion exposed the deficiencies in routine assessment methods when compared to FC. Of the 39 patients with elevated FC levels, FC remained elevated in 49% of patients during the study. While only one patient in our study had a disease complication, longer-term follow-up may uncover complications that occur in the group with persistent elevations of FC levels. Notably, in the CALM multicentre, randomized controlled study in adult patients with CD, escalation to adalimumab based on the tight control (FC ≥ 250 μg/g, CRP ≥ 5 mg/L, CDAI ≥ 150, or prednisone use in the previous week) compared to standard clinical management (symptom-driven decisions alone) resulted in better clinical and endoscopic outcomes at week 48[33].
While this study was limited by sample size and by the limited sensitivity of the PCDAI clinical score this was offset by the inclusion of repeated measurements of biomarkers and PCDAI scores collected prospectively at multiple visits over an 18 mo follow up period. Notable, the PCDAI remains a tool frequently used in clinical care and research studies in children; however, the gold standard assessment tool for disease activity is endoscopic evaluation. Indeed endoscopic guided management of IBD is becoming increasing recommended, although its true value and frequency of use and the role of FC in guiding endoscopic intervention remain to be evaluated prospectively. Although symptom based monitoring remains an important outcome variable, it has become increasingly evident that it correlates poorly with more objective measures of disease activity, necessitating evaluating the predictive value of biochemical markers of inflammation. The inclusion of endoscopy to guide management of IBD is increasingly recommended, although its true value remains to be prospectively evaluated.
In conclusion, this prospective longitudinal pediatric study is the first to demonstrate that routine serial FC measurements are an independent valuable predictor of symptomatic relapse. Moreover, FC elevation was noted up to 3 mo prior to the appearance of symptomatic relapse. Consequently, implementing a 3-monthly test to treat FC monitoring strategy would allow clinicians to make timely therapeutic adjustments in advance of disease relapse. However, future prospective studies are also necessary to evaluate the impact of routine FC monitoring on long-term patient outcomes and healthcare system burdens. Furthermore, research evaluating the relationship between FC levels and infliximab drug trough levels will be necessary to better understand the relationship and to further guide management decisions.
Inflammatory bowel disease (IBD) is a chronic relapsing intestinal inflammatory disorder requiring ongoing monitoring to optimize care. It has become increasing evidence that sustained remission reduces the likelihood of complicated disease. Consequently the incorporation of effective monitoring tools is required to assess disease activity allowing for the escalation of therapy in a timely manner. Fecal calprotectin (FC) has been studied in disease diagnosis and response to therapy but there is limited data evaluating its role in disease relapse in pediatric IBD patients in clinical remission. In the present prospective pediatric IBD study, we show that routine serial FC measurements are a valuable predictor of symptomatic relapse.
The motivation of the study was to evaluate the utility of FC as a predictive marker of disease relapse and in doing so reinforce the use of this strategy in clinical practice. Moreover adopting the strategy outlined in the paper will hopefully lead to better monitoring of disease activity with early optimization of therapy reducing the likelihood of fully blown clinical flare ups. Further research will need to look at the utility of this monitoring strategy and the role and relationship to endoscopic monitoring of disease activity and mucosal healing.
The main objective was to evaluate the accuracy of serial FC measurements to predict clinical flares in pediatric Crohn’s disease (CD) patients on maintenance anti-tumor necrosis factor therapy and in clinical remission. The realization of this objective infers that serial FC done 3-monthly should be incorporated into clinical practice. Future research should also evaluate whether this strategy improves the quality of care, patient outcomes and costs to health care systems.
This was a prospective observational study where serial FC levels were measure in pediatric patients with IBD in clinical remission on Remicade. The responsible clinicians were blinded to the FC results. Patient characteristics included age, sex, age of diagnosis, disease location and behaviour, other medications (both IBD and non-IBD related), IBD-related surgeries, time from diagnosis to infliximab start, and infliximab infusion dosing and interval were collected. The PDCAI was recorded at each visit. FC levels were measure by ELISA within 4-mo of sample extraction. Standard statistical methodology was used. Continuous variables are presented using median values and interquartile range (IQR) and Wilcoxon signed-rank test was used to compare differences between patients who relapsed and remaining in clinical remission. Categorical data are presented as percentages. Kaplan-Meier survival curves (time-to-event data) are used for biomarkers [FC, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), albumin]. One predictor logistic regression and receiver operating characteristic (ROC) curve analyses for biomarkers were used to evaluate the cumulative incidence of flares in a 2-wk time window around (before or after) 3 and 6 mo after the FC and a 4-wk window around 12 mo after the FC. Specificity, sensitivity and optimal threshold values of FC were determined from ROC curve analysis.
Of the 62 patients prospectively enrolled, 53 were analyzed; 68% were male and median follow-up time 1.5 years. A total of 205 stool samples were collected and analyzed (mean of 4 stool samples per patient). Symptomatic relapse was recorded in 18 (34%) patients (mean time from enrolment to clinical flare 7.1 mo, range, 1.5-15 mo). Baseline FC level in the symptomatic relapse group was higher than those remaining in clinical remission [median 723 μg/g (IQR 283-1758) vs 244 μg/g (IQR 61-627), P = 0.02]. The median FC level at the visit prior to relapse was 765 μg/g (IQR 504-1800). Time to clinical relapse curves calculated for the first year of the study using baselines FC levels < or ≥ 250 μg/g showed a significant differences in relapse rates in patients with FC < 250 μg/g compared to those with levels ≥ 250 μg/g (P = 0.004). In addition, FC had good accuracy in predicting clinical disease relapse within 3 mo of collection with a ROC value of 0.86 [95% confidence interval (CI) 0.781-0.937]. Treatment decisions were based on clinical symptoms and standard biomarkers of inflammation (ESR and CRP) and thus did not reflect the potential benefit of the FC result. Consequently prospective evaluation of sequential FC testing is required to evaluate the short and long-term benefits on disease remission and outcome.
FC had good accuracy in predicting clinical disease relapse within 3 mo of collection with a ROC value of 0.86 (95%CI: 0.781-0.937). Prospective monitoring with proactive intervention with either endoscopy assessment of disease activity and extent and, or optimization of medical therapy will reduce the likelihood of clinical flares, reduce the risk of complication and improve patient outcomes. FC is a useful biological tool to evaluate disease activity in patients in clinical remission on a biological agent. The Pediatric CD Activity Index (PCDAI) is not sensitive enough to monitor disease activity in children with CD. The addition of FC monitoring improves the quality of care for children with IBD. Regular 3-monthly FC monitoring should be incorporated into clinical practice. However it is important to follow the trend in level and not rely on a single level. That 3-monthly FC monitoring is a good addition to currently available biomarkers of inflammation. That FC is a good predictor of clinical relapse. That 3-monthly FC monitoring detects patients who will experience a symptomatic flare of disease. The inclusion of regular 3-monthly FC monitoring into clinical practice. The addition of FC monitoring will improves the quality of care and outcomes for children with IBD.
Such studies are important to do. Colonoscopy should be included as an endpoint. A treatment decision can be made based on the FC result with subsequent determination whether the change had positive benefit (i.e., normalization of FC level and evidence of mucosal healing at colonoscopy). To prospective evaluate sequential FC testing and to determine the short and long-term benefits on disease remission, quality of life, patient outcome and related health care-costs. To define the relationship between FC monitoring and endoscopic monitoring of disease activity and mucosal healing. To determine the best cut off for FC for patients in remission. Prospective interventional studies with clearly defined FC levels, endoscopic score and treatment strategies with clearly defined primary and secondary outcomes (i.e., disease in steroid free remission, disease biochemical remission, and mucosal healing).
The authors would like to thank Dr. Mat Provencal and his laboratory staff at the Hôpital Maisonneuve-Rosemont, Quebec for their help with validation of our Elisa method, and to Buhlmann for supplying the ELISA kits. Laboratory support at BC Children’s Hospital was kindly provided by Diana Ralston. In addition, many thanks to our research team including Alam Lakhani and Carlie Penner for assisting with data collection. Dr Foster is supported by a research award provided by the Clinical Investigator program (CIP), University of British Columbia and Dr Jacobson is a Senior Clinician Scientist supported by the Children with Intestinal and Liver Disorders (CHILD) Foundation and the BC Children’s Hospital Research Institute Clinician Scientists Award Program, University of British Columbia. The authors would also like to thank the children and their families who courageously live with IBD on a daily basis and the members of the Division of Gastroenterology, Hepatology and Nutrition at British Columbia Children’s Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kapel N, Matowicka-Karna J, Mesquita J, Talebi Bezmin Abadi A S- Editor: Yan JP L- Editor: A E- Editor: Yin SY
| 1. | Chouliaras G, Margoni D, Dimakou K, Fessatou S, Panayiotou I, Roma-Giannikou E. Disease impact on the quality of life of children with inflammatory bowel disease. World J Gastroenterol. 2017;23:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Makkar R, Bo S. Colonoscopic perforation in inflammatory bowel disease. Gastroenterol Hepatol (NY). 2013;9:573-583. [PubMed] |
| 3. | Nemeth A, Wurm Johansson G, Nielsen J, Thorlacius H, Toth E. Capsule retention related to small bowel capsule endoscopy: A large European single-center 10-year clinical experience. United European Gastroenterol J. 2017;5:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Stoddard PB, Ghazi LJ, Wong-You-Cheong J, Cross RK, Vandermeer FQ. Magnetic resonance enterography: State of the art. Inflamm Bowel Dis. 2015;21:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Alper A, Zhang L, Pashankar DS. Correlation of Erythrocyte Sedimentation Rate and C-Reactive Protein With Pediatric Inflammatory Bowel Disease Activity. J Pediatr Gastroenterol Nutr. 2017;65:e25-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, Sandborn WJ, Feagan BG. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2015;110:802-19; quiz 820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 481] [Article Influence: 48.1] [Reference Citation Analysis (1)] |
| 7. | Sands BE. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology. 2015;149:1275-1285.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 8. | Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000;356:1783-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kostas A, Siakavellas SI, Kosmidis C, Takou A, Nikou J, Maropoulos G, Vlachogiannakos J, Papatheodoridis GV, Papaconstantinou I, Bamias G. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23:7387-7396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, D'Haens G; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463-8; quiz e10-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 644] [Article Influence: 42.9] [Reference Citation Analysis (35)] |
| 11. | Kristensen V, Røseth A, Ahmad T, Skar V, Moum B. Fecal Calprotectin: A Reliable Predictor of Mucosal Healing after Treatment for Active Ulcerative Colitis. Gastroenterol Res Pract. 2017;2017:2098293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Carroccio A, Iacono G, Cottone M, Di Prima L, Cartabellotta F, Cavataio F, Scalici C, Montalto G, Di Fede G, Rini G, Notarbartolo A, Averna MR. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: A prospective study in adults and children. Clin Chem. 2003;49:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Kostakis ID, Cholidou KG, Vaiopoulos AG, Vlachos IS, Perrea D, Vaos G. Fecal calprotectin in pediatric inflammatory bowel disease: A systematic review. Dig Dis Sci. 2013;58:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Fagerberg UL, Lööf L, Myrdal U, Hansson LO, Finkel Y. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;40:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Pavlidis P, Gulati S, Dubois P, Chung-Faye G, Sherwood R, Bjarnason I, Hayee B. Early change in faecal calprotectin predicts primary non-response to anti-TNFα therapy in Crohn's disease. Scand J Gastroenterol. 2016;51:1447-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Hukkinen M, Pakarinen MP, Merras-Salmio L, Koivusalo A, Rintala R, Kolho KL. Fecal calprotectin in the prediction of postoperative recurrence of Crohn's disease in children and adolescents. J Pediatr Surg. 2016;51:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, Hu PJ, Chen MH. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 18. | Chew TS, Mansfield JC. Can faecal calprotectin predict relapse in inflammatory bowel disease: A mini review. Frontline Gastroenterol. 2018;9:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, Dominguez-Muñoz JE. Accuracy of Consecutive Fecal Calprotectin Measurements to Predict Relapse in Inflammatory Bowel Disease Patients Under Maintenance With Anti-TNF Therapy: A Prospective Longitudinal Cohort Study. J Clin Gastroenterol. 2018;52:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Diederen K, Hoekman DR, Leek A, Wolters VM, Hummel TZ, de Meij TG, Koot BG, Tabbers MM, Benninga MA, Kindermann A. Raised faecal calprotectin is associated with subsequent symptomatic relapse, in children and adolescents with inflammatory bowel disease in clinical remission. Aliment Pharmacol Ther. 2017;45:951-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | van Rheenen PF. Role of fecal calprotectin testing to predict relapse in teenagers with inflammatory bowel disease who report full disease control. Inflamm Bowel Dis. 2012;18:2018-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Walkiewicz D, Werlin SL, Fish D, Scanlon M, Hanaway P, Kugathasan S. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Sipponen T, Kolho KL. Faecal calprotectin in children with clinically quiescent inflammatory bowel disease. Scand J Gastroenterol. 2010;45:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 991] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 25. | Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, Fell J, Ruemmele FM, Walters T, Sherlock M, Dubinsky M, Hyams JS. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm Bowel Dis. 2011;17:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1141] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 26. | Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, Griffiths AM, Katz AJ, Grand RJ, Boyle JT. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 803] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6200] [Cited by in RCA: 4932] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 28. | R Core Team (2017). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: URL http://www.R-project.org/. . |
| 29. | Holmes DT. cp-R, an interface the R programming language for clinical laboratory method comparisons. Clin Biochem. 2015;48:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Heida A, Park KT, van Rheenen PF. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm Bowel Dis. 2017;23:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Kallel L, Ayadi I, Matri S, Fekih M, Mahmoud NB, Feki M, Karoui S, Zouari B, Boubaker J, Kaabachi N, Filali A. Fecal calprotectin is a predictive marker of relapse in Crohn's disease involving the colon: A prospective study. Eur J Gastroenterol Hepatol. 2010;22:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Ferreiro-Iglesias R, Barreiro-de Acosta M, Otero Santiago M, Lorenzo Gonzalez A, Alonso de la Peña C, Benitez Estevez AJ, Dominguez-Muñoz JE. Fecal Calprotectin as Predictor of Relapse in Patients With Inflammatory Bowel Disease Under Maintenance Infliximab Therapy. J Clin Gastroenterol. 2016;50:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, Danalioglu A, Novacek G, Armuzzi A, Hébuterne X, Travis S, Danese S, Reinisch W, Sandborn WJ, Rutgeerts P, Hommes D, Schreiber S, Neimark E, Huang B, Zhou Q, Mendez P, Petersson J, Wallace K, Robinson AM, Thakkar RB, D'Haens G. Effect of tight control management on Crohn's disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 668] [Article Influence: 83.5] [Reference Citation Analysis (1)] |